EP1741774A1 - Machine dishwashing compositions and their use - Google Patents
Machine dishwashing compositions and their use Download PDFInfo
- Publication number
- EP1741774A1 EP1741774A1 EP06116118A EP06116118A EP1741774A1 EP 1741774 A1 EP1741774 A1 EP 1741774A1 EP 06116118 A EP06116118 A EP 06116118A EP 06116118 A EP06116118 A EP 06116118A EP 1741774 A1 EP1741774 A1 EP 1741774A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- nonionic surfactant
- charge
- complex
- manganese
- composition according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 68
- 238000004851 dishwashing Methods 0.000 title claims abstract description 9
- 239000002736 nonionic surfactant Substances 0.000 claims abstract description 44
- 239000011572 manganese Substances 0.000 claims abstract description 25
- PWHULOQIROXLJO-UHFFFAOYSA-N Manganese Chemical compound [Mn] PWHULOQIROXLJO-UHFFFAOYSA-N 0.000 claims abstract description 21
- 239000007844 bleaching agent Substances 0.000 claims abstract description 21
- 229910052748 manganese Inorganic materials 0.000 claims abstract description 21
- 239000011148 porous material Substances 0.000 claims abstract description 20
- 150000001875 compounds Chemical class 0.000 claims abstract description 17
- 239000008187 granular material Substances 0.000 claims abstract description 16
- 238000002844 melting Methods 0.000 claims abstract description 9
- 230000008018 melting Effects 0.000 claims abstract description 9
- 125000004433 nitrogen atom Chemical group N* 0.000 claims abstract description 9
- 125000000217 alkyl group Chemical group 0.000 claims abstract description 7
- 239000003446 ligand Substances 0.000 claims abstract description 7
- 230000003647 oxidation Effects 0.000 claims abstract description 6
- 238000007254 oxidation reaction Methods 0.000 claims abstract description 6
- 125000003118 aryl group Chemical group 0.000 claims abstract description 4
- 230000001419 dependent effect Effects 0.000 claims abstract description 4
- 239000000463 material Substances 0.000 claims description 16
- -1 alkali metal percarbonate Chemical class 0.000 claims description 12
- 229910052783 alkali metal Inorganic materials 0.000 claims description 7
- 229910019142 PO4 Inorganic materials 0.000 claims description 6
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims description 4
- 239000010452 phosphate Substances 0.000 claims description 4
- 239000002243 precursor Substances 0.000 claims description 4
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical class OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 claims description 3
- 150000001298 alcohols Chemical class 0.000 claims description 3
- 229910000288 alkali metal carbonate Inorganic materials 0.000 claims description 3
- 150000008041 alkali metal carbonates Chemical class 0.000 claims description 3
- UYXFOIMFLBVYDL-UHFFFAOYSA-N 1,2,4,7-tetramethyl-1,4,7-triazonane Chemical compound CC1CN(C)CCN(C)CCN1C UYXFOIMFLBVYDL-UHFFFAOYSA-N 0.000 claims description 2
- ITWBWJFEJCHKSN-UHFFFAOYSA-N 1,4,7-triazonane Chemical compound C1CNCCNCCN1 ITWBWJFEJCHKSN-UHFFFAOYSA-N 0.000 claims description 2
- WLDGDTPNAKWAIR-UHFFFAOYSA-N 1,4,7-trimethyl-1,4,7-triazonane Chemical compound CN1CCN(C)CCN(C)CC1 WLDGDTPNAKWAIR-UHFFFAOYSA-N 0.000 claims description 2
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 claims description 2
- 239000012190 activator Substances 0.000 claims description 2
- 125000002768 hydroxyalkyl group Chemical group 0.000 claims description 2
- 125000005529 alkyleneoxy group Chemical group 0.000 claims 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 claims 1
- 102000004190 Enzymes Human genes 0.000 description 21
- 108090000790 Enzymes Proteins 0.000 description 21
- 229940088598 enzyme Drugs 0.000 description 21
- 230000003625 amylolytic effect Effects 0.000 description 13
- 229920001577 copolymer Polymers 0.000 description 9
- 229910016887 MnIV Inorganic materials 0.000 description 8
- 108091005804 Peptidases Proteins 0.000 description 8
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 8
- 239000011734 sodium Substances 0.000 description 8
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 8
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 7
- 239000003054 catalyst Substances 0.000 description 7
- 239000011976 maleic acid Substances 0.000 description 7
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical group NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 6
- 229910016884 MnIII Inorganic materials 0.000 description 6
- 102000035195 Peptidases Human genes 0.000 description 6
- WJJMNDUMQPNECX-UHFFFAOYSA-N dipicolinic acid Chemical compound OC(=O)C1=CC=CC(C(O)=O)=N1 WJJMNDUMQPNECX-UHFFFAOYSA-N 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- 229910052708 sodium Inorganic materials 0.000 description 6
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 5
- 239000006172 buffering agent Substances 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- 239000004615 ingredient Substances 0.000 description 5
- 235000021317 phosphate Nutrition 0.000 description 5
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 4
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- 239000011575 calcium Substances 0.000 description 4
- 229910052791 calcium Inorganic materials 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- MMIPFLVOWGHZQD-UHFFFAOYSA-N manganese(3+) Chemical compound [Mn+3] MMIPFLVOWGHZQD-UHFFFAOYSA-N 0.000 description 4
- 238000000034 method Methods 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 229910000029 sodium carbonate Inorganic materials 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- 229910021532 Calcite Inorganic materials 0.000 description 3
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 239000004471 Glycine Substances 0.000 description 3
- 239000004365 Protease Substances 0.000 description 3
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical group [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 3
- 229920002472 Starch Polymers 0.000 description 3
- 108010056079 Subtilisins Proteins 0.000 description 3
- 102000005158 Subtilisins Human genes 0.000 description 3
- BGRWYDHXPHLNKA-UHFFFAOYSA-N Tetraacetylethylenediamine Chemical compound CC(=O)N(C(C)=O)CCN(C(C)=O)C(C)=O BGRWYDHXPHLNKA-UHFFFAOYSA-N 0.000 description 3
- 150000001340 alkali metals Chemical class 0.000 description 3
- 229910000323 aluminium silicate Inorganic materials 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 238000004061 bleaching Methods 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 230000000052 comparative effect Effects 0.000 description 3
- 239000000945 filler Substances 0.000 description 3
- 150000004967 organic peroxy acids Chemical class 0.000 description 3
- 229940024999 proteolytic enzymes for treatment of wounds and ulcers Drugs 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 239000008107 starch Substances 0.000 description 3
- 235000019698 starch Nutrition 0.000 description 3
- 239000004094 surface-active agent Substances 0.000 description 3
- CIOXZGOUEYHNBF-UHFFFAOYSA-N (carboxymethoxy)succinic acid Chemical compound OC(=O)COC(C(O)=O)CC(O)=O CIOXZGOUEYHNBF-UHFFFAOYSA-N 0.000 description 2
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 2
- PQHYOGIRXOKOEJ-UHFFFAOYSA-N 2-(1,2-dicarboxyethylamino)butanedioic acid Chemical compound OC(=O)CC(C(O)=O)NC(C(O)=O)CC(O)=O PQHYOGIRXOKOEJ-UHFFFAOYSA-N 0.000 description 2
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 2
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 2
- CIEZZGWIJBXOTE-UHFFFAOYSA-N 2-[bis(carboxymethyl)amino]propanoic acid Chemical compound OC(=O)C(C)N(CC(O)=O)CC(O)=O CIEZZGWIJBXOTE-UHFFFAOYSA-N 0.000 description 2
- XSVSPKKXQGNHMD-UHFFFAOYSA-N 5-bromo-3-methyl-1,2-thiazole Chemical compound CC=1C=C(Br)SN=1 XSVSPKKXQGNHMD-UHFFFAOYSA-N 0.000 description 2
- 241000194108 Bacillus licheniformis Species 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical class CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 2
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 2
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 2
- KKCBUQHMOMHUOY-UHFFFAOYSA-N Na2O Inorganic materials [O-2].[Na+].[Na+] KKCBUQHMOMHUOY-UHFFFAOYSA-N 0.000 description 2
- KFSLWBXXFJQRDL-UHFFFAOYSA-N Peracetic acid Chemical compound CC(=O)OO KFSLWBXXFJQRDL-UHFFFAOYSA-N 0.000 description 2
- 229920002257 Plurafac® Polymers 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 2
- 229920002125 Sokalan® Polymers 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- 239000003513 alkali Substances 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 238000004140 cleaning Methods 0.000 description 2
- 229910052681 coesite Inorganic materials 0.000 description 2
- 229910052906 cristobalite Inorganic materials 0.000 description 2
- VTIIJXUACCWYHX-UHFFFAOYSA-L disodium;carboxylatooxy carbonate Chemical compound [Na+].[Na+].[O-]C(=O)OOC([O-])=O VTIIJXUACCWYHX-UHFFFAOYSA-L 0.000 description 2
- FJKIXWOMBXYWOQ-UHFFFAOYSA-N ethenoxyethane Chemical compound CCOC=C FJKIXWOMBXYWOQ-UHFFFAOYSA-N 0.000 description 2
- 238000005187 foaming Methods 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 2
- 229940080260 iminodisuccinate Drugs 0.000 description 2
- 238000005342 ion exchange Methods 0.000 description 2
- 229940094522 laponite Drugs 0.000 description 2
- XCOBTUNSZUJCDH-UHFFFAOYSA-B lithium magnesium sodium silicate Chemical compound [Li+].[Li+].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[OH-].[Na+].[Na+].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].O1[Si](O2)([O-])O[Si]3([O-])O[Si]1([O-])O[Si]2([O-])O3.O1[Si](O2)([O-])O[Si]3([O-])O[Si]1([O-])O[Si]2([O-])O3.O1[Si](O2)([O-])O[Si]3([O-])O[Si]1([O-])O[Si]2([O-])O3.O1[Si](O2)([O-])O[Si]3([O-])O[Si]1([O-])O[Si]2([O-])O3.O1[Si](O2)([O-])O[Si]3([O-])O[Si]1([O-])O[Si]2([O-])O3.O1[Si](O2)([O-])O[Si]3([O-])O[Si]1([O-])O[Si]2([O-])O3 XCOBTUNSZUJCDH-UHFFFAOYSA-B 0.000 description 2
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 2
- 150000002696 manganese Chemical class 0.000 description 2
- YDSWCNNOKPMOTP-UHFFFAOYSA-N mellitic acid Chemical compound OC(=O)C1=C(C(O)=O)C(C(O)=O)=C(C(O)=O)C(C(O)=O)=C1C(O)=O YDSWCNNOKPMOTP-UHFFFAOYSA-N 0.000 description 2
- MGFYIUFZLHCRTH-UHFFFAOYSA-N nitrilotriacetic acid Chemical compound OC(=O)CN(CC(O)=O)CC(O)=O MGFYIUFZLHCRTH-UHFFFAOYSA-N 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- VLTRZXGMWDSKGL-UHFFFAOYSA-M perchlorate Inorganic materials [O-]Cl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-M 0.000 description 2
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 2
- 229920000058 polyacrylate Polymers 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 230000001376 precipitating effect Effects 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 239000003352 sequestering agent Substances 0.000 description 2
- 150000004760 silicates Chemical class 0.000 description 2
- 239000000377 silicon dioxide Substances 0.000 description 2
- 229960001922 sodium perborate Drugs 0.000 description 2
- 229940045872 sodium percarbonate Drugs 0.000 description 2
- 159000000000 sodium salts Chemical class 0.000 description 2
- 235000019832 sodium triphosphate Nutrition 0.000 description 2
- YKLJGMBLPUQQOI-UHFFFAOYSA-M sodium;oxidooxy(oxo)borane Chemical compound [Na+].[O-]OB=O YKLJGMBLPUQQOI-UHFFFAOYSA-M 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 238000001694 spray drying Methods 0.000 description 2
- 230000000087 stabilizing effect Effects 0.000 description 2
- 229910052682 stishovite Inorganic materials 0.000 description 2
- 229910021653 sulphate ion Inorganic materials 0.000 description 2
- 229910052905 tridymite Inorganic materials 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 239000010457 zeolite Substances 0.000 description 2
- FXNDIJDIPNCZQJ-UHFFFAOYSA-N 2,4,4-trimethylpent-1-ene Chemical group CC(=C)CC(C)(C)C FXNDIJDIPNCZQJ-UHFFFAOYSA-N 0.000 description 1
- CFPOJWPDQWJEMO-UHFFFAOYSA-N 2-(1,2-dicarboxyethoxy)butanedioic acid Chemical compound OC(=O)CC(C(O)=O)OC(C(O)=O)CC(O)=O CFPOJWPDQWJEMO-UHFFFAOYSA-N 0.000 description 1
- QDDADYIRBDHPRY-UHFFFAOYSA-N 3-(carboxymethoxy)-3-oxopropanoic acid Chemical compound OC(=O)COC(=O)CC(O)=O QDDADYIRBDHPRY-UHFFFAOYSA-N 0.000 description 1
- XTLJJHGQACAZMS-UHFFFAOYSA-N 4-oxo-1h-pyridine-2,6-dicarboxylic acid Chemical compound OC(=O)C1=CC(=O)C=C(C(O)=O)N1 XTLJJHGQACAZMS-UHFFFAOYSA-N 0.000 description 1
- 229920002126 Acrylic acid copolymer Polymers 0.000 description 1
- 108010065511 Amylases Proteins 0.000 description 1
- 102000013142 Amylases Human genes 0.000 description 1
- 241000193830 Bacillus <bacterium> Species 0.000 description 1
- 235000014469 Bacillus subtilis Nutrition 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- DPYZUSDYVFKGKL-UHFFFAOYSA-N C=C.OOP(=O)OP(O)=O Chemical compound C=C.OOP(=O)OP(O)=O DPYZUSDYVFKGKL-UHFFFAOYSA-N 0.000 description 1
- 101100085603 Drosophila melanogaster nclb gene Proteins 0.000 description 1
- 108010083608 Durazym Proteins 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- VQTUBCCKSQIDNK-UHFFFAOYSA-N Isobutene Chemical group CC(C)=C VQTUBCCKSQIDNK-UHFFFAOYSA-N 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- BPQQTUXANYXVAA-UHFFFAOYSA-N Orthosilicate Chemical compound [O-][Si]([O-])([O-])[O-] BPQQTUXANYXVAA-UHFFFAOYSA-N 0.000 description 1
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical class OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 description 1
- 229930182556 Polyacetal Natural products 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 239000004115 Sodium Silicate Substances 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- QYKIQEUNHZKYBP-UHFFFAOYSA-N Vinyl ether Chemical class C=COC=C QYKIQEUNHZKYBP-UHFFFAOYSA-N 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 229920006243 acrylic copolymer Polymers 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 150000004973 alkali metal peroxides Chemical class 0.000 description 1
- 125000003545 alkoxy group Chemical group 0.000 description 1
- 125000002947 alkylene group Chemical group 0.000 description 1
- 235000019418 amylase Nutrition 0.000 description 1
- 229940025131 amylases Drugs 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 150000001642 boronic acid derivatives Chemical class 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 150000007942 carboxylates Chemical class 0.000 description 1
- 229910001914 chlorine tetroxide Inorganic materials 0.000 description 1
- 150000001860 citric acid derivatives Chemical class 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 239000013065 commercial product Substances 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical group OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 1
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- JHUXOSATQXGREM-UHFFFAOYSA-N dodecanediperoxoic acid Chemical compound OOC(=O)CCCCCCCCCCC(=O)OO JHUXOSATQXGREM-UHFFFAOYSA-N 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 229960001484 edetic acid Drugs 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 230000003861 general physiology Effects 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 238000005469 granulation Methods 0.000 description 1
- 230000003179 granulation Effects 0.000 description 1
- 239000003966 growth inhibitor Substances 0.000 description 1
- PMYUVOOOQDGQNW-UHFFFAOYSA-N hexasodium;trioxido(trioxidosilyloxy)silane Chemical group [Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[O-][Si]([O-])([O-])O[Si]([O-])([O-])[O-] PMYUVOOOQDGQNW-UHFFFAOYSA-N 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 239000011147 inorganic material Substances 0.000 description 1
- 150000004966 inorganic peroxy acids Chemical class 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 108010003855 mesentericopeptidase Proteins 0.000 description 1
- JZMJDSHXVKJFKW-UHFFFAOYSA-M methyl sulfate(1-) Chemical compound COS([O-])(=O)=O JZMJDSHXVKJFKW-UHFFFAOYSA-M 0.000 description 1
- XJRBAMWJDBPFIM-UHFFFAOYSA-N methyl vinyl ether Chemical group COC=C XJRBAMWJDBPFIM-UHFFFAOYSA-N 0.000 description 1
- 108010020132 microbial serine proteinases Proteins 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 229910052901 montmorillonite Inorganic materials 0.000 description 1
- 150000001451 organic peroxides Chemical class 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- VLTRZXGMWDSKGL-UHFFFAOYSA-N perchloric acid Chemical compound OCl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-N 0.000 description 1
- 239000002304 perfume Substances 0.000 description 1
- 150000004965 peroxy acids Chemical class 0.000 description 1
- 125000005342 perphosphate group Chemical group 0.000 description 1
- JRKICGRDRMAZLK-UHFFFAOYSA-L persulfate group Chemical group S(=O)(=O)([O-])OOS(=O)(=O)[O-] JRKICGRDRMAZLK-UHFFFAOYSA-L 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 239000004584 polyacrylic acid Substances 0.000 description 1
- 229920005646 polycarboxylate Polymers 0.000 description 1
- 229920001444 polymaleic acid Polymers 0.000 description 1
- 229920006324 polyoxymethylene Polymers 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920005996 polystyrene-poly(ethylene-butylene)-polystyrene Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- USHAGKDGDHPEEY-UHFFFAOYSA-L potassium persulfate Chemical compound [K+].[K+].[O-]S(=O)(=O)OOS([O-])(=O)=O USHAGKDGDHPEEY-UHFFFAOYSA-L 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 239000011814 protection agent Substances 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 229940048086 sodium pyrophosphate Drugs 0.000 description 1
- 235000019351 sodium silicates Nutrition 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- MWNQXXOSWHCCOZ-UHFFFAOYSA-L sodium;oxido carbonate Chemical compound [Na+].[O-]OC([O-])=O MWNQXXOSWHCCOZ-UHFFFAOYSA-L 0.000 description 1
- 239000002689 soil Substances 0.000 description 1
- 108010075550 termamyl Proteins 0.000 description 1
- 150000004685 tetrahydrates Chemical class 0.000 description 1
- RYCLIXPGLDDLTM-UHFFFAOYSA-J tetrapotassium;phosphonato phosphate Chemical compound [K+].[K+].[K+].[K+].[O-]P([O-])(=O)OP([O-])([O-])=O RYCLIXPGLDDLTM-UHFFFAOYSA-J 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- ITMCEJHCFYSIIV-UHFFFAOYSA-M triflate Chemical compound [O-]S(=O)(=O)C(F)(F)F ITMCEJHCFYSIIV-UHFFFAOYSA-M 0.000 description 1
- UNXRWKVEANCORM-UHFFFAOYSA-I triphosphate(5-) Chemical compound [O-]P([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O UNXRWKVEANCORM-UHFFFAOYSA-I 0.000 description 1
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- OHOTVSOGTVKXEL-UHFFFAOYSA-K trisodium;2-[bis(carboxylatomethyl)amino]propanoate Chemical compound [Na+].[Na+].[Na+].[O-]C(=O)C(C)N(CC([O-])=O)CC([O-])=O OHOTVSOGTVKXEL-UHFFFAOYSA-K 0.000 description 1
- AZJYLVAUMGUUBL-UHFFFAOYSA-A u1qj22mc8e Chemical compound [F-].[F-].[F-].[F-].[F-].[F-].[F-].[F-].[F-].[F-].[F-].[F-].[F-].[F-].[F-].[F-].[F-].[F-].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].[Mg+2].O=[Si]=O.O=[Si]=O.O=[Si]=O.O1[Si](O2)([O-])O[Si]3([O-])O[Si]1([O-])O[Si]2([O-])O3.O1[Si](O2)([O-])O[Si]3([O-])O[Si]1([O-])O[Si]2([O-])O3.O1[Si](O2)([O-])O[Si]3([O-])O[Si]1([O-])O[Si]2([O-])O3.O1[Si](O2)([O-])O[Si]3([O-])O[Si]1([O-])O[Si]2([O-])O3.O1[Si](O2)([O-])O[Si]3([O-])O[Si]1([O-])O[Si]2([O-])O3.O1[Si](O2)([O-])O[Si]3([O-])O[Si]1([O-])O[Si]2([O-])O3 AZJYLVAUMGUUBL-UHFFFAOYSA-A 0.000 description 1
- AQLJVWUFPCUVLO-UHFFFAOYSA-N urea hydrogen peroxide Chemical compound OO.NC(N)=O AQLJVWUFPCUVLO-UHFFFAOYSA-N 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 150000003751 zinc Chemical class 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/39—Organic or inorganic per-compounds
- C11D3/3902—Organic or inorganic per-compounds combined with specific additives
- C11D3/3905—Bleach activators or bleach catalysts
- C11D3/3932—Inorganic compounds or complexes
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/66—Non-ionic compounds
- C11D1/72—Ethers of polyoxyalkylene glycols
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/66—Non-ionic compounds
Definitions
- This invention relates to detergent cleaning compositions especially adapted for use in automatic dishwashing machines.
- phosphate or phosphate-free compositions which contain an oxygen bleach and enzymes, especially amylolytic and proteolytic enzymes, such as amylases and proteases.
- the oxygen bleach is typically sodium perborate or sodium percarbonate which advantageously may be used in conjunction with an organic activator or bleach precursor, e.g. N, N, N', N'-tetraacetylethylene diamine (TAED), which upon dissolution will react to form an organic peroxyacid, e.g. peracetic acid, as the bleaching species.
- an organic activator or bleach precursor e.g. N, N, N', N'-tetraacetylethylene diamine (TAED)
- the starch removal properties of an automatic machine dishwashing composition containing a peroxygen bleach can be improved by incorporation of a dinuclear manganese complex.
- products of this kind ofter also contain nonionic surfactants. When the amount of nonionic surfactant is relatively high, particularly at or above 1% by weight of the composition, the manganese complex causes discolouration of the product.
- a first aspect of the present invention now provides an automatic machine dishwashing composition
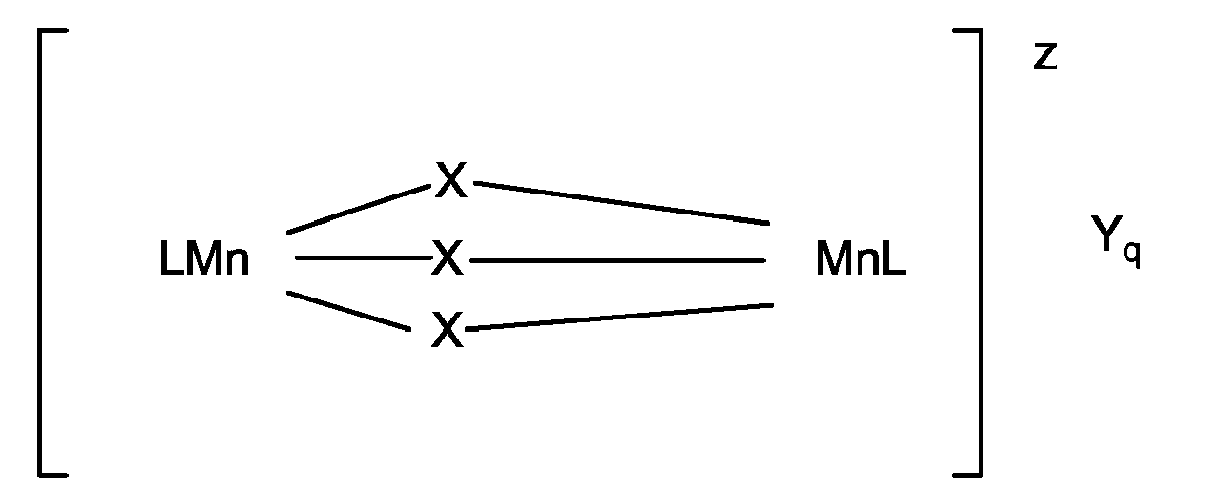
- a peroxygen bleach compound and a dinuclear manganese-complex having the general formula: wherein Mn is manganese which can individually be in the III or IV oxidation state; each x represents a coordinating or bridging species selected from the group consisting of H 2 O, O 2 2- , O 2- , OH - , HO 2 - , SH - , S 2- , >SO, Cl - , N 3- , SCN - , RCOO - , NH 2 - and NR 3 , with R being H, alkyl or aryl, (optionally substituted); L is a ligand which is an organic molecule containing a number of nitrogen atoms which coordinates via all or some of its nitrogen atoms to the manganese centres; z denotes the charge of the complex and is an integer which can
- a second aspect of the present invention provides use of one or more nonionic surfactant components (a) and (b) as defined in the first aspect of the present invention for reducing discolouration in a composition comprising a peroxygen bleach compound and a dinuclear manganese complex as defined in the first aspect of the present invention.
- the machine dishwashing composition is a mildly alkaline product having a solution pH below 12, e.g. from 8-12, preferably from 9-11.
- the solution pH as meant here is the pH as determined from a solution of 3g/l of the compositions in distilled water.
- Preferred manganese-complexes are those wherein x is either CH 3 COO - or O 2 or mixtures thereof, most preferably wherein the manganese is in the IV oxidation state and x is O 2- .
- Preferred ligands are those which coordinate via three nitrogen atoms to one of the manganese centres, preferably being of a macrocyclic nature. Particularly preferred ligands are:
- the type of counter-ion Y for charge neutrality is not critical for the activity of the complex and can be selected from, for example, any of the following counter-ions: chloride; sulphate; nitrate; methylsulphate; surfanctant anions, such as the long-chain alkylsulphates, alkylsulphonates, alkylbenzenesulphonates, tosylate, trifluoromethylsulphonate, perchlorate (ClO 4 - ), BPh 4 - , and PF 6 - ' though some counter-ions are more preferred than others for reasons of product property and safety.
- the preferred manganese complexes useable in the present invention are:
- compositions of the invention must contain at least 1%, preferably at least 2% by weight of nonionic surfactant. At least 50%, preferably at least 65%, optionally up to 90% or up t 100% by weight of the total nonionc surfactant must be selected from
- nonionic surfactants of group (a) are present in the composition of the invention.
- Preferred nonionic surfactants of group (a) include hydroxyalkyl glycolether surfactants having a melting point above 35°C, preferably above 40°C.
- a preferred class of such materials has from 6 to 20, preferably from 8 to 14 carbon atoms in the alkyl chain thereof and from 15 to 50, preferably from 20 to 40 glycol ether units.
- One such material is Dehypon 3697 GRAM, ex Cognis.
- nonionic surfactants of this group are high melting point polyalkoxylated alcohols, optionally with the -OH group endcapped such as with an alkyl group and typically having an average of 10 to 20 alkyl groups and more than 30 alkylene oxide groups, eg ethylene oxide and/or proplylene oxide groups.
- the nonionic surfactants used in group (b) can be any one or more nonionic surfactants such as mentioned anywhere in this specification.
- the granules with number average pore size less than 5 ⁇ m may be granules of suitable inorganic materials such as an alkali used carbonate, bicarbonate, sulphate or any mixtures thereof.
- the inorganic granules themselves may be formed by spray drying or non-spray drying granulation, optionally with use of a small amount of a granulating aid such as a polymer or silicate in aqueous solution.
- a granulating aid such as a polymer or silicate in aqueous solution.
- the process conditions to achieve the required pore size are well known to those skilled in the art.
- the resultant particles can then be sprayed with an aqueous solution of the relevant nonionic(s) and dried, for example in a fluid bed drier.
- one or more other nonionics may be present in the amounts indicated by the foregoing.
- These may includes any alkoxylated nonionic surface-active agent wherein the alkoxy moiety is selected from the group consisting of ethylene oxide, propylene oxide and mixtures thereof, is preferably used to improve the detergency and to suppress excessive foaming due to some protein soil.
- nonionic surfactants for use in the invention are the low- to non-foaming ethoxylated strraightchain alcohols of the Plurafac® RA and LF series, supplied by the BASF, including Plurafac SLF 18B45; of the Lutensol® LF series, supplied by the BASF; of the Tritons DF series, supplied by the Rohm & Hass Company, and of the Synperonic® LF and NCA series, supplied by the ICI, Uniqema company.
- the peroxygen compound bleaches which can be utilized in the present invention include hydrogen peroxide, hydrogen peroxide-liberating compounds, hydrogen peroxide-generating compounds, as well as the organic and inorganic peroxyacids and watersoluble salts thereof, and mixtures thereof.
- the total amount of peroxygen bleach compound(s) is preferably from 5% to 25%, more preferably from 10% to 20% by weight of the composition.
- Hydrogen peroxide sources are well known in the art. They include the alkali metal peroxides, organic peroxide bleaching compounds such as urea peroxide, and inorganic persalt bleaching compounds, such as the alkali metal perborates, percarbonates, perphosphates and persulphates. Mixtures or two or more of such compounds may also be suitable. Particularly preferred is sodium percarbonate. However, sodium perborate, eg in the form sodium perborate monohydrate may also be used. Sodium perborate monohydrate is preferred to tetrahydrate because of its better storage stability while also dissolving very quickly in aqueous solutions. These bleaching agents may be utilied alone or in conjunction with a peroxyacid bleach precursor, such as TAED or any other bleach precursors known in the art, so long as it does not affect the starch-removing properties of the catalyst.
- a peroxyacid bleach precursor such as TAED or any other bleach precursors known in the art
- organic peroxyacids usable in this invention are those compounds known in the art having normally one or more peroxycarboxyl groups: in their molecular structure, e.g. 1,12 - diperoxydodecanedioic acid (DPDA) and phthaloylamido peroxycaproic acid (PAP).
- An inorganic peroxyacid salt usable herein is, for example, potassium monopersulphate.
- Compositions according to the present invention may also normally contain a detergency and water-softening builder.
- Builder materials may be selected from 1) calcium sequestrant materials, 2) precipitating materials, 3) calcium ion-exchange materials and 4) mixtures thereof.
- Examples of calcium sequestrant builder materials include alkali metal polyphospates, such as sodium tripoly phosphate;nitrilotriacetic acid, dipicolinic acid, chelidamic acid and their water-solubel salts; the alkali metal salts of ether polycarboxylates, such as carboxymethyloxy succinic acid, oxydisuccinic acid, mellitic acid; ethylene diamine tetraacetic acid; benzene polycarboxylic acids; citric acid; and polyacetal carboxylates as disclosed in US Patents 4,144,226 and 4,146,495 .
- alkali metal polyphospates such as sodium tripoly phosphate;nitrilotriacetic acid, dipicolinic acid, chelidamic acid and their water-solubel salts
- the alkali metal salts of ether polycarboxylates such as carboxymethyloxy succinic acid, oxydisuccinic acid, mellitic acid
- precipitating builder materials examples include sodium orthophosphate, sodium carbonate and sodium carbonate/calcite.
- Examples of calcium ion-exchange builder materials include the various types of water-insoluble crystalline or amorphous aluminosilicates, of which zeolites are the best-known representatives.
- Other useful materials are, for example, layered silicates, such as SKS®-6 ex Hoechst.
- compositions of the invention may contain any one of the organic or inorganic builder materials, such as sodium or potassium tripolyphosphate, sodium or potassium pyrophosphate, sodium or potassium orthophosphate, sodium carbonate or sodium carbonate/calcite mixtures, the sodium salt of nitrilotriacetic acid, sodium citrate, carboxymethyl malonate, carboxymethyloxy succinate and the water-insoluble crystalline or amorphous aluminosilicate builder materials, or mixtures thereof, although phosphates are less preferred.
- the organic or inorganic builder materials such as sodium or potassium tripolyphosphate, sodium or potassium pyrophosphate, sodium or potassium orthophosphate, sodium carbonate or sodium carbonate/calcite mixtures, the sodium salt of nitrilotriacetic acid, sodium citrate, carboxymethyl malonate, carboxymethyloxy succinate and the water-insoluble crystalline or amorphous aluminosilicate builder materials, or mixtures thereof, although phosphates are less preferred.
- compositions are, however, essentially free of phosphates and will contain, for example, alkali metal citrate, alkali carbonate, alkali metal carbonate/calcite, aluminosilicates (zeolites) or mixtures thereof as preferred builder materials.
- alkali metal is sodium or potassium, preferably sodium.
- the citrates are especially preferred.
- Suitable non-phosphate builders which may be used alone or in combination with any one or more other builders include methyl glycine diacetic acid (MGDA), eg sold as Trilon M, ex BASF, imino disuccinate (IDS) eg sold as Baypure CS, ex Lanxess and dipicolinic acid (DPA), eg available from Raschig.
- MGDA methyl glycine diacetic acid
- IDS imino disuccinate
- DPA dipicolinic acid
- alkali metal salts eg sodium salts
- inorganic detergency builders may have a dual role, eg the carbonates may also act as buffering agents (see below).
- the total amount of detergency builder for example comprising any one or more of any of the foregoing may for example be from 10% to 80%, preferably from 30% to 60% by weight of the total composition.
- An optional but highly desirable additive ingredient with multi-functional characteristics, particularly in non-phosphate compositions is from 1% to 10%, preferably about 5% by weight of a polymeric material having a molecular weight of from 1,000 to 2,000,000 and which can be a homo- or co-polymer of acrylic acid, maleic acid, or salt or anhydride thereof, vinyl pyrrolidone methyl- or ethyl-, vinyl ethers and other polymerizable vinyl monomers.
- polyacrylic acid or polyacrylate are polyacrylic acid or polyacrylate; polymaleic acid/acrylic acid copolymer; 70:30 acrylic acid/hydroxyethyl maleate copolymer; 1:1 styrene/maleic acid copolymer; isobutylene/maleic acid and diisobutylene/maleic acid copolymers; methyl- and ethyl-vinylether/maleic acid copolymers;ethylene/maleic acid copolymer; polyvinyl pyrrolidone; and vinyl pyrrolidone/maleic acid copolymer. These polymers are believed to function as co-builders, although under certain conditions they may also function as main builders.
- Buffering agents may be used in compositions according to the present invention in order to adjust and to maintain the alkalinity and pH at the desired level. These are for example, the alkali metal carbonates and bicarbonates, sometimes also borates, and silicates. If used, sodium silicates preferably have Na 2 O:SiO 2 ratios of from about 2:1 to 1:4. Another possible buffering agent is sodium disilicate having Na 2 O:SiO 2 ratio of about 1:1.8 to 1:2.5.
- the cleaning compositions in which the catalyst is used according to the invention may, as desired, contain an amylolytic enzyme, though conceivably a much smaller amount will now be sufficient.
- amylolytic enzymes for use in the present invention can be those derived from bacteria or fungi.
- Preferred amylolytic enzymes are those prepared and described in British Patent Specification No. 1 296 839 , cultivated from the strains of Bacillus licheniformis NClB 8061, NCIB 8059, ATCC 6334, ATCC 6598, ATCC 11 945, ATCC 8480 and ATCC 9945.
- Examples of such amylolytic enzumes are amylolytic enzymes produced and distributed under the trade name of Sp-95® or Termamyl® by Novo Industri A/S, Copenhagen, Denmark, as well as Termanyl and Duramyl, ex Novozymes and Properase and Purastar ex Genecor.
- amylolytic enzymes are generally presented as granules and may have enzyme activities of from about 2 to 10 Maltose units/milligram. Enzyme granules containing only minor proportions, e.g. less than 30%, particularly not more than 10% by weight of chloride to substantially nil, are preferably used in the compositions of the invention.
- amylolytic activity can be determined by the method as described by P.Bernfeld in "Method of Enzymology” Volume 1 (1955), page 149 .
- composition in which the catalyst is used according to the invention preferably also contains a proteolytic enzyme.
- subtilisins which are obtained from particular strains of B. subtilis and B.licheniformis, such as the commercially available subtilisins Maxatase® supplied by Gist-Brocades N.V., Delft, Holland, and Alcalase®, supplied by Novo Industri A/S, Copenhagen, Denmark.
- protease obtained from a strainof Bacillus having maximum activity throughout the pH range of 8-12, being commercially available from Novo Industri A/S under the registered trade names of Esperase® and Savinase®. The preparation of these and analogous enzymes is described in British Patent No. 1 243 784 .
- Another suitable protease useful herein is a fairly recent commercial product sold by Novo Industry A/S under the trade name "Durazym®" as described in WO-A-89/06279 .
- These enzymes are generally presented as granules, e.g. marumes, prills, T-granulates etc., and may have enzyme activities of from about 500 to 1700 glycine units/milligram.
- Enzyme granules containing only minor proportions, e.g. less than 30%, particularly not more than 10% by weight of chloride to substantially nil, are preferably used in the composition of the invention.
- these enzymes can each be present in a weight percentage amounts of from 0.2 to 5% by weight, such that, for amylolytic enzymes, the final composition will have amylolytic activity of from 10 2 to 10 6 Maltose units/kg, and, for proteolytic enzymes, the final composition will have proteolytic enzyme activity of from about 10 6 to 10 9 Glycine Units/kg.
- compositions according to the present invention may further contain any of the following additional ingredients.
- Stabilizing and anti-scaling agents include those belonging to the class of phosphonates sold under the trade name "Dequest®", such as ethylene diamine tetra-(methylene phosphonate), deithylene triamine penta-(methylene phosphonate) and ethylene hydroxyl diphosphonate.
- Dequest® such as ethylene diamine tetra-(methylene phosphonate), deithylene triamine penta-(methylene phosphonate) and ethylene hydroxyl diphosphonate.
- Another suitable class of anti-scaling agents are the low molecular weight polyacryalates, polymaleates and mixtures thereof or the copolymers thereof, having molecular weights of up to about 6000.
- a further suitable class of anti-scaling agents are polypeptides.
- Benzotriazide may be used as a silver protection agent.
- Zinc salts may be used as glass corrosion inhibitors and sulphonated polymers (eg Alcosperse 240) may be used as anti-scaling polymers.
- Clays such as hectorites and montmorillonites, may be included in the composition of the invention. These assist in reduction of spot formation on glassware, and may be present at from 0.5 to 10% by weight, preferably from 0.5 to 7% by weight. Particularly preferred is the addition of Laponite® clay at about 0.5 to 5% b weight, which is a synthetic hectorite. "Dequest” and “Laponite” are Trade Marks owned by, respectively, Monsanto and Laporte Industries.
- a filler may be required to complete the composition, though, in compacted powdered compositions it should preferably be avoided.
- a preferred filler is sodium sulphate.
- a typical composition according to the present invention may include from 0 to 80%, preferably from 5 to 60% by weight of a detergency and water-softening builder, from 0 to 80%, preferably 5 to 75% by weight of a buffering agent, from 1 to 40%, preferably from 2 to 20% by weight of a peroxygen compound bleach, and optionally an enzyme and fillers and may further comprise a dinuclear manganese complex as defined above, in an amount corresponding to an Mn-content of from 0.0001 to abut 1.0% by weight, preferably from 0.0005 to 0.5% by weight.
- compositions of the present invention may be in any suitable physical form, for example powdered and/or granular compositions or tablets. Tablets may be unitary or comprise two or more layers having differing compositions from each other, the tablet as a whole having the total constituents making-up the composition according to the present invention. Some or all of the dinuclear manganese complex may be in the same or in a different layer from a layer containing some or all of the nonionic surfactant, eg some or all of the nonionic surfactant component(s) (a) - (c) and/or any other non-ionic surfactant. Cavity tablets, eg with a spherical or ball component set into the cavity are also preferred. Again the manganese complex may in this way optionally be at least partially segregated from some or all of any or all of the nonionic surfactant component(s), whether components (a) - (c) or any other nonionic surfactant.
- Example 1 Two tablets were made, respectively designated "Comparative Example A” and “Example 1", the latter being in accordance with the present invention.
- the respective components were incorporated in powdered or granular form and dry-mixed before being compacted to form tablets.
Abstract
wherein at least 50% by weight of the nonionic surfactant is selected from:
(a) at least one nonionic surfactant having a melting point greater than 35°C, preferably greater than 40°C;
(b) at least one nonionic surfactant in the form of a granule having pores with a number average pore size less than 5µm, the pores containing the nonionic surfactant;
and mixtures thereof.
Description
- This invention relates to detergent cleaning compositions especially adapted for use in automatic dishwashing machines.
- Automatic machine dishwashing products are nowadays, normally reduced phosphate or phosphate-free compositions which contain an oxygen bleach and enzymes, especially amylolytic and proteolytic enzymes, such as amylases and proteases. The oxygen bleach is typically sodium perborate or sodium percarbonate which advantageously may be used in conjunction with an organic activator or bleach precursor, e.g. N, N, N', N'-tetraacetylethylene diamine (TAED), which upon dissolution will react to form an organic peroxyacid, e.g. peracetic acid, as the bleaching species.
- As is known from
EP-A-0 530 870 , the starch removal properties of an automatic machine dishwashing composition containing a peroxygen bleach can be improved by incorporation of a dinuclear manganese complex. However, products of this kind ofter also contain nonionic surfactants. When the amount of nonionic surfactant is relatively high, particularly at or above 1% by weight of the composition, the manganese complex causes discolouration of the product. - We have now found that this problem can be overcome by special selection of the type of nonionic surfactant.
- A first aspect of the present invention now provides an automatic machine dishwashing composition comprising at least 1%, preferably at least 2% by weight of nonionic surfactant, a peroxygen bleach compound and a dinuclear manganese-complex having the general formula:
wherein at least 50% by weight of the nonionic surfactant is selected from: - (a) at least one nonionic surfactant having a melting point greater than 35°C, preferably greater than 40°C;
- (b) at least one nonionic surfactant in the form of a granule having pores with a number average pore size less than 5µm, the pores containing the nonionic surfactant;
- A second aspect of the present invention provides use of one or more nonionic surfactant components (a) and (b) as defined in the first aspect of the present invention for reducing discolouration in a composition comprising a peroxygen bleach compound and a dinuclear manganese complex as defined in the first aspect of the present invention.
- Preferably, the machine dishwashing composition is a mildly alkaline product having a solution pH below 12, e.g. from 8-12, preferably from 9-11.
- The solution pH as meant here is the pH as determined from a solution of 3g/l of the compositions in distilled water.
- Preferred manganese-complexes are those wherein x is either CH3COO- or O2 or mixtures thereof, most preferably wherein the manganese is in the IV oxidation state and x is O2-. Preferred ligands are those which coordinate via three nitrogen atoms to one of the manganese centres, preferably being of a macrocyclic nature. Particularly preferred ligands are:
- (1) 1,4,7-trimethyl-1,4,7-triazacyclononane, (Me-TACN); and
- (2) 1,2,4,7-tetramethyl-1,4,7-triazacyclononane, (Me-Me TACN).
- The type of counter-ion Y for charge neutrality is not critical for the activity of the complex and can be selected from, for example, any of the following counter-ions: chloride; sulphate; nitrate; methylsulphate; surfanctant anions, such as the long-chain alkylsulphates, alkylsulphonates, alkylbenzenesulphonates, tosylate, trifluoromethylsulphonate, perchlorate (ClO4 -), BPh4 -, and PF6 -' though some counter-ions are more preferred than others for reasons of product property and safety.
- Consequently, the preferred manganese complexes useable in the present invention are:
- (I) [(Me-TACN)MnIV(µ-0)3MnIV(Me-TACN)]2+(PF6 -)2
- (II) [(Me-MeTACN)MnIV(µ-0)3MnIV(Me-MeTACN)]2+(PF6 -)2
- (III) [(Me-TACN)MnIII(µ-0)(µ-OAc)2MnIII(Me-TACN)]2+(PF6 -)2
- (IV) [(Me-MeTACN)MnIII(µ-0)(µ-OAc)2MnIII(Me-MeTACN)]2+(PF6 -)2
- (I) [MnIV 2(µ-0)3(Me-TACN)2] (PF6)2
- (II) [MnIV 2(µ-0)3(Me-MeTACN)2] (PF6)2
- (III) [MnIII 2(µ-0) (µ-OAc)2(Me-TACN)2] (PF6)2
- (IV) [MnIII 2(µ-0) (µ-OAc)2(Me-TACN) 2](PF6)2
-
-
- It is of note that the manganese complexes are also disclosed in
EP-A-0458397 andEP-A-0458398 as unusually effective bleach and oxidation catalysts. In the further description of this invention they will also be simply referred to as the "catalyst". - These complexes are effective additives for starch removal in mechanical dishwashing compositions, even in the absence of amylolytic enzymes. Whereas amylolytic enzymes are not normally compatible with strong oxidizing and bleaching agents, a bleach system comprising a peroxide compound and the manganese complex bleach catalyst does not seem to attack amylolytic enzymes, so that both systems can be used together to provide a still further improvement of starch removal.
- The compositions of the invention must contain at least 1%, preferably at least 2% by weight of nonionic surfactant. At least 50%, preferably at least 65%, optionally up to 90% or up t 100% by weight of the total nonionc surfactant must be selected from
- (a) at least one nonionic surfactant having a melting point greater than 35°C, preferably greater than 40°C;
- (b) at least one nonionic surfactant in the form of a granule having pores with a number average pore size less than 5µm, the pores containing the nonionic surfactant;
- Preferably, nonionic surfactants of group (a) are present in the composition of the invention. Preferred nonionic surfactants of group (a) include hydroxyalkyl glycolether surfactants having a melting point above 35°C, preferably above 40°C. A preferred class of such materials has from 6 to 20, preferably from 8 to 14 carbon atoms in the alkyl chain thereof and from 15 to 50, preferably from 20 to 40 glycol ether units. One such material is Dehypon 3697 GRAM, ex Cognis. Other suitable nonionic surfactants of this group are high melting point polyalkoxylated alcohols, optionally with the -OH group endcapped such as with an alkyl group and typically having an average of 10 to 20 alkyl groups and more than 30 alkylene oxide groups, eg ethylene oxide and/or proplylene oxide groups.
- The nonionic surfactants used in group (b) can be any one or more nonionic surfactants such as mentioned anywhere in this specification.
- The granules with number average pore size less than 5µm may be granules of suitable inorganic materials such as an alkali used carbonate, bicarbonate, sulphate or any mixtures thereof. Preferably, the inorganic granules themselves may be formed by spray drying or non-spray drying granulation, optionally with use of a small amount of a granulating aid such as a polymer or silicate in aqueous solution. The process conditions to achieve the required pore size are well known to those skilled in the art. The resultant particles can then be sprayed with an aqueous solution of the relevant nonionic(s) and dried, for example in a fluid bed drier.
- Optionally, one or more other nonionics may be present in the amounts indicated by the foregoing. These may includes any alkoxylated nonionic surface-active agent wherein the alkoxy moiety is selected from the group consisting of ethylene oxide, propylene oxide and mixtures thereof, is preferably used to improve the detergency and to suppress excessive foaming due to some protein soil.
- Examples of suitable nonionic surfactants for use in the invention are the low- to non-foaming ethoxylated strraightchain alcohols of the Plurafac® RA and LF series, supplied by the BASF, including Plurafac SLF 18B45; of the Lutensol® LF series, supplied by the BASF; of the Tritons DF series, supplied by the Rohm & Hass Company, and of the Synperonic® LF and NCA series, supplied by the ICI, Uniqema company.
- The peroxygen compound bleaches which can be utilized in the present invention include hydrogen peroxide, hydrogen peroxide-liberating compounds, hydrogen peroxide-generating compounds, as well as the organic and inorganic peroxyacids and watersoluble salts thereof, and mixtures thereof.
- The total amount of peroxygen bleach compound(s) is preferably from 5% to 25%, more preferably from 10% to 20% by weight of the composition.
- Hydrogen peroxide sources are well known in the art. They include the alkali metal peroxides, organic peroxide bleaching compounds such as urea peroxide, and inorganic persalt bleaching compounds, such as the alkali metal perborates, percarbonates, perphosphates and persulphates. Mixtures or two or more of such compounds may also be suitable. Particularly preferred is sodium percarbonate. However, sodium perborate, eg in the form sodium perborate monohydrate may also be used. Sodium perborate monohydrate is preferred to tetrahydrate because of its better storage stability while also dissolving very quickly in aqueous solutions. These bleaching agents may be utilied alone or in conjunction with a peroxyacid bleach precursor, such as TAED or any other bleach precursors known in the art, so long as it does not affect the starch-removing properties of the catalyst.
- The organic peroxyacids usable in this invention are those compounds known in the art having normally one or more peroxycarboxyl groups:
- Compositions according to the present invention may also normally contain a detergency and water-softening builder. Builder materials may be selected from 1) calcium sequestrant materials, 2) precipitating materials, 3) calcium ion-exchange materials and 4) mixtures thereof.
- Examples of calcium sequestrant builder materials include alkali metal polyphospates, such as sodium tripoly phosphate;nitrilotriacetic acid, dipicolinic acid, chelidamic acid and their water-solubel salts; the alkali metal salts of ether polycarboxylates, such as carboxymethyloxy succinic acid, oxydisuccinic acid, mellitic acid; ethylene diamine tetraacetic acid; benzene polycarboxylic acids; citric acid; and polyacetal carboxylates as disclosed in
US Patents 4,144,226 and4,146,495 . - Examples of precipitating builder materials include sodium orthophosphate, sodium carbonate and sodium carbonate/calcite.
- Examples of calcium ion-exchange builder materials include the various types of water-insoluble crystalline or amorphous aluminosilicates, of which zeolites are the best-known representatives. Other useful materials are, for example, layered silicates, such as SKS®-6 ex Hoechst.
- The compositions of the invention may contain any one of the organic or inorganic builder materials, such as sodium or potassium tripolyphosphate, sodium or potassium pyrophosphate, sodium or potassium orthophosphate, sodium carbonate or sodium carbonate/calcite mixtures, the sodium salt of nitrilotriacetic acid, sodium citrate, carboxymethyl malonate, carboxymethyloxy succinate and the water-insoluble crystalline or amorphous aluminosilicate builder materials, or mixtures thereof, although phosphates are less preferred.
- Preferred compositions are, however, essentially free of phosphates and will contain, for example, alkali metal citrate, alkali carbonate, alkali metal carbonate/calcite, aluminosilicates (zeolites) or mixtures thereof as preferred builder materials. In all of these, the alkali metal is sodium or potassium, preferably sodium. The citrates are especially preferred.
- Other suitable non-phosphate builders which may be used alone or in combination with any one or more other builders include methyl glycine diacetic acid (MGDA), eg sold as Trilon M, ex BASF, imino disuccinate (IDS) eg sold as Baypure CS, ex Lanxess and dipicolinic acid (DPA), eg available from Raschig. The alkali metal salts (eg sodium salts) of these are also useable.
- It should be noted that some of the foregoing inorganic detergency builders may have a dual role, eg the carbonates may also act as buffering agents (see below).
- The total amount of detergency builder, for example comprising any one or more of any of the foregoing may for example be from 10% to 80%, preferably from 30% to 60% by weight of the total composition.
- An optional but highly desirable additive ingredient with multi-functional characteristics, particularly in non-phosphate compositions is from 1% to 10%, preferably about 5% by weight of a polymeric material having a molecular weight of from 1,000 to 2,000,000 and which can be a homo- or co-polymer of acrylic acid, maleic acid, or salt or anhydride thereof, vinyl pyrrolidone methyl- or ethyl-, vinyl ethers and other polymerizable vinyl monomers. Preferred examples of such polymeric materials are polyacrylic acid or polyacrylate; polymaleic acid/acrylic acid copolymer; 70:30 acrylic acid/hydroxyethyl maleate copolymer; 1:1 styrene/maleic acid copolymer; isobutylene/maleic acid and diisobutylene/maleic acid copolymers; methyl- and ethyl-vinylether/maleic acid copolymers;ethylene/maleic acid copolymer; polyvinyl pyrrolidone; and vinyl pyrrolidone/maleic acid copolymer. These polymers are believed to function as co-builders, although under certain conditions they may also function as main builders.
- Buffering agents may be used in compositions according to the present invention in order to adjust and to maintain the alkalinity and pH at the desired level. These are for example, the alkali metal carbonates and bicarbonates, sometimes also borates, and silicates. If used, sodium silicates preferably have Na2O:SiO2 ratios of from about 2:1 to 1:4. Another possible buffering agent is sodium disilicate having Na2O:SiO2 ratio of about 1:1.8 to 1:2.5.
- Though not essential, the cleaning compositions in which the catalyst is used according to the invention may, as desired, contain an amylolytic enzyme, though conceivably a much smaller amount will now be sufficient.
- Reduction of the level of once an essential ingredient to even the possibility of omitting such an expensive enzyme ingredient, thereby resulting in improved performance, is one of the major advantages of the present invention, not only in terms technical benefit but also in terms of economy.
- The amylolytic enzymes for use in the present invention can be those derived from bacteria or fungi. Preferred amylolytic enzymes are those prepared and described in
British Patent Specification No. 1 296 839 - The amylolytic activity can be determined by the method as described by P.Bernfeld in "Method of Enzymology" Volume 1 (1955), page 149.
- The composition in which the catalyst is used according to the invention preferably also contains a proteolytic enzyme.
- Examples of suitable proteolytic enzymes are the subtilisins which are obtained from particular strains of B. subtilis and B.licheniformis, such as the commercially available subtilisins Maxatase® supplied by Gist-Brocades N.V., Delft, Holland, and Alcalase®, supplied by Novo Industri A/S, Copenhagen, Denmark.
- Particularly suitable is a protease obtained from a strainof Bacillus having maximum activity throughout the pH range of 8-12, being commercially available from Novo Industri A/S under the registered trade names of Esperase® and Savinase®. The preparation of these and analogous enzymes is described in
British Patent No. 1 243 784 WO-A-89/06279 - Enzyme granules containing only minor proportions, e.g. less than 30%, particularly not more than 10% by weight of chloride to substantially nil, are preferably used in the composition of the invention.
- If used, these enzymes can each be present in a weight percentage amounts of from 0.2 to 5% by weight, such that, for amylolytic enzymes, the final composition will have amylolytic activity of from 102 to 106 Maltose units/kg, and, for proteolytic enzymes, the final composition will have proteolytic enzyme activity of from about 106 to 109 Glycine Units/kg.
- The compositions according to the present invention may further contain any of the following additional ingredients. Stabilizing and anti-scaling agents, crystal-growth inhibitors and threshold agents. Examples of suitable stabilizing and anti-scaling compounds are those belonging to the class of phosphonates sold under the trade name "Dequest®", such as ethylene diamine tetra-(methylene phosphonate), deithylene triamine penta-(methylene phosphonate) and ethylene hydroxyl diphosphonate. Another suitable class of anti-scaling agents are the low molecular weight polyacryalates, polymaleates and mixtures thereof or the copolymers thereof, having molecular weights of up to about 6000. A further suitable class of anti-scaling agents are polypeptides.
- Benzotriazide (BTA) may be used as a silver protection agent. Zinc salts may be used as glass corrosion inhibitors and sulphonated polymers (eg Alcosperse 240) may be used as anti-scaling polymers.
- Clays such as hectorites and montmorillonites, may be included in the composition of the invention. These assist in reduction of spot formation on glassware, and may be present at from 0.5 to 10% by weight, preferably from 0.5 to 7% by weight. Particularly preferred is the addition of Laponite® clay at about 0.5 to 5% b weight, which is a synthetic hectorite. "Dequest" and "Laponite" are Trade Marks owned by, respectively, Monsanto and Laporte Industries.
- Finally, the addition of a filler may be required to complete the composition, though, in compacted powdered compositions it should preferably be avoided. A preferred filler is sodium sulphate.
- A typical composition according to the present invention may include from 0 to 80%, preferably from 5 to 60% by weight of a detergency and water-softening builder, from 0 to 80%, preferably 5 to 75% by weight of a buffering agent, from 1 to 40%, preferably from 2 to 20% by weight of a peroxygen compound bleach, and optionally an enzyme and fillers and may further comprise a dinuclear manganese complex as defined above, in an amount corresponding to an Mn-content of from 0.0001 to abut 1.0% by weight, preferably from 0.0005 to 0.5% by weight.
- The invention will now be further illustrated by the following Examples.
- Compositions of the present invention may be in any suitable physical form, for example powdered and/or granular compositions or tablets. Tablets may be unitary or comprise two or more layers having differing compositions from each other, the tablet as a whole having the total constituents making-up the composition according to the present invention. Some or all of the dinuclear manganese complex may be in the same or in a different layer from a layer containing some or all of the nonionic surfactant, eg some or all of the nonionic surfactant component(s) (a) - (c) and/or any other non-ionic surfactant. Cavity tablets, eg with a spherical or ball component set into the cavity are also preferred. Again the manganese complex may in this way optionally be at least partially segregated from some or all of any or all of the nonionic surfactant component(s), whether components (a) - (c) or any other nonionic surfactant.
- Two tablets were made, respectively designated "Comparative Example A" and "Example 1", the latter being in accordance with the present invention. In each case, the respective components were incorporated in powdered or granular form and dry-mixed before being compacted to form tablets.
Table 1 Comparative example A Example 1 Tri Na Citrate 31.51 31.51 Na Carbonate 20.13 20.13 Na Sulphate 7.00 7.00 Na-silicate 9.96 9.96 Sodium acrylic/maleic copolymer 3.90 3.90 Polyacrylate granule 1.17 1.17 Styrene/Maleic anhydride copolymer 0.23 0.23 EP/PO nonionic* 4.00 1.26 Hydroxy Alkyl Polyglycolether nonionic** 2.74 Na Percarbonate 13.66 13.66 Manganese catalyst cogranule+ 1.22 1.22 Enzyme blend 2.68 2.68 Perfume 0.14 0.14 Dye solution 0.42 0.42 PEG 3.11 3.11 Glycerol 0.86 0.86 100.00 100.00 Total * m.pt. < 20°C
** m.pt. approx 60°C
+ complex of formula (I):(PF6)2 counter ion - After storage at 20°C and at 65% RH, for 2 weeks in a polypropylene bag, the tablets of Comparative Example A showed noticeable formation of brown spots whereas those of Example 1 showed substantially no discolouration.
Claims (11)
- An automatic machine dishwashing composition comprising at least 1%, preferably at least 2% by weight of nonionic surfactant, a peroxygen bleach compound and a dinuclear manganese-complex having the general formula:
wherein at least 50% by weight of the nonionic surfactant is selected from:(a) at least one nonionic surfactant having a melting point greater than 35°C, preferably greater than 40°C;(b) at least one nonionic surfactant in the form of a granule having pores with a number average pore size less than 5µm, the pores containing the nonionic surfactant;and mixtures thereof. - A composition according to claim 1, wherein at least 50% by weight of the non-ionic surfactant is at least one nonionic surfactant having a melting point greater than 35°C, preferably greater than 40°C.
- A composition according to claim 2, wherein the non-ionic surfactant having a melting point greater than 35°C is selected from one or more hydroxyalkyl polyglycolethers and optionally endcapped polyalkoxylated alcohols having at least 30 alkyleneoxy groups and mixtures thereof.
- A composition according to any preceding claim, comprising a nonionic surfactant in the form of a granule having pores with a number average pore size less than 5µm, the pores containing the nonionic surfactant; and mixtures thereof, wherein the granule is formed of one or more alkali metal carbonates, bicarbonates, sulphates and mixtures thereof.
- A composition according to any preceding claim, wherein the peroxygen bleach compound comprises an alkali metal percarbonate.
- A composition according to any preceding claim further comprising a bleach precursor or activator.
- A composition according to any preceding claim, the composition being substantially free of phosphate material.
- A composition according to any preceding claim, further comprising a citrate detergency builder.
- A composition according to any preceding claim, wherein the dinuclear manganese complex comprises a ligand selected from(1) 1,4,7-trimethyl-1,4,7-triazacyclononane, (Me-TACN); and(2) 1,2,4,7-tetramethyl-1,4,7-triazacyclononane, (Me-Me TACN).and mixtures thereof.
- A tablet comprising composition according to any preceding claim.
- Use of one or more nonionic surfactant components (a) and (b) :(a) at least one nonionic surfactant having a melting point greater than 35°C, preferably greater than 40°C;(b) at least one nonionic surfactant in the form of a granule having pores with a number average pore size less than 5µm, the pores containing the nonionic surfactant;and mixtures thereof;
for reducing discolouration in a composition comprising a peroxygen bleach compound and a dinuclear manganese complex having the general formula:
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP06116118A EP1741774B1 (en) | 2005-07-08 | 2006-06-27 | Machine dishwashing compositions and their use |
| PL06116118T PL1741774T3 (en) | 2005-07-08 | 2006-06-27 | Machine dishwashing compositions and their use |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP05076571 | 2005-07-08 | ||
| EP06116118A EP1741774B1 (en) | 2005-07-08 | 2006-06-27 | Machine dishwashing compositions and their use |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1741774A1 true EP1741774A1 (en) | 2007-01-10 |
| EP1741774B1 EP1741774B1 (en) | 2008-08-06 |
Family
ID=35453526
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP06116118A Active EP1741774B1 (en) | 2005-07-08 | 2006-06-27 | Machine dishwashing compositions and their use |
Country Status (6)
| Country | Link |
|---|---|
| EP (1) | EP1741774B1 (en) |
| AT (1) | ATE403711T1 (en) |
| DE (1) | DE602006002075D1 (en) |
| ES (1) | ES2312089T3 (en) |
| PL (1) | PL1741774T3 (en) |
| PT (1) | PT1741774E (en) |
Cited By (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008048537A1 (en) * | 2006-10-16 | 2008-04-24 | Danisco Us, Inc. Genencor Division | Non-phosphate dish detergents |
| EP1967577A1 (en) * | 2007-02-13 | 2008-09-10 | CHT R. BEITLICH GmbH | Catalysed peroxide bleach (catylasator bleach) |
| WO2010010003A2 (en) * | 2008-07-23 | 2010-01-28 | Cht R. Beitlich Gmbh | Catalysed peroxide bleach ("catalyst-bleach variant 3: all-in-one) |
| WO2010116139A1 (en) | 2009-04-09 | 2010-10-14 | Reckitt Benckiser N.V. | Detergent composition |
| WO2011027170A2 (en) | 2009-09-07 | 2011-03-10 | Reckitt Benckiser N.V. | Detergent composition |
| WO2011032868A1 (en) * | 2009-09-21 | 2011-03-24 | Henkel Ag & Co. Kgaa | Dishwasher detergent |
| WO2011042737A1 (en) | 2009-10-09 | 2011-04-14 | Reckitt Benckiser N.V. | Detergent composition |
| WO2011110849A1 (en) | 2010-03-09 | 2011-09-15 | Reckitt Benckiser N.V. | Detergent composition |
| WO2012066344A1 (en) | 2010-11-19 | 2012-05-24 | Reckitt Benckiser N.V. | Dyed coated bleach materials |
| WO2012066341A2 (en) | 2010-11-19 | 2012-05-24 | Reckitt Benckiser N.V. | Coated bleach materials |
| WO2012085534A1 (en) | 2010-12-21 | 2012-06-28 | Reckitt Benckiser N.V. | Bleach catalyst particle |
| WO2012123719A1 (en) | 2011-03-14 | 2012-09-20 | Reckitt Benckiser N.V. | Detergent composition with improved drying performance |
| WO2014060738A1 (en) * | 2012-10-15 | 2014-04-24 | Reckitt & Colman (Overseas) Limited | Ultrasonic method of cleaning |
| WO2015124384A1 (en) | 2014-02-20 | 2015-08-27 | Unilever N.V. | Machine dishwash composition |
| CN105164241A (en) * | 2013-05-02 | 2015-12-16 | 艺康美国股份有限公司 | Concentrated detergent composition for the improved removal of starch in warewashing applications |
| US9249380B2 (en) | 2009-08-07 | 2016-02-02 | Robert McBride Ltd. | Dosage form detergent products |
| US9434915B2 (en) | 2008-11-11 | 2016-09-06 | Danisco Us Inc. | Compositions and methods comprising a subtilisin variant |
| WO2019233696A1 (en) | 2018-06-04 | 2019-12-12 | Reckitt Benckiser Finish B.V. | Composition |
| WO2020043844A1 (en) | 2018-08-31 | 2020-03-05 | Reckitt Benckiser Finish B.V. | Automatic dishwashing product |
| WO2020053132A1 (en) | 2018-09-14 | 2020-03-19 | Reckitt Benckiser Finish B.V. | Granulate for detergent composition |
| WO2020104320A1 (en) | 2018-11-19 | 2020-05-28 | Reckitt Benckiser Finish B.V. | Composition |
| WO2020152044A1 (en) | 2019-01-22 | 2020-07-30 | Reckitt Benckiser Finish B.V. | Method of forming an automatic dishwashing pouch, vacuum forming system and pouch |
| WO2020182656A2 (en) | 2019-03-11 | 2020-09-17 | Reckitt Benckiser Finish B.V. | Product |
| GB202107968D0 (en) | 2021-06-03 | 2021-07-21 | Reckitt Benckiser Finish Bv | Detergent gel composition comprising a fatty alcohol ethoxlate |
| WO2021155135A1 (en) * | 2020-01-31 | 2021-08-05 | Ecolab Usa Inc. | Amylase synergy with oxygen bleach in warewash application |
| WO2021213807A1 (en) | 2020-04-23 | 2021-10-28 | Reckitt Benckiser Finish B.V. | Automatic dishwashing composition |
| WO2022002671A1 (en) | 2020-07-01 | 2022-01-06 | Reckitt Benckiser Finish B.V. | Method for making a gel or a gel-like detergent |
| WO2022002672A1 (en) | 2020-07-01 | 2022-01-06 | Reckitt Benckiser Finish B.V. | Use of a composition as anti-corrosion agent |
| US11266289B2 (en) | 2014-08-05 | 2022-03-08 | Reckitt Benckiser (Brands) Limited | Automatic washing machine and method |
| WO2022189536A1 (en) | 2021-03-12 | 2022-09-15 | Reckitt Benckiser Finish B.V. | Automatic dishwashing composition |
| WO2023156427A1 (en) | 2022-02-15 | 2023-08-24 | Reckitt Benckiser Finish B.V. | Dishwashing detergent composition |
| WO2024002908A1 (en) | 2022-06-29 | 2024-01-04 | Reckitt Benckiser Finish B.V. | A dishwashing detergent composition, a water-soluble container, and an autodosing automatic dishwashing system |
| WO2024002848A1 (en) | 2022-06-29 | 2024-01-04 | Reckitt Benckiser Finish B.V. | Dishwashing, preferably an automatic dishwashing, detergent composition |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0141470A2 (en) * | 1983-11-08 | 1985-05-15 | Unilever N.V. | Manganese adjuncts, their preparation and use |
| WO1994021775A1 (en) * | 1993-03-18 | 1994-09-29 | Unilever Plc | Detergent compositions |
| WO1995006711A1 (en) * | 1993-09-03 | 1995-03-09 | Unilever Plc | Bleach catalyst composition |
| US5622646A (en) * | 1994-04-07 | 1997-04-22 | The Procter & Gamble Company | Bleach compositions comprising metal-containing bleach catalysts and antioxidants |
-
2006
- 2006-06-27 DE DE602006002075T patent/DE602006002075D1/en active Active
- 2006-06-27 AT AT06116118T patent/ATE403711T1/en active
- 2006-06-27 PT PT06116118T patent/PT1741774E/en unknown
- 2006-06-27 EP EP06116118A patent/EP1741774B1/en active Active
- 2006-06-27 PL PL06116118T patent/PL1741774T3/en unknown
- 2006-06-27 ES ES06116118T patent/ES2312089T3/en active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0141470A2 (en) * | 1983-11-08 | 1985-05-15 | Unilever N.V. | Manganese adjuncts, their preparation and use |
| WO1994021775A1 (en) * | 1993-03-18 | 1994-09-29 | Unilever Plc | Detergent compositions |
| WO1995006711A1 (en) * | 1993-09-03 | 1995-03-09 | Unilever Plc | Bleach catalyst composition |
| US5622646A (en) * | 1994-04-07 | 1997-04-22 | The Procter & Gamble Company | Bleach compositions comprising metal-containing bleach catalysts and antioxidants |
Cited By (47)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008048537A1 (en) * | 2006-10-16 | 2008-04-24 | Danisco Us, Inc. Genencor Division | Non-phosphate dish detergents |
| EP1967577A1 (en) * | 2007-02-13 | 2008-09-10 | CHT R. BEITLICH GmbH | Catalysed peroxide bleach (catylasator bleach) |
| WO2010010003A2 (en) * | 2008-07-23 | 2010-01-28 | Cht R. Beitlich Gmbh | Catalysed peroxide bleach ("catalyst-bleach variant 3: all-in-one) |
| WO2010010003A3 (en) * | 2008-07-23 | 2010-07-29 | Cht R. Beitlich Gmbh | Catalysed peroxide bleach ("catalyst-bleach variant 3: all-in-one) |
| US9434915B2 (en) | 2008-11-11 | 2016-09-06 | Danisco Us Inc. | Compositions and methods comprising a subtilisin variant |
| WO2010116139A1 (en) | 2009-04-09 | 2010-10-14 | Reckitt Benckiser N.V. | Detergent composition |
| US9249380B2 (en) | 2009-08-07 | 2016-02-02 | Robert McBride Ltd. | Dosage form detergent products |
| WO2011027170A2 (en) | 2009-09-07 | 2011-03-10 | Reckitt Benckiser N.V. | Detergent composition |
| WO2011032868A1 (en) * | 2009-09-21 | 2011-03-24 | Henkel Ag & Co. Kgaa | Dishwasher detergent |
| EP3255132A1 (en) | 2009-10-09 | 2017-12-13 | Reckitt Benckiser Finish B.V. | Detergent composition |
| WO2011042737A1 (en) | 2009-10-09 | 2011-04-14 | Reckitt Benckiser N.V. | Detergent composition |
| WO2011110849A1 (en) | 2010-03-09 | 2011-09-15 | Reckitt Benckiser N.V. | Detergent composition |
| WO2011110850A1 (en) | 2010-03-09 | 2011-09-15 | Reckitt Benckiser N.V | Compression process for producing a bleach containing product |
| WO2012066341A2 (en) | 2010-11-19 | 2012-05-24 | Reckitt Benckiser N.V. | Coated bleach materials |
| WO2012066344A1 (en) | 2010-11-19 | 2012-05-24 | Reckitt Benckiser N.V. | Dyed coated bleach materials |
| WO2012085534A1 (en) | 2010-12-21 | 2012-06-28 | Reckitt Benckiser N.V. | Bleach catalyst particle |
| US9617500B2 (en) | 2011-03-14 | 2017-04-11 | Reckitt Benckiser Finish B.V. | Detergent composition with improved drying performance |
| US9157050B2 (en) | 2011-03-14 | 2015-10-13 | Reckitt Benckiser N.V. | Detergent composition with improved drying performance |
| WO2012123719A1 (en) | 2011-03-14 | 2012-09-20 | Reckitt Benckiser N.V. | Detergent composition with improved drying performance |
| WO2014060738A1 (en) * | 2012-10-15 | 2014-04-24 | Reckitt & Colman (Overseas) Limited | Ultrasonic method of cleaning |
| CN109181903A (en) * | 2013-05-02 | 2019-01-11 | 艺康美国股份有限公司 | Concentrated cleaning compositions for the improved starch removal in dishwashing detergent is applied |
| JP2016518496A (en) * | 2013-05-02 | 2016-06-23 | エコラボ ユーエスエー インコーポレイティド | Concentrated detergent composition for improved removal of starch in article cleaning applications |
| US10669510B2 (en) | 2013-05-02 | 2020-06-02 | Ecolab Usa Inc. | Concentrated detergent composition for the improved removal of starch in warewashing applications |
| CN105164241A (en) * | 2013-05-02 | 2015-12-16 | 艺康美国股份有限公司 | Concentrated detergent composition for the improved removal of starch in warewashing applications |
| US9969958B2 (en) | 2013-05-02 | 2018-05-15 | Ecolab Usa Inc. | Concentrated detergent composition for the improved removal of starch in warewashing applications |
| US10253278B2 (en) | 2013-05-02 | 2019-04-09 | Ecolab Usa Inc. | Concentrated detergent composition for the improved removal of starch in warewashing applications |
| EP3107987B1 (en) | 2014-02-20 | 2018-10-03 | Unilever N.V. | Machine dishwash composition |
| JP2017507209A (en) * | 2014-02-20 | 2017-03-16 | ユニリーバー・ナームローゼ・ベンノートシヤープ | Machine dishwashing composition |
| WO2015124384A1 (en) | 2014-02-20 | 2015-08-27 | Unilever N.V. | Machine dishwash composition |
| US11266289B2 (en) | 2014-08-05 | 2022-03-08 | Reckitt Benckiser (Brands) Limited | Automatic washing machine and method |
| WO2019233696A1 (en) | 2018-06-04 | 2019-12-12 | Reckitt Benckiser Finish B.V. | Composition |
| WO2020043844A1 (en) | 2018-08-31 | 2020-03-05 | Reckitt Benckiser Finish B.V. | Automatic dishwashing product |
| WO2020053132A1 (en) | 2018-09-14 | 2020-03-19 | Reckitt Benckiser Finish B.V. | Granulate for detergent composition |
| WO2020104320A1 (en) | 2018-11-19 | 2020-05-28 | Reckitt Benckiser Finish B.V. | Composition |
| WO2020152044A1 (en) | 2019-01-22 | 2020-07-30 | Reckitt Benckiser Finish B.V. | Method of forming an automatic dishwashing pouch, vacuum forming system and pouch |
| WO2020182656A2 (en) | 2019-03-11 | 2020-09-17 | Reckitt Benckiser Finish B.V. | Product |
| WO2021155135A1 (en) * | 2020-01-31 | 2021-08-05 | Ecolab Usa Inc. | Amylase synergy with oxygen bleach in warewash application |
| WO2021213807A1 (en) | 2020-04-23 | 2021-10-28 | Reckitt Benckiser Finish B.V. | Automatic dishwashing composition |
| WO2022002671A1 (en) | 2020-07-01 | 2022-01-06 | Reckitt Benckiser Finish B.V. | Method for making a gel or a gel-like detergent |
| WO2022002672A1 (en) | 2020-07-01 | 2022-01-06 | Reckitt Benckiser Finish B.V. | Use of a composition as anti-corrosion agent |
| WO2022189536A1 (en) | 2021-03-12 | 2022-09-15 | Reckitt Benckiser Finish B.V. | Automatic dishwashing composition |
| GB202107968D0 (en) | 2021-06-03 | 2021-07-21 | Reckitt Benckiser Finish Bv | Detergent gel composition comprising a fatty alcohol ethoxlate |
| WO2022253728A1 (en) | 2021-06-03 | 2022-12-08 | Reckitt Benckiser Finish B.V. | Detergent gel composition comprising a fatty alcohol ethoxylate |
| GB2607585A (en) | 2021-06-03 | 2022-12-14 | Reckitt Benckiser Finish Bv | Detergent gel composition comprising a fatty alcohol ethoxylate |
| WO2023156427A1 (en) | 2022-02-15 | 2023-08-24 | Reckitt Benckiser Finish B.V. | Dishwashing detergent composition |
| WO2024002908A1 (en) | 2022-06-29 | 2024-01-04 | Reckitt Benckiser Finish B.V. | A dishwashing detergent composition, a water-soluble container, and an autodosing automatic dishwashing system |
| WO2024002848A1 (en) | 2022-06-29 | 2024-01-04 | Reckitt Benckiser Finish B.V. | Dishwashing, preferably an automatic dishwashing, detergent composition |
Also Published As
| Publication number | Publication date |
|---|---|
| PL1741774T3 (en) | 2009-01-30 |
| ATE403711T1 (en) | 2008-08-15 |
| EP1741774B1 (en) | 2008-08-06 |
| DE602006002075D1 (en) | 2008-09-18 |
| PT1741774E (en) | 2008-11-17 |
| ES2312089T3 (en) | 2009-02-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1741774B1 (en) | Machine dishwashing compositions and their use | |
| EP0530870B1 (en) | Machine dishwashing composition | |
| EP2794836B1 (en) | Detergent composition comprising glutamic-n,n-diacetate, water and bleaching agent | |
| US8173587B2 (en) | Detergent composition | |
| AU632713B2 (en) | Detergent compositions | |
| EP0533239B1 (en) | Aqueous liquid cleaning compositions | |
| JPH05112799A (en) | Concentrated detergent powder composition | |
| US5719112A (en) | Dishwashing composition | |
| WO1989004863A1 (en) | Machine dishwashing compositions | |
| EP3013931B1 (en) | Composition comprising glutamic-n,n-diacetate (glda), water and enzyme | |
| US5527483A (en) | Nonaqueous gelled automatic dishwashing composition containing enzymes | |
| JP2611071B2 (en) | Detergent composition | |
| EP0395333A2 (en) | Detergent compositions | |
| JPH0713238B2 (en) | Detergent composition for dishwasher | |
| JP2002541303A (en) | Bleach-containing detergent | |
| AU2011225893A1 (en) | Detergent composition | |
| EP0313144A2 (en) | Non-phosphorus detergent bleach compositions | |
| WO1994019445A1 (en) | Machine dishwashing composition | |
| EP1556471B1 (en) | Tablet of compacted particulate cleaning composition | |
| EP0266904A2 (en) | Machine dish washing composition containing dipicolinic acid | |
| EP0772671B1 (en) | Method for preparing co-granules, co-granules thus obtained and use thereof as a component in detergent compositions | |
| CA2299831A1 (en) | Dishwasher detergent shaped bodies with specific geometry | |
| EP0319053A2 (en) | Improved phosphate-free detergent bleach compositions | |
| EP0987320A2 (en) | Detergent compositions | |
| EP0319054A2 (en) | Aluminosilicate built detergent bleach compositions |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL BA HR MK YU |
|
| 17P | Request for examination filed |
Effective date: 20061214 |
|
| 17Q | First examination report despatched |
Effective date: 20070222 |
|
| AKX | Designation fees paid |
Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI SK TR |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REF | Corresponds to: |
Ref document number: 602006002075 Country of ref document: DE Date of ref document: 20080918 Kind code of ref document: P |
|
| REG | Reference to a national code |
Ref country code: RO Ref legal event code: EPE |
|
| REG | Reference to a national code |
Ref country code: PT Ref legal event code: SC4A Free format text: AVAILABILITY OF NATIONAL TRANSLATION Effective date: 20081104 |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: TRGR |
|
| REG | Reference to a national code |
Ref country code: GR Ref legal event code: EP Ref document number: 20080403011 Country of ref document: GR |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080806 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20081206 |
|
| REG | Reference to a national code |
Ref country code: PL Ref legal event code: T3 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2312089 Country of ref document: ES Kind code of ref document: T3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080806 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080806 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080806 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20081106 |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080806 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080806 |
|
| PLAX | Notice of opposition and request to file observation + time limit sent |
Free format text: ORIGINAL CODE: EPIDOSNOBS2 |
|
| 26 | Opposition filed |
Opponent name: HENKEL AG & CO. KGAA Effective date: 20090504 |
|
| 26 | Opposition filed |
Opponent name: THE PROCTER & GAMBLE COMMPANY Effective date: 20090506 Opponent name: RECKITT BENCKISER PLC I Effective date: 20090505 Opponent name: HENKEL AG & CO. KGAA Effective date: 20090504 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080806 |
|
| NLR1 | Nl: opposition has been filed with the epo |
Opponent name: THE PROCTER & GAMBLE COMMPANY Opponent name: RECKITT BENCKISER PLC INTELLECTUAL PROPERTY DEPART Opponent name: HENKEL AG & CO. KGAA |
|
| PLAF | Information modified related to communication of a notice of opposition and request to file observations + time limit |
Free format text: ORIGINAL CODE: EPIDOSCOBS2 |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| PLAF | Information modified related to communication of a notice of opposition and request to file observations + time limit |
Free format text: ORIGINAL CODE: EPIDOSCOBS2 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090630 |
|
| PLAK | Information related to reply of patent proprietor to notice(s) of opposition modified |
Free format text: ORIGINAL CODE: EPIDOSCOBS3 |
|
| PLBB | Reply of patent proprietor to notice(s) of opposition received |
Free format text: ORIGINAL CODE: EPIDOSNOBS3 |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| R26 | Opposition filed (corrected) |
Opponent name: THE PROCTER & GAMBLE COMMPANY Effective date: 20090506 Opponent name: RECKITT BENCKISER PLC I Effective date: 20090505 Opponent name: HENKEL AG & CO. KGAA Effective date: 20090504 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| RDAF | Communication despatched that patent is revoked |
Free format text: ORIGINAL CODE: EPIDOSNREV1 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090630 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090630 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20100630 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090627 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20100630 |
|
| APBM | Appeal reference recorded |
Free format text: ORIGINAL CODE: EPIDOSNREFNO |
|
| APBP | Date of receipt of notice of appeal recorded |
Free format text: ORIGINAL CODE: EPIDOSNNOA2O |
|
| APAH | Appeal reference modified |
Free format text: ORIGINAL CODE: EPIDOSCREFNO |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20090207 |
|
| APBQ | Date of receipt of statement of grounds of appeal recorded |
Free format text: ORIGINAL CODE: EPIDOSNNOA3O |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080806 |
|
| APBU | Appeal procedure closed |
Free format text: ORIGINAL CODE: EPIDOSNNOA9O |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| R26 | Opposition filed (corrected) |
Opponent name: THE PROCTER & GAMBLE COMMPANY Effective date: 20090506 Opponent name: HENKEL AG & CO. KGAA Effective date: 20090504 Opponent name: RECKITT BENCKISER PLC INTELLECTUAL PROPERTY DEPART Effective date: 20090505 |
|
| PLCK | Communication despatched that opposition was rejected |
Free format text: ORIGINAL CODE: EPIDOSNREJ1 |
|
| PLBN | Opposition rejected |
Free format text: ORIGINAL CODE: 0009273 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: OPPOSITION REJECTED |
|
| 27O | Opposition rejected |
Effective date: 20130108 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R100 Ref document number: 602006002075 Country of ref document: DE Effective date: 20130108 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 11 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 12 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GR Payment date: 20200611 Year of fee payment: 15 Ref country code: RO Payment date: 20200622 Year of fee payment: 15 Ref country code: IE Payment date: 20200624 Year of fee payment: 15 Ref country code: FI Payment date: 20200622 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20200625 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 20200619 Year of fee payment: 15 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: PD Owner name: UNILEVER IP HOLDINGS B.V.; NL Free format text: DETAILS ASSIGNMENT: CHANGE OF OWNER(S), ASSIGNMENT; FORMER OWNER NAME: UNILEVER N.V. Effective date: 20210413 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: PD Owner name: UNILEVER IP HOLDINGS B.V.; NL Free format text: DETAILS ASSIGNMENT: CHANGE OF OWNER(S), ASSIGNMENT; FORMER OWNER NAME: UNILEVER N.V. Effective date: 20210607 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R081 Ref document number: 602006002075 Country of ref document: DE Owner name: UNILEVER GLOBAL IP LIMITED, WIRRAL, GB Free format text: FORMER OWNER: UNILEVER N.V., ROTTERDAM, NL |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: PC2A Owner name: UNILEVER IP HOLDINGS B.V. Effective date: 20211117 |
|
| REG | Reference to a national code |
Ref country code: FI Ref legal event code: MAE |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: EUG |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RO Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210627 Ref country code: FI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210627 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MM01 Ref document number: 403711 Country of ref document: AT Kind code of ref document: T Effective date: 20210627 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E Free format text: REGISTERED BETWEEN 20220203 AND 20220209 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210627 Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210627 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210628 Ref country code: GR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220105 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PT Payment date: 20220617 Year of fee payment: 17 Ref country code: GB Payment date: 20220622 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: TR Payment date: 20220624 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20230620 Year of fee payment: 18 Ref country code: FR Payment date: 20230622 Year of fee payment: 18 Ref country code: DE Payment date: 20220914 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PL Payment date: 20230615 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20230619 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20230621 Year of fee payment: 18 Ref country code: ES Payment date: 20230828 Year of fee payment: 18 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20231227 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20230627 |