EP1997471B2 - Apparatus for the automatic preparation of a drug and respective method of preparation - Google Patents
Apparatus for the automatic preparation of a drug and respective method of preparation Download PDFInfo
- Publication number
- EP1997471B2 EP1997471B2 EP08157120.0A EP08157120A EP1997471B2 EP 1997471 B2 EP1997471 B2 EP 1997471B2 EP 08157120 A EP08157120 A EP 08157120A EP 1997471 B2 EP1997471 B2 EP 1997471B2
- Authority
- EP
- European Patent Office
- Prior art keywords
- container
- drug
- intake
- control unit
- components
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000003814 drug Substances 0.000 title claims abstract description 77
- 229940079593 drug Drugs 0.000 title claims abstract description 77
- 238000002360 preparation method Methods 0.000 title claims abstract description 38
- 238000000034 method Methods 0.000 title claims description 15
- 238000002347 injection Methods 0.000 claims abstract description 25
- 239000007924 injection Substances 0.000 claims abstract description 25
- 238000012546 transfer Methods 0.000 claims abstract description 7
- 230000004913 activation Effects 0.000 claims abstract description 4
- 230000005540 biological transmission Effects 0.000 claims description 3
- 230000007246 mechanism Effects 0.000 claims description 3
- 238000004891 communication Methods 0.000 claims description 2
- 239000000203 mixture Substances 0.000 claims description 2
- 239000000243 solution Substances 0.000 description 25
- 239000003085 diluting agent Substances 0.000 description 12
- 239000000126 substance Substances 0.000 description 4
- 230000008901 benefit Effects 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- 239000002699 waste material Substances 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 238000002512 chemotherapy Methods 0.000 description 2
- 229940044683 chemotherapy drug Drugs 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 239000007921 spray Substances 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- 230000002588 toxic effect Effects 0.000 description 2
- 238000007792 addition Methods 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 238000012865 aseptic processing Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 230000002354 daily effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 230000003203 everyday effect Effects 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000012212 insulator Substances 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 230000007257 malfunction Effects 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 230000009347 mechanical transmission Effects 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 230000002906 microbiologic effect Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 239000002547 new drug Substances 0.000 description 1
- 230000000474 nursing effect Effects 0.000 description 1
- 230000000771 oncological effect Effects 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01F—MIXING, e.g. DISSOLVING, EMULSIFYING OR DISPERSING
- B01F35/00—Accessories for mixers; Auxiliary operations or auxiliary devices; Parts or details of general application
- B01F35/71—Feed mechanisms
- B01F35/712—Feed mechanisms for feeding fluids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61J—CONTAINERS SPECIALLY ADAPTED FOR MEDICAL OR PHARMACEUTICAL PURPOSES; DEVICES OR METHODS SPECIALLY ADAPTED FOR BRINGING PHARMACEUTICAL PRODUCTS INTO PARTICULAR PHYSICAL OR ADMINISTERING FORMS; DEVICES FOR ADMINISTERING FOOD OR MEDICINES ORALLY; BABY COMFORTERS; DEVICES FOR RECEIVING SPITTLE
- A61J1/00—Containers specially adapted for medical or pharmaceutical purposes
- A61J1/14—Details; Accessories therefor
- A61J1/20—Arrangements for transferring or mixing fluids, e.g. from vial to syringe
- A61J1/2096—Combination of a vial and a syringe for transferring or mixing their contents
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01F—MIXING, e.g. DISSOLVING, EMULSIFYING OR DISPERSING
- B01F33/00—Other mixers; Mixing plants; Combinations of mixers
- B01F33/50—Movable or transportable mixing devices or plants
- B01F33/501—Movable mixing devices, i.e. readily shifted or displaced from one place to another, e.g. portable during use
- B01F33/5011—Movable mixing devices, i.e. readily shifted or displaced from one place to another, e.g. portable during use portable during use, e.g. hand-held
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01F—MIXING, e.g. DISSOLVING, EMULSIFYING OR DISPERSING
- B01F33/00—Other mixers; Mixing plants; Combinations of mixers
- B01F33/50—Movable or transportable mixing devices or plants
- B01F33/501—Movable mixing devices, i.e. readily shifted or displaced from one place to another, e.g. portable during use
- B01F33/5011—Movable mixing devices, i.e. readily shifted or displaced from one place to another, e.g. portable during use portable during use, e.g. hand-held
- B01F33/50112—Movable mixing devices, i.e. readily shifted or displaced from one place to another, e.g. portable during use portable during use, e.g. hand-held of the syringe or cartridge type
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01F—MIXING, e.g. DISSOLVING, EMULSIFYING OR DISPERSING
- B01F33/00—Other mixers; Mixing plants; Combinations of mixers
- B01F33/80—Mixing plants; Combinations of mixers
- B01F33/84—Mixing plants with mixing receptacles receiving material dispensed from several component receptacles, e.g. paint tins
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01F—MIXING, e.g. DISSOLVING, EMULSIFYING OR DISPERSING
- B01F35/00—Accessories for mixers; Auxiliary operations or auxiliary devices; Parts or details of general application
- B01F35/71—Feed mechanisms
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01F—MIXING, e.g. DISSOLVING, EMULSIFYING OR DISPERSING
- B01F35/00—Accessories for mixers; Auxiliary operations or auxiliary devices; Parts or details of general application
- B01F35/71—Feed mechanisms
- B01F35/713—Feed mechanisms comprising breaking packages or parts thereof, e.g. piercing or opening sealing elements between compartments or cartridges
- B01F35/7131—Breaking or perforating packages, containers or vials
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01F—MIXING, e.g. DISSOLVING, EMULSIFYING OR DISPERSING
- B01F35/00—Accessories for mixers; Auxiliary operations or auxiliary devices; Parts or details of general application
- B01F35/71—Feed mechanisms
- B01F35/714—Feed mechanisms for feeding predetermined amounts
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01F—MIXING, e.g. DISSOLVING, EMULSIFYING OR DISPERSING
- B01F35/00—Accessories for mixers; Auxiliary operations or auxiliary devices; Parts or details of general application
- B01F35/71—Feed mechanisms
- B01F35/717—Feed mechanisms characterised by the means for feeding the components to the mixer
- B01F35/7174—Feed mechanisms characterised by the means for feeding the components to the mixer using pistons, plungers or syringes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01F—MIXING, e.g. DISSOLVING, EMULSIFYING OR DISPERSING
- B01F35/00—Accessories for mixers; Auxiliary operations or auxiliary devices; Parts or details of general application
- B01F35/75—Discharge mechanisms
- B01F35/754—Discharge mechanisms characterised by the means for discharging the components from the mixer
- B01F35/75425—Discharge mechanisms characterised by the means for discharging the components from the mixer using pistons or plungers
- B01F35/754251—Discharge mechanisms characterised by the means for discharging the components from the mixer using pistons or plungers reciprocating in the mixing receptacle
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61J—CONTAINERS SPECIALLY ADAPTED FOR MEDICAL OR PHARMACEUTICAL PURPOSES; DEVICES OR METHODS SPECIALLY ADAPTED FOR BRINGING PHARMACEUTICAL PRODUCTS INTO PARTICULAR PHYSICAL OR ADMINISTERING FORMS; DEVICES FOR ADMINISTERING FOOD OR MEDICINES ORALLY; BABY COMFORTERS; DEVICES FOR RECEIVING SPITTLE
- A61J3/00—Devices or methods specially adapted for bringing pharmaceutical products into particular physical or administering forms
- A61J3/002—Compounding apparatus specially for enteral or parenteral nutritive solutions
Definitions
- the present invention concerns an apparatus for the automatic preparation of a drug and the relative method of preparation.
- the present invention is used for the preparation and dosage of liquid homogeneous solutions or dispersions of chemical products, of synthetic or natural origin, in order to prepare solutions for clinical use in the human and veterinary field or for industrial use.
- a first difficulty is connected to the fact that the prescription of a drug to a patient in chemotherapy occurs after his clinical state has been evaluated and the prescribed doses must be confirmed by a medical examination with every cycle. Consequently, the unit responsible for the preparation of the drug with the particular dosage must prepare solutions, which are always personalized and different each time, of the drug with the correct dosage. This has to be done very quickly, and often in great quantities, and so as to allow the nursing staff then to administer the therapies during working hours, often in a day hospital.
- Another difficulty is connected to the variety of molecules used and the need to control the delivery of the preparations, according to how they will be used within the stability period that is characteristic of each preparation.
- the number of solutions prepared a year can be in the order of several tens of thousands, with some dozens of different molecules, of which a large part in everyday use.
- Another difficulty is the need to guarantee absolutely sterile conditions in every step of the preparation cycle.
- the drugs, or their components, are supplied in containers with doses that vary between about 50% and 150% of the average individual daily dose.
- doses that vary between about 50% and 150% of the average individual daily dose.
- the complexity of the therapeutic protocols may even entail the use of five different active principles, distributed over several days.
- the operation to prepare the drug consists in taking a solution containing the drug from a container located in an extractor hood, using a sterile syringe, and controlling visually, on the graduated scale of the syringe, the volume of the solution picked up.
- the accuracy of the assessment depends on the optical aberrations due to the parallax error in the alignment between the operator's eye and the graduated scale of the syringe. This mistake can be accentuated, if the interference of the glass of the extractor hood is taken into account.
- the solution taken is further diluted and infused through an injection point into a sac or container made of deformable plastic material.
- the dosage must be extremely accurate, according to the measuring instruments used, such as sterile syringes and connection sets between the syringe and the containers. Care must also be taken to keep the products sterile by means of methodical disinfection standards. For example, the work place is protected by adequate techniques to prevent the production of spray during the dilution, intake and preparation of the drug.
- extractor hoods and individual protection devices such as gloves, work uniform and mask, allows to protect the operator and the work spaces but, at the same time, it limits spaces available for maneuver and makes the dosing operations not very easy, especially if we consider that these operations are repeated by the operator many times in the course of the working day.
- the repetitiveness of the operation and the familiarity acquired by the operator in handling the components of the drug also lead to a drop in attention of the operator, which increases the probability of mistakes.
- the manual operation could be mistaken due to an exchange of the solutions used or to a mistake in the dosage. In fact, the solutions are mostly colorless or have similar colors and packaging. If a mistake is made, it would be impossible to detect it before the drug is administered.
- the international application WO-A-99/63547 discloses an apparatus for the preparation of radioactive solutions in which a computer controlled syringe pump is used to transfer the solution between reagent vials and to dispense the reagents.
- Purpose of the present invention is to achieve an apparatus and perfect a relative method that allows to prepare a drug automatically, starting from several components, in an accurate and safe way, both for the patient and for the operator, which is totally sterile, quick and with repeatable results in an accurate manner.
- the Applicant has devised, tested and embodied the present invention to overcome the shortcomings of the state of the art and to obtain these and other purposes and advantages.
- an apparatus for the automatic preparation of a drug consisting of at least two components comprises a first container for a first component, a second container for a second component and a third container to receive the drug thus prepared.
- the apparatus also comprises an intermediate container for the preparation of the drug, connected both to the third container and also, by means of valve means selectively openable and closable, to the first and second containers; an intake/injection device, able to be activated in intake mode in order to take in dosed quantities of the first and second component inside the intermediate container, and able to be activated in injection mode in order to transfer the prepared drug from the intermediate container to the third container; and an electronic control unit suitable to manage automatically and in a coordinated manner the selective activation of the valve means and the intake/injection device.
- the electronic unit comprises, or is associated with, memorization means able to memorize data relating to predetermined doses of the drug.
- the apparatus according to the present invention is provided with sensor means able to detect the values of said dosed quantities and to transmit them to the electronic control unit. In this way, from a comparison between the memorized data and the values detected, it is possible to control the correctness of the quantities of the components actually taken in, thus preventing any toxic effect for the patient.
- the whole preparation operation is automated and does not involve the operator directly, except for the preparation of the containers that feed the components and except in the startup and control of the preparation procedure managed by the electronic control unit.
- the present invention thus allows to prepare a drug automatically, starting from several components, in an accurate and safe way, both for the patient and for the operator, which is quick and with repeatable results in an accurate manner.
- the exact quantity of the component taken in is controlled by calibrating the intake/injection device, the containers and the relative connection circuits.
- the calibration values are inserted into the electronic control unit by the operator responsible for starting each operation to prepare the drug or, preferably, memorized once only in the control unit.
- any change in the intake/injection device, the containers and the relative connection circuits is detected by said sensor means and signaled to the control unit which, by means of suitable alarm means, for example, acoustic and/or visual, obliges the operator to insert or recall from the memory the corresponding calibration values.
- suitable alarm means for example, acoustic and/or visual
- the accuracy of intake and dosage that is thus determined also allows to reduce waste in the components of the drug, allowing a considerable saving in costs.
- An advantageous variant of the present invention provides to use a dosing syringe, of the sterile type, as an intake/injection device.
- the apparatus By using sterile containers, syringes and relative connection circuits, the apparatus in its entirety is rendered sterile and prevents contaminations of the drug prepared.
- the apparatus can also be used as an injector-doser in the course of parenteral therapy according to a program controlled by the electronic control unit, by connecting the sterile syringe to an infusion line of suitable length.
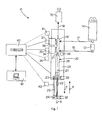
- an apparatus 10 is used for the preparation of chemotherapy drugs formed by two or more components and used in oncological therapies.
- the apparatus 10 comprises two containers 12 and 14, a first container 12 that contains a drug in solution, at a concentration such that it must be suitably diluted so as to be administered to the patient, and a second container 14 that contains a diluent for the drug in solution to be diluted.
- the drug suitably prepared is sent, as shown hereafter, to a medical sac 16.
- the containers 12 and 14 are of the sterile closing type, standard for medical applications, as is the medical sac 16.
- the containers 12 and 14 and the medical sac 16 are put in communication with corresponding circuits 15, 17 and 19, of the sterile type, also standard for medical applications, which in turn converge into a hydraulic connection element 18, for example formed by a two-way Luer-Lock ramp.
- a container 27 Downstream of the connection element 18 a container 27 is provided, selectively connected with the containers 12, 14 and 16, inside which, as will be shown hereafter, the drug is mixed and prepared.
- connection element 18 Connected to the connection element 18 there are two taps 34 and 36, for example two-way or three-way, which are associated respectively with the circuits 15 and 17, in order to regulate the quantity of the stream of drug in solution and of diluent entering the connection element 18.
- the circuits 15 and 17 can consist of needles or ventilated needles which perforate the containers 12 and 14 and which are attached, screwed or inserted into the respective taps 34 and 36.
- the perforation of the containers 12 and 14 is guided by a slide or cradle or hopper on the bottom of which there are packings, either rigid or elastic, in such a number as to regulate the penetration of the points of the needles on the inner side of the containers 12 and 14, allowing maximum recoup of the content thereof.
- the taps 34 and 36 are driven by relative independent motors 35 and 37, to operate in coordination with each other so as to determine all the possible combinations of quantities of drug in solution and diluent.
- the motors 35 and 37 move shaped guides, not shown in the drawings, to house the external wings of the taps 34 and 36.
- the exact dosage quantities of the drug in solution and of diluent are selectively taken in by a dosing syringe 20, whose hollow cylinder 26 defines inside it the container 27. Inside the cylinder 26 the drug in solution and the diluent are mixed, and from here the prepared drug is injected, through the element 18, into the medical sac 16.
- the hollow cylinder 26 of the dosing syringe 20 has a Luer-Lock tip 21, inserted into the connection element 18, and a piston 22 inserted slidingly inside it.
- the piston 22 and the cylinder 26 are able to slide one with respect to the other, along the common longitudinal axis X, to perform the known intake and injection of the syringe 20.
- the piston 22 is provided with a piston-thrust portion 24, which is constrained to suitable retaining fins 32.
- a suitable electric motor 28, shown schematically in fig. 1 is able to act by means of a transmission mechanism 29, on the fins 32, in order to drive the piston-thrust 24 and thus move the piston 22 in alternate mode, along its longitudinal axis X. If the piston 22 is moved in the direction of the arrow F in fig. 1 , the syringe 20 operates in intake mode, whereas if the piston 22 is moved in the direction of the arrow G in fig. 1 , the syringe 20 operates in injection mode.
- the transmission mechanism 29 can consist of a worm screw, a screw pin, metal or polymer telescopic extensions, as required.
- the base 23 of the cylinder 26 is constrained to other fins 30, mounted on the motor 28.
- connection between the fins 30 and 32 and the base 23 and the piston-thrust 24 may be made by means of a screw, an elastic element, an attachment tooth or by means of vacuum.
- the preparation of the drug according to the present invention is made according to updated dosages of the drugs to be administered to each patient.
- the doses are memorized in a database 42 as drug preparation data, together with the chemical-physical characteristics of the components and possible other necessary data.
- the containers 12 and 14 are prepared, block 52 in fig. 2 .
- the medical sac 16 is prepared, into which the drug, prepared and ready to be administered, is introduced.
- a step of opening at least one of the two taps 34 and 36, block 54 in fig. 2 to allow the passage of a determinate dosed quantity of drug in solution and/or diluent.
- the method according to the invention provides an intake step by the syringe 20, block 55 in fig. 2 , which is simultaneous with the step of mixing the drug in solution and the diluent in the cylinder 26 of the syringe 20, so as to prepare the final drug.
- the apparatus 10 In order to coordinate the opening and closing of the taps 34 and 36 with the movement of the piston 22 during the intake and injection steps, and to guarantee the correct dosage of the drug, the apparatus 10 is provided with a control unit 40, which is electronically connected also to the clinical database 42 which contains the dosages.
- the control unit 40 commands the two motors 35 and 37 of the taps 34 and 36, in order to selectively control the opening and closing thereof, simultaneously or in sequence, according to the dosage provided for the preparation of a determinate drug to be administered.
- control unit 40 is also able to command the activation of the electric motor that drives the piston-thrust 24, so as to determine the relative steps of intake and injection.
- the entity of the travel of the piston 22 and the opening of the taps 34 and 36 determines the quantity of drug in solution and of diluent taken in, and is controlled by suitable electro-mechanical and/or electronic sensors 39, 41 and 43, shown schematically in the drawings.
- the sensors 39, 41 and 43 detect signals relating to the dosed quantities and send them to the control unit 40, and also the sequence with which the components are taken in through the taps 34 and 36.
- the signals are used for a safety check, block 57 in fig. 2 , by the control unit 40, which calculates the exact composition of the various components in the drug, and verifies if the doses are correct by comparing them with the pre-set ones in the database 42. If the doses are not correct, then the drug prepared in container 16 is expelled, block 58 in fig. 2 , and eliminated, with the restoration of a new container 16, block 53 in fig. 2 . On the contrary, if the doses are correct, the drug prepared is injected, block 59.
- the quantity and speed of transfer of the drug in solution and diluent through the circuits 15, 17 and 19 depends on the speed of movement of the piston 22, commanded by the electric motor 28 which in turn is commanded by the unit 40.
- the speed in particular, is adjustable according to the doses of drug prescribed, and also according to the nature of the components to be taken in, for example lyophilized drug or in solution, and the viscosity of the solutions prepared or injected, to prevent the formation of instantaneous over-pressure or back flows into the containers 12, 14 and 16.
- the force applied by the motor to move the piston 22 is such as to allow operations in depression and to empty the containers 12 and 14 also without an air valve, with a considerable reduction of waste.
- the depression operation allows to reduce the possibility of leakages in the subsequent operation of the feed container.
- One advantage of the invention is that, all in all, the operating parts of the apparatus 10, that is, the motor that drives the syringe 20, the syringe 20 itself, the taps 34 and 36 with the relative motors 35 and 37 and part of the circuits 15, 17 and 19, have a limited overall bulk, such that they can easily be housed on the work surface of the chemical or microbiological hoods, or hoods for chemotherapy, and the isolators for advanced aseptic processing.
- the containers 12, 14 and 16 used are provided with recognition labels, read optically or electromagnetically, for example a bar code, so that each operation to insert data relating to the components used in the control unit 40 can be validated by means of automatic reading, thus considerably reducing possibilities of mistakes that are found when said data is introduced manually by means of a keyboard.
- the control unit 40 commands a bar code to be printed, which certifies the correct sequence and conformity of preparation and which contains the references of the final destination, the sequence of operations performed and the operators who intervened.
- an RFID tag can be used, which also gives the advantage of traceability.
- the apparatus 10 can be aligned with other identical apparatuses for a battery functioning on the work surface of the hoods, or on a surface suspended by a frame outside the hoods.
- the modular nature of the apparatus 10 allows to associate several lines to prepare the drug in a battery under the same hood or insulator.
- the connections and electric feeds and the electric control boards for the functioning of the apparatuses 10 can be disposed towards the outside of the work surface of the hoods and can converge independently, by means of an electric circuit, in the sole control unit 40. In this way, the simultaneous functioning of the individual lines is possible even in the event of an interruption or malfunction of an apparatus.
- the motor 28 that commands the movement of the piston 22 is of the pneumatic type and that this movement is transmitted directly or by mechanical transmission and that it is controlled electro-mechanically.
- motors 35 and 37 instead of shaped guides, may be provided with grippers or hooks or clamps, in order to drive the external wings of the taps 34 and 36.
- the motor 28 may be conformed so as to collect the possible leaks from the circuits 15 and 17, and may be made of material resistant to corrosion from chemical substances used in the operation to decontaminate the work spaces and/or the chemical neutralization operation.
- a transparent shell may be mounted on the motor 28, which surrounds the syringe 20 and the taps 34 and 36, to reveal and/or confine any sprays that develop.
Abstract
Description
- The present invention concerns an apparatus for the automatic preparation of a drug and the relative method of preparation. In particular, the present invention is used for the preparation and dosage of liquid homogeneous solutions or dispersions of chemical products, of synthetic or natural origin, in order to prepare solutions for clinical use in the human and veterinary field or for industrial use.
- It is known that the preparation of a drug starting from two or more basic components contained in suitable containers, and the relative transfer to a container suitable for the intravenous administration of the drug itself are somewhat difficult, especially in the case of therapies in the oncological field which include the administration of chemotherapy drugs.
- A first difficulty is connected to the fact that the prescription of a drug to a patient in chemotherapy occurs after his clinical state has been evaluated and the prescribed doses must be confirmed by a medical examination with every cycle. Consequently, the unit responsible for the preparation of the drug with the particular dosage must prepare solutions, which are always personalized and different each time, of the drug with the correct dosage. This has to be done very quickly, and often in great quantities, and so as to allow the nursing staff then to administer the therapies during working hours, often in a day hospital.
- Another difficulty is connected to the variety of molecules used and the need to control the delivery of the preparations, according to how they will be used within the stability period that is characteristic of each preparation. The number of solutions prepared a year can be in the order of several tens of thousands, with some dozens of different molecules, of which a large part in everyday use. Another difficulty is the need to guarantee absolutely sterile conditions in every step of the preparation cycle.
- The drugs, or their components, are supplied in containers with doses that vary between about 50% and 150% of the average individual daily dose. The complexity of the therapeutic protocols may even entail the use of five different active principles, distributed over several days.
- Typically, the operation to prepare the drug consists in taking a solution containing the drug from a container located in an extractor hood, using a sterile syringe, and controlling visually, on the graduated scale of the syringe, the volume of the solution picked up.
- The accuracy of the assessment depends on the optical aberrations due to the parallax error in the alignment between the operator's eye and the graduated scale of the syringe. This mistake can be accentuated, if the interference of the glass of the extractor hood is taken into account.
- Subsequently, the solution taken is further diluted and infused through an injection point into a sac or container made of deformable plastic material.
- Since the compounds are often toxic, the dosage must be extremely accurate, according to the measuring instruments used, such as sterile syringes and connection sets between the syringe and the containers. Care must also be taken to keep the products sterile by means of methodical disinfection standards. For example, the work place is protected by adequate techniques to prevent the production of spray during the dilution, intake and preparation of the drug.
- The use of extractor hoods and individual protection devices, such as gloves, work uniform and mask, allows to protect the operator and the work spaces but, at the same time, it limits spaces available for maneuver and makes the dosing operations not very easy, especially if we consider that these operations are repeated by the operator many times in the course of the working day. The repetitiveness of the operation and the familiarity acquired by the operator in handling the components of the drug also lead to a drop in attention of the operator, which increases the probability of mistakes. For example, the manual operation could be mistaken due to an exchange of the solutions used or to a mistake in the dosage. In fact, the solutions are mostly colorless or have similar colors and packaging. If a mistake is made, it would be impossible to detect it before the drug is administered.
- Such mistakes can have very serious consequences, also considering the often critical clinical situation of the patient.
- Furthermore, this type of manual preparation requires maximum precision in order to limit to a minimum the waste of the drug which is usually very expensive.
- The international application
WO-A-99/63547 - Purpose of the present invention is to achieve an apparatus and perfect a relative method that allows to prepare a drug automatically, starting from several components, in an accurate and safe way, both for the patient and for the operator, which is totally sterile, quick and with repeatable results in an accurate manner.
- The Applicant has devised, tested and embodied the present invention to overcome the shortcomings of the state of the art and to obtain these and other purposes and advantages.
- The present invention is set forth and characterized in the independent claims, while the dependent claims describe other characteristics of the invention or variants to the main inventive idea.
- In accordance with the above purpose, an apparatus for the automatic preparation of a drug consisting of at least two components comprises a first container for a first component, a second container for a second component and a third container to receive the drug thus prepared.
- According to a characteristic feature of the present invention, the apparatus also comprises an intermediate container for the preparation of the drug, connected both to the third container and also, by means of valve means selectively openable and closable, to the first and second containers; an intake/injection device, able to be activated in intake mode in order to take in dosed quantities of the first and second component inside the intermediate container, and able to be activated in injection mode in order to transfer the prepared drug from the intermediate container to the third container; and an electronic control unit suitable to manage automatically and in a coordinated manner the selective activation of the valve means and the intake/injection device.
- According to an advantageous variant of the present invention, the electronic unit comprises, or is associated with, memorization means able to memorize data relating to predetermined doses of the drug. Advantageously, furthermore, the apparatus according to the present invention is provided with sensor means able to detect the values of said dosed quantities and to transmit them to the electronic control unit. In this way, from a comparison between the memorized data and the values detected, it is possible to control the correctness of the quantities of the components actually taken in, thus preventing any toxic effect for the patient.
- Advantageously, the whole preparation operation is automated and does not involve the operator directly, except for the preparation of the containers that feed the components and except in the startup and control of the preparation procedure managed by the electronic control unit.
- The present invention thus allows to prepare a drug automatically, starting from several components, in an accurate and safe way, both for the patient and for the operator, which is quick and with repeatable results in an accurate manner.
- Advantageously, the exact quantity of the component taken in is controlled by calibrating the intake/injection device, the containers and the relative connection circuits. The calibration values are inserted into the electronic control unit by the operator responsible for starting each operation to prepare the drug or, preferably, memorized once only in the control unit.
- Any change in the intake/injection device, the containers and the relative connection circuits is detected by said sensor means and signaled to the control unit which, by means of suitable alarm means, for example, acoustic and/or visual, obliges the operator to insert or recall from the memory the corresponding calibration values.
- The accuracy of intake and dosage that is thus determined also allows to reduce waste in the components of the drug, allowing a considerable saving in costs.
- An advantageous variant of the present invention provides to use a dosing syringe, of the sterile type, as an intake/injection device.
- By using sterile containers, syringes and relative connection circuits, the apparatus in its entirety is rendered sterile and prevents contaminations of the drug prepared.
- In an unclaimed example the apparatus can also be used as an injector-doser in the course of parenteral therapy according to a program controlled by the electronic control unit, by connecting the sterile syringe to an infusion line of suitable length.
- These and other characteristics of the present invention will become apparent from the following description of a preferential form of embodiment, given as a non-restrictive example with reference to the attached drawings wherein:
-
fig. 1 is a schematic representation of an apparatus for the automatic preparation of a drug according to the present invention; -
fig. 2 is a schematic representation of a method for the automatic preparation of a drug according to the present invention - With reference to
fig. 1 , anapparatus 10 according to the present invention is used for the preparation of chemotherapy drugs formed by two or more components and used in oncological therapies. - The
apparatus 10 comprises twocontainers first container 12 that contains a drug in solution, at a concentration such that it must be suitably diluted so as to be administered to the patient, and asecond container 14 that contains a diluent for the drug in solution to be diluted. - The drug suitably prepared is sent, as shown hereafter, to a
medical sac 16. - The
containers medical sac 16. - The
containers medical sac 16 are put in communication withcorresponding circuits hydraulic connection element 18, for example formed by a two-way Luer-Lock ramp. - Downstream of the connection element 18 a
container 27 is provided, selectively connected with thecontainers - Connected to the
connection element 18 there are twotaps circuits connection element 18. Thecircuits containers respective taps containers containers - The
taps independent motors motors taps - The exact dosage quantities of the drug in solution and of diluent are selectively taken in by a
dosing syringe 20, whosehollow cylinder 26 defines inside it thecontainer 27. Inside thecylinder 26 the drug in solution and the diluent are mixed, and from here the prepared drug is injected, through theelement 18, into themedical sac 16. - In particular, the
hollow cylinder 26 of thedosing syringe 20 has a Luer-Locktip 21, inserted into theconnection element 18, and apiston 22 inserted slidingly inside it. - The
piston 22 and thecylinder 26 are able to slide one with respect to the other, along the common longitudinal axis X, to perform the known intake and injection of thesyringe 20. - The
piston 22 is provided with a piston-thrust portion 24, which is constrained tosuitable retaining fins 32. - A suitable
electric motor 28, shown schematically infig. 1 , is able to act by means of atransmission mechanism 29, on thefins 32, in order to drive the piston-thrust 24 and thus move thepiston 22 in alternate mode, along its longitudinal axis X. If thepiston 22 is moved in the direction of the arrow F infig. 1 , thesyringe 20 operates in intake mode, whereas if thepiston 22 is moved in the direction of the arrow G infig. 1 , thesyringe 20 operates in injection mode. - The
transmission mechanism 29 can consist of a worm screw, a screw pin, metal or polymer telescopic extensions, as required. - The
base 23 of thecylinder 26 is constrained toother fins 30, mounted on themotor 28. - The connection between the
fins base 23 and the piston-thrust 24 may be made by means of a screw, an elastic element, an attachment tooth or by means of vacuum. - The preparation of the drug according to the present invention is made according to updated dosages of the drugs to be administered to each patient. In a first step of the method to prepare the drug, indicated by
block 51 infig. 2 , the doses are memorized in adatabase 42 as drug preparation data, together with the chemical-physical characteristics of the components and possible other necessary data. - In a second step of the method according to the invention, the
containers fig. 2 . - Moreover, in the second step, block 53 in
fig. 2 , themedical sac 16 is prepared, into which the drug, prepared and ready to be administered, is introduced. - According to the invention, we then have a step of opening at least one of the two taps 34 and 36, block 54 in
fig. 2 , to allow the passage of a determinate dosed quantity of drug in solution and/or diluent. In the typical step of preparing the drug, we find both thetaps tap 36 is open, this corresponds to the step when the circuits and the syringe are washed by the diluents, which is propaedeutic to the preparation of a new drug. - Subsequently, the method according to the invention provides an intake step by the
syringe 20, block 55 infig. 2 , which is simultaneous with the step of mixing the drug in solution and the diluent in thecylinder 26 of thesyringe 20, so as to prepare the final drug. - Afterwards, we have a step of closing the
taps containers syringe 20, block 59, of the drug prepared, formed by the drug in solution and the diluent, into theappropriate sac 16. - In order to coordinate the opening and closing of the
taps piston 22 during the intake and injection steps, and to guarantee the correct dosage of the drug, theapparatus 10 is provided with acontrol unit 40, which is electronically connected also to theclinical database 42 which contains the dosages. - The
control unit 40 commands the twomotors taps - Furthermore, the
control unit 40 is also able to command the activation of the electric motor that drives the piston-thrust 24, so as to determine the relative steps of intake and injection. - The entity of the travel of the
piston 22 and the opening of thetaps electronic sensors - In particular, during the intake step, the
sensors control unit 40, and also the sequence with which the components are taken in through thetaps - The signals are used for a safety check, block 57 in
fig. 2 , by thecontrol unit 40, which calculates the exact composition of the various components in the drug, and verifies if the doses are correct by comparing them with the pre-set ones in thedatabase 42. If the doses are not correct, then the drug prepared incontainer 16 is expelled, block 58 infig. 2 , and eliminated, with the restoration of anew container 16, block 53 infig. 2 . On the contrary, if the doses are correct, the drug prepared is injected, block 59. - The quantity and speed of transfer of the drug in solution and diluent through the
circuits piston 22, commanded by theelectric motor 28 which in turn is commanded by theunit 40. The speed, in particular, is adjustable according to the doses of drug prescribed, and also according to the nature of the components to be taken in, for example lyophilized drug or in solution, and the viscosity of the solutions prepared or injected, to prevent the formation of instantaneous over-pressure or back flows into thecontainers - Advantageously, furthermore, the force applied by the motor to move the
piston 22 is such as to allow operations in depression and to empty thecontainers - Another safety and precision measure is given by a
spirit level device 38. - One advantage of the invention is that, all in all, the operating parts of the
apparatus 10, that is, the motor that drives thesyringe 20, thesyringe 20 itself, thetaps relative motors circuits - Advantageously, the
containers control unit 40 can be validated by means of automatic reading, thus considerably reducing possibilities of mistakes that are found when said data is introduced manually by means of a keyboard. In particular, at the end of the preparation of the drug, thecontrol unit 40 commands a bar code to be printed, which certifies the correct sequence and conformity of preparation and which contains the references of the final destination, the sequence of operations performed and the operators who intervened. Instead of the bar code, an RFID tag can be used, which also gives the advantage of traceability. - Advantageously, furthermore, the
apparatus 10 can be aligned with other identical apparatuses for a battery functioning on the work surface of the hoods, or on a surface suspended by a frame outside the hoods. The modular nature of theapparatus 10 allows to associate several lines to prepare the drug in a battery under the same hood or insulator. In this case, the connections and electric feeds and the electric control boards for the functioning of theapparatuses 10 can be disposed towards the outside of the work surface of the hoods and can converge independently, by means of an electric circuit, in thesole control unit 40. In this way, the simultaneous functioning of the individual lines is possible even in the event of an interruption or malfunction of an apparatus. - It is clear that modifications and/or additions of parts and/or steps may be made to the apparatus for the automatic preparation of a drug and the relative method of preparation as described heretofore, without departing from the field and scope of the present invention.
- For example, it comes within the field of the present invention to provide that the
motor 28 that commands the movement of thepiston 22 is of the pneumatic type and that this movement is transmitted directly or by mechanical transmission and that it is controlled electro-mechanically. - Moreover, the
motors taps - Furthermore, the
motor 28 may be conformed so as to collect the possible leaks from thecircuits - Advantageously, moreover, a transparent shell may be mounted on the
motor 28, which surrounds thesyringe 20 and thetaps - It is also clear that, although the present invention has been described with reference to specific examples, a person of skill in the art shall certainly be able to achieve many other equivalent forms of apparatus for the preparation of a drug and the relative method of preparation, having the characteristics as set forth in the claims and hence all coming within the field of protection defined thereby.
Claims (10)
- Apparatus for the automatic preparation of a drug consisting of at least two components, comprising a first container (12) for a first of said components, a second container (14) for a second of said components, a third container (16) to receive the prepared drug, characterized in that it also comprises:- an intake/injection device able to be activated in intake mode to simultaneously take in and mix dosed quantities of said first and second component, and activated in injection mode to transfer the mixed drug to said third container (16) and consisting of a sterile dosing syringe (20) comprising a hollow cylinder (26), which defines an intermediate container (27) for the mixing of said at least two components to obtain said drug, connected both to said third container (16) and also, by means of valve means (34, 36) driven by relative independent motors (35, 37) and selectively openable and closable, to said first container (12) and to said second container (14), and a piston (22) able to slide inside the hollow cylinder (26) to perform the intake/injection operation of the dosing syringe (20);- a first (15) and a second (17) sterile circuit which respectively connect, by means of said valve means (34, 36), a hydraulic connection element formed by a two-way Luer-Lock ramp (18) to the respective first container (12) and second container (14), inside said two-way Luer-Lock ramp. (18) dosed quantities of said at least two components, taken in from said first container (12) and second container (14), being made to pass before entering in said intermediate container (27);- a third (19) sterile circuit being provided to connect said two-way Luer-Lock ramp (18) with the third container (16),- said hollow cylinder (26) of said dosing syringe (20) comprising a Luer-Lock tip (21) inserted into the two-way Luer-Lock ramp (18) for the connection of the dosing syringe (20) to the two-way Luer-Lock ramp (18);- an electronic control unit (40) suitable to manage automatically and in a coordinated manner the selective activation of said valve means (34, 36) and of said intake/injection device (20).
- Apparatus as in claim 1, characterized in that the electronic control unit (40) is associated with, or comprises, memorization means (42) able to memorize data relating to pre-determined preparation doses of the drug.
- Apparatus as in claim 1 or 2, characterized in that it also comprises sensor means (39, 41, 43) able to detect the values of the quantities dosed and to transmit them to the electronic control unit (40).
- Apparatus as in any claim hereinbefore, characterized in that it comprises drive means (28), controlled by the electronic control unit (40), which is able to drive the intake/injection device (20).
- Apparatus as in claims 4, characterized in that said drive means (28) is able to apply a force to move the piston (22) of the dosing syringe (20) such as to allow operations in depression and to empty the first container (12) and the second container (14).
- Apparatus as in claims 4 and 5 or 6, characterized in that said piston (22) is provided with a piston-thrust portion (24), which is constrained to suitable retaining fins (32), said drive means comprising an electric motor (28) being able to act by means of a transmission mechanism (29) on the fins (32) in order to drive the piston-thrust (24) and thus move the piston (22) in alternate mode, so as to determine the intake or injection operation mode of the dosing syringe (20).
- Method for the automatic preparation of a drug consisting of at least two components, characterized in that it comprises:- a first step in which the data relating to pre-determined preparation doses of said drug are memorized in memorization means (42) associated with an electronic control unit (40);- a second step in which at least a first container (12) is prepared for a first of said components, a second container (14) for a second of said components and a third container (16) to receive the drug prepared;- a third step in which, by means of said electronic control unit (40), valve means (34, 36) driven by relative independent motors (35, 37) are selectively opened, associated with an intermediate container (27) for the mixing of said at least two components to obtain said drug, so as to put the first container (12) and the second container (14) in communication with said intermediate container (27), a hydraulic connection element formed by a two-way Luer-Lock ramp (18) being used to connect, by means of said valve means (34, 36), a first (15) and a second (17) sterile circuit to the respective first container (12) and second container (14), inside which two-way Luer-Lock ramp (18) dosed quantities of said at least two components, taken in from said first container (12) and second container (14), are made to pass before entering in said intermediate container (27);- a fourth step in which, by means of the electronic control unit (40), an intake/injection device consisting of a sterile dosing syringe (20) comprising a hollow cylinder (26), which defines said intermediate container (27) for the mixing of said at least two components to obtain said drug and comprises a Luer-Lock tip (21) inserted into the two-way Luer-Lock ramp (18) for the connection of the dosing syringe (20) to the two-way Luer-Lock ramp (18), is activated in intake mode in order to take in, selectively, dosed quantities of the first component and the second component from said two-way Luer-Lock ramp (18) inside the intermediate container (27), and in which, simultaneously with the intake, said components are mixed and the drug prepared;- a fifth step in which, by means of said electronic control unit (40), said valve means (34, 36) are closed;- a sixth step in which, by means of said electronic control unit (40), the intake/injection device (20) is activated in injection mode in order to transfer the prepared drug from the intermediate container (27) through the two-way Luer-Lock ramp (18) to the third container (16).
- Method as in claim 7, characterized in that in the fourth step it provides to detect the values of the dosed quantities by means of sensor means (39, 41, 43) and to transmit the values to the electronic control unit (40) and in that, before the sixth step, a step is provided to verify the dosed quantities according to the data memorized in the memorization means (42).
- Method as in claim 7 or 8, characterized by the use of a sterile dosing syringe (20) as the intake/injection device, said sterile dosing syringe (20) comprising a hollow cylinder (26), which defines said intermediate container (27) for the preparation of said drug, and a piston (22) able to slide inside the hollow cylinder (26).
- Method as in claim 9, characterized in that the dosing syringe (20) is driven by means of drive means (28), controlled by the electronic control unit (40), applying a force to move the piston (22) of the dosing syringe (20) such as to allow operations in depression and to empty the first container (12) and the second container (14).
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IT000093A ITUD20070093A1 (en) | 2007-05-30 | 2007-05-30 | EQUIPMENT FOR THE AUTOMATIC PREPARATION OF A DRUG AND ITS PROCEDURE FOR PREPARATION |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP1997471A1 EP1997471A1 (en) | 2008-12-03 |
| EP1997471B1 EP1997471B1 (en) | 2012-03-14 |
| EP1997471B2 true EP1997471B2 (en) | 2015-02-25 |
Family
ID=39744037
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP08157120.0A Active EP1997471B2 (en) | 2007-05-30 | 2008-05-28 | Apparatus for the automatic preparation of a drug and respective method of preparation |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP1997471B2 (en) |
| AT (1) | ATE549008T1 (en) |
| ES (1) | ES2384193T5 (en) |
| IT (1) | ITUD20070093A1 (en) |
Families Citing this family (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102724946B (en) * | 2009-07-29 | 2015-06-10 | Icu医学有限公司 | Fluid transfer devices and methods of use |
| EP2446875B1 (en) * | 2010-10-27 | 2013-10-02 | Fresenius Kabi Deutschland GmbH | Mixing device and method for reconstructing or mixing a medicinal liquid |
| CN102430363B (en) * | 2011-09-20 | 2014-03-26 | 中国科学院深圳先进技术研究院 | Automatic dispensing device and automatic dispensing method |
| ES2945322T3 (en) * | 2011-12-22 | 2023-06-30 | Icu Medical Inc | Fluid Transfer Devices and Methods of Use |
| US9475019B2 (en) | 2013-03-15 | 2016-10-25 | Baxter Corporation Englewood | Systems and methods for compounding a preparation using a premix solution |
| EP3073982B1 (en) | 2013-11-25 | 2020-04-08 | ICU Medical, Inc. | Methods and system for filling iv bags with therapeutic fluid |
| EP3169925A4 (en) * | 2014-07-14 | 2018-01-31 | ICU Medical, Inc. | Fluid transfer devices and methods of use |
| ITUB20150371A1 (en) * | 2015-05-05 | 2016-11-05 | Masmec S P A | SYSTEM FOR THE PREPARATION AND DOSAGE OF CHEMOTHERAPY DRUGS |
| US20180257051A1 (en) * | 2015-09-22 | 2018-09-13 | Medical Dispensing Systems | Method and device for making up a pharmaceutical preparation |
| JP6710758B2 (en) | 2015-12-04 | 2020-06-17 | アイシーユー・メディカル・インコーポレーテッド | Electronic medical fluid transfer device for transferring medical fluid |
| USD851745S1 (en) | 2016-07-19 | 2019-06-18 | Icu Medical, Inc. | Medical fluid transfer system |
| CA3031529A1 (en) | 2016-07-25 | 2018-02-01 | Icu Medical, Inc. | Systems, methods, and components for trapping air bubbles in medical fluid transfer modules and systems |
| US11793722B2 (en) | 2017-08-23 | 2023-10-24 | Fresenius Kabi Deutschland Gmbh | Valve unit for a system for producing a medical preparation |
| US11590057B2 (en) | 2020-04-03 | 2023-02-28 | Icu Medical, Inc. | Systems, methods, and components for transferring medical fluids |
| WO2023075600A1 (en) * | 2021-11-01 | 2023-05-04 | The Compounding Company B.V. | Compounding system |
| NL2031054B1 (en) * | 2021-11-01 | 2023-05-31 | The Compounding Company B V | Compounding system |
| WO2023204699A1 (en) * | 2022-04-17 | 2023-10-26 | The Compounding Company B.V. | Compounding system |
| NL2033305B1 (en) * | 2022-04-17 | 2023-11-06 | The Compounding Company B V | Compounding system |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1074222A2 (en) † | 1999-08-04 | 2001-02-07 | Grupo Grifols, S.A. | Device for the injection of co2 for angiography |
| EP1236644A1 (en) † | 2001-02-28 | 2002-09-04 | Probitas Pharma, S.A. | Apparatus for filling containers for pharmaceutical uses and the like |
| US6699232B2 (en) † | 2001-03-01 | 2004-03-02 | Scimed Life Systems, Inc. | Fluid injection apparatus with improved contrast visualization |
| WO2006001913A1 (en) † | 2004-06-15 | 2006-01-05 | Mallinckrodt Inc. | Automated dispensing system and associated method of use |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3602075C1 (en) * | 1986-01-24 | 1987-07-23 | Fresenius Ag | Method and device for producing mixtures of pharmaceutical liquids |
| US5911252A (en) * | 1997-04-29 | 1999-06-15 | Cassel; Douglas | Automated syringe filling system for radiographic contrast agents and other injectable substances |
| WO1999063547A2 (en) | 1998-06-02 | 1999-12-09 | The Dow Chemical Company | Apparatus for the preparation of radioactive solutions |
| CA2560719A1 (en) * | 2004-04-07 | 2005-10-20 | Forhealth Technologies, Inc. | Reconstituting a drug vial and medication dose underfill detection system in an automated syringe preparing system |
-
2007
- 2007-05-30 IT IT000093A patent/ITUD20070093A1/en unknown
-
2008
- 2008-05-28 ES ES08157120.0T patent/ES2384193T5/en active Active
- 2008-05-28 EP EP08157120.0A patent/EP1997471B2/en active Active
- 2008-05-28 AT AT08157120T patent/ATE549008T1/en active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1074222A2 (en) † | 1999-08-04 | 2001-02-07 | Grupo Grifols, S.A. | Device for the injection of co2 for angiography |
| EP1236644A1 (en) † | 2001-02-28 | 2002-09-04 | Probitas Pharma, S.A. | Apparatus for filling containers for pharmaceutical uses and the like |
| US6699232B2 (en) † | 2001-03-01 | 2004-03-02 | Scimed Life Systems, Inc. | Fluid injection apparatus with improved contrast visualization |
| WO2006001913A1 (en) † | 2004-06-15 | 2006-01-05 | Mallinckrodt Inc. | Automated dispensing system and associated method of use |
Also Published As
| Publication number | Publication date |
|---|---|
| ATE549008T1 (en) | 2012-03-15 |
| ES2384193T3 (en) | 2012-07-02 |
| EP1997471B1 (en) | 2012-03-14 |
| EP1997471A1 (en) | 2008-12-03 |
| ITUD20070093A1 (en) | 2008-11-30 |
| ES2384193T5 (en) | 2015-06-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1997471B2 (en) | Apparatus for the automatic preparation of a drug and respective method of preparation | |
| CN108093624B (en) | Composite device, system, kit, software and method | |
| WO2016196810A1 (en) | Compounding device, system, kit, software, and method | |
| CA2983610C (en) | Compounding device, system, kit, software and method | |
| WO2016191210A2 (en) | Compounding device, system, kit, software and method |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL BA MK RS |
|
| 17P | Request for examination filed |
Effective date: 20090529 |
|
| AKX | Designation fees paid |
Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT RO SE SI SK TR |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: FRISAN, ANDREA Owner name: CADEL, DANIELE Owner name: SNIDERO, PAOLA Owner name: DE MARCO, LUIGINO Owner name: LAZZARINI, RENZO Owner name: PERIN, ANDREA |
|
| 17Q | First examination report despatched |
Effective date: 20091228 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61J 3/00 20060101ALN20110916BHEP Ipc: B01F 15/02 20060101ALN20110916BHEP Ipc: B01F 13/00 20060101ALN20110916BHEP Ipc: B01F 13/10 20060101ALN20110916BHEP Ipc: A61J 1/20 20060101AFI20110916BHEP |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: ISITEC S.R.L. |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 549008 Country of ref document: AT Kind code of ref document: T Effective date: 20120315 Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602008014066 Country of ref document: DE Effective date: 20120510 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2384193 Country of ref document: ES Kind code of ref document: T3 Effective date: 20120702 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: VDEP Effective date: 20120314 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120614 |
|
| LTIE | Lt: invalidation of european patent or patent extension |
Effective date: 20120314 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120615 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 549008 Country of ref document: AT Kind code of ref document: T Effective date: 20120314 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 Ref country code: BE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120714 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120716 |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120531 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| 26 | Opposition filed |
Opponent name: GRIFOLS, S.A. Effective date: 20121214 |
|
| PLAX | Notice of opposition and request to file observation + time limit sent |
Free format text: ORIGINAL CODE: EPIDOSNOBS2 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120531 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120531 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R026 Ref document number: 602008014066 Country of ref document: DE Effective date: 20121214 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120528 |
|
| PLBB | Reply of patent proprietor to notice(s) of opposition received |
Free format text: ORIGINAL CODE: EPIDOSNOBS3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120614 Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120314 |
|
| PLBP | Opposition withdrawn |
Free format text: ORIGINAL CODE: 0009264 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120528 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080528 |
|
| PUAH | Patent maintained in amended form |
Free format text: ORIGINAL CODE: 0009272 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT MAINTAINED AS AMENDED |
|
| 27A | Patent maintained in amended form |
Effective date: 20150225 |
|
| AK | Designated contracting states |
Kind code of ref document: B2 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R102 Ref document number: 602008014066 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R102 Ref document number: 602008014066 Country of ref document: DE Effective date: 20150225 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: DC2A Ref document number: 2384193 Country of ref document: ES Kind code of ref document: T5 Effective date: 20150605 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150225 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 9 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 10 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: TR Payment date: 20190522 Year of fee payment: 12 Ref country code: FR Payment date: 20190523 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20200520 Year of fee payment: 13 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200531 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20210617 Year of fee payment: 14 Ref country code: GB Payment date: 20210401 Year of fee payment: 14 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 602008014066 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20211201 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200528 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20220528 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220528 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20230314 Year of fee payment: 16 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20230630 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220529 |