US20040141944A1 - Inhibition of xenoreactive antibodies - Google Patents
Inhibition of xenoreactive antibodies Download PDFInfo
- Publication number
- US20040141944A1 US20040141944A1 US10/402,845 US40284503A US2004141944A1 US 20040141944 A1 US20040141944 A1 US 20040141944A1 US 40284503 A US40284503 A US 40284503A US 2004141944 A1 US2004141944 A1 US 2004141944A1
- Authority
- US
- United States
- Prior art keywords
- polymer
- αgal
- gal
- antibodies
- xenoantigen
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 230000005764 inhibitory process Effects 0.000 title description 18
- 238000000034 method Methods 0.000 claims abstract description 133
- 239000000203 mixture Substances 0.000 claims abstract description 93
- 241001465754 Metazoa Species 0.000 claims abstract description 66
- 230000004044 response Effects 0.000 claims abstract description 13
- 238000004519 manufacturing process Methods 0.000 claims abstract description 9
- 229920000642 polymer Polymers 0.000 claims description 249
- 101000718529 Saccharolobus solfataricus (strain ATCC 35092 / DSM 1617 / JCM 11322 / P2) Alpha-galactosidase Proteins 0.000 claims description 191
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 claims description 141
- 229920001223 polyethylene glycol Polymers 0.000 claims description 85
- 238000006243 chemical reaction Methods 0.000 claims description 64
- 239000002202 Polyethylene glycol Substances 0.000 claims description 63
- 210000004027 cell Anatomy 0.000 claims description 63
- 210000001519 tissue Anatomy 0.000 claims description 62
- -1 phosphoryl halide Chemical class 0.000 claims description 54
- 230000000694 effects Effects 0.000 claims description 53
- 229920001542 oligosaccharide Polymers 0.000 claims description 51
- 241000282898 Sus scrofa Species 0.000 claims description 49
- GVJXGCIPWAVXJP-UHFFFAOYSA-N 2,5-dioxo-1-oxoniopyrrolidine-3-sulfonate Chemical compound ON1C(=O)CC(S(O)(=O)=O)C1=O GVJXGCIPWAVXJP-UHFFFAOYSA-N 0.000 claims description 46
- 210000000056 organ Anatomy 0.000 claims description 46
- 125000000524 functional group Chemical group 0.000 claims description 45
- WQZGKKKJIJFFOK-FPRJBGLDSA-N beta-D-galactose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-FPRJBGLDSA-N 0.000 claims description 43
- 125000003277 amino group Chemical group 0.000 claims description 42
- 125000005647 linker group Chemical group 0.000 claims description 32
- 210000004369 blood Anatomy 0.000 claims description 30
- 239000008280 blood Substances 0.000 claims description 30
- 150000001720 carbohydrates Chemical class 0.000 claims description 30
- 239000003795 chemical substances by application Substances 0.000 claims description 30
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 claims description 28
- 210000000628 antibody-producing cell Anatomy 0.000 claims description 27
- 150000002482 oligosaccharides Chemical class 0.000 claims description 27
- 241000288906 Primates Species 0.000 claims description 25
- 210000002216 heart Anatomy 0.000 claims description 24
- 230000027455 binding Effects 0.000 claims description 23
- 239000003153 chemical reaction reagent Substances 0.000 claims description 23
- 241000894007 species Species 0.000 claims description 23
- 150000001412 amines Chemical class 0.000 claims description 21
- 238000006880 cross-coupling reaction Methods 0.000 claims description 20
- 239000003814 drug Substances 0.000 claims description 20
- 230000002163 immunogen Effects 0.000 claims description 19
- 210000003734 kidney Anatomy 0.000 claims description 19
- 238000002360 preparation method Methods 0.000 claims description 19
- 210000003719 b-lymphocyte Anatomy 0.000 claims description 18
- 239000002253 acid Substances 0.000 claims description 17
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 17
- 239000003431 cross linking reagent Substances 0.000 claims description 17
- 125000000717 hydrazino group Chemical group [H]N([*])N([H])[H] 0.000 claims description 17
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 17
- 230000008878 coupling Effects 0.000 claims description 16
- 238000010168 coupling process Methods 0.000 claims description 16
- 238000005859 coupling reaction Methods 0.000 claims description 16
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 16
- 108060003951 Immunoglobulin Proteins 0.000 claims description 15
- 230000003213 activating effect Effects 0.000 claims description 15
- 150000001350 alkyl halides Chemical class 0.000 claims description 15
- 238000009472 formulation Methods 0.000 claims description 15
- 102000018358 immunoglobulin Human genes 0.000 claims description 15
- 229910052717 sulfur Inorganic materials 0.000 claims description 15
- 229910052760 oxygen Inorganic materials 0.000 claims description 13
- 230000036470 plasma concentration Effects 0.000 claims description 13
- 108010005774 beta-Galactosidase Proteins 0.000 claims description 12
- 230000000269 nucleophilic effect Effects 0.000 claims description 12
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 claims description 12
- 150000001408 amides Chemical class 0.000 claims description 10
- 238000005406 washing Methods 0.000 claims description 10
- 206010052779 Transplant rejections Diseases 0.000 claims description 9
- 150000001299 aldehydes Chemical class 0.000 claims description 9
- 238000004132 cross linking Methods 0.000 claims description 9
- 210000004185 liver Anatomy 0.000 claims description 9
- 230000001404 mediated effect Effects 0.000 claims description 9
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 claims description 9
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 9
- 239000000376 reactant Substances 0.000 claims description 8
- 230000003248 secreting effect Effects 0.000 claims description 8
- 230000002194 synthesizing effect Effects 0.000 claims description 8
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide Chemical compound CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 claims description 7
- 150000007513 acids Chemical class 0.000 claims description 7
- 150000002576 ketones Chemical class 0.000 claims description 7
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims description 6
- XYFCBTPGUUZFHI-UHFFFAOYSA-N Phosphine Chemical compound P XYFCBTPGUUZFHI-UHFFFAOYSA-N 0.000 claims description 6
- 239000000872 buffer Substances 0.000 claims description 6
- 230000014509 gene expression Effects 0.000 claims description 6
- 210000003958 hematopoietic stem cell Anatomy 0.000 claims description 6
- 150000003573 thiols Chemical class 0.000 claims description 6
- 230000003614 tolerogenic effect Effects 0.000 claims description 6
- 125000003172 aldehyde group Chemical group 0.000 claims description 5
- 210000001185 bone marrow Anatomy 0.000 claims description 5
- RAABOESOVLLHRU-UHFFFAOYSA-N diazene Chemical group N=N RAABOESOVLLHRU-UHFFFAOYSA-N 0.000 claims description 5
- 229910000071 diazene Inorganic materials 0.000 claims description 5
- 210000004072 lung Anatomy 0.000 claims description 5
- 231100001274 therapeutic index Toxicity 0.000 claims description 5
- DQJCDTNMLBYVAY-ZXXIYAEKSA-N (2S,5R,10R,13R)-16-{[(2R,3S,4R,5R)-3-{[(2S,3R,4R,5S,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-5-(ethylamino)-6-hydroxy-2-(hydroxymethyl)oxan-4-yl]oxy}-5-(4-aminobutyl)-10-carbamoyl-2,13-dimethyl-4,7,12,15-tetraoxo-3,6,11,14-tetraazaheptadecan-1-oic acid Chemical compound NCCCC[C@H](C(=O)N[C@@H](C)C(O)=O)NC(=O)CC[C@H](C(N)=O)NC(=O)[C@@H](C)NC(=O)C(C)O[C@@H]1[C@@H](NCC)C(O)O[C@H](CO)[C@H]1O[C@H]1[C@H](NC(C)=O)[C@@H](O)[C@H](O)[C@@H](CO)O1 DQJCDTNMLBYVAY-ZXXIYAEKSA-N 0.000 claims description 4
- 102000030902 Galactosyltransferase Human genes 0.000 claims description 4
- 108060003306 Galactosyltransferase Proteins 0.000 claims description 4
- 108010015899 Glycopeptides Proteins 0.000 claims description 4
- 102000002068 Glycopeptides Human genes 0.000 claims description 4
- HSCJRCZFDFQWRP-UHFFFAOYSA-N Uridindiphosphoglukose Natural products OC1C(O)C(O)C(CO)OC1OP(O)(=O)OP(O)(=O)OCC1C(O)C(O)C(N2C(NC(=O)C=C2)=O)O1 HSCJRCZFDFQWRP-UHFFFAOYSA-N 0.000 claims description 4
- 230000004913 activation Effects 0.000 claims description 4
- 150000001298 alcohols Chemical class 0.000 claims description 4
- 150000007942 carboxylates Chemical group 0.000 claims description 4
- 150000002148 esters Chemical class 0.000 claims description 4
- 239000001963 growth medium Substances 0.000 claims description 4
- 150000002632 lipids Chemical class 0.000 claims description 4
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 4
- 239000007848 Bronsted acid Substances 0.000 claims description 3
- 239000003341 Bronsted base Substances 0.000 claims description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 3
- 102000008100 Human Serum Albumin Human genes 0.000 claims description 3
- 108091006905 Human Serum Albumin Proteins 0.000 claims description 3
- 239000002841 Lewis acid Substances 0.000 claims description 3
- 239000002879 Lewis base Substances 0.000 claims description 3
- OVRNDRQMDRJTHS-KEWYIRBNSA-N N-acetyl-D-galactosamine Chemical compound CC(=O)N[C@H]1C(O)O[C@H](CO)[C@H](O)[C@@H]1O OVRNDRQMDRJTHS-KEWYIRBNSA-N 0.000 claims description 3
- MBLBDJOUHNCFQT-UHFFFAOYSA-N N-acetyl-D-galactosamine Natural products CC(=O)NC(C=O)C(O)C(O)C(O)CO MBLBDJOUHNCFQT-UHFFFAOYSA-N 0.000 claims description 3
- OVRNDRQMDRJTHS-FMDGEEDCSA-N N-acetyl-beta-D-glucosamine Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O OVRNDRQMDRJTHS-FMDGEEDCSA-N 0.000 claims description 3
- 229910019142 PO4 Inorganic materials 0.000 claims description 3
- 150000001266 acyl halides Chemical class 0.000 claims description 3
- 229940045714 alkyl sulfonate alkylating agent Drugs 0.000 claims description 3
- 150000008052 alkyl sulfonates Chemical class 0.000 claims description 3
- 150000001732 carboxylic acid derivatives Chemical class 0.000 claims description 3
- 150000001805 chlorine compounds Chemical class 0.000 claims description 3
- 150000002256 galaktoses Chemical class 0.000 claims description 3
- 150000007517 lewis acids Chemical class 0.000 claims description 3
- 150000007527 lewis bases Chemical class 0.000 claims description 3
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims description 3
- 239000010452 phosphate Substances 0.000 claims description 3
- 150000003003 phosphines Chemical class 0.000 claims description 3
- OJMIONKXNSYLSR-UHFFFAOYSA-N phosphorous acid Chemical compound OP(O)O OJMIONKXNSYLSR-UHFFFAOYSA-N 0.000 claims description 3
- 229910000073 phosphorus hydride Inorganic materials 0.000 claims description 3
- 150000003958 selenols Chemical class 0.000 claims description 3
- 150000003568 thioethers Chemical class 0.000 claims description 3
- 230000019970 B cell anergy Effects 0.000 claims description 2
- 230000000903 blocking effect Effects 0.000 claims description 2
- 238000002372 labelling Methods 0.000 claims description 2
- 229940124531 pharmaceutical excipient Drugs 0.000 claims description 2
- HSCJRCZFDFQWRP-ABVWGUQPSA-N UDP-alpha-D-galactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1OP(O)(=O)OP(O)(=O)OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](N2C(NC(=O)C=C2)=O)O1 HSCJRCZFDFQWRP-ABVWGUQPSA-N 0.000 claims 1
- 229920006037 cross link polymer Polymers 0.000 claims 1
- 238000002203 pretreatment Methods 0.000 claims 1
- 230000002265 prevention Effects 0.000 claims 1
- 210000000987 immune system Anatomy 0.000 abstract description 8
- 230000002401 inhibitory effect Effects 0.000 abstract description 7
- 230000001419 dependent effect Effects 0.000 abstract description 6
- 230000028993 immune response Effects 0.000 abstract description 4
- PFAXACNYGZVKMX-UHFFFAOYSA-N fenethazine Chemical compound C1=CC=C2N(CCN(C)C)C3=CC=CC=C3SC2=C1 PFAXACNYGZVKMX-UHFFFAOYSA-N 0.000 abstract description 2
- 230000028327 secretion Effects 0.000 abstract description 2
- 238000011282 treatment Methods 0.000 description 62
- 239000000243 solution Substances 0.000 description 48
- 241001504519 Papio ursinus Species 0.000 description 42
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 42
- 238000003786 synthesis reaction Methods 0.000 description 37
- 230000015572 biosynthetic process Effects 0.000 description 36
- 238000002054 transplantation Methods 0.000 description 36
- OVRNDRQMDRJTHS-UHFFFAOYSA-N N-acelyl-D-glucosamine Natural products CC(=O)NC1C(O)OC(CO)C(O)C1O OVRNDRQMDRJTHS-UHFFFAOYSA-N 0.000 description 35
- MBLBDJOUHNCFQT-LXGUWJNJSA-N N-acetylglucosamine Natural products CC(=O)N[C@@H](C=O)[C@@H](O)[C@H](O)[C@H](O)CO MBLBDJOUHNCFQT-LXGUWJNJSA-N 0.000 description 35
- OVRNDRQMDRJTHS-RTRLPJTCSA-N N-acetyl-D-glucosamine Chemical compound CC(=O)N[C@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1O OVRNDRQMDRJTHS-RTRLPJTCSA-N 0.000 description 34
- 210000002966 serum Anatomy 0.000 description 34
- 239000000427 antigen Substances 0.000 description 28
- 102000036639 antigens Human genes 0.000 description 28
- 108091007433 antigens Proteins 0.000 description 28
- 241000282693 Cercopithecidae Species 0.000 description 27
- 239000007987 MES buffer Substances 0.000 description 26
- 241000282520 Papio Species 0.000 description 25
- 235000000346 sugar Nutrition 0.000 description 25
- FHIVAFMUCKRCQO-UHFFFAOYSA-N diazinon Chemical compound CCOP(=S)(OCC)OC1=CC(C)=NC(C(C)C)=N1 FHIVAFMUCKRCQO-UHFFFAOYSA-N 0.000 description 23
- 125000000837 carbohydrate group Chemical group 0.000 description 22
- 238000004458 analytical method Methods 0.000 description 21
- 238000001802 infusion Methods 0.000 description 21
- 150000001875 compounds Chemical class 0.000 description 20
- 230000000295 complement effect Effects 0.000 description 19
- 239000011541 reaction mixture Substances 0.000 description 19
- 238000002965 ELISA Methods 0.000 description 18
- NNISLDGFPWIBDF-MPRBLYSKSA-N alpha-D-Gal-(1->3)-beta-D-Gal-(1->4)-D-GlcNAc Chemical compound O[C@@H]1[C@@H](NC(=O)C)C(O)O[C@H](CO)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O2)O)[C@@H](O)[C@@H](CO)O1 NNISLDGFPWIBDF-MPRBLYSKSA-N 0.000 description 16
- 238000003556 assay Methods 0.000 description 16
- 239000000843 powder Substances 0.000 description 16
- 239000000047 product Substances 0.000 description 16
- 238000000502 dialysis Methods 0.000 description 15
- 210000003038 endothelium Anatomy 0.000 description 15
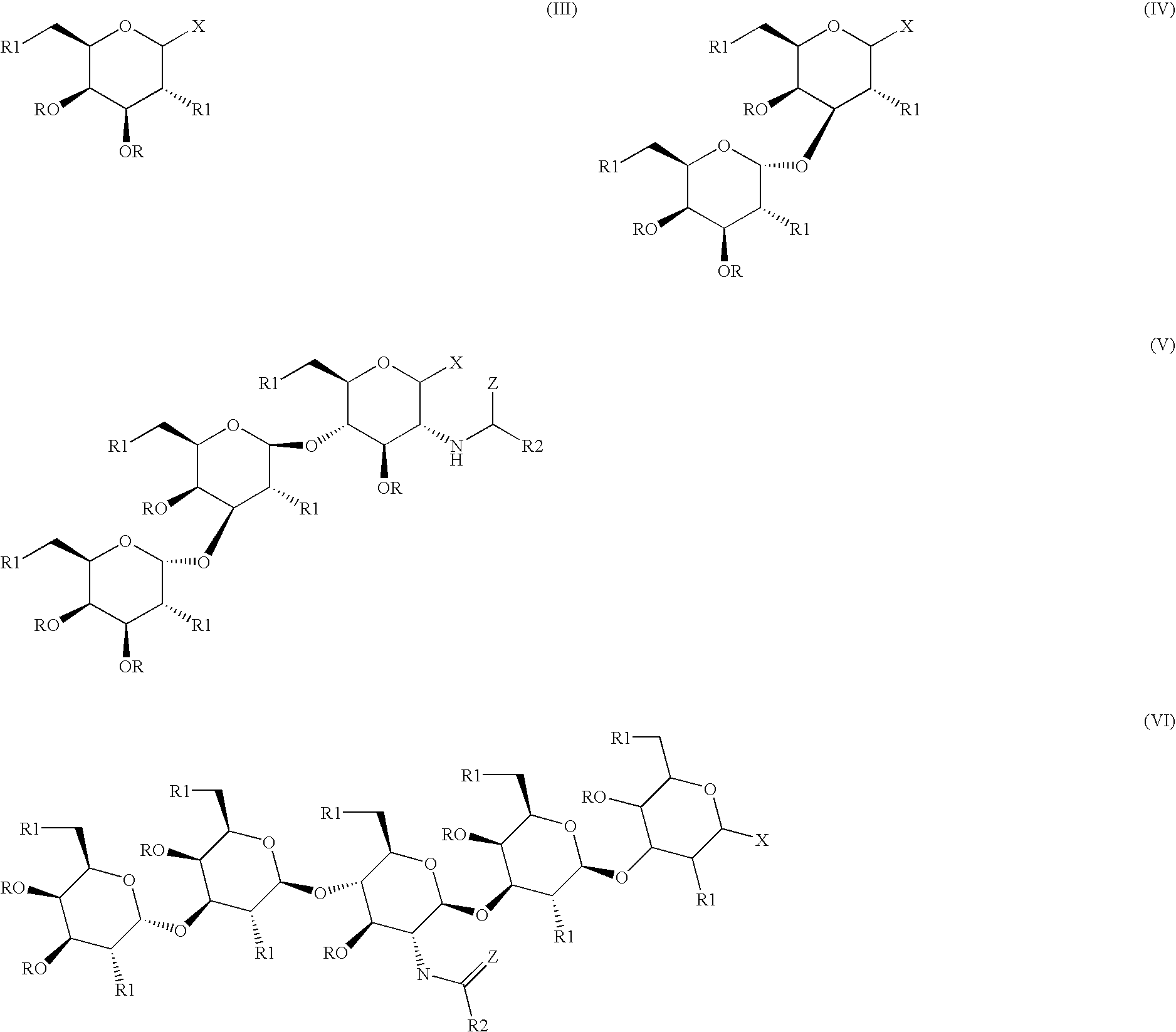
- 0 [1*]CC1OC(C)C([1*])C(O[C@@H]2O[C@H](C[1*])[C@H](C)[C@H](O[C@@H]3O[C@H](C[1*])[C@@H](O[C@@H]4O[C@H](C[1*])[C@H](C)[C@H](O[C@H]5O[C@H](C[1*])[C@H](C)[C@H](C)[C@H]5[1*])[C@H]4[1*])[C@H](C)[C@H]3NC([2*])=C)[C@H]2[1*])C1C.[1*]C[C@H]1OC(C)[C@H](NC([2*])C)[C@@H](C)[C@@H]1O[C@@H]1O[C@H](C[1*])[C@H](C)[C@H](O[C@H]2O[C@H](C[1*])[C@H](C)[C@H](C)[C@H]2[1*])[C@H]1[1*].[1*]C[C@H]1OC(C)[C@H]([1*])[C@@H](C)[C@H]1C.[1*]C[C@H]1OC(C)[C@H]([1*])[C@@H](O[C@H]2O[C@H](C[1*])[C@H](C)[C@H](C)[C@H]2[1*])[C@H]1C Chemical compound [1*]CC1OC(C)C([1*])C(O[C@@H]2O[C@H](C[1*])[C@H](C)[C@H](O[C@@H]3O[C@H](C[1*])[C@@H](O[C@@H]4O[C@H](C[1*])[C@H](C)[C@H](O[C@H]5O[C@H](C[1*])[C@H](C)[C@H](C)[C@H]5[1*])[C@H]4[1*])[C@H](C)[C@H]3NC([2*])=C)[C@H]2[1*])C1C.[1*]C[C@H]1OC(C)[C@H](NC([2*])C)[C@@H](C)[C@@H]1O[C@@H]1O[C@H](C[1*])[C@H](C)[C@H](O[C@H]2O[C@H](C[1*])[C@H](C)[C@H](C)[C@H]2[1*])[C@H]1[1*].[1*]C[C@H]1OC(C)[C@H]([1*])[C@@H](C)[C@H]1C.[1*]C[C@H]1OC(C)[C@H]([1*])[C@@H](O[C@H]2O[C@H](C[1*])[C@H](C)[C@H](C)[C@H]2[1*])[C@H]1C 0.000 description 14
- 239000003018 immunosuppressive agent Substances 0.000 description 14
- 235000014633 carbohydrates Nutrition 0.000 description 13
- 239000000463 material Substances 0.000 description 13
- 230000008569 process Effects 0.000 description 13
- 230000004083 survival effect Effects 0.000 description 13
- 230000006870 function Effects 0.000 description 11
- 239000008194 pharmaceutical composition Substances 0.000 description 11
- ODDPRQJTYDIWJU-UHFFFAOYSA-N 3'-beta-D-galactopyranosyl-lactose Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(OC2C(OC(O)C(O)C2O)CO)OC(CO)C1O ODDPRQJTYDIWJU-UHFFFAOYSA-N 0.000 description 10
- 238000002474 experimental method Methods 0.000 description 10
- 230000001506 immunosuppresive effect Effects 0.000 description 10
- 108090000623 proteins and genes Proteins 0.000 description 10
- 230000009261 transgenic effect Effects 0.000 description 10
- 241000282412 Homo Species 0.000 description 9
- 206010062016 Immunosuppression Diseases 0.000 description 9
- 210000001744 T-lymphocyte Anatomy 0.000 description 9
- 238000007792 addition Methods 0.000 description 9
- 125000003169 alpha-Gal epitope group Chemical group [C@H]1([C@H](O)[C@@H](O)[C@@H](O)[C@H](O1)CO)O[C@@H]1[C@H]([C@@H](O[C@@H]([C@@H]1O)CO)O[C@H]1[C@@H]([C@H](C(O[C@@H]1CO)*)NC(C)=O)O)O 0.000 description 9
- 125000004429 atom Chemical group 0.000 description 9
- 238000002347 injection Methods 0.000 description 9
- 239000007924 injection Substances 0.000 description 9
- 230000007774 longterm Effects 0.000 description 9
- PVVTWNMXEHROIA-UHFFFAOYSA-N 2-(3-hydroxypropyl)-1h-quinazolin-4-one Chemical compound C1=CC=C2NC(CCCO)=NC(=O)C2=C1 PVVTWNMXEHROIA-UHFFFAOYSA-N 0.000 description 8
- 229920002307 Dextran Polymers 0.000 description 8
- 229920000768 polyamine Polymers 0.000 description 8
- 239000000725 suspension Substances 0.000 description 8
- NQTADLQHYWFPDB-UHFFFAOYSA-N N-Hydroxysuccinimide Chemical compound ON1C(=O)CCC1=O NQTADLQHYWFPDB-UHFFFAOYSA-N 0.000 description 7
- 230000000890 antigenic effect Effects 0.000 description 7
- 230000004087 circulation Effects 0.000 description 7
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 description 7
- 238000005580 one pot reaction Methods 0.000 description 7
- 230000002829 reductive effect Effects 0.000 description 7
- 239000000523 sample Substances 0.000 description 7
- 230000002792 vascular Effects 0.000 description 7
- YGEHCIVVZVBCLE-FSYGUOKUSA-N 3alpha-galactobiose Chemical compound OC[C@@H](O)[C@H](O)[C@@H]([C@@H](O)C=O)O[C@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O YGEHCIVVZVBCLE-FSYGUOKUSA-N 0.000 description 6
- PYTHFOVVUNVIKF-FPVMNMOTSA-N CCC1OC(CC)C(C)C(O[C@H]2O[C@H](CC)[C@H](C)[C@H](C)[C@H]2C)C1C Chemical compound CCC1OC(CC)C(C)C(O[C@H]2O[C@H](CC)[C@H](C)[C@H](C)[C@H]2C)C1C PYTHFOVVUNVIKF-FPVMNMOTSA-N 0.000 description 6
- 241000283707 Capra Species 0.000 description 6
- 230000005875 antibody response Effects 0.000 description 6
- 239000002775 capsule Substances 0.000 description 6
- 239000000969 carrier Substances 0.000 description 6
- 238000010790 dilution Methods 0.000 description 6
- 239000012895 dilution Substances 0.000 description 6
- 229940079593 drug Drugs 0.000 description 6
- 238000003018 immunoassay Methods 0.000 description 6
- 229960003444 immunosuppressant agent Drugs 0.000 description 6
- 229940125721 immunosuppressive agent Drugs 0.000 description 6
- 239000003446 ligand Substances 0.000 description 6
- 239000012466 permeate Substances 0.000 description 6
- 229920003023 plastic Polymers 0.000 description 6
- 239000004033 plastic Substances 0.000 description 6
- 235000018102 proteins Nutrition 0.000 description 6
- 102000004169 proteins and genes Human genes 0.000 description 6
- 230000009467 reduction Effects 0.000 description 6
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 230000001629 suppression Effects 0.000 description 6
- 238000002560 therapeutic procedure Methods 0.000 description 6
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 5
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 5
- 108010010803 Gelatin Proteins 0.000 description 5
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 5
- 229920001213 Polysorbate 20 Polymers 0.000 description 5
- 229920002472 Starch Polymers 0.000 description 5
- 208000007536 Thrombosis Diseases 0.000 description 5
- 239000004480 active ingredient Substances 0.000 description 5
- 230000001413 cellular effect Effects 0.000 description 5
- 238000003114 enzyme-linked immunosorbent spot assay Methods 0.000 description 5
- 239000000499 gel Substances 0.000 description 5
- 229920000159 gelatin Polymers 0.000 description 5
- 239000008273 gelatin Substances 0.000 description 5
- 235000019322 gelatine Nutrition 0.000 description 5
- 235000011852 gelatine desserts Nutrition 0.000 description 5
- 239000011521 glass Substances 0.000 description 5
- 230000001900 immune effect Effects 0.000 description 5
- 230000001861 immunosuppressant effect Effects 0.000 description 5
- 239000008101 lactose Substances 0.000 description 5
- 238000006386 neutralization reaction Methods 0.000 description 5
- 230000036961 partial effect Effects 0.000 description 5
- 210000005259 peripheral blood Anatomy 0.000 description 5
- 239000011886 peripheral blood Substances 0.000 description 5
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 5
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 5
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 5
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 5
- 125000006239 protecting group Chemical group 0.000 description 5
- 239000003826 tablet Substances 0.000 description 5
- 239000003981 vehicle Substances 0.000 description 5
- 230000035899 viability Effects 0.000 description 5
- 238000002689 xenotransplantation Methods 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 4
- 108010036949 Cyclosporine Proteins 0.000 description 4
- SHZGCJCMOBCMKK-UHFFFAOYSA-N D-mannomethylose Natural products CC1OC(O)C(O)C(O)C1O SHZGCJCMOBCMKK-UHFFFAOYSA-N 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 4
- 208000032843 Hemorrhage Diseases 0.000 description 4
- 101000897400 Homo sapiens CD59 glycoprotein Proteins 0.000 description 4
- 241000699666 Mus <mouse, genus> Species 0.000 description 4
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical group [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 229960002685 biotin Drugs 0.000 description 4
- 239000011616 biotin Substances 0.000 description 4
- 229960001265 ciclosporin Drugs 0.000 description 4
- 229930182912 cyclosporin Natural products 0.000 description 4
- 230000000779 depleting effect Effects 0.000 description 4
- 150000004985 diamines Chemical class 0.000 description 4
- 239000008298 dragée Substances 0.000 description 4
- 239000003937 drug carrier Substances 0.000 description 4
- 238000000338 in vitro Methods 0.000 description 4
- 238000010348 incorporation Methods 0.000 description 4
- 238000001990 intravenous administration Methods 0.000 description 4
- 210000004153 islets of langerhan Anatomy 0.000 description 4
- 239000012528 membrane Substances 0.000 description 4
- 239000003068 molecular probe Substances 0.000 description 4
- 239000000178 monomer Substances 0.000 description 4
- 210000000822 natural killer cell Anatomy 0.000 description 4
- 230000037361 pathway Effects 0.000 description 4
- 229940068917 polyethylene glycols Drugs 0.000 description 4
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 4
- 239000012465 retentate Substances 0.000 description 4
- 150000003839 salts Chemical class 0.000 description 4
- 239000003381 stabilizer Substances 0.000 description 4
- 235000019698 starch Nutrition 0.000 description 4
- 210000000130 stem cell Anatomy 0.000 description 4
- 239000011593 sulfur Substances 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- 150000004043 trisaccharides Chemical class 0.000 description 4
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 3
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 description 3
- 108010009575 CD55 Antigens Proteins 0.000 description 3
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 3
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 3
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 3
- 206010011968 Decreased immune responsiveness Diseases 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 229930186217 Glycolipid Natural products 0.000 description 3
- 108090000288 Glycoproteins Proteins 0.000 description 3
- 102000003886 Glycoproteins Human genes 0.000 description 3
- 108010031186 Glycoside Hydrolases Proteins 0.000 description 3
- 102000005744 Glycoside Hydrolases Human genes 0.000 description 3
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 description 3
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 3
- 239000004472 Lysine Substances 0.000 description 3
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 3
- 241000124008 Mammalia Species 0.000 description 3
- 102100039373 Membrane cofactor protein Human genes 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- 206010030113 Oedema Diseases 0.000 description 3
- 108010038807 Oligopeptides Proteins 0.000 description 3
- 102000015636 Oligopeptides Human genes 0.000 description 3
- 241001415846 Procellariidae Species 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 description 3
- USAZACJQJDHAJH-KDEXOMDGSA-N [[(2r,3s,4r,5s)-5-(2,4-dioxo-1h-pyrimidin-6-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(2r,3r,4s,5r,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] hydrogen phosphate Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1OP(O)(=O)OP(O)(=O)OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](C=2NC(=O)NC(=O)C=2)O1 USAZACJQJDHAJH-KDEXOMDGSA-N 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 238000010171 animal model Methods 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 125000003118 aryl group Chemical group 0.000 description 3
- 230000004888 barrier function Effects 0.000 description 3
- MSWZFWKMSRAUBD-UHFFFAOYSA-N beta-D-galactosamine Natural products NC1C(O)OC(CO)C(O)C1O MSWZFWKMSRAUBD-UHFFFAOYSA-N 0.000 description 3
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 3
- 229910002092 carbon dioxide Inorganic materials 0.000 description 3
- 230000024203 complement activation Effects 0.000 description 3
- 125000004122 cyclic group Chemical group 0.000 description 3
- 229960004397 cyclophosphamide Drugs 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 238000011026 diafiltration Methods 0.000 description 3
- 150000002016 disaccharides Chemical class 0.000 description 3
- 210000002889 endothelial cell Anatomy 0.000 description 3
- 229930182830 galactose Natural products 0.000 description 3
- 125000002519 galactosyl group Chemical group C1([C@H](O)[C@@H](O)[C@@H](O)[C@H](O1)CO)* 0.000 description 3
- 238000003209 gene knockout Methods 0.000 description 3
- 239000008103 glucose Substances 0.000 description 3
- 230000002414 glycolytic effect Effects 0.000 description 3
- 230000003394 haemopoietic effect Effects 0.000 description 3
- 210000002865 immune cell Anatomy 0.000 description 3
- 238000002513 implantation Methods 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 230000001939 inductive effect Effects 0.000 description 3
- 208000015181 infectious disease Diseases 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 239000003120 macrolide antibiotic agent Substances 0.000 description 3
- 210000005087 mononuclear cell Anatomy 0.000 description 3
- 230000003472 neutralizing effect Effects 0.000 description 3
- IJGRMHOSHXDMSA-UHFFFAOYSA-N nitrogen Substances N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 3
- 244000052769 pathogen Species 0.000 description 3
- 230000010412 perfusion Effects 0.000 description 3
- 125000004309 pyranyl group Chemical group O1C(C=CC=C1)* 0.000 description 3
- 230000035484 reaction time Effects 0.000 description 3
- 239000000600 sorbitol Substances 0.000 description 3
- 239000008223 sterile water Substances 0.000 description 3
- 229960005322 streptomycin Drugs 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 150000008163 sugars Chemical class 0.000 description 3
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 description 3
- NHJVRSWLHSJWIN-UHFFFAOYSA-N 2,4,6-trinitrobenzenesulfonic acid Chemical compound OS(=O)(=O)C1=C([N+]([O-])=O)C=C([N+]([O-])=O)C=C1[N+]([O-])=O NHJVRSWLHSJWIN-UHFFFAOYSA-N 0.000 description 2
- 101710098620 Alpha-1,2-fucosyltransferase Proteins 0.000 description 2
- 206010002091 Anaesthesia Diseases 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 102100022002 CD59 glycoprotein Human genes 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 2
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 description 2
- 108020004414 DNA Proteins 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 238000012286 ELISA Assay Methods 0.000 description 2
- 238000011510 Elispot assay Methods 0.000 description 2
- ULGZDMOVFRHVEP-RWJQBGPGSA-N Erythromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 ULGZDMOVFRHVEP-RWJQBGPGSA-N 0.000 description 2
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 2
- 102000006395 Globulins Human genes 0.000 description 2
- 108010044091 Globulins Proteins 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- FWKQNCXZGNBPFD-UHFFFAOYSA-N Guaiazulene Chemical compound CC(C)C1=CC=C(C)C2=CC=C(C)C2=C1 FWKQNCXZGNBPFD-UHFFFAOYSA-N 0.000 description 2
- 101000942118 Homo sapiens C-reactive protein Proteins 0.000 description 2
- 101100386242 Homo sapiens CD55 gene Proteins 0.000 description 2
- 101000922020 Homo sapiens Cysteine and glycine-rich protein 1 Proteins 0.000 description 2
- 101000961414 Homo sapiens Membrane cofactor protein Proteins 0.000 description 2
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 2
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 2
- 229930182816 L-glutamine Natural products 0.000 description 2
- SHZGCJCMOBCMKK-JFNONXLTSA-N L-rhamnopyranose Chemical compound C[C@@H]1OC(O)[C@H](O)[C@H](O)[C@H]1O SHZGCJCMOBCMKK-JFNONXLTSA-N 0.000 description 2
- PNNNRSAQSRJVSB-UHFFFAOYSA-N L-rhamnose Natural products CC(O)C(O)C(O)C(O)C=O PNNNRSAQSRJVSB-UHFFFAOYSA-N 0.000 description 2
- 239000007993 MOPS buffer Substances 0.000 description 2
- 241000282567 Macaca fascicularis Species 0.000 description 2
- 108010054377 Mannosidases Proteins 0.000 description 2
- 102000001696 Mannosidases Human genes 0.000 description 2
- 108010090665 Mannosyl-Glycoprotein Endo-beta-N-Acetylglucosaminidase Proteins 0.000 description 2
- 229930182555 Penicillin Natural products 0.000 description 2
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 2
- 108010039918 Polylysine Proteins 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- 241000282887 Suidae Species 0.000 description 2
- 230000006044 T cell activation Effects 0.000 description 2
- YPWFISCTZQNZAU-UHFFFAOYSA-N Thiane Chemical group C1CCSCC1 YPWFISCTZQNZAU-UHFFFAOYSA-N 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- 230000004308 accommodation Effects 0.000 description 2
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 2
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 125000001931 aliphatic group Chemical group 0.000 description 2
- 125000003342 alkenyl group Chemical group 0.000 description 2
- 229940100198 alkylating agent Drugs 0.000 description 2
- 239000002168 alkylating agent Substances 0.000 description 2
- 125000000304 alkynyl group Chemical group 0.000 description 2
- 108010030291 alpha-Galactosidase Proteins 0.000 description 2
- 102000005840 alpha-Galactosidase Human genes 0.000 description 2
- 230000037005 anaesthesia Effects 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 230000003110 anti-inflammatory effect Effects 0.000 description 2
- 230000000340 anti-metabolite Effects 0.000 description 2
- 230000000692 anti-sense effect Effects 0.000 description 2
- 230000001494 anti-thymocyte effect Effects 0.000 description 2
- 229940088710 antibiotic agent Drugs 0.000 description 2
- 229940100197 antimetabolite Drugs 0.000 description 2
- 239000002256 antimetabolite Substances 0.000 description 2
- 210000002403 aortic endothelial cell Anatomy 0.000 description 2
- 239000000010 aprotic solvent Substances 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 229960002170 azathioprine Drugs 0.000 description 2
- LMEKQMALGUDUQG-UHFFFAOYSA-N azathioprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC=NC2=C1NC=N2 LMEKQMALGUDUQG-UHFFFAOYSA-N 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- CNBGNNVCVSKAQZ-UHFFFAOYSA-N benzydamine Chemical compound C12=CC=CC=C2C(OCCCN(C)C)=NN1CC1=CC=CC=C1 CNBGNNVCVSKAQZ-UHFFFAOYSA-N 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 230000006037 cell lysis Effects 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- MYSWGUAQZAJSOK-UHFFFAOYSA-N ciprofloxacin Chemical compound C12=CC(N3CCNCC3)=C(F)C=C2C(=O)C(C(=O)O)=CN1C1CC1 MYSWGUAQZAJSOK-UHFFFAOYSA-N 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 239000000356 contaminant Substances 0.000 description 2
- 238000011109 contamination Methods 0.000 description 2
- 238000013270 controlled release Methods 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 125000000392 cycloalkenyl group Chemical group 0.000 description 2
- 125000000753 cycloalkyl group Chemical group 0.000 description 2
- 231100000135 cytotoxicity Toxicity 0.000 description 2
- 230000003013 cytotoxicity Effects 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- 238000007865 diluting Methods 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 230000003828 downregulation Effects 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 230000003511 endothelial effect Effects 0.000 description 2
- 238000006911 enzymatic reaction Methods 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 239000010685 fatty oil Substances 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- 229960000390 fludarabine Drugs 0.000 description 2
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- 238000002523 gelfiltration Methods 0.000 description 2
- 102000034356 gene-regulatory proteins Human genes 0.000 description 2
- 108091006104 gene-regulatory proteins Proteins 0.000 description 2
- 238000010353 genetic engineering Methods 0.000 description 2
- 210000004907 gland Anatomy 0.000 description 2
- 230000035931 haemagglutination Effects 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 244000144980 herd Species 0.000 description 2
- 125000004404 heteroalkyl group Chemical group 0.000 description 2
- 125000001072 heteroaryl group Chemical group 0.000 description 2
- 102000051442 human CD59 Human genes 0.000 description 2
- 102000051143 human CRP Human genes 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 150000002430 hydrocarbons Chemical class 0.000 description 2
- 230000008105 immune reaction Effects 0.000 description 2
- 230000002998 immunogenetic effect Effects 0.000 description 2
- 229940124589 immunosuppressive drug Drugs 0.000 description 2
- 230000001976 improved effect Effects 0.000 description 2
- 238000011065 in-situ storage Methods 0.000 description 2
- 230000002779 inactivation Effects 0.000 description 2
- 230000000977 initiatory effect Effects 0.000 description 2
- 238000010255 intramuscular injection Methods 0.000 description 2
- 239000007927 intramuscular injection Substances 0.000 description 2
- 238000010253 intravenous injection Methods 0.000 description 2
- 208000028867 ischemia Diseases 0.000 description 2
- 210000003292 kidney cell Anatomy 0.000 description 2
- 210000005228 liver tissue Anatomy 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 230000002101 lytic effect Effects 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 230000000116 mitigating effect Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000010369 molecular cloning Methods 0.000 description 2
- 239000000537 myeloablative agonist Substances 0.000 description 2
- 230000001400 myeloablative effect Effects 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 235000019198 oils Nutrition 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 238000007911 parenteral administration Methods 0.000 description 2
- 230000001717 pathogenic effect Effects 0.000 description 2
- 229940049954 penicillin Drugs 0.000 description 2
- 125000001151 peptidyl group Chemical group 0.000 description 2
- 239000000825 pharmaceutical preparation Substances 0.000 description 2
- 239000002504 physiological saline solution Substances 0.000 description 2
- 125000003386 piperidinyl group Chemical group 0.000 description 2
- 238000002616 plasmapheresis Methods 0.000 description 2
- 210000004180 plasmocyte Anatomy 0.000 description 2
- 229920000962 poly(amidoamine) Polymers 0.000 description 2
- 229920000656 polylysine Polymers 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 229960004618 prednisone Drugs 0.000 description 2
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- 230000001698 pyrogenic effect Effects 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 2
- 230000009257 reactivity Effects 0.000 description 2
- 230000001739 rebound effect Effects 0.000 description 2
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical group O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 description 2
- 238000013207 serial dilution Methods 0.000 description 2
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 2
- 229960002930 sirolimus Drugs 0.000 description 2
- 210000003491 skin Anatomy 0.000 description 2
- 239000002002 slurry Substances 0.000 description 2
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 2
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 238000001179 sorption measurement Methods 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 150000003431 steroids Chemical class 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 239000011550 stock solution Substances 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 125000000020 sulfo group Chemical group O=S(=O)([*])O[H] 0.000 description 2
- 239000000829 suppository Substances 0.000 description 2
- 238000010189 synthetic method Methods 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 229960001967 tacrolimus Drugs 0.000 description 2
- 239000000454 talc Substances 0.000 description 2
- 229910052623 talc Inorganic materials 0.000 description 2
- 235000012222 talc Nutrition 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 230000002992 thymic effect Effects 0.000 description 2
- WYWHKKSPHMUBEB-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 2
- 238000011269 treatment regimen Methods 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 1
- FDWRIIDFYSUTDP-KVTDHHQDSA-N (2r,4r,5s,6r)-6-methyloxane-2,4,5-triol Chemical compound C[C@H]1O[C@@H](O)C[C@@H](O)[C@@H]1O FDWRIIDFYSUTDP-KVTDHHQDSA-N 0.000 description 1
- NTBYIQWZAVDRHA-KCDKBNATSA-N (2s,3s,4r,5s)-2-amino-3,4,5-trihydroxyhexanal Chemical compound C[C@H](O)[C@@H](O)[C@@H](O)[C@H](N)C=O NTBYIQWZAVDRHA-KCDKBNATSA-N 0.000 description 1
- QRXMUCSWCMTJGU-UHFFFAOYSA-L (5-bromo-4-chloro-1h-indol-3-yl) phosphate Chemical compound C1=C(Br)C(Cl)=C2C(OP([O-])(=O)[O-])=CNC2=C1 QRXMUCSWCMTJGU-UHFFFAOYSA-L 0.000 description 1
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 1
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 1
- DDMOUSALMHHKOS-UHFFFAOYSA-N 1,2-dichloro-1,1,2,2-tetrafluoroethane Chemical compound FC(F)(Cl)C(F)(F)Cl DDMOUSALMHHKOS-UHFFFAOYSA-N 0.000 description 1
- UYBWIEGTWASWSR-UHFFFAOYSA-N 1,3-diaminopropan-2-ol Chemical compound NCC(O)CN UYBWIEGTWASWSR-UHFFFAOYSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- FDWRIIDFYSUTDP-UHFFFAOYSA-N 102850-49-7 Natural products CC1OC(O)CC(O)C1O FDWRIIDFYSUTDP-UHFFFAOYSA-N 0.000 description 1
- IDINUJSAMVOPCM-UHFFFAOYSA-N 15-Deoxyspergualin Natural products NCCCNCCCCNC(=O)C(O)NC(=O)CCCCCCN=C(N)N IDINUJSAMVOPCM-UHFFFAOYSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- XUDSQIDNHJMBBW-FOWTUZBSSA-N 2-[4-[(e)-n-hydroxy-c-methylcarbonimidoyl]phenoxy]-1-piperidin-1-ylethanone Chemical compound C1=CC(C(=N/O)/C)=CC=C1OCC(=O)N1CCCCC1 XUDSQIDNHJMBBW-FOWTUZBSSA-N 0.000 description 1
- MSWZFWKMSRAUBD-GASJEMHNSA-N 2-amino-2-deoxy-D-galactopyranose Chemical compound N[C@H]1C(O)O[C@H](CO)[C@H](O)[C@@H]1O MSWZFWKMSRAUBD-GASJEMHNSA-N 0.000 description 1
- MSWZFWKMSRAUBD-IVMDWMLBSA-N 2-amino-2-deoxy-D-glucopyranose Chemical compound N[C@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1O MSWZFWKMSRAUBD-IVMDWMLBSA-N 0.000 description 1
- MSWZFWKMSRAUBD-CBPJZXOFSA-N 2-amino-2-deoxy-D-mannopyranose Chemical compound N[C@@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1O MSWZFWKMSRAUBD-CBPJZXOFSA-N 0.000 description 1
- GOJUJUVQIVIZAV-UHFFFAOYSA-N 2-amino-4,6-dichloropyrimidine-5-carbaldehyde Chemical group NC1=NC(Cl)=C(C=O)C(Cl)=N1 GOJUJUVQIVIZAV-UHFFFAOYSA-N 0.000 description 1
- 125000000022 2-aminoethyl group Chemical group [H]C([*])([H])C([H])([H])N([H])[H] 0.000 description 1
- HZLCGUXUOFWCCN-UHFFFAOYSA-N 2-hydroxynonadecane-1,2,3-tricarboxylic acid Chemical compound CCCCCCCCCCCCCCCCC(C(O)=O)C(O)(C(O)=O)CC(O)=O HZLCGUXUOFWCCN-UHFFFAOYSA-N 0.000 description 1
- ZWROXKPAXPBUNI-UHFFFAOYSA-N 2-oxononanal Chemical compound CCCCCCCC(=O)C=O ZWROXKPAXPBUNI-UHFFFAOYSA-N 0.000 description 1
- MWDGNKGKLOBESZ-UHFFFAOYSA-N 2-oxooctanal Chemical compound CCCCCCC(=O)C=O MWDGNKGKLOBESZ-UHFFFAOYSA-N 0.000 description 1
- 125000000094 2-phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 description 1
- NZNJQXHLVGOHMX-UHFFFAOYSA-N 3-(sulfoamino)propanoic acid Chemical group OC(=O)CCNS(O)(=O)=O NZNJQXHLVGOHMX-UHFFFAOYSA-N 0.000 description 1
- QIGJYVCQYDKYDW-UHFFFAOYSA-N 3-O-alpha-D-mannopyranosyl-D-mannopyranose Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(CO)OC(O)C1O QIGJYVCQYDKYDW-UHFFFAOYSA-N 0.000 description 1
- HVCOBJNICQPDBP-UHFFFAOYSA-N 3-[3-[3,5-dihydroxy-6-methyl-4-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyoxan-2-yl]oxydecanoyloxy]decanoic acid;hydrate Chemical compound O.OC1C(OC(CC(=O)OC(CCCCCCC)CC(O)=O)CCCCCCC)OC(C)C(O)C1OC1C(O)C(O)C(O)C(C)O1 HVCOBJNICQPDBP-UHFFFAOYSA-N 0.000 description 1
- WOVTUUKKGNHVFZ-UHFFFAOYSA-N 4-(fluoren-9-ylidenemethyl)benzenecarboximidamide Chemical compound C1=CC(C(=N)N)=CC=C1C=C1C2=CC=CC=C2C2=CC=CC=C21 WOVTUUKKGNHVFZ-UHFFFAOYSA-N 0.000 description 1
- DVEQCIBLXRSYPH-UHFFFAOYSA-N 5-butyl-1-cyclohexylbarbituric acid Chemical compound O=C1C(CCCC)C(=O)NC(=O)N1C1CCCCC1 DVEQCIBLXRSYPH-UHFFFAOYSA-N 0.000 description 1
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 1
- 210000002925 A-like Anatomy 0.000 description 1
- 244000215068 Acacia senegal Species 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 229920000936 Agarose Polymers 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 108020005544 Antisense RNA Proteins 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 238000012935 Averaging Methods 0.000 description 1
- 102000019260 B-Cell Antigen Receptors Human genes 0.000 description 1
- 108010012919 B-Cell Antigen Receptors Proteins 0.000 description 1
- 230000003844 B-cell-activation Effects 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 102100032487 Beta-mannosidase Human genes 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- VZCADWXAFWGPMI-UHFFFAOYSA-N CC(=O)NCC(C(C)=O)S(=O)(=O)O.O=C1CC(SOOO)C(=O)N1O Chemical compound CC(=O)NCC(C(C)=O)S(=O)(=O)O.O=C1CC(SOOO)C(=O)N1O VZCADWXAFWGPMI-UHFFFAOYSA-N 0.000 description 1
- PYTHFOVVUNVIKF-IPQHYSPZSA-N CCC1O[C@H](CC)[C@H](C)[C@H](O[C@H]2O[C@H](CC)[C@H](C)[C@H](C)[C@H]2C)[C@H]1C Chemical compound CCC1O[C@H](CC)[C@H](C)[C@H](O[C@H]2O[C@H](CC)[C@H](C)[C@H](C)[C@H]2C)[C@H]1C PYTHFOVVUNVIKF-IPQHYSPZSA-N 0.000 description 1
- FVLVBPDQNARYJU-XAHDHGMMSA-N C[C@H]1CCC(CC1)NC(=O)N(CCCl)N=O Chemical compound C[C@H]1CCC(CC1)NC(=O)N(CCCl)N=O FVLVBPDQNARYJU-XAHDHGMMSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 1
- 241000700199 Cavia porcellus Species 0.000 description 1
- 229930186147 Cephalosporin Natural products 0.000 description 1
- 229920001661 Chitosan Polymers 0.000 description 1
- 102100025680 Complement decay-accelerating factor Human genes 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 239000004971 Cross linker Substances 0.000 description 1
- 229920000858 Cyclodextrin Polymers 0.000 description 1
- 229930105110 Cyclosporin A Natural products 0.000 description 1
- JWFRNGYBHLBCMB-UHFFFAOYSA-N D-Canaytose Natural products CC(O)C(O)C(O)CC=O JWFRNGYBHLBCMB-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-CUHNMECISA-N D-Cellobiose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-CUHNMECISA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- YVECGMZCTULTIS-HSUXUTPPSA-N D-galactal Chemical compound OC[C@H]1OC=C[C@@H](O)[C@H]1O YVECGMZCTULTIS-HSUXUTPPSA-N 0.000 description 1
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 description 1
- AEMOLEFTQBMNLQ-AQKNRBDQSA-N D-glucopyranuronic acid Chemical compound OC1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O AEMOLEFTQBMNLQ-AQKNRBDQSA-N 0.000 description 1
- QWIZNVHXZXRPDR-UHFFFAOYSA-N D-melezitose Natural products O1C(CO)C(O)C(O)C(O)C1OC1C(O)C(CO)OC1(CO)OC1OC(CO)C(O)C(O)C1O QWIZNVHXZXRPDR-UHFFFAOYSA-N 0.000 description 1
- 108010092160 Dactinomycin Proteins 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 239000004338 Dichlorodifluoromethane Substances 0.000 description 1
- RPNUMPOLZDHAAY-UHFFFAOYSA-N Diethylenetriamine Chemical compound NCCNCCN RPNUMPOLZDHAAY-UHFFFAOYSA-N 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- URJQOOISAKEBKW-UHFFFAOYSA-N Emorfazone Chemical compound C1=NN(C)C(=O)C(OCC)=C1N1CCOCC1 URJQOOISAKEBKW-UHFFFAOYSA-N 0.000 description 1
- 241000792859 Enema Species 0.000 description 1
- 241000305071 Enterobacterales Species 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- 241000195522 Fucales Species 0.000 description 1
- PNNNRSAQSRJVSB-SLPGGIOYSA-N Fucose Natural products C[C@H](O)[C@@H](O)[C@H](O)[C@H](O)C=O PNNNRSAQSRJVSB-SLPGGIOYSA-N 0.000 description 1
- IAJILQKETJEXLJ-UHFFFAOYSA-N Galacturonsaeure Natural products O=CC(O)C(O)C(O)C(O)C(O)=O IAJILQKETJEXLJ-UHFFFAOYSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 102000051366 Glycosyltransferases Human genes 0.000 description 1
- 108700023372 Glycosyltransferases Proteins 0.000 description 1
- 206010048748 Graft loss Diseases 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- 206010019280 Heart failures Diseases 0.000 description 1
- 101000856022 Homo sapiens Complement decay-accelerating factor Proteins 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 1
- XDXDZDZNSLXDNA-TZNDIEGXSA-N Idarubicin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XDXDZDZNSLXDNA-TZNDIEGXSA-N 0.000 description 1
- XDXDZDZNSLXDNA-UHFFFAOYSA-N Idarubicin Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XDXDZDZNSLXDNA-UHFFFAOYSA-N 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102000004560 Interleukin-12 Receptors Human genes 0.000 description 1
- 108010017515 Interleukin-12 Receptors Proteins 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- 108090000978 Interleukin-4 Proteins 0.000 description 1
- AYRXSINWFIIFAE-SCLMCMATSA-N Isomaltose Natural products OC[C@H]1O[C@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)[C@@H](O)[C@@H](O)[C@@H]1O AYRXSINWFIIFAE-SCLMCMATSA-N 0.000 description 1
- 241000588748 Klebsiella Species 0.000 description 1
- OKPQBUWBBBNTOV-UHFFFAOYSA-N Kojibiose Natural products COC1OC(O)C(OC2OC(OC)C(O)C(O)C2O)C(O)C1O OKPQBUWBBBNTOV-UHFFFAOYSA-N 0.000 description 1
- SHZGCJCMOBCMKK-DHVFOXMCSA-N L-fucopyranose Chemical compound C[C@@H]1OC(O)[C@@H](O)[C@H](O)[C@@H]1O SHZGCJCMOBCMKK-DHVFOXMCSA-N 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- GQYIWUVLTXOXAJ-UHFFFAOYSA-N Lomustine Chemical compound ClCCN(N=O)C(=O)NC1CCCCC1 GQYIWUVLTXOXAJ-UHFFFAOYSA-N 0.000 description 1
- 235000019759 Maize starch Nutrition 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 101710146216 Membrane cofactor protein Proteins 0.000 description 1
- FQISKWAFAHGMGT-SGJOWKDISA-M Methylprednisolone sodium succinate Chemical compound [Na+].C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)COC(=O)CCC([O-])=O)CC[C@H]21 FQISKWAFAHGMGT-SGJOWKDISA-M 0.000 description 1
- VFKZTMPDYBFSTM-KVTDHHQDSA-N Mitobronitol Chemical compound BrC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CBr VFKZTMPDYBFSTM-KVTDHHQDSA-N 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 1
- 229910020889 NaBH3 Inorganic materials 0.000 description 1
- BLXXJMDCKKHMKV-UHFFFAOYSA-N Nabumetone Chemical compound C1=C(CCC(C)=O)C=CC2=CC(OC)=CC=C21 BLXXJMDCKKHMKV-UHFFFAOYSA-N 0.000 description 1
- AYRXSINWFIIFAE-UHFFFAOYSA-N O6-alpha-D-Galactopyranosyl-D-galactose Natural products OCC1OC(OCC(O)C(O)C(O)C(O)C=O)C(O)C(O)C1O AYRXSINWFIIFAE-UHFFFAOYSA-N 0.000 description 1
- 206010053159 Organ failure Diseases 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 102000016979 Other receptors Human genes 0.000 description 1
- 241000282577 Pan troglodytes Species 0.000 description 1
- 241000282516 Papio anubis Species 0.000 description 1
- 241000009328 Perro Species 0.000 description 1
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 1
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- MUPFEKGTMRGPLJ-RMMQSMQOSA-N Raffinose Natural products O(C[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O[C@@]2(CO)[C@H](O)[C@@H](O)[C@@H](CO)O2)O1)[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 MUPFEKGTMRGPLJ-RMMQSMQOSA-N 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 239000006146 Roswell Park Memorial Institute medium Substances 0.000 description 1
- 241000607142 Salmonella Species 0.000 description 1
- 239000002262 Schiff base Substances 0.000 description 1
- 150000004753 Schiff bases Chemical class 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- HIWPGCMGAMJNRG-ACCAVRKYSA-N Sophorose Natural products O([C@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O)[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HIWPGCMGAMJNRG-ACCAVRKYSA-N 0.000 description 1
- 241000191967 Staphylococcus aureus Species 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- 101000895926 Streptomyces plicatus Endo-beta-N-acetylglucosaminidase H Proteins 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 108091008874 T cell receptors Proteins 0.000 description 1
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 206010060872 Transplant failure Diseases 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- UATJOMSPNYCXIX-UHFFFAOYSA-N Trinitrobenzene Chemical compound [O-][N+](=O)C1=CC([N+]([O-])=O)=CC([N+]([O-])=O)=C1 UATJOMSPNYCXIX-UHFFFAOYSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- GLNADSQYFUSGOU-GPTZEZBUSA-J Trypan blue Chemical compound [Na+].[Na+].[Na+].[Na+].C1=C(S([O-])(=O)=O)C=C2C=C(S([O-])(=O)=O)C(/N=N/C3=CC=C(C=C3C)C=3C=C(C(=CC=3)\N=N\C=3C(=CC4=CC(=CC(N)=C4C=3O)S([O-])(=O)=O)S([O-])(=O)=O)C)=C(O)C2=C1N GLNADSQYFUSGOU-GPTZEZBUSA-J 0.000 description 1
- MUPFEKGTMRGPLJ-UHFFFAOYSA-N UNPD196149 Natural products OC1C(O)C(CO)OC1(CO)OC1C(O)C(O)C(O)C(COC2C(C(O)C(O)C(CO)O2)O)O1 MUPFEKGTMRGPLJ-UHFFFAOYSA-N 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 229940122803 Vinca alkaloid Drugs 0.000 description 1
- 230000003187 abdominal effect Effects 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- 150000001241 acetals Chemical class 0.000 description 1
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 125000002015 acyclic group Chemical group 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 229940040563 agaric acid Drugs 0.000 description 1
- 238000007818 agglutination assay Methods 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 230000000735 allogeneic effect Effects 0.000 description 1
- 238000011316 allogeneic transplantation Methods 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- FJGXDMQHNYEUHI-LRFIHEIOSA-N alpha-D-GalNpAc-(1->3)-beta-D-GalpNAc Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@H](O)[C@@H]1O[C@@H]1[C@H](NC(C)=O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 FJGXDMQHNYEUHI-LRFIHEIOSA-N 0.000 description 1
- 102000019199 alpha-Mannosidase Human genes 0.000 description 1
- 108010012864 alpha-Mannosidase Proteins 0.000 description 1
- 229940126575 aminoglycoside Drugs 0.000 description 1
- ISRODTBNJUAWEJ-UHFFFAOYSA-N amixetrine Chemical compound C=1C=CC=CC=1C(OCCC(C)C)CN1CCCC1 ISRODTBNJUAWEJ-UHFFFAOYSA-N 0.000 description 1
- 229950001993 amixetrine Drugs 0.000 description 1
- LSQZJLSUYDQPKJ-NJBDSQKTSA-N amoxicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=C(O)C=C1 LSQZJLSUYDQPKJ-NJBDSQKTSA-N 0.000 description 1
- 229960003022 amoxicillin Drugs 0.000 description 1
- 229940051880 analgesics and antipyretics pyrazolones Drugs 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 229940045687 antimetabolites folic acid analogs Drugs 0.000 description 1
- 229940045719 antineoplastic alkylating agent nitrosoureas Drugs 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000008135 aqueous vehicle Substances 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- BYFMCKSPFYVMOU-UHFFFAOYSA-N bendazac Chemical compound C12=CC=CC=C2C(OCC(=O)O)=NN1CC1=CC=CC=C1 BYFMCKSPFYVMOU-UHFFFAOYSA-N 0.000 description 1
- 229960005149 bendazac Drugs 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229960000333 benzydamine Drugs 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 102000005936 beta-Galactosidase Human genes 0.000 description 1
- 108010055059 beta-Mannosidase Proteins 0.000 description 1
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 description 1
- HIWPGCMGAMJNRG-UHFFFAOYSA-N beta-sophorose Natural products OC1C(O)C(CO)OC(O)C1OC1C(O)C(O)C(O)C(CO)O1 HIWPGCMGAMJNRG-UHFFFAOYSA-N 0.000 description 1
- 230000002146 bilateral effect Effects 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 229910021538 borax Inorganic materials 0.000 description 1
- PHEZJEYUWHETKO-UHFFFAOYSA-N brequinar Chemical compound N1=C2C=CC(F)=CC2=C(C(O)=O)C(C)=C1C(C=C1)=CC=C1C1=CC=CC=C1F PHEZJEYUWHETKO-UHFFFAOYSA-N 0.000 description 1
- 229950010231 brequinar Drugs 0.000 description 1
- 229950003872 bucolome Drugs 0.000 description 1
- 239000007975 buffered saline Substances 0.000 description 1
- 229960002092 busulfan Drugs 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- BQRGNLJZBFXNCZ-UHFFFAOYSA-N calcein am Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC(CN(CC(=O)OCOC(C)=O)CC(=O)OCOC(C)=O)=C(OC(C)=O)C=C1OC1=C2C=C(CN(CC(=O)OCOC(C)=O)CC(=O)OCOC(=O)C)C(OC(C)=O)=C1 BQRGNLJZBFXNCZ-UHFFFAOYSA-N 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 125000002837 carbocyclic group Chemical group 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229960004424 carbon dioxide Drugs 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 229960005243 carmustine Drugs 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000011748 cell maturation Effects 0.000 description 1
- 239000013553 cell monolayer Substances 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 229940124587 cephalosporin Drugs 0.000 description 1
- 150000001780 cephalosporins Chemical class 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000013043 chemical agent Substances 0.000 description 1
- 229910052729 chemical element Inorganic materials 0.000 description 1
- 238000001311 chemical methods and process Methods 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 229960004630 chlorambucil Drugs 0.000 description 1
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 208000020832 chronic kidney disease Diseases 0.000 description 1
- 229960003405 ciprofloxacin Drugs 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 230000015271 coagulation Effects 0.000 description 1
- 238000005345 coagulation Methods 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 230000006957 competitive inhibition Effects 0.000 description 1
- 230000003750 conditioning effect Effects 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 230000000139 costimulatory effect Effects 0.000 description 1
- 238000011262 co‐therapy Methods 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- ATDGTVJJHBUTRL-UHFFFAOYSA-N cyanogen bromide Chemical compound BrC#N ATDGTVJJHBUTRL-UHFFFAOYSA-N 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- 230000002559 cytogenic effect Effects 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 230000002435 cytoreductive effect Effects 0.000 description 1
- 230000001086 cytosolic effect Effects 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 238000002784 cytotoxicity assay Methods 0.000 description 1
- 231100000263 cytotoxicity test Toxicity 0.000 description 1
- 229960003901 dacarbazine Drugs 0.000 description 1
- 229960000640 dactinomycin Drugs 0.000 description 1
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 239000012024 dehydrating agents Substances 0.000 description 1
- 239000003405 delayed action preparation Substances 0.000 description 1
- 150000008266 deoxy sugars Chemical class 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 230000000994 depressogenic effect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 235000019404 dichlorodifluoromethane Nutrition 0.000 description 1
- 229940042935 dichlorodifluoromethane Drugs 0.000 description 1
- 229940087091 dichlorotetrafluoroethane Drugs 0.000 description 1
- PWHROYKAGRUWDQ-UHFFFAOYSA-N difenpiramide Chemical compound C=1C=CC=NC=1NC(=O)CC(C=C1)=CC=C1C1=CC=CC=C1 PWHROYKAGRUWDQ-UHFFFAOYSA-N 0.000 description 1
- 229960001536 difenpiramide Drugs 0.000 description 1
- JDZNTUQRMDAIRO-UHFFFAOYSA-N dimethylmyleran Chemical compound CS(=O)(=O)OC(C)CCC(C)OS(C)(=O)=O JDZNTUQRMDAIRO-UHFFFAOYSA-N 0.000 description 1
- KPUWHANPEXNPJT-UHFFFAOYSA-N disiloxane Chemical class [SiH3]O[SiH3] KPUWHANPEXNPJT-UHFFFAOYSA-N 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- UUCMDZWCRNZCOY-UHFFFAOYSA-N ditazole Chemical compound O1C(N(CCO)CCO)=NC(C=2C=CC=CC=2)=C1C1=CC=CC=C1 UUCMDZWCRNZCOY-UHFFFAOYSA-N 0.000 description 1
- 229960005067 ditazole Drugs 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 238000000921 elemental analysis Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 229950010243 emorfazone Drugs 0.000 description 1
- 208000028208 end stage renal disease Diseases 0.000 description 1
- 201000000523 end stage renal failure Diseases 0.000 description 1
- 230000008497 endothelial barrier function Effects 0.000 description 1
- 239000002158 endotoxin Substances 0.000 description 1
- 239000007920 enema Substances 0.000 description 1
- 229940079360 enema for constipation Drugs 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 229960003276 erythromycin Drugs 0.000 description 1
- GTSMOYLSFUBTMV-UHFFFAOYSA-N ethidium homodimer Chemical compound [H+].[H+].[Cl-].[Cl-].[Cl-].[Cl-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2C(C)=[N+]1CCCNCCNCCC[N+](C1=CC(N)=CC=C1C1=CC=C(N)C=C11)=C1C1=CC=CC=C1 GTSMOYLSFUBTMV-UHFFFAOYSA-N 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- UPCIBFUJJLCOQG-UHFFFAOYSA-L ethyl-[2-[2-[ethyl(dimethyl)azaniumyl]ethyl-methylamino]ethyl]-dimethylazanium;dibromide Chemical compound [Br-].[Br-].CC[N+](C)(C)CCN(C)CC[N+](C)(C)CC UPCIBFUJJLCOQG-UHFFFAOYSA-L 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- WQMLFJWIKARBFW-BKKMTDGVSA-N evomonoside Chemical compound O[C@@H]1[C@H](O)[C@@H](O)[C@H](C)O[C@H]1O[C@@H]1C[C@@H](CC[C@H]2[C@]3(CC[C@@H]([C@@]3(C)CC[C@H]32)C=2COC(=O)C=2)O)[C@]3(C)CC1 WQMLFJWIKARBFW-BKKMTDGVSA-N 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 238000013401 experimental design Methods 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- ZNOLGFHPUIJIMJ-UHFFFAOYSA-N fenitrothion Chemical compound COP(=S)(OC)OC1=CC=C([N+]([O-])=O)C(C)=C1 ZNOLGFHPUIJIMJ-UHFFFAOYSA-N 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- 239000012847 fine chemical Substances 0.000 description 1
- 238000000799 fluorescence microscopy Methods 0.000 description 1
- 229940124307 fluoroquinolone Drugs 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 150000002224 folic acids Chemical class 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 238000012817 gel-diffusion technique Methods 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- DLRVVLDZNNYCBX-CQUJWQHSSA-N gentiobiose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)C(O)O1 DLRVVLDZNNYCBX-CQUJWQHSSA-N 0.000 description 1
- YVECGMZCTULTIS-PBXRRBTRSA-N glucal Chemical compound OC[C@H]1OC=C[C@@H](O)[C@@H]1O YVECGMZCTULTIS-PBXRRBTRSA-N 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 239000000174 gluconic acid Substances 0.000 description 1
- 235000012208 gluconic acid Nutrition 0.000 description 1
- 229960002442 glucosamine Drugs 0.000 description 1
- 229940097043 glucuronic acid Drugs 0.000 description 1
- 125000005456 glyceride group Chemical group 0.000 description 1
- 125000003827 glycol group Chemical group 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 229960002350 guaiazulen Drugs 0.000 description 1
- 244000005709 gut microbiome Species 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000005003 heart tissue Anatomy 0.000 description 1
- 238000011134 hematopoietic stem cell transplantation Methods 0.000 description 1
- 150000002386 heptoses Chemical class 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 230000006801 homologous recombination Effects 0.000 description 1
- 238000002744 homologous recombination Methods 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 102000044446 human CD46 Human genes 0.000 description 1
- 230000008348 humoral response Effects 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 1
- 229960000908 idarubicin Drugs 0.000 description 1
- 210000001822 immobilized cell Anatomy 0.000 description 1
- 230000000984 immunochemical effect Effects 0.000 description 1
- 230000000951 immunodiffusion Effects 0.000 description 1
- 230000016784 immunoglobulin production Effects 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 230000002134 immunopathologic effect Effects 0.000 description 1
- 238000001114 immunoprecipitation Methods 0.000 description 1
- 230000004957 immunoregulator effect Effects 0.000 description 1
- 238000002650 immunosuppressive therapy Methods 0.000 description 1
- 238000009399 inbreeding Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 238000007914 intraventricular administration Methods 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- DLRVVLDZNNYCBX-RTPHMHGBSA-N isomaltose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)C(O)O1 DLRVVLDZNNYCBX-RTPHMHGBSA-N 0.000 description 1
- 229960003299 ketamine Drugs 0.000 description 1
- VCMGMSHEPQENPE-UHFFFAOYSA-N ketamine hydrochloride Chemical compound [Cl-].C=1C=CC=C(Cl)C=1C1([NH2+]C)CCCCC1=O VCMGMSHEPQENPE-UHFFFAOYSA-N 0.000 description 1
- PZDOWFGHCNHPQD-OQPGPFOOSA-N kojibiose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](C=O)O[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O PZDOWFGHCNHPQD-OQPGPFOOSA-N 0.000 description 1
- 239000004922 lacquer Substances 0.000 description 1
- QIGJYVCQYDKYDW-LCOYTZNXSA-N laminarabiose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1O[C@H]1[C@H](O)[C@@H](CO)OC(O)[C@@H]1O QIGJYVCQYDKYDW-LCOYTZNXSA-N 0.000 description 1
- 230000002045 lasting effect Effects 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 229920006008 lipopolysaccharide Polymers 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 229940057995 liquid paraffin Drugs 0.000 description 1
- 229960002247 lomustine Drugs 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 229940041033 macrolides Drugs 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 210000001161 mammalian embryo Anatomy 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000010534 mechanism of action Effects 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- QWIZNVHXZXRPDR-WSCXOGSTSA-N melezitose Chemical compound O([C@@]1(O[C@@H]([C@H]([C@@H]1O[C@@H]1[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O1)O)O)CO)CO)[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O QWIZNVHXZXRPDR-WSCXOGSTSA-N 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 229960001810 meprednisone Drugs 0.000 description 1
- PIDANAQULIKBQS-RNUIGHNZSA-N meprednisone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)CC2=O PIDANAQULIKBQS-RNUIGHNZSA-N 0.000 description 1
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 1
- 229960001428 mercaptopurine Drugs 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 229960004584 methylprednisolone Drugs 0.000 description 1
- 229960000282 metronidazole Drugs 0.000 description 1
- VAOCPAMSLUNLGC-UHFFFAOYSA-N metronidazole Chemical compound CC1=NC=C([N+]([O-])=O)N1CCO VAOCPAMSLUNLGC-UHFFFAOYSA-N 0.000 description 1
- 244000309715 mini pig Species 0.000 description 1
- CFCUWKMKBJTWLW-BKHRDMLASA-N mithramycin Chemical compound O([C@@H]1C[C@@H](O[C@H](C)[C@H]1O)OC=1C=C2C=C3C[C@H]([C@@H](C(=O)C3=C(O)C2=C(O)C=1C)O[C@@H]1O[C@H](C)[C@@H](O)[C@H](O[C@@H]2O[C@H](C)[C@H](O)[C@H](O[C@@H]3O[C@H](C)[C@@H](O)[C@@](C)(O)C3)C2)C1)[C@H](OC)C(=O)[C@@H](O)[C@@H](C)O)[C@H]1C[C@@H](O)[C@H](O)[C@@H](C)O1 CFCUWKMKBJTWLW-BKHRDMLASA-N 0.000 description 1
- 229960005485 mitobronitol Drugs 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 230000002107 myocardial effect Effects 0.000 description 1
- BLUYEPLOXLPVCJ-INIZCTEOSA-N n-[(1s)-2-[4-(3-aminopropylamino)butylamino]-1-hydroxyethyl]-7-(diaminomethylideneamino)heptanamide Chemical compound NCCCNCCCCNC[C@H](O)NC(=O)CCCCCCNC(N)=N BLUYEPLOXLPVCJ-INIZCTEOSA-N 0.000 description 1
- 229950006780 n-acetylglucosamine Drugs 0.000 description 1
- 229960004270 nabumetone Drugs 0.000 description 1
- 239000006199 nebulizer Substances 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- HYWYRSMBCFDLJT-UHFFFAOYSA-N nimesulide Chemical compound CS(=O)(=O)NC1=CC=C([N+]([O-])=O)C=C1OC1=CC=CC=C1 HYWYRSMBCFDLJT-UHFFFAOYSA-N 0.000 description 1
- 229960000965 nimesulide Drugs 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 1
- 230000036963 noncompetitive effect Effects 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000007899 nucleic acid hybridization Methods 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 238000002515 oligonucleotide synthesis Methods 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 239000003791 organic solvent mixture Substances 0.000 description 1
- 229960004534 orgotein Drugs 0.000 description 1
- 108010070915 orgotein Proteins 0.000 description 1
- 229960005113 oxaceprol Drugs 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- LSQZJLSUYDQPKJ-UHFFFAOYSA-N p-Hydroxyampicillin Natural products O=C1N2C(C(O)=O)C(C)(C)SC2C1NC(=O)C(N)C1=CC=C(O)C=C1 LSQZJLSUYDQPKJ-UHFFFAOYSA-N 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- XKFIQZCHJUUSBA-UHFFFAOYSA-N perisoxal Chemical compound C1=C(C=2C=CC=CC=2)ON=C1C(O)CN1CCCCC1 XKFIQZCHJUUSBA-UHFFFAOYSA-N 0.000 description 1
- 229950005491 perisoxal Drugs 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 230000035479 physiological effects, processes and functions Effects 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 229950006452 pifoxime Drugs 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 238000009832 plasma treatment Methods 0.000 description 1
- 239000004014 plasticizer Substances 0.000 description 1
- 229960003171 plicamycin Drugs 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 229920000724 poly(L-arginine) polymer Polymers 0.000 description 1
- 229920000083 poly(allylamine) Polymers 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 108010011110 polyarginine Proteins 0.000 description 1
- 108010052780 polyasparagine Proteins 0.000 description 1
- 108010040003 polyglutamine Proteins 0.000 description 1
- 229920000155 polyglutamine Polymers 0.000 description 1
- 229920002714 polyornithine Polymers 0.000 description 1
- 108010055896 polyornithine Proteins 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 150000004804 polysaccharides Chemical class 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 238000010837 poor prognosis Methods 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- AOHJOMMDDJHIJH-UHFFFAOYSA-N propylenediamine Chemical compound CC(N)CN AOHJOMMDDJHIJH-UHFFFAOYSA-N 0.000 description 1
- 229960002466 proquazone Drugs 0.000 description 1
- JTIGKVIOEQASGT-UHFFFAOYSA-N proquazone Chemical compound N=1C(=O)N(C(C)C)C2=CC(C)=CC=C2C=1C1=CC=CC=C1 JTIGKVIOEQASGT-UHFFFAOYSA-N 0.000 description 1
- 235000004252 protein component Nutrition 0.000 description 1
- 239000003586 protic polar solvent Substances 0.000 description 1
- OLTAWOVKGWWERU-UHFFFAOYSA-N proxazole Chemical compound C=1C=CC=CC=1C(CC)C1=NOC(CCN(CC)CC)=N1 OLTAWOVKGWWERU-UHFFFAOYSA-N 0.000 description 1
- 229960001801 proxazole Drugs 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- 150000003214 pyranose derivatives Chemical group 0.000 description 1
- JEXVQSWXXUJEMA-UHFFFAOYSA-N pyrazol-3-one Chemical class O=C1C=CN=N1 JEXVQSWXXUJEMA-UHFFFAOYSA-N 0.000 description 1
- 150000003217 pyrazoles Chemical class 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 150000007660 quinolones Chemical class 0.000 description 1
- 238000003127 radioimmunoassay Methods 0.000 description 1
- MUPFEKGTMRGPLJ-ZQSKZDJDSA-N raffinose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O2)O)O1 MUPFEKGTMRGPLJ-ZQSKZDJDSA-N 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000010410 reperfusion Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 229940100486 rice starch Drugs 0.000 description 1
- 238000007363 ring formation reaction Methods 0.000 description 1
- 229940058287 salicylic acid derivative anticestodals Drugs 0.000 description 1
- 150000003872 salicylic acid derivatives Chemical class 0.000 description 1
- 229960003440 semustine Drugs 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 238000009097 single-agent therapy Methods 0.000 description 1
- 238000004513 sizing Methods 0.000 description 1
- 210000004927 skin cell Anatomy 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- 239000012279 sodium borohydride Substances 0.000 description 1
- 229910000033 sodium borohydride Inorganic materials 0.000 description 1
- JQWHASGSAFIOCM-UHFFFAOYSA-M sodium periodate Chemical compound [Na+].[O-]I(=O)(=O)=O JQWHASGSAFIOCM-UHFFFAOYSA-M 0.000 description 1
- 235000010339 sodium tetraborate Nutrition 0.000 description 1
- 239000007901 soft capsule Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000012439 solid excipient Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- PZDOWFGHCNHPQD-VNNZMYODSA-N sophorose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](C=O)O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O PZDOWFGHCNHPQD-VNNZMYODSA-N 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 238000010911 splenectomy Methods 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 229920006301 statistical copolymer Polymers 0.000 description 1
- 239000008174 sterile solution Substances 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 125000000446 sulfanediyl group Chemical group *S* 0.000 description 1
- 150000003462 sulfoxides Chemical class 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- 239000002511 suppository base Substances 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 229940037128 systemic glucocorticoids Drugs 0.000 description 1
- 229960003676 tenidap Drugs 0.000 description 1
- LXIKEPCNDFVJKC-QXMHVHEDSA-N tenidap Chemical compound C12=CC(Cl)=CC=C2N(C(=O)N)C(=O)\C1=C(/O)C1=CC=CS1 LXIKEPCNDFVJKC-QXMHVHEDSA-N 0.000 description 1
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 description 1
- 229960001278 teniposide Drugs 0.000 description 1
- TUGDLVFMIQZYPA-UHFFFAOYSA-N tetracopper;tetrazinc Chemical compound [Cu+2].[Cu+2].[Cu+2].[Cu+2].[Zn+2].[Zn+2].[Zn+2].[Zn+2] TUGDLVFMIQZYPA-UHFFFAOYSA-N 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- 229960001196 thiotepa Drugs 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- 239000004408 titanium dioxide Substances 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- 150000004654 triazenes Chemical class 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- CYRMSUTZVYGINF-UHFFFAOYSA-N trichlorofluoromethane Chemical compound FC(Cl)(Cl)Cl CYRMSUTZVYGINF-UHFFFAOYSA-N 0.000 description 1
- 229940029284 trichlorofluoromethane Drugs 0.000 description 1
- FYZXEMANQYHCFX-UHFFFAOYSA-K tripotassium;2-[2-[bis(carboxylatomethyl)amino]ethyl-(carboxymethyl)amino]acetate Chemical compound [K+].[K+].[K+].OC(=O)CN(CC([O-])=O)CCN(CC([O-])=O)CC([O-])=O FYZXEMANQYHCFX-UHFFFAOYSA-K 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- BSVBQGMMJUBVOD-UHFFFAOYSA-N trisodium borate Chemical compound [Na+].[Na+].[Na+].[O-]B([O-])[O-] BSVBQGMMJUBVOD-UHFFFAOYSA-N 0.000 description 1
- 239000013598 vector Substances 0.000 description 1
- 230000002861 ventricular Effects 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 229940100445 wheat starch Drugs 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/001—Preparations to induce tolerance to non-self, e.g. prior to transplantation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/59—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/59—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes
- A61K47/60—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes the organic macromolecular compound being a polyoxyalkylene oligomer, polymer or dendrimer, e.g. PEG, PPG, PEO or polyglycerol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/61—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule the organic macromolecular compound being a polysaccharide or a derivative thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/64—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent
- A61K47/645—Polycationic or polyanionic oligopeptides, polypeptides or polyamino acids, e.g. polylysine, polyarginine, polyglutamic acid or peptide TAT
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/60—Medicinal preparations containing antigens or antibodies characteristics by the carrier linked to the antigen
- A61K2039/6093—Synthetic polymers, e.g. polyethyleneglycol [PEG], Polymers or copolymers of (D) glutamate and (D) lysine
Definitions
- This invention is in the general field of pharmaceuticals for treatment of conditions caused by xenoreactive antibodies.
- Organ transplantation is now widely viewed as the preferred treatment for end stage organ failure, based on both a quality of life and cost basis.
- One year graft survival for kidney and heart transplantation at the major transplant centers is now greater than 90% with acute rejection rates less than 30%.
- the major limitation to more widespread utilization of this procedure is a shortage of available organs. This can be seen by looking at the transplant waiting list, which shows that more than twice the number of people are waiting for an organ than could ever receive one. Additionally, 10 people per day die while waiting for a transplant.
- the demand for organs is much greater than the number of people on the transplant waiting list.
- There are 125,000 patients in end stage renal failure who theoretically could benefit from a transplant. It is also been estimated that 25,000 to 40,000 people could benefit from a heart transplant if a donor organ were available.
- the preferred animal species for transplantation to humans is the pig.
- the closely related non-human primate species are immunologically more similar to humans, and therefore the rejection process which is seen upon transplantation could likely be more easily controlled by current immunosuppressive drugs, a number of reasons make this possibility remote.
- a non-human primate of appropriate size for a heart transplant into an adult human would be a chimpanzee or equivalent large animal. These animals are on the endangered species list and thus would raise significant ethical questions even if it were possible to breed large numbers in captivity.
- the most abundant non-human primate, which is the baboon is small and could not provide hearts for transplantation into adult humans although conceivably kidneys or livers would be possible.
- the baboon contains a number of potentially pathogenic organisms which could present a problem if transmitted to a human, and the generation of large numbers of specific pathogen free animals would be extremely costly and time consuming.
- the pig a domesticated farm animal, on the other hand is of an appropriate size, can be raised to obtain specific pathogen free animals and is available in large numbers.
- the immunological problems could be overcome the pig would be the ideal donor to solve the organ shortage problem.
- the initial barrier to transplantation of a pig organ to primates is a process of hyperacute rejection which results in the loss of the graft within a few minutes to hours of transplantation and is characterized histologically by thrombosis, hemorrhage, edema and a lack of a cellular infiltrate (Platt, J. L., et al., Immunology Today 11:450-456, 1990).
- This process is initiated by the binding of antibody, which is already present in the recipient, to the graft endothelium, and the resulting activation of the complement cascade.
- a similar process can be seen in allografts when antibody is present in the recipient prior to transplantation. In thinking about the pig as a donor, it is useful to review the effect of pre-existing antibodies on allograft survival and what strategies are important in obtaining prolonged graft survival.
- the presence of antibody in the serum of an allograft recipient which recognizes antigen present on the donor endothelium, can be an indicator of a poor prognosis for long-term graft survival. Indeed, in a percentage of these allografts a rapid process of rejection, as described above for pig to primate xenografts, in which the graft is lost within a few minutes to hours after reperfusion, can occur. An example of this situation is seen when the donor and recipient are incompatible with regard to the blood group ABO system.
- the blood group antigen which is a carbohydrate, is present on the donor endothelium.
- the xenoreactive antibody which is found in the recipient prior to transplant predominantly if not exclusively recognizes the unique carbohydrate structure, ⁇ Gal(1,3)Gal. This structure is found as the terminal sugar on glycolipids and glycoproteins present on the donor pig endothelium. The pig and many other mammals but not humans or Old World monkeys synthesize this epitope. This scenario is in many ways reminiscent of the ABO blood group mismatched allografts described above. In a similar fashion to the mammalian blood group antigens such as the human blood group A, the lack of the antigen in an animal results in the synthesis of antibodies which recognize it.
- Galili et. el (1988) showed that anti-Gal antibodies bound to a variety of enterobacteria such as E. coli , klebsiella and salmonella.
- the Gal reactive structure varied between the bacteria and was either the lipopolysaccharide or the capsular polysaccharide (Hamadeh et. el. 1992). Both of these classes of antigens are well known as T-cell independent antigens.
- the isotype of the ABO and ⁇ -Gal antibodies are similar, the levels of antibody in the serum are similar and the density of the carbohydrate antigens on the donor endothelium are also within the same range.
- the graft is almost always rejected within a few minutes to hours with the very occasional graft lasting at most several days. This is in direct contrast with what happens in the allograft where the occasional graft is hyperacutely rejected and the majority survive for prolonged periods of time (weeks to months). A significant difference between the survival of allografts and xenografts under these conditions is most likely due to the incompatibility of the complement regulatory proteins.
- antibody binds to the graft endothelium and results in the activation of the complement cascade.
- the allograft there are a series of proteins present on the donor endothelium which regulate the activity of complement and can be thought of as a primitive self/non-self-recognition process whereby if complement is inadvertently activated on the endothelium it can be inactivated before damage occurs.
- These proteins include Decay Accelerating Factor (DAF: CD55), Membrane Cofactor Protein (MCP: CD46) and CD59.
- DAF Decay Accelerating Factor
- MCP Membrane Cofactor Protein
- vascular rejection is characterized histologically like hyperacute rejection by hemorrhage, thrombosis, and a lack of a cellular infiltrate; however, unlike hyperacute rejection there are significant regions of ischaemia. Immunopathological analysis reveals that antibody is bound to the graft but very little complement components are deposited.
- ⁇ Gal(1,3)Gal antibodies A similar rise in ⁇ Gal(1,3)Gal antibodies is seen in humans who are the recipients of cellular pig xenografts such as pancreatic islets or who have been exposed to a pig liver in an ex vivo perfusion circuit. These data suggest that the grafts are lost due to the enhanced production of ⁇ Gal(1,3)Gal antibodies.
- One aspect of the present invention provides a polymer, synthesized by a cross-coupling reaction of
- polymer scaffold units comprising a plurality of nucleophilic or electrophilic functional groups
- reaction is carried out in the presence of a crosslinking reagent under conditions wherein polymer scaffold units are coupled with xenoantigens, and separate polymer scaffold units are cross-linked to one another.
- the functional groups for the polymer scaffold units and xenoantigens are selected from the group consisting of amine and carboxylic acid, alcohol and alkyl halide or sulfonate, thiol and alkyl halide or sulfonate, phosphine and alkyl halide or sulfonate, phosphite and alkyl halide or sulfonate, and aldehyde or ketone and amine.
- the functional groups produce an amide, ether, thioether, or phosphate linkage between the polymer scaffold unit and xenoantigen upon cross-coupling.
- the cross-coupling may be carried out, e.g., in the presence of an activating agent that activates the functional groups of the polymer scaffold units and xenoantigens for forming a covalent bond there between.
- the activating group may be a dehydrating agents, Bronsted acid, Bronsted base, Lewis acid, Lewis base, acyl halide, or a phosphoryl halide.
- the activating agent is a diimide, e.g., dicyclohexyldiimide [DCC] or ethyl-3-(dimethylamino)propyldiimide [EDC].
- the polymer scaffold units are pharmacologically-acceptable, non-immunogenic molecules.
- the polymer scaffold units can be polyethylene glycol, an oligopeptide, or oligosaccharide (e.g., dextran, dextrin or a cyclodextrin) bearing nucleophilic or electrophilic functional groups.
- Suitable nucleophilic or electrophilic functional groups include amines, alcohols, thiols, selenols, phosphines, aldehydes, ketones, acid chlorides, acids, esters, alkyl halides, and alkyl sulfonates.
- the functional group on the polymer scaffold unit is an amino or carboxylate moiety, and the functional group of the xenoantigen reacts therewith to form an amide linkage.
- the polymer scaffold unit is a multiply-aminated polyethylene glycol, e.g., octa(amino)polyethylene glycol.
- the polymer scaffold unit is aminated dextran or an aminated cyclodextrin.
- the polymer scaffold is a dendridic polyamine.
- the xenoantigen can be, to illustrate, a carbohydrate, a peptide, a glycopeptide or a lipid.
- xenoantigen includes an epitope which is cross-reactive with an xenoreactive antibody against ⁇ -galactosyl moieties, and more preferably the xenoantigen is an oligosaccharide, e.g., having an ⁇ Gal(1,3)Gal moiety represented by the general formula:
- R independently for each occurrence, represents H or a C 1 -C 6 alkyl or other hydroxyl protecting group
- R′ is absent or represents an oligosacchride consisting of 1-8 saccharide residues
- X represents a bond or linker moiety linking the oligosacchride moiety to the polymer backbone, e.g., preferably being a cyclic, branched or straight chain aliphatic group of 2-10 bonds in length, cycloalkyl, alkenyls, cycloalkenyl, alkynyl, aryl, heteroalkyl, or heteroaryl moiety.
- the xenoantigen can include an oligosacchride having represented by one of the general formula:
- R1 independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
- R independently for each occurrence, represents H or a C 1 -C 6 alkyl or other hydroxyl protecting group
- R2 is a C 1 -C 6 alkyl
- Y independently for each occurrence, is absent, or —C(S)—, —S(O) 2 —, —C(O)-A-;
- X represents a bond or linker moiety linking the oligosaccbride moiety to the polymer backbone
- Z is O or S
- A is —NH—, —NH—(C 1 -C 6 alkyl)-, —NH—(C 1 -C 6 alkenyl)-, —O—, —O—(C 1 -C 6 alkyl)-, —O—(C 1 -C 6 alkenyl)-, —S—, —S—(C 1 -C 6 alkyl)-, —S—(C 1 -C 6 alkenyl)-.
- the xenoantigen includes one or more of: ⁇ Gal(1,3) ⁇ Gal; ⁇ Gal(1,3) ⁇ Gal(1,4) ⁇ GlcNAc; or ⁇ Gal(1,3) ⁇ Gal(1,4) ⁇ Glc moieties.
- the xenoantigen may include one or more of: ⁇ Gal(1,2)Gal, ⁇ Gal(1,4)Gal, ⁇ Gal(1,3)GalNAc or 3-O-sulphated galactose (SO 4 -3Gal).
- the xenoantigen can be a di-, tri-, tetra- and penta-oligosacchrides.
- the polymer is homogenous with respect to the xenoantigen displayed thereon.
- the polymer includes two or more different xenoantigens, e.g., on the same molecule.
- the crosslinking agent has at least two functional groups capable of reacting with the polymer scaffold units, the xenoantigen, the covalent linkage of the scaffold with the xenoantigen, or a combination thereof.
- the crosslinking agent can be derived from N-hydroxysulfosuccinimide.
- the cross-coupling reaction is carried out in the pH range of 4 to 7.
- the cross-coupling reaction is carried out in the temperature range of 0° C. and 40° C.
- the cross-coupling reaction is carried out a temperature, pH, reactant concentration and for a time sufficient to yield a polymer having a nominal molecular weight of 100,000-500,000 daltons.
- the cross-coupling reaction is carried out a temperature, pH, reactant concentration and for a time sufficient to yield a polymer having a nominal molecular weight less than 100,000 daltons.
- the xenopolymer of the instant invention induces B cell anergy for the xenoantigen.
- the subject xenopolymer is formulated with a pharmaceutical excipient, and is preferably formulated as a sterile formulation.
- Another aspect of the present invention is a method for manufacture of a medicament using the subject xenopolymer, the medicament being administered to a patient which is to receive, or has received, a discordant tissue graft, and in an amount sufficient to reduce the severity of rejection of the graft.
- the medicament is formulated with a xenopolymer having a therapeutic index for preventing discordant graft rejection of at least 10.
- kits including two or more of the subject xenopolymers, each polymer isolated from the other and homogenous with respect to the xenoantigen displayed thereon but different from at least one other polymer of the kit.
- Another aspect of the present invention provides a polymer comprising a plurality of subunits interconnected by crosslinking moieties. See generally, FIG. 25.
- Each crosslinking moiety is covalently bound to at least two subunits, although the individual bonds between a crosslinker and a subunit need not be of the same type or comprise the same atom(s) of the subunits.
- the individual subunits comprise a backbone or scaffold, and a plurality of xenoantigens.
- the xenoantigens are covalently tethered to the backbone or scaffold, typically via a tether consisting of between 10 and 20 non-hydrogen atoms.
- the backbone or scaffold may itself be a polymer, e.g., an oligoethylene glycol, oligosaccharide, or oligopeptide.
- the synthesis of a polymer of this embodiment of the present invention may be achieved in a single reaction or in a series of reactions.
- the process comprises combining the backbone or scaffold with the crosslinking agent and the xenoantigens to give a product comprising subunits, i.e., individual backbone or scaffold groups to which xenoantigens have been tethered, that are covalently interconnected via crosslinking moieties. See FIG. 25.
- the overall process comprises: combining the backbone or scaffold with the xenoantigens under conditions where the two react to generate subunits bearing a plurality of tethered xenoantigens; and combining the subunits bearing a plurality of tethered xenoantigens with the crosslinking agent to form crosslinks between the individual subunits.
- Certain embodiments of this aspect of the present invention provide a polymer, comprising a polymer backbone of polyethylene glycol subunits, at least a portion of which have attached thereto a saccharide moiety represented in the following general formula:
- R1 independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
- R5 is H, —OR, NH—Y—R2, or -L-E;
- Y independently for each occurrence is absent, —C(O)—, —C(S)—, —C(NR)—, O, S, or Se;
- B for one occurrence is —R, and for the other occurrence is —R or from 1-9 saccharide residues;
- R independently for each occurrence, represents H or a C 1 -C 6 alkyl, a protecting group, or -L-E;
- R2 is a C 1 -C 6 alkyl
- X2 represents a linker of 1-10 atoms in the length
- L represents a linker of 1-20 atoms in length, e.g., preferably being a cyclic, branched or straight chain aliphatic group of 2-15 bonds in length, cycloalkyl, alkenyls, cycloalkenyl, alkynyl, aryl, heteroalkyl, or heteroaryl moiety; and
- E represents a second PEG subunit of the polymer, wherein, independently for each occurrence of E, the linker group L is covalently attached via R5 or B of a saccharide moiety of the second PEG subunit, or via an amine of the second PEG subunit or via an amide moiety in the tether between the saccharide and the corresponding polyethylene glycol subunit.
- Certain embodiments of this aspect of the present invention provide a polymer, comprising a polymer backbone of polyethylene glycol subunits, at least a portion of which have attached thereto a saccharide moiety represented in the general formula (VII):
- R1 independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
- R5 is H, —OR, NH—Y—R2, or -L-E;
- Y independently for each occurrence is absent, —C(O)—, —C(S)—, —C(NR)—, O, S, or Se;
- B for one occurrence is —R, and for the other occurrence is —R or from 1-9 saccharide residues;
- R independently for each occurrence, represents H or a C 1 -C 6 alkyl or other hydroxyl protecting group
- R2 is a C 1 -C 6 alkyl
- X2 represents a linker of 1-10 atoms in the length
- D represents H, or -L-E
- L represents a linker of 1-20 atoms in length
- E represents a second PEG subunit of the polymer, wherein, independently for each occurrence of E, the linker group L is covalently attached via R5 or B of a saccharide moiety of the second PEG subunit, or via an amine of the second PEG subunit.
- Still another aspect of the present invention is directed to a polymer, represented by the general formula
- R1 independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
- R5 is H, —OR, NH—Y—R2, or -L-E;
- Y independently for each occurrence is absent, —C(O)—, —C(S)—, —C(NR)—, O, S, or Se;
- B for one occurrence is —R, and for the other occurrence is —R or from 1-9 saccharide residues;
- R independently for each occurrence, represents H or a C 1 -C 6 alkyl or other hydroxyl protecting group
- R2 is a C 1 -C 6 alkyl
- X2 represents a linker of 1-10 atoms in the length D represents H, or -L-E;
- L represents a linker a linker of 1-20 atoms in length
- E represents another polyethylene glycol subunit of the polymer having a saccharide moiety VII attached thereto, wherein, independently for each occurrence of E, the linker group L is covalently attached via R5 or B in the saccharide moiety.
- PEG represents a polyethylene glycol subunit
- X3 represents one or more additional polyethylene glycol subunit which may be derivatived with a ⁇ saccharide moiety of formula VII,
- the polymer has an average molecular weight in the range of 10,000 daltons to 1,000,000 daltons.
- Still another aspect of the invention relates to a composition including: a non-immunogenic pharmacologically acceptable carrier; and multiple ⁇ Gal(1,3)Gal moieties covalently linked to said carrier.
- the invention is also directed to a composition useful for reducing plasma levels of anti-( ⁇ Gal(1,3)Gal) antibodies in a primate subject in need of a pig organ transplant.
- the term “primate” is understood to include humans. Reduction of levels of circulating anti-( ⁇ Gal(1,3)Gal) antibodies is expected to prevent or ameliorate hyperacute rejection of the donated pig organ, thereby allowing long-term function of the transplanted organ.
- composition of the invention comprises a non-immunogenic pharmacologically acceptable carrier covalently linked to multiple ⁇ Gal(1,3)Gal moieties.
- the invention is also directed to methods for synthesizing the composition.
- a preferred method is to react in a solution at a pH of 4-7 the following reagents:
- branched polymer exemplified by polyethylene glycol, which has at least three arms and a minimum of 6 active groups such as amino groups or hydrazino group at its free ends,
- a second preferred method is conducted as above, except the branched polymer has covalently linked carboxy groups, and the ⁇ Gal(1,3)Gal has a covalently linked active group such as an amino or hydrazino group.
- a third preferred method is to react in a solution at a pH range of 4 to 7 the following reagents:
- a branched polymer having at least three arms, and at least six active groups such as amino or hydrazino groups covalently linked to the free ends of the arms, and - ⁇ Gal(1,3)Gal having a covalently linked aldehyde.
- a fourth preferred method is conducted as the third method above, except that the branched polymer has at least six aldehydes covalently linked to its arms, and the ⁇ Gal(1,3)Gal has a covalently linked amino group or hydrazino group.
- a fifth preferred method is to react in a solution at a pH range of 4 to 7 the following reagents:
- branched polymer having at least three arms and at least three covalently linked active groups such as amino or hydrazino groups,
- N-hydroxysulfosuccinimide (NHSS).
- a sixth preferred method is conducted as the fifth method above, except that the branched polymer has covalently linked carboxy groups and the ⁇ Gal(1,3)Gal has covalently linked amino or hydrazino groups.
- the invention also provides methods for reducing plasma levels of anti-( ⁇ Gal(1,3)Gal) antibodies in primate subjects, including human subjects, by administering the composition of the invention.
- the invention is also directed to a kit and methods for detecting and quantifying anti-( ⁇ Gal(1,3)Gal) antibody secreting cells in the blood of a subject.
- Yet another aspect of the invention provides a method for attenuating rejection of tissues from a donor animal of one species transplanted to a recipient animal of another species, comprising administering to the recipient animal an amount of a polymer reactive with xenoreactive antibodies of the recipient animal effective for delaying or lessening the severity of a graft rejection response, wherein the polymer has epitopes cross-reactive with an xenoantigen of the graft, and rejection of the tissue is mediated at least in part by the expression of the xenoantigen on the tissue.
- the subject method can be used as part of a pretreatment program for a patient who is to be transplanted with tissue or cells from a discordant species, and/or for treatment of a patient who has been transplanted with tissue or cells from a discordant species.
- the method reduces or otherwise attenuates the severity of hyperacute rejection of the transplanted tissues.
- the polymer is administered in an amount sufficient to neutralize host antibodies immunoreactive with the xenoantigen.
- the receipient does not possess an endogenous UDP galactose: ⁇ -D-galactosyl-1,4-N-acetyl-D-glucosminide ⁇ (1,3) galactosyltransferase ( ⁇ -1,3-GT) activity or does not otherwise produce or display the ⁇ Gal(1,3)Gal xenoantigen on its cells, tissues or organs.
- the recipient can be a human or old world primate.
- the xenograft is of pig origin, more preferably minature swine.
- the xenotransplanted tissue can be an organ, e.g., a vascularized organ, such as a kidney, heart, lung or liver.
- the xenotransplant tissue may also be in the form of parts of organs, cell clusters and glands, such as pancreatic islet cells, skin, corneal tissue and bone marrow or other preparations of hematopoietic cells.
- a composition comprising:
- Still another aspect of the present invention provides a complex comprising one or more B-cells associated with a composition comprising:
- Yet another aspect of the present invention provides a complex comprising one or more antibodies associated with a composition comprising:
- kits for detecting and quantifying anti-( ⁇ Gal(1,3)Gal) antibody-secreting cells in the blood of a subject comprising:
- blocking solution comprising a buffer and human serum albumin
- [0130] means for labeling said anti-human immunoglobulin antibodies bound to the plate.
- Still another feature to the instant invention is a method for detecting and quantifying anti-( ⁇ Gal(1,3)Gal) antibody-secreting cells in the blood of a subject comprising:
- FIG. 1 depicts the chemical structures of 4-arm and 8-arm polyethylene glycol with active groups at the end of each arm.
- TPC synthesizing an exemplary composition of the invention (TPC)
- ⁇ Gal(1,3)Gal ⁇ 1-4 GlcNAc is covalently bound at amino sites on the ends of the arms.
- FIG. 2 depicts the chemical structure of ⁇ Gal(1,3)Gal ⁇ 1-4 GlcNAc (sugar) covalently bound to one arm of a multi-armed polyethylene glycol (PEG-NH 2 ).
- FIG. 3 shows the concentration of various compositions required for 50% inhibition of xenoreactive antibody activity.
- FIG. 4 shows the effect of TPC (410S) administration on anti-Gal IgM levels in cynomologous monkeys.
- FIG. 5 shows the effect of TPC (410S) administration on anti-Gal IgG levels in cynomologous monkeys.
- FIG. 6 shows xenoreactive antibody levels in a cynomologous monkey that received control vehicle instead of TPC (410S). “Tx” indicates day of heterotopic heart transplant.
- FIG. 7 shows suppression of xenoreactive antibody levels in a cynomologous monkey that received 250 mg TPC (410S) by infusion according to the schedule described in Example 17. “Tx” indicates day of heterotopic heart transplant.
- FIG. 8 likewise shows suppression of xenoreactive antibody levels in a second monkey treated as described in Example 17.
- FIG. 9 likewise shows suppression of xenoreactive antibody levels in a third monkey treated as described in Example 17.
- FIG. 10 shows the inhibition of anti-Gal IgM secreting B cells achieved with an exemplary composition of the invention.
- TPC 410S shows the inhibition of anti-Gal IgG secreting B cells by TPC (410S).
- FIG. 12 shows suppression of xenoreactive IgM levels in a baboon treated with TPC (410S) as described in Example 19.
- FIG. 13 likewise shows suppression of xenoreactive IgM levels in a second baboon treated with TPC (410S) as described in Example 19.
- FIGS. 14 - 23 depict selected oligosaccharide moieties.
- FIG. 24 presents the IC 50 values of various molecules in the inhibition assay.
- FIG. 25 depicts a schematic representation of one possible structure for the products produced by the instant processes.
- FIG. 26 is a graph of IgM XNA Levels: 810S vs IP-treated baboons. Baboons were either treated with 810S (50 mg/kg) every 3 days for a total of 6 treatments, or underwent a total of 6 immunoapheresis treatments using ⁇ -gal columns every 3 days. Serum from each baboon was analyzed by ELISA for IgM antibodies directed against the ⁇ -gal epitope. The percent IgM XNAs remaining were calculated using baseline values from each animal as 100%.
- FIG. 27 is a graph of IgG XNA Levels: 810S vs IP-treated baboons. Baboons were either treated with 810S (50 mg/kg) every 3 days for a total of 6 treatments, or underwent a total of 6 immunoapheresis treatments using ⁇ -gal columns every 3 days. Serum from each baboon was analyzed by ELISA for IgG antibodies directed against the a -gal epitope. The percent IgM XNAs remaining were calculated using baseline values from each animal as 100%.
- FIG. 28 is a graph of baboon 46-98 antibody response to 810S.
- Baboon 46-98 was treated with 810S (50 mg/kg) every 3 days for a total of 6 treatments.
- Serum was analyzed for IgM and IgG antibodies directed against the ⁇ -gal epitope (XIgM or XIgG), as well as for total IgM (TotIgM) or total IgG (TotIgG) by ELISA. The percent antibody remaining was calculated using baseline values as 100%.
- FIG. 29 is a graph of baboon 52-98 antibody response to 810S.
- Baboon 52-98 was treated with 810S (50 mg/kg) every 3 days for a total of 6 treatments.
- Serum was analyzed for IgM and IgG antibodies directed against the ⁇ -gal epitope (XIgM or XIgG), as well as for total IgM (TotIgM) or total IgG (TotIgG) by ELISA. The percent antibody remaining was calculated using baseline values as 100%.
- FIG. 30 is a graph of baboon 50-98 antibody response to 810S.
- Baboon 50-98 was treated with 810S (50 mg/kg) every 3 days for a total of 6 treatments.
- Serum was analyzed for IgM and IgG antibodies directed against the ⁇ -gal epitope (XIgM or XIgG), as well as for total IgM (TotIgM) or total IgG (TotIgG) by ELISA. The percent antibody remaining was calculated using baseline values as 100%.
- FIG. 31 is a graph of baboon 51-98 antibody response to IP.
- Baboon 51-98 underwent a total of 6 immunoapheresis treatments using ⁇ -gal columns every 3 days. Serum was analyzed for IgM and IgG antibodies directed against the ⁇ -gal epitope (XIgM or XIgG), as well as for total IgM (TotIgM) or total IgG (TotIgG) by ELISA. The percent antibody remaining was calculated using baseline values as 100%.
- FIG. 32 is a graph of baboon 48-98 antibody response to IP.
- Baboon 48-98 underwent a total of 6 immunoapheresis treatments using ⁇ -gal columns every 3 days. Serum was analyzed for IgM and IgG antibodies directed against the ⁇ -gal epitope (XIgM or XIgG), as well as for total IgM (TotIgM) or total IgG (TotIgG) by ELISA. The percent antibody remaining was calculated using baseline values as 100%.
- FIG. 33 is a graph of baboon 47-98 antibody response to IP.
- Baboon 47-98 underwent a total of 6 immunoapheresis treatments using ⁇ -gal columns every 3 days. Serum was analyzed for IgM and IgG antibodies directed against the ⁇ -gal epitope (XIgM or XIgG), as well as for total IgM (TotIgM) or total IgG (TotIgG) by ELISA. The percent antibody remaining was calculated using baseline values as 100%.
- FIG. 34 compares the IgG response between IP and TPC (810) treated baboons.
- Baboons were either treated with 810S (50 mg/kg) every 3 days for a total of 6 treatments, or underwent a total of 6 immunoapheresis treatments using ⁇ -gal columns every 3 days.
- Peripheral blood mononuclear cells were collected and analyzed for the number of cells secreting IgG antibodies directed against ⁇ -gal using an ELISPOT analysis.
- the fold-change in antibody secreting cells (ASCs) was calculated by normalizing to baseline values. Values are represented as the mean from 3 baboons per group.
- FIG. 35 compares the IgM response between IP and TPC (810) treated baboons.
- Baboons were either treated with 810S (50 mg/kg) every 3 days for a total of 6 treatments, or underwent a total of 6 immunoapheresis treatments using ⁇ -gal columns every 3 days.
- Peripheral blood mononuclear cells were collected and analyzed for the number of cells secreting IgM antibodies directed against ⁇ -gal using an ELISPOT analysis.
- the fold-change in antibody secreting cells (ASCs) was calculated by normalizing to baseline values. Values are represented as the mean from 3 baboons per group.
- Pigs are top candidates for xenotransplant donors because their physiology and anatomy parallel that of humans, and because they are plentiful and can be raised under clean conditions to minimize risk of infection.
- hyperacute rejection is associated at least in part with host antibodies reactive with galactosyl antigens present on the pig tissue, such as ⁇ galactosyl (1,3) galactose moieties (the “ ⁇ Gal(1,3)Gal” or “ ⁇ Gal” epitope). Inhibiting the interaction between host antibodies reactive with the ⁇ Gal(1,3)Gal epitopes of xenotransplant tissue can be used to prevent rejection.
- One aspect of the present invention relates to methods and compositions for attenuating xenograft rejection by administering, to an animal receiving the xenograft, an amount of a polymer-derivatized xenoantigen (hereinafter “xenopolymer”) effective for inhibiting or lessening the severity of hyperacute rejection response (HAR), or other immunological response to the graft, that is dependent on the presence of the xenoantigen on the grafted tissues or cells.
- xenopolymer is administered in an amount sufficient to neutralize host antibodies (“xenoreactive antibodies” or “XNA”) immunoreactive with the xenoantigen.
- the xenopolymer may additionally, or alternatively, be used as a tolerogen (or anergen) for the xenoantigen, e.g., able to suppress, to some degree, the production/secretion of XNAs by the immune system of the host.
- a tolerogen or anergen
- the xenoantigen can be, to illustrate, a carbohydrate, a protein (or peptidyl portion thereof), a lipid or other fatty acid moitey, or a nucleic acid.
- the xenoantigen(s) that is disposed on the polymer is immunoreactive with host XNAs against carbohydrate epitopes of the xenograft, and more preferably is immunoreactive with host antibodies against ⁇ -galactosyl epitopes, such as an ⁇ Gal(1,3)Gal epitope.
- the polymer component e.g., the backbone of the subject xenoploymers
- the polymer component is preferably derived from a polymer that is water-soluble, and substantially non-toxic and non-immunogenic.
- Illustrative polymers of this type include polyethyleneglycols, polyvinylpyrrolidones, polyacrylamides, dextrans, and polyglycomers.
- the xenopolymer is formed from a polyamine, such as aminated polyethyleneglycols, polyalkylamines, polypeptides (such as polylysine, polyornithine, polyarginine, polyasparagine, polyglutamine), polyallylamines, poly[N(2-aminoethyl)]methacrylamides, and polyaminocarbohydrates such as aminodextran or chitosan.
- the polymer may be homopolymeric or copolymeric, and if the latter, may be a block or statistical copolymer.
- the subject method can be used to promote tolerance to the ⁇ Gal(1,3)Gal antigen in a recipient mammal which does not possess an endogenous UDP galactose: ⁇ -D-galactosyl-1,4-N-acetyl-D-glucosminide ⁇ (1,3) galactosyltransferase ( ⁇ 1,3-GT) activity or which otherwise does not produce or display the ⁇ Gal(1,3)Gal antigen on its cells, tissues or organs.
- the recipient animal is a human or old world primate, e.g., a baboon (such as Papio anubis ) or cynomolgus monkey (such as Macaca fascicularis ).
- a human or old world primate e.g., a baboon (such as Papio anubis ) or cynomolgus monkey (such as Macaca fascicularis ).
- Xenografted tissue is preferably of pig origin, though tissues from other non-human mammals are also contemplated for use in this invention.
- the xenotransplanted tissue is in the form of an organ, and more preferably a vascularized organ, as for example, kidney, heart, lung or liver.
- Xenotransplant tissue may also be in the form of parts of organs, cell clusters, glands and the like. Examples include lenses, pancreatic islet cells, skin, corneal tissue and bone marrow or other preparations of hematopoietic cells.
- the xenopolymers of the present invention have interesting properties. In particular, they have affinity for human polyclonal XNAs present in body fluids, e.g., blood, and are useful as depleting agents.
- the subject xenopolymers can be used for intracorporal depletion of XNAs, e.g., by injection, infusion or perfusion of the polymer into a xenograft recipient prior to and/or after transplantation.
- the xenopolymers of the present invention in certain embodiments, may also be used as a pharmaceutical for inducing tolerance or anergy towards xenoantigenic epitopes or to specifically target B cells with xenoantigen receptors. The administration may be repeated as required to achieve tolerance.
- the method of the present invention includes a step of pre-treating a patient, who is to be transplanted with tissue or cells from a discordant species, with an effective amount of the subject xeno-polymer so as to reduce the risk of hyperacute rejection or other adverse immunological reaction to the xenograft, upon transplantation.
- the subject method includes a step of treating a patient, who has been transplanted with tissue or cells from a discordant species, with an effective amount of the subject xeno-polymer so as to reduce the risk of hyperacute rejection or other adverse immunological reaction to the xenograft.
- Treatment according to the method of this invention will not necessarily prevent all aspects of rejection, but it will be effective to suppress the antibody-dependent component of transplant rejection. Even partial suppression of rejection is beneficial, because mitigation of the rejection phenomena will allow rescue of some of the tissue in the graft in a viable state. For example, even a partially functioning kidney may permit the recipient to avoid dialysis or prolong the periods between dialysis, even if some cell lysis has occurred. Similarly, a fully functional heart graft is preferable, but mitigation of rejection sufficient to permit some increase in exertion by the recipient, relative to the recipient's state before transplant, is valuable. Partial rescue of liver tissue in a liver graft undergoing rejection is also valuable because the remaining viable liver tissue can recover and regenerate while the patient is supported with coagulation proteins and other substances normally produced by the liver.
- the subject method can be combined with other transplantation therapies, including immunoaphoresis and other treatment protocols for inducing accommodation, immunosuppressive therapy and the use of genetically engineered donor tissue.
- compositions including the subject xenopolymers, especially polymers displaying xenoantigens immunoreactive with host antibodies against ⁇ -galactosyl epitopes such as ⁇ Gal(1-3)Gal.
- the polymer preparations are formulated with a pharmaceutically acceptable excipient, are sterile, and are substantially non-pyrogenic.
- compositions of the invention are the combined properties of activity at a very low molar concentration plus long-term stability that make the compositions of the invention practical and useful as pharmaceuticals. These properties allow for effectiveness at low doses, which are expected to be well tolerated by patients in need of chronic reduction of circulating anti-Gal antibodies.
- stability of the compositions allows for long-tern storage at temperatures achievable in a household setting, thus opening the possibility that patients may self-administer the pharmaceuticals over the long term, perhaps even over their entire lifetime after receiving a pig organ transplant.
- Another aspect of the present invention relates to the use of the subject xenopolymers in the manufacture of a medicament for the treatment of a discordant graft rejection, wherein the medicament is administered to a patient either as a pre-transplant conditioning or for maintenance after transplantation, or both, and in an amount sufficient to reduce the severity of rejection of the graft.
- Gal refers to galactose
- Glc refers to glucose
- GlcNAc refers to N-acetylglucosamine
- sugar monomers or “saccharide subunits”, which are used interchangeably herein, refer to naturally occurring sugars, such as galactose, glucose and the like, as well as sugar monomers which are modifications thereof.
- the term includes protected, partially protected or unprotected deoxysugars of the D- and L-configurations, preferably 2-, 3-, 4-, 5- and 6-deoxyaldoses, such as fucose, rhamnose and digitoxose, 1,2-dideoxyaldoses, such as glucal, galactal and fucal, and 1-, 3-, 4-, 5- and 6-deoxyketoses, 2-, 3-, 4-, 5- and 6-deoxyaminosugars of the D and L configurations, such as glucosamine, mannosamine, galactosamine and fucosamine, and deoxyacylaminosugars, such as N-acylglucosamine, N-acylmannosamine, N-acylgalactosamine
- aldonic, aldaric and uronic acids such as gluconic acid or glucuronic acid, and also ascorbic acid and amino acid-carrying sugar monomers.
- Modified sugar monomers are also understood to mean those having a carbon chain which is longer than 6 C atoms, such as heptoses, octoses, nonoses, heptuloses, octuloses and nonuloses, and also their representatives which are substituted in accordance with the above-listed criteria, such as ketodeoxyoctanic acid, ketodeoxynonanic acid, N-acylneuraminic acids and N-acylmuraminic acids.
- Oligosaccharides are considered to have a reducing end and a non-reducing end, whether or not the sacchride at the reducing end is in fact a reducing sugar. In accordance with accepted nomenclature, oligosacchrides are depicted herein with the non-reducing end on the left and the reducing end on the right.
- the linkage is preferably ⁇ -O-glycosidic or ⁇ -O-glycosidic, e.g., (1 ⁇ 2)-, (1 ⁇ 3)-, (1 ⁇ 4)-, (1 ⁇ 5)-, (1 ⁇ 6)-, (2 ⁇ 3)- and (2 ⁇ 6)-glycosidic linkages.
- disaccharides examples include trehalose, sophorose, kojibiose, laminaribiose, maltose, cellobiose, isomaltose, gentibiose, sucrose and lactose, and their derivatives.
- trisaccharides examples include raffinose and melezitose.
- the oligosaccharides may be linked via S-, N- and C-glycosidic linkages, e.g.
- each sacchride subunit is a pyranose.
- ⁇ Gal(1,3)Gal moiety refers to two galactosyl residues, the Gal residue at the reducing end being an ⁇ -galactosyl moiety, that are covalently joined by a 1-3 glycosidic linkage.
- ⁇ Gal oligosaccharide refers to oligosaccharides (e.g., containing two or more sacchride moieties) including the ⁇ Gal(1,3)Gal moiety.
- XNA refers to xenoreactive natural antibodies, such as human antibodies which are immunoreactive with the carbohydrate ⁇ Gal(1,3)Gal epitope on pig tissues.
- hypertension refers to rapid graft rejection, beginning minutes after implantation, and which is mediated by pre-existing antibodies to the graft.
- a “xenograft” may be an organ, tissue, aggregates of cells, or cells, collectively referred to herein as “tissue”.
- the graft may be selected from any appropriate tissue of the body of the tissue donor. These tissues include, but are not limited to, heart, kidney, lung, islet cells, liver, bowel, skin and hematopoietic cells.
- xenogeneic and xenograft refer to cells or tissue which originates with or is derived from a species other than that of the recipient.
- autologous refers to tissue or cells which originate with or are derived from the recipient, whereas the terms “allogeneic” and “allograft” refer to cells and tissue which originate with or are derived from a donor of the same species as the recipient.
- an “immunosuppressive agent”, as used herein, is an agent, e.g., a chemical agent, e.g., a drug, which, when administered at an appropriate dosage, results in the inhibition of T cells.
- agents e.g., cyclosporine, FK-506, and rapamycin.
- Hematopoietic space-creating irradiation refers to irradiation directed to the hematopoietic tissue, i.e., to tissue in which stem cells are found, e.g., the bone marrow. It is of sufficient intensity to kill or inactivate a substantial number of hematopoietic cells. It is often given as whole body irradiation.
- Tolerance refers to an inhibition of a graft recipient's immune response which would otherwise occur, e.g., in response to the introduction of a nonself antigen into the recipient. Tolerance can involve humoral, cellular, or both humoral and cellular responses. Tolerance, as used herein, refers not only to complete immunologic tolerance to an antigen, but to partial immunologic tolerance, i.e., a degree of tolerance to an antigen which is greater than what would be seen if a method of the invention were not employed. Moreover, tolerance, as used herein, refers to a donor antigen-specific inhibition of the immune system as opposed to the broad spectrum inhibition of the immune system seen with immunosuppressants.
- “Inhibiting immune cell activity” refers to reducing the number of active immune cells, e.g., thymocytes, T cells, B cells, or NK cells, preferably donor reactive cells, or precursor donor reactive cells, in a subject. Inhibition can include partial inhibition, or partial reduction (as opposed to total elimination) of the number of active immune cells, e.g., T cells.
- Discordant species combination refers to two species in which hyperacute rejection occurs when a graft is grafted from one to the other. Generally, discordant species are from different orders, while non-discordant species are from the same order. For example, rats and mice are non-discordant concordant species. Concordant species combinations do not exhibit hyperacute rejection. In xenogeneic method of the invention, the donor and recipient (subject) can be a discordant species combination.
- Miniature swine refers to a miniature pig which is preferably wholly or partially inbred at at least one MHC locus.
- the coefficient of inbreeding of the herd which supplies the miniature swine should be at least, 0.70 and more preferably at least 0.82.
- the herd from which donor animals are drawn should be homozygous at the SLA genes.
- myeloablative refers to a treatment in which death, due to marrow failure, in a significant number of recipients, will occur if hematopoietic stem cell transplantation is not given.
- non-myeloablative refers to a treatment which kills marrow cells but will not, in a significant number of recipients, lead to death from marrow failure.
- subject refers to a mammal, e.g., a human.
- ED 50 means the dose of a drug, e.g., a subject polymer, which produces 50% of its maximum response or effect. Alternatively, the dose which produces a pre-determined response in 50% of test subjects or preparations.
- LD 50 means the dose of a drug which is lethal in 50% of test subjects.
- therapeutic index refers to the therapeutic index of a drug defined as LD 50 /ED 50 .
- the chemical elements are identified in accordance with the Periodic Table of the Elements, CAS version, Handbook of Chemistry and Physics, 67th Ed., 1986-87, inside cover.
- the term “hydrocarbon” is contemplated to include all permissible compounds having at least one hydrogen and one carbon atom.
- the permissible hydrocarbons include acyclic and cyclic, branched and unbranched, carbocyclic and heterocyclic, aromatic and nonaromatic organic compounds which can be substituted or unsubstituted.
- protecting group means temporary substituents which protect a potentially reactive functional group from undesired chemical transformations.
- protecting groups include esters of carboxylic acids, silyl ethers of alcohols, and acetals and ketals of aldehydes and ketones, respectively.
- the field of protecting group chemistry has been reviewed (Greene, T. W.; Wuts, P. G. M. Protective Groups in Organic Synthesis, 2 nd ed.; Wiley: New York, 1991).
- this invention provides pharmaceutical compositions capable of reducing plasma levels of anti- ⁇ Gal(1,3)Gal (“anti- ⁇ Gal”) antibodies in primate subjects, including human patients. While the instant invention contemplates methods and compositions for reducing xenoreactive antibodies to other antigens, for clarity, most of the discussion to follow focuses on oligosaccharide xenoantigens.
- compositions are intended for administration to patients being prepared for transplant of a tissue or cells from a discordant donor, such as a pig organ, a kidney or heart, or patients who have already received such transplants and are in need of reduction of anti- ⁇ Gal antibodies in order to prevent or reduce rejection or other antibody-dependent degradation in performance of the pig organ, e.g., to reduce hyperacute rejection of the organ.
- a discordant donor such as a pig organ, a kidney or heart
- a preferred composition of the invention is a polymer derived from the reaction of a polymer backbone comprising a plurality of nucleophilic or electrophilic functional groups with a xenoantigen comprising a functional group capable of reacting with the functional groups presented on the structural backbone in the presence of a reagent capable of activating one set of the aforementioned functional groups for reaction with the other set of functional groups, all in the presence of a crosslinking reagent (see FIG. 25).
- the complementary pairs of functional groups are selected from the group consisting of amine/carboxylic acid, alcohol/alkyl halide or sulfonate, thiol/alkyl halide or sulfonate, phosphine/alkyl halide or sulfonate, phosphite/alkyl halide or sulfonate, and aldehyde or ketone/amine.
- Suitable complementary pairs of functional groups are those that produce a linkage between the antigen and the scaffold that is robust under physiological conditions, e.g., an amide, ether, thioether, or phosphate.
- the linkage between the scaffold and the antigen comprises an amide moiety.
- the activating reagent i.e., the reagent that facilitates the formation of the covalent bond between the scaffold and the xenoantigen, is any reagent capable of activating a nucleophilic or electrophilic functional group for reaction with a complementary electrophilic or nucleophilic functional group to form a new covalent bond.
- the activating reagent is a dehydrating agent, diimide, Bronsted acid or base, Lewis acid or base, acyl halide, or phosphoryl halide.
- the activating reagent is a diimide, e.g., dicyclohexyldiimide [DCC] or ethyl-3-(dimethylamino)propyldiimide [EDC]. In certain embodiments, the activating agent is EDC.
- the composition has a structural backbone comprising a non-immunogenic, pharmacologically-acceptable scaffold, e.g., polyethylene glycol bearing multiple nucleophilic or electrophilic functional groups.
- the scaffold is preferrably in the molecular weight range of 1,000 Da to 100,000 Da, more preferably in the range of 3,000 to 70,000 Da, most preferably in the range of 5,000 to 30,000 Da.
- the molecular weight of the final polymeric product can be much larger, primarily due to cross-linking of the scaffold units.
- the nucleophilic or electrophilic functional groups on the scaffold are selected from the group comprising amines, alcohols, thiols, selenols, phosphines, aldehydes, ketones, acid chlorides, acids, esters, alkyl halides, and alkyl sulfonates.
- the functional group on the scaffold is an amino or carboxylate moiety, and the functional group comprised by the antigen reacts therewith to form an amide linkage.
- the antigen of the present invention may comprise a carbohydrate, peptide, glycopeptide, lipid, or any small organic or inorganic moiety known to be xenoantigenic, i.e., presented by the xenograft and antigenic to the subject's immune system.
- Xenoantigenic groups can be readily identified by methods well known in the art. For instance, XNAs from human blood can be isolated by perfusing the blood over xenograft material, isolating from the graft those XNAs which specifically bound to it, and using those antibodies to screen candidate epitopes, e.g., by immunoassay.
- the antigen is an oligosaccharide, oligopeptide or glycopeptide.
- human xenoreactive antibodies can be polymorphic with respect to binding specificity for oligosacchrides including the ⁇ Gal(1,3)Gal epitope, e.g., including species which bind to di-, tri-, tetra-, penta- and high order oligosacchrides including the ⁇ Gal(1,3)Gal moiety.
- the invention contemplates xenoantigen-polymers derived with ⁇ Gal(1,3)Gal terminated oligosaccharides, e.g., including from 1-10 saccharide residues, more preferably 1-5 saccharide residues.
- ⁇ Gal oligosaccharides of the invention include those saccharide compositions comprising two or more saccharide moieties in which a galactose moiety is joined by an ⁇ (1,3) glycosidic linkage to another galactose moiety.
- the ⁇ Gal motif (Gal ⁇ 1-3Gal) is located within 15, 10, 5, 4, 3, 2, or 1 saccharide unit(s) from the non-reducing end of the oligosaccharide.
- the ⁇ Gal motif represents the terminus (i.e., the non-reducing end) of the oligosaccharide.
- the ⁇ Gal oligosaccharides may comprise one, or a plurality of ⁇ Gal motifs.
- the ⁇ Gal oligosaccharide may be a branched carbohydrate having multiple terminal Gal ⁇ 1-3Gal residues or a linear oligosaccharide containing both terminal and internal Gal ⁇ 1-3Gal residues.
- the subject polymers can include xenoantigens represented by the general formula (I):
- R independently for each occurrence, represents H or a C 1 -C 6 alkyl or other hydroxyl protecting group, and more preferably represents H for each occurrence;
- R′ is absent or represents an oligosacchride, e.g., having from 1-8 saccharide residues; and
- X represents a bond or linker moiety linking the oligosacchride moiety to the polymer backbone.
- the xenoantigen and an ⁇ Gal terminating saccharide or oligosaccharide e.g., represented in one of the general formulas (III-VI):
- R1 independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
- R independently for each occurrence, represents H or a C 1 -C 6 alkyl or other hydroxyl protecting group, and more preferably represents H for each occurrence;
- R2 is a C 1 -C 6 alkyl, and more preferably represents CH 3 ;
- Y independently for each ocurrence, is absent, or —C(S)—, —S(O) 2 —, —C(O)-A-;
- X represents a bond or linker moiety linking the oligosacchride moiety to the polymer backbone
- Z is O or S
- A is —NH—, —NH—(C 1 -C 6 alkyl)-, —NH—(C 1 -C 6 alkenyl)-, —O—, —O—(C 1 -C 6 alkyl)-, —O—(C 1 -C 6 alkenyl)-, —S—, —S—(C 1 -C 6 alkyl)-, —S—(C 1 -C 6 alkenyl)-.
- the ⁇ Gal oligosaccharides of the invention comprise a saccharide sequence corresponding to the antigenic glycolipid expressed on pig endothelial cell membrances, e.g., vascular endothelial or other endothelial tissue of heart, kidney, liver or lung tissue.
- ⁇ Gal oligosaccharides include, but are not limited to Gal ⁇ 1-3Gal; Gal ⁇ 1-3Gal ⁇ 1-4GlcNAc; Gal ⁇ 1-3Gal ⁇ 1-4GlcNAc ⁇ 1-3Gal; and Gal ⁇ 1-3Gal ⁇ 1-4GlcNAc ⁇ 1-3Gal ⁇ 1-4Glc.
- FIGS. 14 - 23 Other exemplary oligosacchrides for incorporation in the subject xenoantigen-polymers are shown in FIGS. 14 - 23 .
- ⁇ Gal(1,3)Gal epitope several other pig carbohydrate epitopes have been identified as immunoreactive with natural antibodies in human serum, including ⁇ Gal(1,2)Gal, ⁇ Gal(1,4)Gal, ⁇ Gal(1,3)GalNAc and 3-O-sulphated galactose (SO4-3Gal), each of which can be used as a xenoantigen for incorporation into the subject polymers.
- SO4-3Gal 3-O-sulphated galactose
- oligosaccharide epitopes for human xenoreactive antibodies as including A or A-like carbohydrates (namely A disaccharide, A trisacchride, A tetrasacchrides and linear A type 6), Forssman disaccharide and trisaccharide, ⁇ -L-rhamnose and rhamnose-containing oligosaccharides, and ⁇ GlcNac-containing oligosacchrides. See, Cooper et al. (1993) Transplan Immunol 1:198; and Good et al. (1992) Transplan Proc 24:559.
- carbohydrate xenoantigens are also suggested by the fact that other species such as the pig, goat, dog, rat, etc—which do not produce anti- ⁇ Gal(1,3)Gal antibodies—have xenoreactive natural antibodies which presumably recognize other epitopes.
- the subject xenoantigen-polymers can be generated using xenoantigenic oligosaccharides other than ⁇ Gal, e.g., which are cross-reactive with natural human xenoreactive antibodies.
- modified ⁇ Gal oligosaccharides include, but are not limited to, salts and sulfate substitutes of ⁇ Gal oligosaccharides, as well as ⁇ Gal oligosaccharides in which one or more or the pyran rings has been substituted with a piperidine ring system and/or a tetrahydrothiopyran ring system.
- ⁇ Gal oligosaccharide aza sugars in which the oxygen of one or more of the pyran rings of the oligosaccharide is replaced with nitrogen to form a piperidine ring system may be prepared by enzymatic methods known in the art using the appropriate aza saccharide as the acceptor substrate.
- aza sugar donor moieties may be transferred by the corresponding glycosyltransferase for the natural sugar.
- Aza glucose can be isolated from natural sources and converted to the aza lactose by the action of a galactosyltransferase in the presence of a galactose donor such as for example, UDP-galactose.
- the polymeric composition of the invention may also include ⁇ Gal oligosaccharide thio sugars, e.g., in which the is oxygen of one or more of the pyran rings of the oligosaccharide is replaced with sulfur to form a tetrahydrothiopyran ring system.
- the monothiosaccharide may be prepared by known organic chemical techniques from the corresponding monosaccharide and the ⁇ Gal oligosaccharide derivative thio sugar may be prepared applying enzymatic methods and using the appropriate thio saccharide as the acceptor substrate.
- libraries of oligosaccharides can be readily generated, and the ability of individual members of the library, alone or displayed on a polymer, to bind to human xenoreactive antibodies can be readily assessed. Moreover, such libraries can be used to identify oligosaccharides which selectively bind xenoreactive antibodies, but which are not substantially immunoreactive with human (or other host) antibodies for, e.g., bacterial carbohydrate epitopes. In such a manner, the xenoantigen(s) of subject polymers can be optimized.
- the subject polymers can be derived such that single molecules of polymer simultaneously display two or more different xenoantigens.
- the polymeric compositions can be a mixture of different, discrete xenoantigen-polymers to provide a broader spectrum of XNA-neutralizing activity.
- the subject method can include a step of determining the anti- ⁇ Gal phenotype of the patient, and administering a xenoantigen-polymer of the present invention which includes ⁇ Gal oligosaccharides, e.g., di-, tri-, tetra and pentasaccharides, in a ratio which is determined by the phenotype of the individual to be treated, e.g., wherein the ratio approximates the phenotypic ration of anti- ⁇ Gal antibodies.
- a xenoantigen-polymer of the present invention which includes ⁇ Gal oligosaccharides, e.g., di-, tri-, tetra and pentasaccharides, in a ratio which is determined by the phenotype of the individual to be treated, e.g., wherein the ratio approximates the phenotypic ration of anti- ⁇ Gal antibodies.
- the xenoantigen is a protein, or peptidyl portion thereof, which competes with a xenograft for human xenoreactive antibodies.
- Anti-pig antibodies for example, have been identified to bind to protein components on the surface of the graft. For instance, HLA-specific antibodies in highly sensitized patients can cause a positive crossmatch against pig lymphocytes. Taylor et al. (1998) Transplantation 65:1634.
- the crosslinking agent used to generate the subject polymers will preferably include at least two functional groups capable of reacting with the scaffold, the antigen, the functional group formed by the covalent linkage of the scaffold with the antigen, or any or all of them.
- the crosslinking agent is sterically or electronically predisposed, or both, to crosslinking as opposed to reacting with two sites within the same molecule (actually not a crosslinking reaction, but rather a cyclization reaction).
- the crosslinking agent is generated in situ from a precursor.
- the crosslinking agent (48; wherein X represents a leaving group) is generated in situ from N-hydroxysulfosuccinimide (47).
- crosslinking agent reacts with the amide linkages formed by the reaction of amines on the scaffold with carboxylates on the antigens; crosslinking agents represented by 48 are particularly effective in these embodiments.
- the average molecular weight of the oligomers is proportional to the reaction time even though all of the functional groups on the scaffolds react with functional groups on the antigens within the first fifteen minutes of the reaction time. The significance of molecular weight is discussed infra. In these embodiments, crosslinking continues after formation of the covalent bonds between the scaffolds and antigens is complete.
- the polymers of the present invention may be synthesized in a single chemical reaction, i.e., by including all necessary reagents in a single reaction vessel, or they may be synthesized in two or more chemical reactions conducted in series.
- the intermediate compounds may be purified before being subjected to the next reaction in the series, or they may be used without purification.
- chemical reactions conducted in series may be completed in a single reaction vessel, e.g., by allowing a certain group of reagents to react in the vessel for a desired period, followed by introduction into the vessel of the reagents required for the next reaction in the series.
- the products obtained via these various approaches will comprise the same molecular components, but will differ in their properties or activity in a subject or assay due to differences in their microscopic structures, e.g., the degree of crosslinking, or the identities, locations or ratios of the functional groups involved in the crosslinks.
- the reaction protocol and conditions necessary to produce a product of the present invention with properties optimized for a specific assay or subject will be able to ascertain using no more than ordinary experimentation the reaction protocol and conditions necessary to produce a product of the present invention with properties optimized for a specific assay or subject.
- the polymer may include a polymer backbone comprised of polyethylene glycol (PEG) subunits, some of which have attached thereto a saccharide moiety represented in the general formula (VII):
- PEG polyethylene glycol
- R1 independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
- R5 is H, —OR, NH—Y—R2, or -L-E;
- Y independently for each occurrence is absent, —C(O)—, —C(S)—, —C(NR)—, O, S, or SC;
- B for one occurrence is —R, and for the other occurrence is —R or from 1-9 saccharide residues;
- R independently for each occurrence, represents H or a C 1 -C 6 alkyl or other hydroxyl protecting group, and more preferably represents H for each occurrence;
- R2 is a C 1 -C 6 alkyl, and more preferably represents CH 3 ;
- X2 represents a linker of 1-10 atoms in length, preferably —O-alkyl, more preferably —O—(CH 2 ) 5 —;
- D represents H, or -L-E
- L represents a linker a linker of 1-20 atoms in length, preferably
- E represents a second PEG subunit of the polymer, wherein, independently for each occurrence of E, the linker group L is covalently attached via R5 or B of a saccharide moiety of the second PEG subunit, or via an amine of the second PEG subunit.
- the polymer may be represented by the general formula
- PEG represents a polyethylene glycol (PEG) subunit of the polymer
- X3 represents one or more additional PEG subunits of the polymer, each of which may be derivatived with a saccharide moiety of formula VII,
- polymer has an average molecular weight in the range of 10,000 daltons to 1,000,000 daltons.
- An exemplary composition of the invention is derived from an octa(amino)polyethylene glycol scaffold to which several ⁇ Gal(1,3)Gal moieties are linked via a covalent tether, wherein the individual molecules of the scaffold bearing the disaccharide moieties are crosslinked to yield an oligomeric material comprising two or more scaffold-disaccharide molecules.
- the reactions of the polyamino-PEG scaffold with oligosaccharides will be carried out in the pH range of 4 to 7, and most preferably in the pH range of 5-6.
- the preferred temperature range for the reactions is between 0° C. and 40° C., and more preferably is in the range of 4° C. to 27° C.
- Examples 23 and 26 highlight the relationship between the pH and reaction temperature and the activity of the composition produced.
- a polar, protic or aprotic solvent may be used. As described in the examples, the reactions can be run in aqueous solutions. Alternatively polar, aprotic solvents such as a nitrogen-dialkyluted carboxymide, lactum, sulfoxide or sulfore, as for example dimethyl formamide, may be used. In those instances, the reactions may be effected without or with the additions of water, for example, up to quantities at which the polymer remains in solution. Non-limiting examples of preparations are discussed in the examples.
- the molecular weight of the resulting polymer can depend on such factors as reaction time, pH, temperature and reactant concentrations.
- larger molecular weight polymers may be used, e.g. polymer compositions having an average molecular weight of 100,000-1,000,000 daltons, though more preferable in the range of 100,000-500,000 daltons, and even more preferably 300,000-500,000 daltons.
- the resulting xenopolymer is to be used at least in part as a tolerogen, e.g. to induce tolerance or anergy to the xenoantigens
- polymer formulations at lower molecular weight, e.g. having an average molecular weight of 10,000-300,000 daltons, more preferably having an average molecular weight less than 100,000 daltons, e.g., 25,000-100,000 daltons.
- Such lower molecular weight polymers even if not tolerogenic, will have reduced immunogenic activity.
- compositions according to this aspect of the invention can be identified according to the following criteria. First, they are preferably non-immunogenic when administered to a primate, including a human. As a practical matter, this can be evaluated by comparing the level of antibodies specific for a candidate polymer in peripheral blood of a primate, including a human, prior to and after administration of the candidate polymer.
- compositions according to this aspect of the invention may also include pharmaceutically acceptable carriers, diluents, and/or controlled release agents.
- pharmaceutically acceptable carriers, diluents, and/or controlled release agents include buffered saline, oils, implantable pumps and encapsulated beads.
- the composition in certain instances, such as for formulations of the polymer for human therapeutic use, it will be desirable for the composition to be relatively homogenous with respect to polymer size. That is, the polymers can be formulated to have narrow molecular distributions or polydispersity, as defined by the ratio Mw:Mn, where Mw is the weight average molecular weight of the polymer and Mn is the number average molecular weight of the polymer.
- the subject polymer compositions will have a polydispersity, for the xenopolymer, of 20 or less, more preferably 10 or less, and even more preferably 5 or less.
- the subject method for generating the xenopolymers of the present invention is part of an overall method for manufacturing a medicament.
- the xenopolymer is formulated with one or more pharmaceutically acceptable excipients, such as described below, in an amount and concentrations to provide at least the efffective dose (ED 50 ) for depleting xenoreactive antibodies in the patient, inducing tolerance or anergy to the xenoantigen.
- the polymer can be formulated such that, for its mode of delivery, a dose of at least the ED 50 for inhibitions of HAR can be administered to the patient.
- the factor VIII-polymers have a therapeutic index of at least 10, and more preferably at least 100, 1000, or even 10,000, for the particular mode of administration.
- the polymer can be synthesized and handled under sterile conditions, or can be sterilized prior to formulation. Likewise, contaminants can be removed, including the unreacted reactants and synthesis by-products. The resulting medicament should be sterile, and substantially free of pyrogenic or other toxic contaminants.
- a branched polymer having at least three arms and at least six covalently linked active groups such as amino or hydrazino groups is reacted in the presence of N-(3-dimethlyaminopropyl)-N′-ethylcarbodiimide (EDC) with Gal ⁇ 1-3 Gal having a covalently linked carboxy group.
- EDC N-(3-dimethlyaminopropyl)-N′-ethylcarbodiimide
- the branched polymer has covalently linked carboxy groups while the Gal ⁇ 1-3 Gal has a covalently linked amino or hyrazino group.
- the branched polymer has at least three arms and at least six active groups such as amino or hydrazino groups, and the ⁇ Gal(1,3)Gal has a covalently linked aldehyde group.
- the converse of the third method the branched polymer has at least six aldehyde groups while the ⁇ Gal(1,3)Gal has a covalently linked amino or hydrazino group.
- EDC is not required for the reaction to take place.
- the branched polymer has at least three arms, with at least three active groups such as amino or hydrazino groups covalently linked to the free ends of the arms, and the ⁇ Gal(1,3)Gal has a covalently linked carboxy group.
- the converse of the fifth method the branched polymer has at least three covalently linked carboxy groups while the ⁇ Gal(1,3)Gal has a covalently linked amino or hydrazino group.
- the fifth and sixth methods are conducted in the presence of EDC and N-hydroxysulfosuccinimide (NHSS).
- the compositions resulting from the fifth and sixth methods have a covalently linked activation group which is N-hydroxysulfosuccinimide.
- “Mixed arm” syntheses can also be conducted with all sorts of combinations of groups on the ends of the arms, for instance 7 amino/1 carboxy, or 5/3, or 4/4. Given the guidance of the experimental examples below, one of ordinary skill in the art of chemical synthesis will be able to devise various combinations of reagents that will yield functional compositions within the scope of the present invention.
- compositions of the invention are designated in FIG. 3 and throughout according to the following nomenclature:
- N denominates a synthesis that used both EDC and N-hydroxysuccinimide (NHS).
- the letter L denominates a lysine coupled to the ends of branched PEG-amino, and EDC for coupling.
- the first number in the numbering system denominates the number of branches on the PEG, and the second number (10 or 20) denominates the molecular weight of the starting PEG-amine (10,000 or 20,000 Da).
- IC 50 0.5 mg/mL for 410L versus 50 mg/mL for the free sugar.
- the compositions which were synthesized using N-hydroxysulfosuccinimide (NHSS; NHS with a sulfo group) achieved 50% inhibition of anti-Gal antibody reactivity at very low concentrations of approximately 10 ⁇ 5 mg/mL to 10 ⁇ 7 mg/mL.
- these compositions were stable for 12 months when stored at a pH in the range of 4 to 7 at temperatures ranging from 1-10° C.
- the subject xenopolymer is formed by derivatizing an aminodextran with the xenoantigen.
- a dextran polymer In an illustrative process for preparing a xenoantigen-conjugate with an aminodextran, one can begin with a dextran polymer. The dextran is reacted with an oxidizing agent to effect a controlled oxidation of a portion of its carbohydrate rings to generate aldehyde groups. The oxidation is conveniently effected with glycolytic chemical reagents, e.g., NaIO 4 , according to conventional procedures. The oxidized dextran is then reacted with a polyamine, such as a diamine.
- a polyamine such as a diamine.
- Suitable amines include, e.g., ethylene diamine, propylene diamine or similar polymethylene diamines, diethylene triamine or like polyamines, 1,3-diamino-2-hydroxypropane or other like hydroxylated diamines or polyamines, and the like.
- Reductive stabilization of the resultant intermediate is effected by reacting the Schiff base intermediate with a reducing agent, e.g., NaBH 4 , NaBH 3 CN, or the like.
- the resultant adduct can be further purified by passage through a conventional sizing column to remove cross-linked dextrans.
- An estimate of the number of available primary amino groups on the aminodextran can be effected by reaction of a weighed sample with trinitrobenzenesulfonic acid and correlation of the optical density at 420 nm with a standard.
- the dextran can be derivatized by conventional methods for introducing amine functions, e.g., by reaction with cyanogen bromide, followed by reaction with a diamine.
- Quantification of circulating anti- ⁇ Gal antibodies in the serum of an individual and the ability of polymers to remove, bind, and/or neutralize anti- ⁇ Gal antibodies can be routinely determined applying immunoassays known in the art which may be routinely adapted for such determination.
- Such immunoassays include, but are not limited to, competitive and noncompetitive assay systems using techniques such as western blots, radioimmunoassays, ELISA (enzyme linked immunosorbent assay), “sandwich” immunoassays, immunoprecipitation assays, precipitin reactions, gel diffusion precipitin reactions, immunodiffusion assays, agglutination assays (e.g., hemagglutination), complement-fixation assays, immunoradiometric assays, fluorescent immunoassays and protein A immunoassays.
- competitive and noncompetitive assay systems using techniques such as western blots, radioimmunoassays, ELISA (enzyme linked immunosorbent assay), “sandwich” immunoassays, immunoprecipitation assays, precipitin reactions, gel diffusion precipitin reactions, immunodiffusion assays, agglutination assays (e.g., hemagglut
- These assays may further be applied to determine the dosage of the ⁇ Gal oligosaccharide polymers of the invention to be administered, as well as to monitor the neutralization and/or removal of anti- ⁇ Gal antibodies by the ⁇ Gal oligosaccharide polymers of the invention.
- ELISA assays While assays for measuring circulating anti- ⁇ Gal antibodies can be accomplished by various immunological methods, ELISA assays have the advantage in that they can be standardized for the immobilized ligand, are reproducibly quantitative, and can be scored for different immunoglobulin isotypes by the use of appropriate secondary detection reagents.
- ELISA capture ligands for testing human or old world monkey serum antibodies can employ fixed cells, glycolipids, glycoproteins, or oligosaccharides. Such capture ligands include but are not limited to mouse laminin, PK-15 pig kidney cells, immobilized BSA- ⁇ Gal neoglycoconjugates, and immobilized ⁇ Gal oligosaccharides.
- Covalent immobilization of a specific ⁇ Gal oligosaccharide(s) onto the ELISA immobilized surface permits the predetermined deposition of known antigenic ligands by covalent chemistry.
- an ELISA is also useful in clinical monitoring of anti- ⁇ Gal antibodies in blood of patients in advance of, and following xenotransplantation of organs from animals that express the ⁇ Gal epitope.
- quantification of anti- ⁇ Gal antibodies and/or the extent of xenoreactive antibody neutralization or removal upon treatment with the ⁇ Gal oligosaccharide polymers of the invention is measured using a cell cytotoxicity assay.
- a cell cytotoxicity assay may comprise contacting serum, isolated from a patient using techniques known in the art, with ⁇ Gal expressing cell monolayers (e.g., pig kidney cells (PK-15), pig aortic endothelial cells, or mouse aortic endothelial cells (MAE).
- PK-15 pig kidney cells
- MAE mouse aortic endothelial cells
- these cells are from the same species as the donor of the xenograft, and most preferably from the same tissue-type as the tissue to be transplanted).
- cytotoxicity is mediated by either endogenous complement, or the sera are heat inactivated (e.g., through heating the sera at 56° C. for 30 minutes) and exogenous complement (e.g., from rabbit or guinea pig) is added. After washing away excess serum, the cells are treated with viable dye mix “live-dead” (calcein AM/ethidium homodimer) which is commercially available in the form of a Live/Dead cytotoxicity kit (Molecular Probes Inc., Eugene, Oreg.) and scored for viability using fluorescence microscopy.
- live-dead viable dye mix “live-dead” (calcein AM/ethidium homodimer) which is commercially available in the form of a Live/Dead cytotoxicity kit (Molecular Probes Inc., Eugene, Oreg.) and scored for viability using fluorescence microscopy.
- Staining with the “live/dead” dye mix allows for clear distinction between live cells, which show cytoplasmic green fluorescence.and dead cells, which show dark cytoplasm land red fluorescent nuclei.
- the extent of cell lysis that is complement-mediated may be determined by comparing the results observed in the control for which there has been no inactivation of complement, with the lysis observed with complement that has been inactivated through heat treatment.
- the invention also encompasses animal-based model systems, which may include baboon, other old world monkeys, or other animals having serum that contains anti- ⁇ Gal antibodies.
- animal models may assess the ability of compositions containing an ⁇ Gal oligosaccharide polymer as an active ingredient to bind to and/or neutralize anti- ⁇ Gal antibodies, attenuate the rejection of a xenotransplant expressing the ⁇ Gal epitope and/or to suppress B lymphocytes expressing anti- ⁇ Gal idiotypes.
- this assay may involve exposing animal models to the ⁇ Gal oligosaccharide polymer, or pharmaceutically acceptable derivative as an active ingredient, at a sufficient concentration and for a time sufficient to elicit the desired effect in the exposed animals.
- the response of the animals to the exposure may be monitored by assessing the level of anti- ⁇ Gal reactive antibodies in the serum of the animal, evaluating the appearance of rejections of the xenografted organ, and/or quantitating B lymphocytes expressing anti- ⁇ Gal idiotypes. Dosages of test agents may be determined by deriving dose-response curves.
- the relative anti- ⁇ Gal binding activity that a ⁇ Gal oligosaccharide polymer exhibits against the anti- ⁇ Gal antibody profile of the serum of an individual may be determined, and the polymer formulation best suited for neutralizing and/or binding the anti- ⁇ Gal profile of an individual can be determined.
- compositions comprising the subject xenopolymers, or pharmaceutically acceptable derivatives thereof, can be administered to a patient, preferably a human or old world monkey, by itself, or in pharmaceutical compositions where it is mixed with suitable carriers or excipient(s) at doses to ameliorate symptoms associated with xenograft rejection, prolong xenograft survival in a patient or to direct complement-mediated lytic attack of targeted tissue or cell types.
- compositions of the present invention in particular, those formulated as solutions, may be administered parenterally, such as by intravenous injection.
- the compounds can be formulated readily using pharmaceutically acceptable carriers well known in the art into dosages suitable for the form of administration desired.
- compositions of the invention may be administered using techniques well known to those in the art.
- agents are formulated and administered systemically.
- Techniques for formulation and administration of the compounds of the invention may be found in “Remington's Pharmaceutical Sciences,” 18th ed., 1990, Mack Publishing Co., Easton, Pa., latest edition.
- Suitable routes of administration may, for example, include oral, rectal, transmucosal, or intestinal administration; parenteral delivery, including intramuscular, subcutaneous, intramedullary injections, as well as intrathecal, direct intraventricular, intravenous, intraperitoneal, intranasal, or intraocular injections; transdermal, topical, vaginal and the like.
- compositions for use in accordance with the present invention thus may be formulated in conventional manner using one or more physiologically acceptable carriers comprising excipients and auxiliaries which facilitate processing of the activ6 compounds into preparations which can be used pharmaceutically. Proper formulation is dependent upon the route of administration chosen.
- the agents of the invention may be formulated in aqueous solutions, preferably in physiologically compatible buffers such as Hanks's solution, Ringer's solution, or physiological saline buffer.
- physiologically compatible buffers such as Hanks's solution, Ringer's solution, or physiological saline buffer.
- penetrants appropriate to the barrier to be permeated are used in the formulation. Such penetrants are generally known in the art.
- the compounds can be formulated readily by combining the active compounds with pharmaceutically acceptable carriers well known in the art.
- Such carriers enable the compounds of the invention to be formulated as tablets, pills, dragees, capsules, liquids, gels, syrups, slurries, suspensions and the like, for oral ingestion by a patient to be treated.
- Pharmaceutical preparations for oral use can be obtained in the form of a solid excipient, optionally by grinding a resulting mixture, and processing the mixture of granules, after adding suitable auxiliaries, if desired, to obtain tablets or dragee cores.
- Suitable excipients are, in particular, fillers such as sugars, including lactose, sucrose, mannitol, or sorbitol; cellulose preparations such as, for example, maize starch, wheat starch, rice starch, potato starch, gelatin, gum tragacanth, methyl cellulose, hydroxypropylmethyl-cellulose, sodium carboxymethylcellulose, and/or polyvinylpyrrolidone (PVP).
- disintegrating agents may be added, such as the cross-linked polyvinyl pyrrolidone, agar, or alginic acid or a salt thereof such as sodium alginate.
- Dragee cores are provided with suitable coatings.
- suitable coatings may be used, which may optionally contain gum arabic, talc, polyvinyl pyrrolidone, carbopol gel, polyethylene glycol, and/or titanium dioxide, lacquer solutions, and suitable organic solvents or solvent mixtures.
- Dyestuffs or pigments may be added to the tablets or dragee coatings for identification or to characterize different combinations of active compound doses.
- compositions which can be used orally include push-fit capsules made of gelatin, as well as soft, sealed capsules made of gelatin and a plasticizer, such as glycerol or sorbitol.
- the push-fit capsules can contain the active ingredients in admixture with filler such as lactose, binders such as starches, and/or lubricants such as talc or magnesium stearate and, optionally, stabilizers.
- the active compounds may be dissolved or suspended in suitable liquids, such as fatty oils, liquid paraffin, or liquid polyethylene glycols.
- stabilizers may be added. All formulations for oral administration should be in dosages suitable for such administration.
- compositions may take the form of tablets or lozenges formulated in conventional manner.
- the compounds for use according to the present invention are conveniently delivered in the form of an aerosol spray presentation from pressurized packs or a nebulizer, with the use of a suitable propellant, e.g., dichlorodifluoromethane, trichlorofluoromethane, dichlorotetrafluoroethane, carbon dioxide or other suitable gas.
- a suitable propellant e.g., dichlorodifluoromethane, trichlorofluoromethane, dichlorotetrafluoroethane, carbon dioxide or other suitable gas.
- the dosage unit may be determined by providing a valve to deliver a metered amount.
- Capsules and cartridges of e.g., gelatin for use in an inhaler or insufflator may be formulated containing a powder mix of the compound and a suitable powder base such as lactose or starch.
- the compounds may be formulated for parenteral administration by injection, e.g., by bolus injection or continuous infusion.
- Formulations for injection may be presented in unit dosage form, e.g., in ampoules or in multidose containers, with an added preservative.
- compositions may take such forms as suspensions, solutions or emulsions in oily or aqueous vehicles, and may contain formulatory agents such as suspending, stabilizing and/or dispersing agents.
- compositions for parenteral administration include aqueous solutions of the active compounds in water-soluble form. Additionally, suspensions of the active compounds may be prepared as appropriate oily injection suspensions. Suitable lipophilic solvents or vehicles include fatty oils such as sesame oil, or synthetic fatty acid esters, such as ethyl oleate or triglycerides, or liposomes. Aqueous injection suspensions may contain substances which increase the viscosity of the suspension, such as sodium carboxymethyl cellulose, sorbitol, or dextran. Optionally, the suspension may also contain suitable stabilizers or agents which increase the solubility of the compounds to allow for the preparation of highly concentrated solutions.
- the active ingredient may be in powder form for constitution with a suitable vehicle, e.g., sterile pyrogen-free water, before use.
- a suitable vehicle e.g., sterile pyrogen-free water
- the compounds may also be formulated in rectal compositions such as suppositories or retention enemas, e.g., containing conventional suppository bases such as cocoa butter or other glycerides.
- the compounds may also be formulated as a depot preparation. Such long acting formulations may be administered by implantation (for example subcutaneously or intramuscularly) or by intramuscular injection.
- the compounds may be formulated with suitable polymeric or hydrophobic materials (for example as an emulsion in an acceptable oil) or ion exchange resins, or as sparingly soluble derivatives, for example, as a sparingly soluble salt.
- compositions also may comprise suitable solid or gel phase carriers or excipients.
- suitable solid or gel phase carriers or excipients include but are not limited to calcium carbonate, calcium phosphate, various sugars, starches, cellulose derivatives, gelatin, and polymers such as polyethylene glycols.
- Pharmaceutical compositions suitable for use in the present invention include compositions wherein the active ingredients are contained in an effective amount to achieve its intended purpose. Determination of the effective amounts is well within the capability of those skilled in the art, especially in light of the detailed disclosure provided herein.
- a preferred method of neutralizing anti- ⁇ Gal antibodies involves the administration of the subject ⁇ Gal oligosaccharide polymers, or pharmaceutically acceptable derivative(s) thereof, in sufficient quantity to block binding of the circulating antibody to the donor endothelium and thereby prevent anti- ⁇ Gal antibody directed complement-mediated lytic attack of the transplanted tissue.
- the therapeutic method of the invention is carried out as monotherapy, i.e., as the only agent provided for attenuating xenograft rejection.
- administration of the ⁇ Gal oligosaccharide polymer of the invention is combined with other regimens that may accompany neutralization and/or depletion of anti- ⁇ Gal antibodies using the subject ⁇ Gal oligosaccharides polymers, including, but not limited to, extracorporeal treatment with column immobilized human antianimal idiotypic antibodies (for example, see U.S. Pat. No.
- plasmapheresis in which all antibodies or specifically, one or more antibody types specific for antigenic epitopes on the surface of donor endothelial cells have been removed from the plasma
- perfusion of blood to be administered to the patient through organs, tissue or cells expressing ⁇ Gal antigens (such as, for example, hearts, kidneys, erythrocytes, and cell lines derived from pig kidney, pig aortic endothelium, mouse endothelium, etc.).
- organs, tissue or cells expressing ⁇ Gal antigens such as, for example, hearts, kidneys, erythrocytes, and cell lines derived from pig kidney, pig aortic endothelium, mouse endothelium, etc.
- the subject polymer is selected for its ability to induce clonal tolerance to the xenoantigen.
- the polymer can also be used in a step for neutralizing and/or depleting circulating XNAs.
- the subject polymer can be used principally as a tolerogen, and circulating XNAs can be removed by immunoaphoresis or other regimen for antibody removal.
- the xenopolymers of the present invention may be administered alone or in combination with other agents useful in attenuating xenograft rejection, including conventional nonspecific immunosuppressive agents, including but not limited to, steroids, cyclosporine, cyclosporine analogs, cyclophosphamide methylprednisone, prednisone, azathioprine, FK-506, 15-deoxyspergualin, and other immunosuppressive agents known in the art.
- conventional nonspecific immunosuppressive agents including but not limited to, steroids, cyclosporine, cyclosporine analogs, cyclophosphamide methylprednisone, prednisone, azathioprine, FK-506, 15-deoxyspergualin, and other immunosuppressive agents known in the art.
- the pharmaceutical compositions comprise an antibiotic agent selected from the group consisting of tetracycline, metronidazole, amoxicillin, P lactamases, aminoglycosides, macrolides, quinolones, fluoroquinolones, cephalosporins, erythromycin, ciprofloxacin, and streptomycin.
- the pharmaceutical composition comprises an anti-inflammatory.
- Such anti-inflammatories include, but are not limited to, glucocorticoids and the nonsteroidal anti-inflammatories, aminoarylcarboxylic acid derivatives, arylacetic acid derivatives, arylbutyric acid derivatives, arylcarboxylic acids, arylpropionic acid derivatives, pyrazoles, pyrazolones, salicylic acid derivatives, thiazinecarboxamides, c-acetamidocaproic acid, Sadenosylmethionine, 3-amino-4-hydroxybutyric acid, amixetrine, bendazac, benzydamine, bucolome, difenpiramide, ditazol, emorfazone, guaiazulene, nabumetone, nimesulide, orgotein, oxaceprol, paranyline, perisoxal, pifoxime, proquazone, proxazole, and tenidap.
- Combinations may be administered either concomitantly, e.g., as an admixture, separately but simultaneously or concurrently; or sequentially.
- Administration “in combination” further includes the separate administration of one of the agents given first, followed by the second.
- the administration of the subject xenopolymers can be part of a treatment regimen which includes total-body irradiation and splenectomy. See, for example, Cooper et al. (1997) World J Surg 21:901.
- the subject therapies include one or more steps of myeloreductive treatment, especially where the xenograft is bone marrow or other hematopoietic stem cell preparations.
- a method may include, e.g., treating the subject, prior to introduction of the donor stem cells, with an cytoreductive agent selected from one or more of alkylating agents (e.g., nitrogen mustards [such as mechloretamine], cyclophosphamide, melphalan and chlorambucil), alkyl sulphonates (e.g., busulphan), nitrosoureas (e.g., carmustine, lomustine, semustine and streptozocine), triazenes (e.g., dacarbazine), antimetabolites (e.g., folic acid analogs such as methotrexate), pyrimidine analogs (e.g.
- alkylating agents e.g., nitrogen mustards [such as mechloretamine],
- fluorouracil and cytarabine purine analogs (e.g., fludarabine, idarubicin, cytosine arabinoside, mercaptopurine and thioguanine), vinca alkaloids (e.g., vinblastine, vincristine and vendesine), epipodophyllotoxins (e.g., etoposide and teniposide), antibiotics (e.g., dactinomycin, daunorubicin, doxorubicin, bleomycin, plicamycin and mitomycin), dibromomannitol, deoxyspergualine, dimethyl myleran and thiotepa.
- Preferred myeloreductive non-myeloablative agents are alkylating agents, e.g., cyclophosphamide, or fludarabine or similar substances, however, hematopoietic space creating antibodies or drugs, e.g., inhibitors of cell proliferation, e.g., DSG, or an anti-metabolite, e.g. brequinar, or an anti-T cell antibody, e.g., one or both of an anti-CD4 or anti-CD8 antibody can be used as a myeloreductive non-myeloablative agent.
- alkylating agents e.g., cyclophosphamide, or fludarabine or similar substances
- hematopoietic space creating antibodies or drugs e.g., inhibitors of cell proliferation, e.g., DSG, or an anti-metabolite, e.g. brequinar, or an anti-T cell antibody, e.g., one or both of an anti
- anti-T cell antibodies e.g., an ATG preparation, polyclonal or monoclonal antibody directed against CD4, CD8, or CD2
- an anti-CD2 antibody e.g., the anti-CD2 monoclonal antibody BTI-322 or a humanized version thereof, or an antibody which overlaps or binds the epitope recognized by BTI-322, are particularly useful
- an anti-CD2 antibody e.g., the anti-CD2 monoclonal antibody BTI-322 or a humanized version thereof, or an antibody which overlaps or binds the epitope recognized by BTI-322, are particularly useful
- an agent e.g., an antibody, which blocks or otherwise inhibits a pathway, e.g., a costimulatory pathway, of T cell activation
- agents e.g., antibodies, which block the CD28-B7 pathway, e.g., a CTLA4-IgG fusion protein, or agents, e.g., an antibody which blocks the CD40-gp39 pathway, e.g., an anti-gp39 antibody, are particularly suited for use in the method
- a treatment which down modulates or otherwise inhibits one or more of the T cell receptor, CD4 co-receptor, CD8 co-receptor or other receptor or co-receptor which promotes T cell activation or maturation;
- an immunosuppressive agent e.g., a macrolide, e.g., cyclosporine, FK506, or rapamycin; and
- the myeloreductive treatment includes treating the subject with an immunosuppressant regimen, prior to introduction of the donor stem cells, in an amount sufficient to prevent rejection of the donor stem cells by the host immune system.
- immunosuppressant regimens can include, independently for pre- and post-transplantation is both are carried out, a treatment of the subject which inactivates and/or depletes host T-lymphocytes and/or natural killer (NK) cells in the subject.
- the immunosuppressant regimen includes treatment with T cell-depleting anti-CD4 and/or CD8 antibodies, such as anti-thymocyte globulin (ATG), OKT3, LO-CD2a, or Minnesota anti-lymphoblast globulin (MALG).
- TAG anti-thymocyte globulin
- OKT3, LO-CD2a OKT3, LO-CD2a
- MALG Minnesota anti-lymphoblast globulin
- the immunosuprrelich regimen both before and after transplantation, includes administration of ATG.
- the immunosuppressant regimen includes treatment with one or more of a macrolide immunosuppressant, azathioprine, steroids (e.g., prednisone, methyl prednisolone), or sub-lethal nonmyleoablative irradiation of lymphocyte-containing tissue.
- a macrolide immunosuppressant e.g., azathioprine, steroids (e.g., prednisone, methyl prednisolone), or sub-lethal nonmyleoablative irradiation of lymphocyte-containing tissue.
- the method may also include inhibiting natural killer cells of the subject preferably prior to introducing the xenograft, e.g., by introducing into the subject an antibody capable of binding to natural killer cells of the subject.
- One source of anti-NK antibody is anti-human thymocyte polyclonal anti-serum.
- a second anti-mature T cell antibody can be administered as well, which inhibits T cells as well as NK cells.
- Anti-T cell antibodies are present, along with anti-NK antibodies, in anti-thymocyte anti-serum. Repeated doses of anti-NK or anti-T cell antibody may be preferable.
- Monoclonal preparations can be used in the methods of the invention.
- the subject method of administering a xenopolymer can be combined with treatment of the donor and/or donor tissue.
- the xenograft can be treated with glycolytic enzymes prior to implantation in order to reduce the presence of the ⁇ Gal epitope.
- the ⁇ Gal epitopes can be enzymatically removed by specific endo- or exo-glycosidases and mannosidases.
- Such glycolytic enzymes and general methods for their use are well known in the art.
- glycosidases and mannosidases suitable for use in this aspect of the invention include ⁇ - or ⁇ -galactosidase, ⁇ -N-acetylhexosamimidase, ⁇ - or ⁇ -mannosidase, endoglycosidase H or F, and peptide-N-glycosidase F.
- These enzymes are all commercially available from Oxford Glycosystems (Rosedale, N.Y.), and are typically used according to the manufacturer's instructions.
- Antisense RNAs can be used to specifically inhibit gene expression of, e.g., ⁇ 1,3-GT activity in the donor animal.
- antisense may be used to reduce the human serum reactivity to porcine endothelial cells by antisense-mediated down-regulation of GpIIIa expression (Keams-Jonker et al. (1997) Transplantation 63:588), and tissue from such animals could be used in combinations with, inter alia, the subject xenoploymers.
- Gene knockout is a method of genetic manipulation via homologous recombination. These techniques may allow for the use of specially designed DNA molecules (gene knockout constructions) to achieve targeted inactivation (knockout) of a particular gene upon introduction of the construction into a cell.
- treatment of a xenograft recipient with the subject polymers may be combined with transplantation of an organ or tissue derived from such transgenic “knockout” animals may be described in, e.g., U.S. Pat. No. 5,821,117 entitled “Xenotransplantation Therapies”.
- the subject polymers may be used as part of a treatment regimen for recipients of xenogenic grafts from transgenic animals engineered to express: human decay-accelerating factor, such as may be isolated from the animals described by Cozzi et al. (1994) Transplant Proc 26:1402; or which express the human complement regulatory protein CD59 and DAF, as taught by Byrne et al. (1996) Transplant Proc 1996 April; 28 (2):759; human ⁇ 1,2-fucosyltransferase, as might be generated according to Chen et al.
- human decay-accelerating factor such as may be isolated from the animals described by Cozzi et al. (1994) Transplant Proc 26:1402; or which express the human complement regulatory protein CD59 and DAF, as taught by Byrne et al. (1996) Transplant Proc 1996 April; 28 (2):759
- human ⁇ 1,2-fucosyltransferase as might be generated according to Chen et al.
- the reaction mixture was filled into a dialysis tube of molecular weight cut-off of 3,500 and dialyzed five times against 4 liters of DI water.
- the dialyzed material was freeze-dried to a white powder.
- a sample of the freeze-dried powder was dissolved in 20 mL of 0.1M sodium borate and transferred to a 50 mL volumetric flask.
- 100 ul of TNBS (trinitrobenzene sufonic acid) (Pierce, Rockford, Ill. 61105) was added to the volumetric flask, capped and the flask inverted for mixing. Water was added to 50 mL and the solution was mixed by inversion.
- the sample was incubated for one hour before reading the OD at 421 nm.
- the amount of free amino groups was calculated by comparing the OD of the sample to a standard curve generated with glycine as standard. Amino group analysis revealed that more than 98% of amino groups were modified.
- PEG-(NH 2 ) 8 was synthesized by covalently linking a lysine residue to each of the amine ends of the 4-arm 10,000 mw PEG. Since a lysine contains 2 amino groups and a carboxy group, the carboxy group was coupled to the amine group of the PEG, thereby doubling the number of amine groups on the PEG.
- 810S was dissolved in three different buffers and the optical density was measured at 270 nm. At a pH of 3, the OD was 1.4, at pH 7 the OD was 1.7, and at pH 10 the OD was 4.8, indicating incorporation of sulfo-beta-alanine units. Also, gel filtration analysis using an Aquagel-OH (Polymer Labs, Amhearst, Mass.) gel filtration column, showed all products synthesized with NHSS, to possess a strong adsorption at 270 nm which was associated with the molecular weight of the modified PEG. This excluded the possibility that the adsorption observed stemmed from contamination of the final product from the NHSS used. Elemental analysis of 810S indicated a sulfur content of 2.34%.
- a competition ELISA was set up in such a way that Gal-molecules linked to human serum albumin and immobilized onto a microtiter plate and Gal-molecules linked to PEG compete for anti-Gal antibodies in baboon serum.
- the antibodies binding to the Gal-HSA were visualized using secondary antibodies to these antibodies with appropriate color development agents.
- the color in the case in which no Gal-PEG was added was defined as 100%.
- the IC50 was defined, for FIG. 3, as the concentration of Gal-PEG needed to inhibit 50% of the color development when trying to detect antibodies immobilized onto the Gal-HSA plate.
- the IC50 is defined in terms of mass per volume, e.g. mg/mL, of polymer.
- a 96 well microtiter was coated with 100 ul/well of a 30 ug/mL solution of ⁇ Gal(1,3)Gal ⁇ 1-4 GlcNAc-human serum albumin (Gal-HSA) and incubated for 90 minutes at room temperature. After incubation with Gal-HSA, the plate was blocked with 1% HSA in PBS for 60 minutes.
- Gal-HSA ⁇ Gal(1,3)Gal ⁇ 1-4 GlcNAc-human serum albumin
- samples were prepared as follows: Fifteen different dilution solutions were prepared for 1 plate.
- the first dilution solution was 1% HSA in PBS.
- Dilution solutions 3-5 were obtained by preparing a 2.5 mL stock solution of 10 mM ⁇ Gal(1,3)Gal ⁇ 1-4 GlcNAc in 1% HSA/PBS and then serially diluting 1/10 three times.
- Dilution solutions 6-15 were obtained by preparing a 2.5 mL stock solution of the first concentration of modified PEG and then serially diluting 1/10 nine times in 1% HSA in PBS.
- the plate was incubated for 90 minutes at 4° C. The plate was washed five times with PBS containing 0.5% Tween 20 and blot dried.
- the biotinylated secondary antibody was incubated for one hour at 4° C. and afterwards the plate was washed five times with PBS containing 0.5% Tween 20 and blot dried.
- 100 ul/well streptavidin-alkaline phosphatase conjugate was added at a dilution of 1/4000 in 1% HSA/PBS and incubated for 30 minutes at room temperature.
- the microtiter plate was washed five times with PBS containing 0.5% Tween 20 and blot dried.
- 150 ul developer per well was added and the plate incubated for 30 minutes at room temperature.
- the color development was stopped by addition of 40 ul/well of 3 M NaOH.
- the plate was read in a plate reader at wavelength of 405 nm.
- the IC50 data was calculated from the percent inhibition from the dilution of baboon control in 1% HSA/PBS which gave this highest point within the linear range.
- results As shown in FIG. 24, the molecules made using NHSS achieved 50% inhibition of xenoreactive antibody activity at much lower concentrations than those made using EDC alone.
- FIG. 24 also provides IC50 values for a variety of different polymeric formulations. In terms of experimental protocol, the only significant difference between the experiements producing the data of FIGS. 3 and 24 was to use a new pipette tip for additions of ⁇ Gal preparations to the wells of the ELISA plate, which should prevent any inadvertant contamination with higher concentrations of the ⁇ Gal sample. Furthermore, the values reported in FIG. 24 are in mass per volume, rather than molar. According to FIG.
- the 810S preparation had an IC50 of 5 ⁇ 10 4 mg/mL, compared to the 810E preparation with an IC50 of 0.1 mg/mL.
- the 810S molecule achieved 50% inhibition at 1 ⁇ 10 ⁇ 5 M, compared to the 801E molecule which required 1 ⁇ 10 ⁇ 1 M for the same effect.
- Peripheral blood mononuclear cells were isolated by Ficoll-Hypaque (specific gravity, 1.077 g/mL; Pharmacia Fine Chemicals, Piscatawy, N.J.) density gradients at 400 g for 30 min. at 22° C. Low density mononuclear cells at the interfaces were harvested, washed twice by centrifugation (400 g for 10 min.) and resuspended in serum-free complete culture medium consisting of RPMI 1640 (GIBCO, Grand Island, N.Y.) supplemented with 1% HSA, 2 mM L-glutamine, and 100 U/mL penicillin/100 ug/mL streptomycin (GIBCO, Grand Island, N.Y.).
- RPMI 1640 Gib Island, N.Y.
- TPC short-term in vitro effects of TPC on peripheral blood-derived ASCs were assessed by incubating (2 hours at 37° C., with rotation at 6 rpm) heparinized peripheral blood from a normal donor in the presence or absence of 50 ug/mL TPC (410S). After 2 hours, mononuclear cells from both the untreated and the TPC treated blood samples were obtained and assayed for ASCs. TPC alone appeared to have no effect on total cell yield and viability or on the number of detectable ASCs (data not shown).
- TPC is not apparently cytotoxic to any particular peripheral blood mononuclear cell population (T cells, B cells and monocytes/macrophages) and has no direct inhibitory or stimulatory effect on anti-Gal ASC.
- CD19+B cells were obtained by positive selection with the use of anti-CD19+ immunomagnetic beads (Pan-B (CD19); Dynal, Lake Success, N.Y.). The B cell population obtained was >95% reactive with the anti-CD20 mAb (CD20-PE, clone L27; Becton Dickinson, San Jose Calif.).
- Purified CD19+B cells (1 ⁇ 10 5 /well) were cultured with formalinized Staphylococcus aureus cells (SAC, Cowan strain, Calbiochem) (1:10,000) plus IL-2 (50 ng/mL), IL-4 (50 ng/mL) (R&D Systems, Minneapolis, Minn.) and anti-CD40 (1 ug/mL, Cat#33070D; Pharmingen, San Diego, Calif.), in 96-well round bottom culture plates (Corning) in 0.2 mL of RPMI 140 medium supplemented with 10% human AB serum (anti-Gal depleted), 2 mM L-glutamine, 25 mM Hepes, and 100 U/mL penicillin/100 ug/mL streptomycin (GIBCO, Grand Island, N.Y.) at 37° C.
- SAC formalinized Staphylococcus aureus cells
- IL-2 50 ng/mL
- IL-4 50 ng/mL
- TPC xenoreactive ASC
- B cells were pretreated with TPC (410S; 1 mg/mL) for 30 min. at 37° C. and then added to the cultures after extensive washing.
- 50 ug/mL of TPC (410S) was added directly to the cultures at initiation and daily thereafter. Following the 5 day culture period, cells were washed extensively, counted, viability determined and the number of ASC determined using the anti-Gal ELISPOT assay.
- TPC appeared to have no effect on total cell yield and viability or on the number of detectable ASCs (data not shown).
- TPC molecule 410S was prepared as described in Example 5. 410S or PEG control was administered to cynomolgous monkeys in doses of either 50 mg or 250 mg every 3 days for 2 weeks. Blood was drawn for serum before and after each administration. During the subsequent 2 week period, blood was drawn each Monday and Friday. Serum samples were analyzed for xenoreactive IgG (X IgG) and xenoreactive IgM (X IgM).
- Results are shown for IgM in FIG. 4 and for IgG in FIG. 5.
- both the 50 mg and the 250 mg doses dramatically reduced the level compared to control animals treated with the blocked PEG.
- the IgM level in the 250 mg dose was approximately 5% of the initial level and the IgM level in the 50 mg dose was approximately 15% of the initial level.
- This low level of anti-Gal IgM was maintained even after dosing with TPC had stopped. Indeed, at 15 days post-treatment the level in the 250 mg animals was averaging 15% of the predosing level. In the 50 mg animals the level was aproximately 40% of the initial level.
- the TPC molecule 410S was prepared as described above in Example 5. Three monkeys received 250 mg TPC (# 19-97, 18-97, 21-97), and two monkeys received 250 mg control vehicle (#28-97, 23-97) every 3 days for 2 weeks (total 6 doses/animal) via IV infusion. 250 mg/5 mL per vial was supplied as sterile solution to be injected into 50 ML sterile physiological saline in an IV bag and administered IV at a rate of approximately 2-3 mLs/min. Prior to administration and 15-60 minutes after administration was complete, 1-3 mLs of blood was drawn for serum from each monkey at a site distant from the site of administration.
- each monkey underwent a heterotopic heart transplant following drug infusion.
- a heart from a transgenic pig which expresses human CD59 and human CD55 was placed in the abdominal position as described (Byrne, et al., 1997 , Transplantation 63:149-155) and remained in place up to 6 hours before being explanted.
- the monkeys continued infusion of either TPC or control vehicle, as above, once per day for 9 days. Blood was drawn for serum analysis before and after each administration. The monkeys were monitored for at least 15 days after cessation of TPC administration, and blood was drawn 2 times per week for serum analysis. One monkey died 2 days post-transplant.
- Results are shown in FIGS. 6 to 9 .
- the control animal (Cyno 23-97, see FIG. 6) experienced a 28-fold increase in IgG XNA levels, and a 25-fold increase in IgM XNA levels post-transplant. Most likely, this was due to stimulation by the pig heart tissue of B cells which produce IgM anti-Gal antibodies to differentiate into IgM-XNA producing plasma cells, and due to stimulation of B cells which produce IgM anti-Gal antibodies to class switch to become IgG XNA-producing B cells and then to differentiate into IgG XNA-producing plasma cells. In contrast, the monkeys which received TPC maintained low levels of xenoreactive antibodies during the 9 days of TPC administration following explant of the pig heart. Following cessation of TPC, XNA levels rose, but not as dramatically as in the non-TPC-treated control.
- Mononuclear cells were isolated via Ficoll-Hypaque gradient centrifugation and assayed for anti-Gal IgM and IgG ASC using the ELISPOT assay as described in Example 15. Circulating anti-Gal ASC (IgM and IgG) numbers were inhibited 70-99% following 6 dosages of TPC over 18 days; whereas PEG treatment resulted in a modest (9-35%) decrease in IgM ASC with no significant changes in the number of IgG ASC (FIGS. 2&3). At day 7 post transplantation (porcine transgenic heart), circulating IgM and IgG ASC levels decreased 98.5-100% and 70-99.7%, respectively in monkeys treated daily with TPC.
- TPC molecule 410S was synthesized as described in Example 5 above. Baboons were first immunoapheresed using ⁇ -gal-columns in order to remove circulating ⁇ Gal antibodies, and to set a basis for comparison of the ability of TPC to remove ⁇ Gal antibodies from the circulation.
- the plasma was asceptically conducted to an ⁇ -gal column; effluent from the column was pumped and remixed on-line with the cells and platelets, and reinfused to the subject. This procedure was carried out continuously for 2-3 plasma volumes.
- the blood flow rate of 75-100 cc/min (with a plasma flow rate of 35-50 cc/min), was well tolerated by the subject.
- the entire column procedure lasted 1-1.5 hours.
- Immunoapheresis #1 removed 75% of IgM ⁇ Gal antibodies from the circulation. Five days later, IgM XNAs rebounded to about 80% of pretreatment levels. A second immunoapheresis procedure brought circulating IgM XNAs to 25% of pretreatment levels. One day later, XNAs rebounded to about 55% of original levels. A third immunoapheresis brought XNA levels to 30% baseline values, and a subsequent infusion of 250 mg TPC post-immunoapheresis resulted in undetectable levels of IgM XNAs. Three days later IgM XNA levels had rebounded to about 15% pretreatment levels. The animal developed an infection at the catheter site and the experiment was terminated. This data suggested that TPC was at least as efficient, and perhaps more efficient at reducing circulating IGM XNA levels compared to immunoapheresis.
- Immunoapheresis #1 reduced circulating IgM XNA levels to 40% of pretreatment levels. Two days later, a rebound to about 50% of pretreatment levels was observed, and after a second immunoapheresis plus administration of 250 mg TPC, circulating IgM xXNA levels were undetectable. Three days later, a rebound in IgM XNA to 35% pretreatment levels occurred, and administration of 250 mg TPC brought these levels to about 5%. Six subsequent administrations of 250 mg TPC every 3 or 4 days brought circulating levels of IgM XNA lower each time, and a less dramatic rebound effect was noted.
- a kidney from a transgenic pig expressing human CD59, human CD46 and human DAF was transplanted into the baboon, and a bilateral nephrectomey was performed on the baboon native kidneys.
- the baboon was dependent upon the porcine kidneys for physiological function.
- the transplanted kidney exhibited primary nonfunction and the baboon was euthanized on post-op day 4.
- Pathology analysis of the explanted pig kidney revealed no evidence of hyperacute rejection.
- the diafiltration apparatus is set up and sterilized according to the manufacturer's instructions.
- the diafiltration system is drained and rinsed with sterile water until the pH of both the permeate and retentate streams are between pH 5.5 to 7.0.
- a minimum of 5 buffer exchanges is performed against sterile saline.
- the carboy connection is clamped off and the air filter line is opened.
- the diafiltered solution is concentrated to a third of the original volume. The volume is brought up to 750 mL sterile saline and sterile filtered through a 0.2% filter and stored at 4° C.
- IC 50 [mg/mL] 210 5 ⁇ 10 ⁇ 6 mg/mL 150 5 ⁇ 10 ⁇ 6 mg/mL 125 4 ⁇ 10 ⁇ 5 mg/mL 100 8 ⁇ 10 ⁇ 4 mg/mL 75 1 ⁇ 10 ⁇ 3 mg/mL
- pH IC50 [mg/mL] 5 5 ⁇ 10 ⁇ 6 mg/mL 5.5 5 ⁇ 10 ⁇ 6 mg/mL 6 5 ⁇ 10 ⁇ 6 mg/mL 7 9 ⁇ 10 ⁇ 4 mg/mL 7.5 6 ⁇ 10 ⁇ 3 mg/mL DI water 1 ⁇ 10 ⁇ 5 mg/mL
- EDC [mg] IC50 [mg/mL] 100 9 ⁇ 10 ⁇ 6 mg/mL 200 9 ⁇ 10 ⁇ 6 mg/mL 300 7 ⁇ 10 ⁇ 6 mg/mL 400 5 ⁇ 10 ⁇ 6 mg/mL 500 5 ⁇ 10 ⁇ 6 mg/mL 625 5 ⁇ 10 ⁇ 6 mg/mL 800 5 ⁇ 10 ⁇ 6 mg/mL
- pH IC50 [mg/mL] 5 5 ⁇ 10 ⁇ 5 mg/mL 5.5 5 ⁇ 10 ⁇ 5 mg/mL 6 8 ⁇ 10 ⁇ 5 mg/mL 6.5 5 ⁇ 10 ⁇ 4 mg/mL 7 5 ⁇ 10 ⁇ 4 mg/mL 7.5 8 ⁇ 10 ⁇ 3 mg/mL
- EDC In three 15 mL centrifuge tubes, 250 mg 820E, 70 mg NHSS, 100 mg, 200 mg and 375 mg EDC, were dissolved in 5 mL of 50 mM MES buffer, pH 5.5, respectively. The reactions were carried out overnight. The respective solutions were dialyzed against DI water with four exchanges and lyophilized. The approximate IC 50 s were measured as described above and are listed below. EDC [mg] IC50 [mg/mL] 100 8 ⁇ 10 ⁇ 3 mg/mL 200 4 ⁇ 10 ⁇ 4 mg/mL 375 8 ⁇ 10 ⁇ 4 mg/mL
- 810 S was fractionated by diafiltration through successively higher molecular weight cut-off membranes. 50 grams of 810 S (lot#808003) was first diafiltered 6 times against sterile water to remove salt through a nominally 3,000 MW cut-off membrane. The retentate was then diafiltered six times against sterile water successively through nominally 30,000 MW, 100,000 MW, 300,000 MW and 500,000 MW cut-off membranes. The permeates and the final retentate were lyophilized. The dry powders were weighed and a mass balance established as listed below. The approximate IC 50 s were measured as described above and are listed below.
- the reaction mixture was filled into dialysis tubes of molecular weight cut off 3,500 and dialyzed five times against 20 liters of DI water.
- the dialyzed material was freeze-dried to a yellowish powder. Amino group analysis revealed that more than 98% of amino groups were modified.
- a total of 6 baboons were used.
- Three baboons (designated 51-98, 48-98 and 47-98) underwent immunoapheresis (IP) using ⁇ -gal columns, with plasma from 3 blood volumes being passed over the columns every 3 days for a total of 6 treatments.
- Three other baboons (designated 46-98, 52-98, 50-98) received IV infusions of 810S at 50 mg/kg every 3 days for a total of 6 treatments. No immunosuppression or exposure to pig organs was included in the protocol.
- Each IP treatment removed circulating IgM and IgG XNAs to 10-20% of initial levels, with a rebound of 50% of initial levels occurring in between each treatment. This is slightly different from what is usually observed, although our experience is with animals under immunosuppression.
- XNAs are undetectable in the post-treatment serum.
- a rebound of XNAs to 20-60% of the initial levels is usually observed after the 1st treatment, and this rebound effect generally diminishes with each IP treatment.
- IgG and IgM XNA levels were at 7-17% and 3-15% of initial baseline levels, respectively. See FIGS. 26 and 31- 33 .
- FIGS. 27 and 31- 33 Total IgM and IgG levels in all 3 animals remained relatively constant throughout the experiment. See FIGS. 31 - 33 .
- FIGS. 34 and 35 show the average fold-change in IgG and IgM response, respectively, for each of the IP and 810S treated animals.
- IgG XNA levels in 2 810S-treated baboons were still at 30-38% of baseline values, whereas in one baboon IgG XNA levels had returned to baseline.
- the IgM XNA levels in this 3rd baboon reached 60% of baseline levels by day 184, while IgM XNA levels in the other 2 baboons reached 24-39% of baseline values.
- Total IgM and IgG levels in all 3 animals remained relatively constant throughout the experiment.
- IP physically removes circulating antibodies, and their return to the circulation is expected due to re-synthesis. While the mode of action of 810S is less clear, it is apparent from in vitro ELISA experiments that 810S can bind XNAs and prevent them from binding ⁇ Gal epitopes. The sustained reduction on XNA levels several weeks after 810S treatment is discontinued, and the downregulation of ⁇ Gal ASCs, implies that 810S has an immunoregulatory function, e.g., is tolerogenic, as well.
Abstract
One aspect of the present invention relates to methods and compositions for attenuating xenograft rejection by administering, to an animal receiving the xenograft, an amount of a polymer-derivatized xenoantigen (hereinafter “xenopolymer”) effective for inhibiting or lessening the severity of hyperacute rejection response (HAR), or other immunological response to the graft, that is dependent on the presence of the xenoantigen on the grafted tissues or cells. In certain embodiments, the xenopolymer is administered in an amount sufficient to neutralize host antibodies (“xenoreactive antibodies” or “XNA”) immunoreactive with the xenoantigen. The xenopolymer may additionally, or alternatively, be used as a tolerogen (or anergen) for the xenoantigen, e.g., able to suppress, to some degree, the production/secretion of XNAs by the immune system of the host.
Description
- This application claims the benefit of the filing date of U.S. patent application Ser. No. 09/060,525, filed Apr. 15, 1998.
- This invention is in the general field of pharmaceuticals for treatment of conditions caused by xenoreactive antibodies.
- Organ Transplantation:
- Organ transplantation is now widely viewed as the preferred treatment for end stage organ failure, based on both a quality of life and cost basis. One year graft survival for kidney and heart transplantation at the major transplant centers is now greater than 90% with acute rejection rates less than 30%. There are approximately 2500 heart transplants and 12,500 kidney transplants performed every year in the USA. The major limitation to more widespread utilization of this procedure is a shortage of available organs. This can be seen by looking at the transplant waiting list, which shows that more than twice the number of people are waiting for an organ than could ever receive one. Additionally, 10 people per day die while waiting for a transplant. The demand for organs, however, is much greater than the number of people on the transplant waiting list. There are 125,000 patients in end stage renal failure who theoretically could benefit from a transplant. It is also been estimated that 25,000 to 40,000 people could benefit from a heart transplant if a donor organ were available.
- The availability of human organs is not likely to increase in any significant way over the next few years and even if all of the potential human donor organs could be utilized this would only increase the number of organs transplanted by about 2 fold, far less than the actual demand. Possible mechanical solutions to this problem such as left ventricular assist devices for cardiac failure while adequate at least for temporary support appear unlikely to provide for a long-term solution and as yet no implantable device exists for a kidney. Indeed, renal transplantation is viewed as superior and preferred in terms of the quality of life compared to dialysis. Given these alternatives the medical and scientific communities have turned to animals to provide for a solution to the organ shortage problem.
- Animals as Donors:
- The preferred animal species for transplantation to humans is the pig. Although the closely related non-human primate species are immunologically more similar to humans, and therefore the rejection process which is seen upon transplantation could likely be more easily controlled by current immunosuppressive drugs, a number of reasons make this possibility remote. First, a non-human primate of appropriate size for a heart transplant into an adult human would be a chimpanzee or equivalent large animal. These animals are on the endangered species list and thus would raise significant ethical questions even if it were possible to breed large numbers in captivity. The most abundant non-human primate, which is the baboon, is small and could not provide hearts for transplantation into adult humans although conceivably kidneys or livers would be possible. However, the baboon contains a number of potentially pathogenic organisms which could present a problem if transmitted to a human, and the generation of large numbers of specific pathogen free animals would be extremely costly and time consuming. The pig, a domesticated farm animal, on the other hand is of an appropriate size, can be raised to obtain specific pathogen free animals and is available in large numbers. Clearly, if the immunological problems could be overcome the pig would be the ideal donor to solve the organ shortage problem.
- The Immunological Problem:
- The initial barrier to transplantation of a pig organ to primates is a process of hyperacute rejection which results in the loss of the graft within a few minutes to hours of transplantation and is characterized histologically by thrombosis, hemorrhage, edema and a lack of a cellular infiltrate (Platt, J. L., et al., Immunology Today 11:450-456, 1990). This process is initiated by the binding of antibody, which is already present in the recipient, to the graft endothelium, and the resulting activation of the complement cascade. A similar process can be seen in allografts when antibody is present in the recipient prior to transplantation. In thinking about the pig as a donor, it is useful to review the effect of pre-existing antibodies on allograft survival and what strategies are important in obtaining prolonged graft survival.
- The presence of antibody in the serum of an allograft recipient, which recognizes antigen present on the donor endothelium, can be an indicator of a poor prognosis for long-term graft survival. Indeed, in a percentage of these allografts a rapid process of rejection, as described above for pig to primate xenografts, in which the graft is lost within a few minutes to hours after reperfusion, can occur. An example of this situation is seen when the donor and recipient are incompatible with regard to the blood group ABO system. The blood group antigen, which is a carbohydrate, is present on the donor endothelium. When a graft from a blood group A donor is transplanted into a blood group O recipient, in which antibody against the blood group A antigen is present, antibody is deposited in the graft. In the case of these grafts in which hyperacute rejection occurs the binding of antibody to the graft endothelium causes complement to be fully activated which results in damage to the endothelium and subsequent loss of the endothelial barrier function resulting in thrombosis, hemorrhage, edema and irreversible graft failure. If hyperacute rejection does not occur many, though clearly not all, of the grafts are lost due to vascular rejection in the subsequent weeks to months. These transplants were initially performed in the pre-cyclosporin era and led to the suggestion that matching for ABO compatibility should be performed prior to transplantation.
- As the success of allografts improved, dramatic shortages in the availability of organs led a number of groups to reassess the possibility of transplanting across the ABO barrier particularly in the case of living related kidney donors. These investigators found that in order to routinely prevent hyperacute rejection and achieve optimal long term graft survival, comparable to that obtained with ABO matched grafts, the antibody present at the time of transplant has to be temporarily removed usually by physical means such as immunoapheresis. A standard or in some cases an enhanced immunosuppression protocol is then applied. Under these circumstances the long-term outcome for these grafts is as good as would be obtained with ABO compatible grafts. In a percentage of these ABO incompatible grafts antibody apparently returns to the circulation but the graft is not rejected and this phenomenon has been termed “accommodation”. However, it is still far from clear that this antibody as detected in standard haemagglutination reactions is actually capable of binding to the graft. In many circumstances the antibody does not return in a detectable form to the circulation, although it is certainly conceivable that antibody is bound to the graft.
- In a pig to primate xenograft, the xenoreactive antibody which is found in the recipient prior to transplant predominantly if not exclusively recognizes the unique carbohydrate structure, αGal(1,3)Gal. This structure is found as the terminal sugar on glycolipids and glycoproteins present on the donor pig endothelium. The pig and many other mammals but not humans or Old World monkeys synthesize this epitope. This scenario is in many ways reminiscent of the ABO blood group mismatched allografts described above. In a similar fashion to the mammalian blood group antigens such as the human blood group A, the lack of the antigen in an animal results in the synthesis of antibodies which recognize it. This antibody is probably induced and stimulated on an ongoing basis by gut bacteria, which possess a related structure. Indeed, Galili et. el (1988) showed that anti-Gal antibodies bound to a variety of enterobacteria such as E. coli , klebsiella and salmonella. The Gal reactive structure varied between the bacteria and was either the lipopolysaccharide or the capsular polysaccharide (Hamadeh et. el. 1992). Both of these classes of antigens are well known as T-cell independent antigens. Additionally, the isotype of the ABO and α-Gal antibodies are similar, the levels of antibody in the serum are similar and the density of the carbohydrate antigens on the donor endothelium are also within the same range. However, when a pig to primate organ transplant is performed the graft is almost always rejected within a few minutes to hours with the very occasional graft lasting at most several days. This is in direct contrast with what happens in the allograft where the occasional graft is hyperacutely rejected and the majority survive for prolonged periods of time (weeks to months). A significant difference between the survival of allografts and xenografts under these conditions is most likely due to the incompatibility of the complement regulatory proteins. In the xenografts, antibody binds to the graft endothelium and results in the activation of the complement cascade. In the case of the allograft there are a series of proteins present on the donor endothelium which regulate the activity of complement and can be thought of as a primitive self/non-self-recognition process whereby if complement is inadvertently activated on the endothelium it can be inactivated before damage occurs. These proteins include Decay Accelerating Factor (DAF: CD55), Membrane Cofactor Protein (MCP: CD46) and CD59. In the case of the pig, the pig equivalent of the human CRP's does not function effectively to limit the action of human complement. Therefore, when complement is activated in the xenograft due to the functional lack of the CRP's the endothelium is severely damaged resulting in thrombosis, hemorrhage, edema and graft loss. This hypothesis has been confirmed by the generation of transgenic pigs, which express human CRP's and routinely overcome hyperacute rejection.
- If this were the only difference between an allograft and a xenograft then one might assume that with the addition of immunosuppression or antibody removal plus immunosuppression and the use of transgenic animals that one would observe xenograft survival with a similar time course as in an allograft. However, the simple addition of immmunosuppression does not substantially prolong graft survival and the organ invariably succumbs to vascular rejection. Antibody removal at the time of transplant combined with immunosuppression can prolong survival but not reach survival levels comparable with an allograft and again vascular rejection inevitably ensues. This vascular rejection is characterized histologically like hyperacute rejection by hemorrhage, thrombosis, and a lack of a cellular infiltrate; however, unlike hyperacute rejection there are significant regions of ischaemia. Immunopathological analysis reveals that antibody is bound to the graft but very little complement components are deposited.
- There are exceptions to this course of events and these are found when exceptionally high doses of immunosuppressive agents, whose mechanism of action is related to their anti-proliferate effect, are utilized or the physical removal of antibody by immunoapheresis in the post transplant period is aggressively continued. Taken together with the lack of a cellular infiltrate these results suggest that the organs are being lost due to a vascular rejection process which is most likely an antibody mediated event although the mechanism whereby antibody causes this rejection process are not yet clear.
- When the graft is present in the recipient, it is difficult to determine the level and specificity of xenoreactive antibody because the graft can act as a sink and bind up the antibody. We sought to circumvent this problem by following the antibodies in the animal after the removal of a rejected non life supporting heterotopic heart transplant and at the same time maintain the immunosuppressive regime after the graft is removed. Under these circumstances soon after the graft removal and in the following few days we observe a rapid rise in xenoreactive antibody levels in the animal. This is characterized by a 4-10 fold increase in IgM and a 100-200-fold increase in IgG. These antibodies almost exclusively recognize the αGal(1,3)Gal structure. A similar rise in αGal(1,3)Gal antibodies is seen in humans who are the recipients of cellular pig xenografts such as pancreatic islets or who have been exposed to a pig liver in an ex vivo perfusion circuit. These data suggest that the grafts are lost due to the enhanced production of αGal(1,3)Gal antibodies.
- The observation that the antibodies which we hypothesize mediate graft rejection in the pig to primate species combination predominantly recognize a single chemically defined epitope raises the possibility to control this antibody production in a specific manner. Specificity in the control of the immune response would be advantageous because it would reduce the risk of infectious complications associated with generalized immunosuppression. As discussed above, these responses are not easily controlled by current immunosuppression at clinically relevant levels making the rationale for a greater specificity even more important.
- In vivo neutralization of antibody by the administration of soluble ligand has been proposed for both ABO incompatible allografts and pig to primate xenografts. In both systems the infusion of large amounts of sugar are required due to a combination of low affinity of the ligand for antibody and short half life in the serum. In experimental ABO incompatible baboon to baboon heart allografts, if sugar infusion is maintained for a number of days in the presence of immunosuppressive agents the infusion can be stopped and long term graft survival can be achieved. In many ways this is reminiscent of the observation that the temporary removal of antibody by physical means, for example plasma exchange or specific immunopheresis in combination with immunosuppression, can result in long-term graft survival.
- In the case of the pig to primate xenograft the administration of soluble forms of the αGal(1,3)Gal carbohydrate has prevented hyperacute rejection, however, as soon as the sugar infusion is stopped the graft is rejected. To date the infusion has not been maintained for a prolonged enough period of time to assess when, if at all, the infusion can be stopped, as was the case with the allograft. It is conceivable that there is a substantial difference between allograft and xenograft rejection such that infusion cannot be stopped in the vast majority of cases. This experiment could be at least conceptually improved if it were possible to either dramatically improve the half-life of the sugar or increase the affinity or both such that much less was required or dosing was less frequent.
- One aspect of the present invention provides a polymer, synthesized by a cross-coupling reaction of
- (i) polymer scaffold units comprising a plurality of nucleophilic or electrophilic functional groups, and
- (ii) xenoantigens comprising a functional group capable of reacting with the functional groups presented on the polymer scaffold unit,
- wherein the reaction is carried out in the presence of a crosslinking reagent under conditions wherein polymer scaffold units are coupled with xenoantigens, and separate polymer scaffold units are cross-linked to one another.
- In certain embodiments, the functional groups for the polymer scaffold units and xenoantigens are selected from the group consisting of amine and carboxylic acid, alcohol and alkyl halide or sulfonate, thiol and alkyl halide or sulfonate, phosphine and alkyl halide or sulfonate, phosphite and alkyl halide or sulfonate, and aldehyde or ketone and amine. In preferred embodiments, the functional groups produce an amide, ether, thioether, or phosphate linkage between the polymer scaffold unit and xenoantigen upon cross-coupling.
- The cross-coupling may be carried out, e.g., in the presence of an activating agent that activates the functional groups of the polymer scaffold units and xenoantigens for forming a covalent bond there between. For instance, the activating group may be a dehydrating agents, Bronsted acid, Bronsted base, Lewis acid, Lewis base, acyl halide, or a phosphoryl halide. In certain preferred embodiments, the activating agent is a diimide, e.g., dicyclohexyldiimide [DCC] or ethyl-3-(dimethylamino)propyldiimide [EDC].
- In certain embodiments, the polymer scaffold units are pharmacologically-acceptable, non-immunogenic molecules. For instance, the polymer scaffold units can be polyethylene glycol, an oligopeptide, or oligosaccharide (e.g., dextran, dextrin or a cyclodextrin) bearing nucleophilic or electrophilic functional groups. Suitable nucleophilic or electrophilic functional groups include amines, alcohols, thiols, selenols, phosphines, aldehydes, ketones, acid chlorides, acids, esters, alkyl halides, and alkyl sulfonates. In particular embodiments, the functional group on the polymer scaffold unit is an amino or carboxylate moiety, and the functional group of the xenoantigen reacts therewith to form an amide linkage.
- In certain preferred embodiments, the polymer scaffold unit is a multiply-aminated polyethylene glycol, e.g., octa(amino)polyethylene glycol. In additional embodiments, the polymer scaffold unit is aminated dextran or an aminated cyclodextrin. In further embodiments, the polymer scaffold is a dendridic polyamine.
- The xenoantigen can be, to illustrate, a carbohydrate, a peptide, a glycopeptide or a lipid. In certain preferred embodiments, xenoantigen includes an epitope which is cross-reactive with an xenoreactive antibody against α-galactosyl moieties, and more preferably the xenoantigen is an oligosaccharide, e.g., having an αGal(1,3)Gal moiety represented by the general formula:
- wherein:
- R, independently for each occurrence, represents H or a C 1-C6 alkyl or other hydroxyl protecting group;
- R′ is absent or represents an oligosacchride consisting of 1-8 saccharide residues; and
- X represents a bond or linker moiety linking the oligosacchride moiety to the polymer backbone, e.g., preferably being a cyclic, branched or straight chain aliphatic group of 2-10 bonds in length, cycloalkyl, alkenyls, cycloalkenyl, alkynyl, aryl, heteroalkyl, or heteroaryl moiety.
-
- wherein,
- R1, independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
- R, independently for each occurrence, represents H or a C 1-C6 alkyl or other hydroxyl protecting group;
- R2 is a C 1-C6 alkyl;
- Y, independently for each occurrence, is absent, or —C(S)—, —S(O) 2—, —C(O)-A-;
- X represents a bond or linker moiety linking the oligosaccbride moiety to the polymer backbone;
- Z is O or S; and
- A is —NH—, —NH—(C 1-C6 alkyl)-, —NH—(C1-C6 alkenyl)-, —O—, —O—(C1-C6 alkyl)-, —O—(C1-C6 alkenyl)-, —S—, —S—(C1-C6 alkyl)-, —S—(C1-C6 alkenyl)-.
- In certain preferred embodiments, the xenoantigen includes one or more of: αGal(1,3) βGal; αGal(1,3)βGal(1,4)βGlcNAc; or αGal(1,3)βGal(1,4)βGlc moieties. In other embodiments, the xenoantigen may include one or more of: αGal(1,2)Gal, αGal(1,4)Gal, βGal(1,3)GalNAc or 3-O-sulphated galactose (SO 4-3Gal).
- The xenoantigen can be a di-, tri-, tetra- and penta-oligosacchrides.
- In certain embodiments, the polymer is homogenous with respect to the xenoantigen displayed thereon. In other embodiments, the polymer includes two or more different xenoantigens, e.g., on the same molecule.
- In particular preferred embodiments, the crosslinking agent has at least two functional groups capable of reacting with the polymer scaffold units, the xenoantigen, the covalent linkage of the scaffold with the xenoantigen, or a combination thereof. For instance, the crosslinking agent can be derived from N-hydroxysulfosuccinimide.
- In certain preferred embodiments, the cross-coupling reaction is carried out in the pH range of 4 to 7.
- In certain preferred embodiments, the cross-coupling reaction is carried out in the temperature range of 0° C. and 40° C.
- In certain preferred embodiments, the cross-coupling reaction is carried out a temperature, pH, reactant concentration and for a time sufficient to yield a polymer having a nominal molecular weight of 100,000-500,000 daltons. In certain embodiments, e.g., for tolerogenic forms of the xenopolymer, the cross-coupling reaction is carried out a temperature, pH, reactant concentration and for a time sufficient to yield a polymer having a nominal molecular weight less than 100,000 daltons.
- In certain preferred embodiments, the xenopolymer of the instant invention induces B cell anergy for the xenoantigen.
- For many applications, the subject xenopolymer is formulated with a pharmaceutical excipient, and is preferably formulated as a sterile formulation.
- Another aspect of the present invention is a method for manufacture of a medicament using the subject xenopolymer, the medicament being administered to a patient which is to receive, or has received, a discordant tissue graft, and in an amount sufficient to reduce the severity of rejection of the graft.
- In preferred embodiments, the medicament is formulated with a xenopolymer having a therapeutic index for preventing discordant graft rejection of at least 10.
- Another aspect of the present invention provides a kit, including two or more of the subject xenopolymers, each polymer isolated from the other and homogenous with respect to the xenoantigen displayed thereon but different from at least one other polymer of the kit.
- Another aspect of the present invention provides a polymer comprising a plurality of subunits interconnected by crosslinking moieties. See generally, FIG. 25. Each crosslinking moiety is covalently bound to at least two subunits, although the individual bonds between a crosslinker and a subunit need not be of the same type or comprise the same atom(s) of the subunits. The individual subunits comprise a backbone or scaffold, and a plurality of xenoantigens. The xenoantigens are covalently tethered to the backbone or scaffold, typically via a tether consisting of between 10 and 20 non-hydrogen atoms. The backbone or scaffold may itself be a polymer, e.g., an oligoethylene glycol, oligosaccharide, or oligopeptide. The synthesis of a polymer of this embodiment of the present invention may be achieved in a single reaction or in a series of reactions. When the synthesis of the polymer occurs in a single reaction, the process comprises combining the backbone or scaffold with the crosslinking agent and the xenoantigens to give a product comprising subunits, i.e., individual backbone or scaffold groups to which xenoantigens have been tethered, that are covalently interconnected via crosslinking moieties. See FIG. 25. When the synthesis of the polymer occurs in a series of reactions, the overall process comprises: combining the backbone or scaffold with the xenoantigens under conditions where the two react to generate subunits bearing a plurality of tethered xenoantigens; and combining the subunits bearing a plurality of tethered xenoantigens with the crosslinking agent to form crosslinks between the individual subunits.
-
- wherein
- R1, independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
- R5 is H, —OR, NH—Y—R2, or -L-E;
- Y independently for each occurrence is absent, —C(O)—, —C(S)—, —C(NR)—, O, S, or Se;
- B, for one occurrence is —R, and for the other occurrence is —R or from 1-9 saccharide residues;
- R, independently for each occurrence, represents H or a C 1-C6 alkyl, a protecting group, or -L-E;
- R2 is a C 1-C6 alkyl;
- X2 represents a linker of 1-10 atoms in the length;
- L represents a linker of 1-20 atoms in length, e.g., preferably being a cyclic, branched or straight chain aliphatic group of 2-15 bonds in length, cycloalkyl, alkenyls, cycloalkenyl, alkynyl, aryl, heteroalkyl, or heteroaryl moiety; and
- E represents a second PEG subunit of the polymer, wherein, independently for each occurrence of E, the linker group L is covalently attached via R5 or B of a saccharide moiety of the second PEG subunit, or via an amine of the second PEG subunit or via an amide moiety in the tether between the saccharide and the corresponding polyethylene glycol subunit.
-
- wherein,
- R1, independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
- R5 is H, —OR, NH—Y—R2, or -L-E;
- Y independently for each occurrence is absent, —C(O)—, —C(S)—, —C(NR)—, O, S, or Se;
- B, for one occurrence is —R, and for the other occurrence is —R or from 1-9 saccharide residues;
- R, independently for each occurrence, represents H or a C 1-C6 alkyl or other hydroxyl protecting group;
- R2 is a C 1-C6 alkyl;
- X2 represents a linker of 1-10 atoms in the length;
- D represents H, or -L-E;
- L represents a linker of 1-20 atoms in length; and
- E represents a second PEG subunit of the polymer, wherein, independently for each occurrence of E, the linker group L is covalently attached via R5 or B of a saccharide moiety of the second PEG subunit, or via an amine of the second PEG subunit.
-
- wherein
- R1, independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
- R5 is H, —OR, NH—Y—R2, or -L-E;
- Y independently for each occurrence is absent, —C(O)—, —C(S)—, —C(NR)—, O, S, or Se;
- B, for one occurrence is —R, and for the other occurrence is —R or from 1-9 saccharide residues;
- R, independently for each occurrence, represents H or a C 1-C6 alkyl or other hydroxyl protecting group;
- R2 is a C 1-C6 alkyl;
- X2 represents a linker of 1-10 atoms in the length D represents H, or -L-E;
- L represents a linker a linker of 1-20 atoms in length;
- E represents another polyethylene glycol subunit of the polymer having a saccharide moiety VII attached thereto, wherein, independently for each occurrence of E, the linker group L is covalently attached via R5 or B in the saccharide moiety.
- PEG represents a polyethylene glycol subunit; and
- X3 represents one or more additional polyethylene glycol subunit which may be derivatived with a α saccharide moiety of formula VII,
- wherein the polymer has an average molecular weight in the range of 10,000 daltons to 1,000,000 daltons.
- Still another aspect of the invention relates to a composition including: a non-immunogenic pharmacologically acceptable carrier; and multiple αGal(1,3)Gal moieties covalently linked to said carrier.
- The invention is also directed to a composition useful for reducing plasma levels of anti-(αGal(1,3)Gal) antibodies in a primate subject in need of a pig organ transplant. Herein, the term “primate” is understood to include humans. Reduction of levels of circulating anti-(αGal(1,3)Gal) antibodies is expected to prevent or ameliorate hyperacute rejection of the donated pig organ, thereby allowing long-term function of the transplanted organ.
- The composition of the invention comprises a non-immunogenic pharmacologically acceptable carrier covalently linked to multiple αGal(1,3)Gal moieties.
- The invention is also directed to methods for synthesizing the composition.
- A preferred method is to react in a solution at a pH of 4-7 the following reagents:
- a branched polymer, exemplified by polyethylene glycol, which has at least three arms and a minimum of 6 active groups such as amino groups or hydrazino group at its free ends,
- αGal(1,3)Gal having a covalently linked carboxy group, and
- N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC).
- A second preferred method is conducted as above, except the branched polymer has covalently linked carboxy groups, and the αGal(1,3)Gal has a covalently linked active group such as an amino or hydrazino group.
- A third preferred method is to react in a solution at a pH range of 4 to 7 the following reagents:
- a branched polymer having at least three arms, and at least six active groups such as amino or hydrazino groups covalently linked to the free ends of the arms, and -αGal(1,3)Gal having a covalently linked aldehyde.
- A fourth preferred method is conducted as the third method above, except that the branched polymer has at least six aldehydes covalently linked to its arms, and the αGal(1,3)Gal has a covalently linked amino group or hydrazino group.
- A fifth preferred method is to react in a solution at a pH range of 4 to 7 the following reagents:
- a branched polymer having at least three arms and at least three covalently linked active groups such as amino or hydrazino groups,
- αGal(1,3)Gal having a covalently linked carboxy group,
- EDC, and
- N-hydroxysulfosuccinimide (NHSS).
- A sixth preferred method is conducted as the fifth method above, except that the branched polymer has covalently linked carboxy groups and the αGal(1,3)Gal has covalently linked amino or hydrazino groups.
- The invention also provides methods for reducing plasma levels of anti-(αGal(1,3)Gal) antibodies in primate subjects, including human subjects, by administering the composition of the invention.
- The invention is also directed to a kit and methods for detecting and quantifying anti-(αGal(1,3)Gal) antibody secreting cells in the blood of a subject.
- Yet another aspect of the invention provides a method for attenuating rejection of tissues from a donor animal of one species transplanted to a recipient animal of another species, comprising administering to the recipient animal an amount of a polymer reactive with xenoreactive antibodies of the recipient animal effective for delaying or lessening the severity of a graft rejection response, wherein the polymer has epitopes cross-reactive with an xenoantigen of the graft, and rejection of the tissue is mediated at least in part by the expression of the xenoantigen on the tissue.
- The subject method can be used as part of a pretreatment program for a patient who is to be transplanted with tissue or cells from a discordant species, and/or for treatment of a patient who has been transplanted with tissue or cells from a discordant species. In preferred embodiments, the method reduces or otherwise attenuates the severity of hyperacute rejection of the transplanted tissues. In certain embodiments, the polymer is administered in an amount sufficient to neutralize host antibodies immunoreactive with the xenoantigen.
- In preferred embodiments, the receipient does not possess an endogenous UDP galactose: β-D-galactosyl-1,4-N-acetyl-D-glucosminide α(1,3) galactosyltransferase (α-1,3-GT) activity or does not otherwise produce or display the αGal(1,3)Gal xenoantigen on its cells, tissues or organs. For instance, the recipient can be a human or old world primate.
- In certain preferred embodiments, the xenograft is of pig origin, more preferably minature swine.
- The xenotransplanted tissue can be an organ, e.g., a vascularized organ, such as a kidney, heart, lung or liver. The xenotransplant tissue may also be in the form of parts of organs, cell clusters and glands, such as pancreatic islet cells, skin, corneal tissue and bone marrow or other preparations of hematopoietic cells.
- In one embodiment, there is provided a method for reducing plasma levels of anti-(αGal(1,3)Gal) antibodies in a primate subject, said method comprising administering to said subject a composition comprising:
- a non-immunogenic pharmacologically acceptable carrier; and
- multiple αGal(1,3)Gal moieties covalently linked to said carrier.
- Still another aspect of the present invention provides a complex comprising one or more B-cells associated with a composition comprising:
- a non-immunogenic pharmacologically acceptable carrier; and
- multiple αGal(1,3)Gal moieties covalently linked to the carrier.
- Yet another aspect of the present invention provides a complex comprising one or more antibodies associated with a composition comprising:
- a non-immunogenic pharmacologically acceptable carrier; and
- multiple αGal(1,3)Gal moieties covalently linked to said carrier.
- Another feature of the instant invention provides a kit for detecting and quantifying anti-(αGal(1,3)Gal) antibody-secreting cells in the blood of a subject comprising:
- a multi-well plate to which ≢Gal(1,3)Gal is covalently coupled;
- blocking solution comprising a buffer and human serum albumin;
- serum-free complete culture medium;
- anti-human immunoglobulin antibodies; and
- means for labeling said anti-human immunoglobulin antibodies bound to the plate.
- Still another feature to the instant invention is a method for detecting and quantifying anti-(αGal(1,3)Gal) antibody-secreting cells in the blood of a subject comprising:
- providing peripheral blood mononuclear cells isolated from a blood sample of said subject;
- providing a multi-well plate to which αGal(1,3)Gal is covalently coupled;
- incubating said peripheral blood mononuclear cells on said plate for a sufficient time and under appropriate conditions to allow anti-(αGal(1,3)Gal) antibody-secreting cells and their antibodies to bind specifically to said αGal(1,3)Gal coated on said plate;
- washing said plate to remove non-binding cells;
- incubating said anti-αGal(1,3)Gal) antibody-secreting cells and their antibodies bound to said αGal(1,3)Gal coated on said plate with anti-human immunoglobulin antibodies;
- washing said plate to remove non-binding anti-human immunoglobulin antibodies;
- applying a means to label anti-human immunoglobulin antibodies bound to said plate; and,
- quantifying single anti-(αGal(1,3)Gal) antibody-secreting, spot forming cells indicated by spots formed on said plate by the binding of said labelled anti-human immunoglobulin antibodies.
- FIG. 1 depicts the chemical structures of 4-arm and 8-arm polyethylene glycol with active groups at the end of each arm. In synthesizing an exemplary composition of the invention (TPC), αGal(1,3)Gal β 1-4 GlcNAc is covalently bound at amino sites on the ends of the arms.
- FIG. 2 depicts the chemical structure of αGal(1,3)Gal β1-4 GlcNAc (sugar) covalently bound to one arm of a multi-armed polyethylene glycol (PEG-NH 2).
- FIG. 3 shows the concentration of various compositions required for 50% inhibition of xenoreactive antibody activity.
- FIG. 4 shows the effect of TPC (410S) administration on anti-Gal IgM levels in cynomologous monkeys.
- FIG. 5 shows the effect of TPC (410S) administration on anti-Gal IgG levels in cynomologous monkeys.
- FIG. 6 shows xenoreactive antibody levels in a cynomologous monkey that received control vehicle instead of TPC (410S). “Tx” indicates day of heterotopic heart transplant.
- FIG. 7 shows suppression of xenoreactive antibody levels in a cynomologous monkey that received 250 mg TPC (410S) by infusion according to the schedule described in Example 17. “Tx” indicates day of heterotopic heart transplant.
- FIG. 8 likewise shows suppression of xenoreactive antibody levels in a second monkey treated as described in Example 17.
- FIG. 9 likewise shows suppression of xenoreactive antibody levels in a third monkey treated as described in Example 17.
- FIG. 10 shows the inhibition of anti-Gal IgM secreting B cells achieved with an exemplary composition of the invention.
TPC 410S FIG. 11 shows the inhibition of anti-Gal IgG secreting B cells by TPC (410S). - FIG. 12 shows suppression of xenoreactive IgM levels in a baboon treated with TPC (410S) as described in Example 19.
- FIG. 13 likewise shows suppression of xenoreactive IgM levels in a second baboon treated with TPC (410S) as described in Example 19.
- FIGS. 14-23 depict selected oligosaccharide moieties.
- FIG. 24 presents the IC 50 values of various molecules in the inhibition assay.
- FIG. 25 depicts a schematic representation of one possible structure for the products produced by the instant processes.
- FIG. 26 is a graph of IgM XNA Levels: 810S vs IP-treated baboons. Baboons were either treated with 810S (50 mg/kg) every 3 days for a total of 6 treatments, or underwent a total of 6 immunoapheresis treatments using α-gal columns every 3 days. Serum from each baboon was analyzed by ELISA for IgM antibodies directed against the α-gal epitope. The percent IgM XNAs remaining were calculated using baseline values from each animal as 100%.
- FIG. 27 is a graph of IgG XNA Levels: 810S vs IP-treated baboons. Baboons were either treated with 810S (50 mg/kg) every 3 days for a total of 6 treatments, or underwent a total of 6 immunoapheresis treatments using α-gal columns every 3 days. Serum from each baboon was analyzed by ELISA for IgG antibodies directed against the a -gal epitope. The percent IgM XNAs remaining were calculated using baseline values from each animal as 100%.
- FIG. 28 is a graph of baboon 46-98 antibody response to 810S. Baboon 46-98 was treated with 810S (50 mg/kg) every 3 days for a total of 6 treatments. Serum was analyzed for IgM and IgG antibodies directed against the α-gal epitope (XIgM or XIgG), as well as for total IgM (TotIgM) or total IgG (TotIgG) by ELISA. The percent antibody remaining was calculated using baseline values as 100%.
- FIG. 29 is a graph of baboon 52-98 antibody response to 810S. Baboon 52-98 was treated with 810S (50 mg/kg) every 3 days for a total of 6 treatments. Serum was analyzed for IgM and IgG antibodies directed against the α-gal epitope (XIgM or XIgG), as well as for total IgM (TotIgM) or total IgG (TotIgG) by ELISA. The percent antibody remaining was calculated using baseline values as 100%.
- FIG. 30 is a graph of baboon 50-98 antibody response to 810S. Baboon 50-98 was treated with 810S (50 mg/kg) every 3 days for a total of 6 treatments. Serum was analyzed for IgM and IgG antibodies directed against the α-gal epitope (XIgM or XIgG), as well as for total IgM (TotIgM) or total IgG (TotIgG) by ELISA. The percent antibody remaining was calculated using baseline values as 100%.
- FIG. 31 is a graph of baboon 51-98 antibody response to IP. Baboon 51-98 underwent a total of 6 immunoapheresis treatments using α-gal columns every 3 days. Serum was analyzed for IgM and IgG antibodies directed against the α-gal epitope (XIgM or XIgG), as well as for total IgM (TotIgM) or total IgG (TotIgG) by ELISA. The percent antibody remaining was calculated using baseline values as 100%.
- FIG. 32 is a graph of baboon 48-98 antibody response to IP. Baboon 48-98 underwent a total of 6 immunoapheresis treatments using α-gal columns every 3 days. Serum was analyzed for IgM and IgG antibodies directed against the α-gal epitope (XIgM or XIgG), as well as for total IgM (TotIgM) or total IgG (TotIgG) by ELISA. The percent antibody remaining was calculated using baseline values as 100%.
- FIG. 33 is a graph of baboon 47-98 antibody response to IP. Baboon 47-98 underwent a total of 6 immunoapheresis treatments using α-gal columns every 3 days. Serum was analyzed for IgM and IgG antibodies directed against the α-gal epitope (XIgM or XIgG), as well as for total IgM (TotIgM) or total IgG (TotIgG) by ELISA. The percent antibody remaining was calculated using baseline values as 100%.
- FIG. 34 compares the IgG response between IP and TPC (810) treated baboons. Baboons were either treated with 810S (50 mg/kg) every 3 days for a total of 6 treatments, or underwent a total of 6 immunoapheresis treatments using α-gal columns every 3 days. Peripheral blood mononuclear cells were collected and analyzed for the number of cells secreting IgG antibodies directed against α-gal using an ELISPOT analysis. The fold-change in antibody secreting cells (ASCs) was calculated by normalizing to baseline values. Values are represented as the mean from 3 baboons per group.
- FIG. 35 compares the IgM response between IP and TPC (810) treated baboons. Baboons were either treated with 810S (50 mg/kg) every 3 days for a total of 6 treatments, or underwent a total of 6 immunoapheresis treatments using α-gal columns every 3 days. Peripheral blood mononuclear cells were collected and analyzed for the number of cells secreting IgM antibodies directed against α-gal using an ELISPOT analysis. The fold-change in antibody secreting cells (ASCs) was calculated by normalizing to baseline values. Values are represented as the mean from 3 baboons per group.
- (i) Overview
- The primary obstacle to all forms of organ transplantation is the rejection of the transplant by the recipient's immune system. The more closely related the donor and recipient are, the less likely the transplanted organ will be rejected. In allotransplantation, the transplantation of organs between the same species, the problem of immune rejection can be largely overcome by immunosuppressive drugs. The current shortage of human tissues for human transplantation, however, has led to use of xenografts as a source of organs. A problem which arises when tissues from non human-species are grafted to humans is one of hyperacute rejection, which occurs due to the existence of natural antibodies in human serum which react with antigens present in these species. This phenomenon depends, in general, on the presence of some or all of antibody, complement, neutrophils, platelets and other mediators of inflammation.
- Pigs are top candidates for xenotransplant donors because their physiology and anatomy parallel that of humans, and because they are plentiful and can be raised under clean conditions to minimize risk of infection. In the context of transplantation of pig tissue into humans, hyperacute rejection is associated at least in part with host antibodies reactive with galactosyl antigens present on the pig tissue, such as α galactosyl (1,3) galactose moieties (the “αGal(1,3)Gal” or “αGal” epitope). Inhibiting the interaction between host antibodies reactive with the αGal(1,3)Gal epitopes of xenotransplant tissue can be used to prevent rejection.
- One aspect of the present invention relates to methods and compositions for attenuating xenograft rejection by administering, to an animal receiving the xenograft, an amount of a polymer-derivatized xenoantigen (hereinafter “xenopolymer”) effective for inhibiting or lessening the severity of hyperacute rejection response (HAR), or other immunological response to the graft, that is dependent on the presence of the xenoantigen on the grafted tissues or cells. In certain embodiments, the xenopolymer is administered in an amount sufficient to neutralize host antibodies (“xenoreactive antibodies” or “XNA”) immunoreactive with the xenoantigen. The xenopolymer may additionally, or alternatively, be used as a tolerogen (or anergen) for the xenoantigen, e.g., able to suppress, to some degree, the production/secretion of XNAs by the immune system of the host.
- As described in further detail below, the xenoantigen can be, to illustrate, a carbohydrate, a protein (or peptidyl portion thereof), a lipid or other fatty acid moitey, or a nucleic acid. In certain preferred embodiments, the xenoantigen(s) that is disposed on the polymer is immunoreactive with host XNAs against carbohydrate epitopes of the xenograft, and more preferably is immunoreactive with host antibodies against α-galactosyl epitopes, such as an αGal(1,3)Gal epitope.
- The polymer component, e.g., the backbone of the subject xenoploymers, is preferably derived from a polymer that is water-soluble, and substantially non-toxic and non-immunogenic. Illustrative polymers of this type include polyethyleneglycols, polyvinylpyrrolidones, polyacrylamides, dextrans, and polyglycomers. In preferred embodiments, however, the xenopolymer is formed from a polyamine, such as aminated polyethyleneglycols, polyalkylamines, polypeptides (such as polylysine, polyornithine, polyarginine, polyasparagine, polyglutamine), polyallylamines, poly[N(2-aminoethyl)]methacrylamides, and polyaminocarbohydrates such as aminodextran or chitosan. The polymer may be homopolymeric or copolymeric, and if the latter, may be a block or statistical copolymer.
- In certain embodiments, the subject method can be used to promote tolerance to the αGal(1,3)Gal antigen in a recipient mammal which does not possess an endogenous UDP galactose:β-D-galactosyl-1,4-N-acetyl-D-glucosminide α(1,3) galactosyltransferase (α1,3-GT) activity or which otherwise does not produce or display the αGal(1,3)Gal antigen on its cells, tissues or organs. Thus, in preferred embodiments, the recipient animal is a human or old world primate, e.g., a baboon (such as Papio anubis) or cynomolgus monkey (such as Macaca fascicularis).
- Xenografted tissue is preferably of pig origin, though tissues from other non-human mammals are also contemplated for use in this invention. Preferably the xenotransplanted tissue is in the form of an organ, and more preferably a vascularized organ, as for example, kidney, heart, lung or liver. Xenotransplant tissue may also be in the form of parts of organs, cell clusters, glands and the like. Examples include lenses, pancreatic islet cells, skin, corneal tissue and bone marrow or other preparations of hematopoietic cells.
- The xenopolymers of the present invention have interesting properties. In particular, they have affinity for human polyclonal XNAs present in body fluids, e.g., blood, and are useful as depleting agents. In certain embodiments, the subject xenopolymers can be used for intracorporal depletion of XNAs, e.g., by injection, infusion or perfusion of the polymer into a xenograft recipient prior to and/or after transplantation. The xenopolymers of the present invention, in certain embodiments, may also be used as a pharmaceutical for inducing tolerance or anergy towards xenoantigenic epitopes or to specifically target B cells with xenoantigen receptors. The administration may be repeated as required to achieve tolerance.
- Thus, in one embodiment the method of the present invention includes a step of pre-treating a patient, who is to be transplanted with tissue or cells from a discordant species, with an effective amount of the subject xeno-polymer so as to reduce the risk of hyperacute rejection or other adverse immunological reaction to the xenograft, upon transplantation.
- In another embodiment, the subject method includes a step of treating a patient, who has been transplanted with tissue or cells from a discordant species, with an effective amount of the subject xeno-polymer so as to reduce the risk of hyperacute rejection or other adverse immunological reaction to the xenograft.
- Treatment according to the method of this invention will not necessarily prevent all aspects of rejection, but it will be effective to suppress the antibody-dependent component of transplant rejection. Even partial suppression of rejection is beneficial, because mitigation of the rejection phenomena will allow rescue of some of the tissue in the graft in a viable state. For example, even a partially functioning kidney may permit the recipient to avoid dialysis or prolong the periods between dialysis, even if some cell lysis has occurred. Similarly, a fully functional heart graft is preferable, but mitigation of rejection sufficient to permit some increase in exertion by the recipient, relative to the recipient's state before transplant, is valuable. Partial rescue of liver tissue in a liver graft undergoing rejection is also valuable because the remaining viable liver tissue can recover and regenerate while the patient is supported with coagulation proteins and other substances normally produced by the liver.
- As described in further detail below, the subject method can be combined with other transplantation therapies, including immunoaphoresis and other treatment protocols for inducing accommodation, immunosuppressive therapy and the use of genetically engineered donor tissue.
- Still another aspect of the invention relates to compositions including the subject xenopolymers, especially polymers displaying xenoantigens immunoreactive with host antibodies against α-galactosyl epitopes such as αGal(1-3)Gal. In preferred embodiments, the polymer preparations are formulated with a pharmaceutically acceptable excipient, are sterile, and are substantially non-pyrogenic.
- For certain of the subject xenopolymers, it is the combined properties of activity at a very low molar concentration plus long-term stability that make the compositions of the invention practical and useful as pharmaceuticals. These properties allow for effectiveness at low doses, which are expected to be well tolerated by patients in need of chronic reduction of circulating anti-Gal antibodies. The stability of the compositions allows for long-tern storage at temperatures achievable in a household setting, thus opening the possibility that patients may self-administer the pharmaceuticals over the long term, perhaps even over their entire lifetime after receiving a pig organ transplant.
- Another aspect of the present invention relates to the use of the subject xenopolymers in the manufacture of a medicament for the treatment of a discordant graft rejection, wherein the medicament is administered to a patient either as a pre-transplant conditioning or for maintenance after transplantation, or both, and in an amount sufficient to reduce the severity of rejection of the graft.
- (ii) Definitions
- For convenience, certain terms employed in the specification and appended claims are collected here.
- The term “Gal” refers to galactose; “Glc” refers to glucose and “GlcNAc” refers to N-acetylglucosamine.
- The terms “sugar monomers” or “saccharide subunits”, which are used interchangeably herein, refer to naturally occurring sugars, such as galactose, glucose and the like, as well as sugar monomers which are modifications thereof. For example, the term includes protected, partially protected or unprotected deoxysugars of the D- and L-configurations, preferably 2-, 3-, 4-, 5- and 6-deoxyaldoses, such as fucose, rhamnose and digitoxose, 1,2-dideoxyaldoses, such as glucal, galactal and fucal, and 1-, 3-, 4-, 5- and 6-deoxyketoses, 2-, 3-, 4-, 5- and 6-deoxyaminosugars of the D and L configurations, such as glucosamine, mannosamine, galactosamine and fucosamine, and deoxyacylaminosugars, such as N-acylglucosamine, N-acylmannosamine, N-acylgalactosamine and N-acyl-fucosamine, preferably their C 1-C4alkyl esters. In addition, these modifications are understood to mean aldonic, aldaric and uronic acids, such as gluconic acid or glucuronic acid, and also ascorbic acid and amino acid-carrying sugar monomers. Modified sugar monomers are also understood to mean those having a carbon chain which is longer than 6 C atoms, such as heptoses, octoses, nonoses, heptuloses, octuloses and nonuloses, and also their representatives which are substituted in accordance with the above-listed criteria, such as ketodeoxyoctanic acid, ketodeoxynonanic acid, N-acylneuraminic acids and N-acylmuraminic acids.
- Oligosaccharides are considered to have a reducing end and a non-reducing end, whether or not the sacchride at the reducing end is in fact a reducing sugar. In accordance with accepted nomenclature, oligosacchrides are depicted herein with the non-reducing end on the left and the reducing end on the right.
- All oligosacchrides described herein with the name or abbreviation for the non-reducing sacchride (e.g., Gal), followed by the configuration of the glycosidic bond (α or β), the ring bond, the ring position of the reducing sacchride involved in the bond, and the name or abbreviation of the reducing sacchride (e.g., GlcNAc). The linkage is preferably α-O-glycosidic or β-O-glycosidic, e.g., (1→2)-, (1→3)-, (1→4)-, (1→5)-, (1→6)-, (2→3)- and (2→6)-glycosidic linkages. Examples of disaccharides are e.g. trehalose, sophorose, kojibiose, laminaribiose, maltose, cellobiose, isomaltose, gentibiose, sucrose and lactose, and their derivatives. Examples of trisaccharides are raffinose and melezitose. Furthermore, the oligosaccharides may be linked via S-, N- and C-glycosidic linkages, e.g. —S—, —NR 12-, —NR12C(O)— or —CR13R14—, wherein R12, R13 and R14 independently of each other are H,C1-C12alkyl, C5- or C6cycloalkyl, C5- or C6cycloalkylmethyl or -ethyl, phenyl, benzyl or phenethyl. Generally, each sacchride subunit is a pyranose.
- An “αGal(1,3)Gal moiety”, or αGal moiety, refers to two galactosyl residues, the Gal residue at the reducing end being an α-galactosyl moiety, that are covalently joined by a 1-3 glycosidic linkage.
- The term “αGal oligosaccharide” refers to oligosaccharides (e.g., containing two or more sacchride moieties) including the αGal(1,3)Gal moiety.
- The term “XNA” refers to xenoreactive natural antibodies, such as human antibodies which are immunoreactive with the carbohydrate αGal(1,3)Gal epitope on pig tissues.
- As used herein, “hyperacute rejection” refers to rapid graft rejection, beginning minutes after implantation, and which is mediated by pre-existing antibodies to the graft.
- As defined herein, a “xenograft” may be an organ, tissue, aggregates of cells, or cells, collectively referred to herein as “tissue”. The graft may be selected from any appropriate tissue of the body of the tissue donor. These tissues include, but are not limited to, heart, kidney, lung, islet cells, liver, bowel, skin and hematopoietic cells.
- The phrases “reduce xenograft rejection” and “attenuate xenograft rejection”, which are used interchangeably herein, mean to inhibit, interfere with or otherwise reduce the severity of processes resulting in hyperacute rejection, such as, but not limited to, ischemia, thrombosis, myocardial congestion and tissue necrosis.
- The terms “xenogeneic” and “xenograft” refer to cells or tissue which originates with or is derived from a species other than that of the recipient.
- The term “autologous” refers to tissue or cells which originate with or are derived from the recipient, whereas the terms “allogeneic” and “allograft” refer to cells and tissue which originate with or are derived from a donor of the same species as the recipient.
- An “immunosuppressive agent”, as used herein, is an agent, e.g., a chemical agent, e.g., a drug, which, when administered at an appropriate dosage, results in the inhibition of T cells. Examples of such agents are cyclosporine, FK-506, and rapamycin.
- “Hematopoietic space-creating irradiation”, as used herein, refers to irradiation directed to the hematopoietic tissue, i.e., to tissue in which stem cells are found, e.g., the bone marrow. It is of sufficient intensity to kill or inactivate a substantial number of hematopoietic cells. It is often given as whole body irradiation.
- “Tolerance”, as used herein, refers to an inhibition of a graft recipient's immune response which would otherwise occur, e.g., in response to the introduction of a nonself antigen into the recipient. Tolerance can involve humoral, cellular, or both humoral and cellular responses. Tolerance, as used herein, refers not only to complete immunologic tolerance to an antigen, but to partial immunologic tolerance, i.e., a degree of tolerance to an antigen which is greater than what would be seen if a method of the invention were not employed. Moreover, tolerance, as used herein, refers to a donor antigen-specific inhibition of the immune system as opposed to the broad spectrum inhibition of the immune system seen with immunosuppressants.
- “Inhibiting immune cell activity” refers to reducing the number of active immune cells, e.g., thymocytes, T cells, B cells, or NK cells, preferably donor reactive cells, or precursor donor reactive cells, in a subject. Inhibition can include partial inhibition, or partial reduction (as opposed to total elimination) of the number of active immune cells, e.g., T cells.
- “Discordant species combination”, as used herein, refers to two species in which hyperacute rejection occurs when a graft is grafted from one to the other. Generally, discordant species are from different orders, while non-discordant species are from the same order. For example, rats and mice are non-discordant concordant species. Concordant species combinations do not exhibit hyperacute rejection. In xenogeneic method of the invention, the donor and recipient (subject) can be a discordant species combination.
- “Miniature swine”, as used herein, refers to a miniature pig which is preferably wholly or partially inbred at at least one MHC locus. The coefficient of inbreeding of the herd which supplies the miniature swine should be at least, 0.70 and more preferably at least 0.82. The herd from which donor animals are drawn should be homozygous at the SLA genes.
- As used herein, “myeloablative” refers to a treatment in which death, due to marrow failure, in a significant number of recipients, will occur if hematopoietic stem cell transplantation is not given.
- As used herein, “non-myeloablative” refers to a treatment which kills marrow cells but will not, in a significant number of recipients, lead to death from marrow failure.
- The term “subject”, as used herein, refers to a mammal, e.g., a human.
- The term “ED 50” means the dose of a drug, e.g., a subject polymer, which produces 50% of its maximum response or effect. Alternatively, the dose which produces a pre-determined response in 50% of test subjects or preparations.
- The term “LD 50” means the dose of a drug which is lethal in 50% of test subjects.
- The term “therapeutic index” refers to the therapeutic index of a drug defined as LD 50/ED50.
- For purposes of this invention, the chemical elements are identified in accordance with the Periodic Table of the Elements, CAS version, Handbook of Chemistry and Physics, 67th Ed., 1986-87, inside cover. Also for purposes of this invention, the term “hydrocarbon” is contemplated to include all permissible compounds having at least one hydrogen and one carbon atom. In a broad aspect, the permissible hydrocarbons include acyclic and cyclic, branched and unbranched, carbocyclic and heterocyclic, aromatic and nonaromatic organic compounds which can be substituted or unsubstituted.
- The phrase “protecting group” as used herein means temporary substituents which protect a potentially reactive functional group from undesired chemical transformations. Examples of such protecting groups include esters of carboxylic acids, silyl ethers of alcohols, and acetals and ketals of aldehydes and ketones, respectively. The field of protecting group chemistry has been reviewed (Greene, T. W.; Wuts, P. G. M. Protective Groups in Organic Synthesis, 2nd ed.; Wiley: New York, 1991).
- The practice of the present invention will employ, unless otherwise indicated, conventional techniques of cell biology, cell culture, molecular biology, transgenic biology, microbiology, recombinant DNA, and immunology, which are within the skill of the art. Such techniques are explained fully in the literature. See, for example, Molecular Cloning A Laboratory Manual, 2nd Ed., ed. by Sambrook, Fritsch and Maniatis (Cold Spring Harbor Laboratory Press: 1989); DNA Cloning, Volumes I and II (D. N. Glover ed., 1985); Oligonucleotide Synthesis (M. J. Gait ed., 1984); Mullis et al. U.S. Pat. No. 4,683,195; Nucleic Acid Hybridization (B. D. Hames & S. J. Higgins eds. 1984); Transcription And Translation (B. D. Hames & S. J. Higgins eds. 1984); Culture Of Animal Cells (R. I. Freshney, Alan R. Liss, Inc., 1987); Immobilized Cells And Enzymes (IRL Press, 1986); B. Perbal, A Practical Guide To Molecular Cloning (1984); the treatise, Methods In Enzymology (Academic Press, Inc., N.Y.); Gene Transfer Vectors For Mammalian Cells (J. H. Miller and M. P. Calos eds., 1987, Cold Spring Harbor Laboratory); Methods In Enzymology, Vols. 154 and 155 (Wu et al. eds.), Immunochemical Methods In Cell And Molecular Biology (Mayer and Walker, eds., Academic Press, London, 1987); Handbook Of Experimental Immunology, Volumes I-IV (D. M. Weir and C. C. Blackwell, eds., 1986); Manipulating the Mouse Embryo, (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., 1986).
- (iii) Illustrative Embodiments
- In one aspect, this invention provides pharmaceutical compositions capable of reducing plasma levels of anti-αGal(1,3)Gal (“anti-αGal”) antibodies in primate subjects, including human patients. While the instant invention contemplates methods and compositions for reducing xenoreactive antibodies to other antigens, for clarity, most of the discussion to follow focuses on oligosaccharide xenoantigens. The compositions are intended for administration to patients being prepared for transplant of a tissue or cells from a discordant donor, such as a pig organ, a kidney or heart, or patients who have already received such transplants and are in need of reduction of anti-αGal antibodies in order to prevent or reduce rejection or other antibody-dependent degradation in performance of the pig organ, e.g., to reduce hyperacute rejection of the organ.
- A. Illustrative Xenopolymers
- A preferred composition of the invention is a polymer derived from the reaction of a polymer backbone comprising a plurality of nucleophilic or electrophilic functional groups with a xenoantigen comprising a functional group capable of reacting with the functional groups presented on the structural backbone in the presence of a reagent capable of activating one set of the aforementioned functional groups for reaction with the other set of functional groups, all in the presence of a crosslinking reagent (see FIG. 25). In certain embodiments, the complementary pairs of functional groups are selected from the group consisting of amine/carboxylic acid, alcohol/alkyl halide or sulfonate, thiol/alkyl halide or sulfonate, phosphine/alkyl halide or sulfonate, phosphite/alkyl halide or sulfonate, and aldehyde or ketone/amine. Suitable complementary pairs of functional groups are those that produce a linkage between the antigen and the scaffold that is robust under physiological conditions, e.g., an amide, ether, thioether, or phosphate. In certain embodiments, the linkage between the scaffold and the antigen comprises an amide moiety.
- The activating reagent, i.e., the reagent that facilitates the formation of the covalent bond between the scaffold and the xenoantigen, is any reagent capable of activating a nucleophilic or electrophilic functional group for reaction with a complementary electrophilic or nucleophilic functional group to form a new covalent bond. In certain embodiments, the activating reagent is a dehydrating agent, diimide, Bronsted acid or base, Lewis acid or base, acyl halide, or phosphoryl halide. In certain embodiments, the activating reagent is a diimide, e.g., dicyclohexyldiimide [DCC] or ethyl-3-(dimethylamino)propyldiimide [EDC]. In certain embodiments, the activating agent is EDC.
- In preferred embodiments the composition has a structural backbone comprising a non-immunogenic, pharmacologically-acceptable scaffold, e.g., polyethylene glycol bearing multiple nucleophilic or electrophilic functional groups. The scaffold is preferrably in the molecular weight range of 1,000 Da to 100,000 Da, more preferably in the range of 3,000 to 70,000 Da, most preferably in the range of 5,000 to 30,000 Da. However, as described in the appended examples, the molecular weight of the final polymeric product can be much larger, primarily due to cross-linking of the scaffold units.
- The nucleophilic or electrophilic functional groups on the scaffold are selected from the group comprising amines, alcohols, thiols, selenols, phosphines, aldehydes, ketones, acid chlorides, acids, esters, alkyl halides, and alkyl sulfonates. In certain embodiments, the functional group on the scaffold is an amino or carboxylate moiety, and the functional group comprised by the antigen reacts therewith to form an amide linkage.
- The antigen of the present invention may comprise a carbohydrate, peptide, glycopeptide, lipid, or any small organic or inorganic moiety known to be xenoantigenic, i.e., presented by the xenograft and antigenic to the subject's immune system. Xenoantigenic groups can be readily identified by methods well known in the art. For instance, XNAs from human blood can be isolated by perfusing the blood over xenograft material, isolating from the graft those XNAs which specifically bound to it, and using those antibodies to screen candidate epitopes, e.g., by immunoassay. In certain embodiments, the antigen is an oligosaccharide, oligopeptide or glycopeptide.
- It has been observed that human xenoreactive antibodies can be polymorphic with respect to binding specificity for oligosacchrides including the αGal(1,3)Gal epitope, e.g., including species which bind to di-, tri-, tetra-, penta- and high order oligosacchrides including the αGal(1,3)Gal moiety. Thus, in certain embodiments, the invention contemplates xenoantigen-polymers derived with αGal(1,3)Gal terminated oligosaccharides, e.g., including from 1-10 saccharide residues, more preferably 1-5 saccharide residues. αGal oligosaccharides of the invention include those saccharide compositions comprising two or more saccharide moieties in which a galactose moiety is joined by an α(1,3) glycosidic linkage to another galactose moiety. In preferred embodiments the αGal motif (Galα1-3Gal) is located within 15, 10, 5, 4, 3, 2, or 1 saccharide unit(s) from the non-reducing end of the oligosaccharide. In a most preferred embodiment, the αGal motif represents the terminus (i.e., the non-reducing end) of the oligosaccharide. The αGal oligosaccharides may comprise one, or a plurality of αGal motifs. For example, the αGal oligosaccharide may be a branched carbohydrate having multiple terminal Galα1-3Gal residues or a linear oligosaccharide containing both terminal and internal Galα1-3Gal residues. To further illustrate, the subject polymers can include xenoantigens represented by the general formula (I):
- wherein: R, independently for each occurrence, represents H or a C 1-C6 alkyl or other hydroxyl protecting group, and more preferably represents H for each occurrence; R′ is absent or represents an oligosacchride, e.g., having from 1-8 saccharide residues; and X represents a bond or linker moiety linking the oligosacchride moiety to the polymer backbone.
- Certain experiments have indicated the strongest binding of human xenoreactive antibodies to oligosaccharides with an α-galactosyl terminal residue included the oligosaccharides αGal(1,3)Galβ, αGal(1,3)Galβ(1,4)GlcNAcβ and αGal(1,3)Galβ(1,4)Glcβ. See, for example, Cooper et al. (1994) Immunol Rev 141:31. Thus, in certain embodiments, the xenoantigen is represented by the general formula (II):
- wherein R and R′ are as defined above.
-
- wherein,
- R1, independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
- R, independently for each occurrence, represents H or a C 1-C6 alkyl or other hydroxyl protecting group, and more preferably represents H for each occurrence;
- R2 is a C 1-C6 alkyl, and more preferably represents CH3;
- Y, independently for each ocurrence, is absent, or —C(S)—, —S(O) 2—, —C(O)-A-;
- X represents a bond or linker moiety linking the oligosacchride moiety to the polymer backbone;
- Z is O or S; and
- A is —NH—, —NH—(C 1-C6 alkyl)-, —NH—(C1-C6 alkenyl)-, —O—, —O—(C1-C6 alkyl)-, —O—(C1-C6 alkenyl)-, —S—, —S—(C1-C6 alkyl)-, —S—(C1-C6 alkenyl)-.
- In preferred embodiments, the αGal oligosaccharides of the invention comprise a saccharide sequence corresponding to the antigenic glycolipid expressed on pig endothelial cell membrances, e.g., vascular endothelial or other endothelial tissue of heart, kidney, liver or lung tissue. Such αGal oligosaccharides include, but are not limited to Galα1-3Gal; Galα1-3Galβ1-4GlcNAc; Galα1-3Galβ1-4GlcNAcβ1-3Gal; and Galα1-3Galβ1-4GlcNAcβ1-3Galβ1-4Glc.
- Other exemplary oligosacchrides for incorporation in the subject xenoantigen-polymers are shown in FIGS. 14-23.
- Moreover, while findings in the art indicate that most human xenoreactive IgM and some human xenoreactive IgG is specific for the αGal(1,3)Gal epitope, some human xenoreactive antibodies directed against other determinants may also be responsible for hyperacute rejection. See, for example, Parker et al. (1995) Transplant Immunol 3:181; Ye et al. (1994) Transplantation 58:330; and Collins et al. (1994) Xenotransplantation 1:36. Thus far, in addition to the αGal(1,3)Gal epitope, several other pig carbohydrate epitopes have been identified as immunoreactive with natural antibodies in human serum, including αGal(1,2)Gal, αGal(1,4)Gal, βGal(1,3)GalNAc and 3-O-sulphated galactose (SO4-3Gal), each of which can be used as a xenoantigen for incorporation into the subject polymers. See, e.g., Holgersson et al. (1990) J Biochem 108:766; Holgersson et al. (1991) Glycoconj J 8:172; and Good et al. (1992) Transplant Proc 24:559. Others report oligosaccharide epitopes for human xenoreactive antibodies as including A or A-like carbohydrates (namely A disaccharide, A trisacchride, A tetrasacchrides and linear A type 6), Forssman disaccharide and trisaccharide, α-L-rhamnose and rhamnose-containing oligosaccharides, and βGlcNac-containing oligosacchrides. See, Cooper et al. (1993) Transplan Immunol 1:198; and Good et al. (1992) Transplan Proc 24:559. The possibility of other carbohydrate xenoantigens is also suggested by the fact that other species such as the pig, goat, dog, rat, etc—which do not produce anti-αGal(1,3)Gal antibodies—have xenoreactive natural antibodies which presumably recognize other epitopes. Cameron et al. (1983) J Surg Oncol 22:157. Accordingly, the subject xenoantigen-polymers can be generated using xenoantigenic oligosaccharides other than αGal, e.g., which are cross-reactive with natural human xenoreactive antibodies.
- Examples of modified αGal oligosaccharides (i.e., derivatives) also encompassed by the invention include, but are not limited to, salts and sulfate substitutes of αGal oligosaccharides, as well as αGal oligosaccharides in which one or more or the pyran rings has been substituted with a piperidine ring system and/or a tetrahydrothiopyran ring system.
- αGal oligosaccharide aza sugars in which the oxygen of one or more of the pyran rings of the oligosaccharide is replaced with nitrogen to form a piperidine ring system may be prepared by enzymatic methods known in the art using the appropriate aza saccharide as the acceptor substrate. Alternatively, aza sugar donor moieties may be transferred by the corresponding glycosyltransferase for the natural sugar. Aza glucose can be isolated from natural sources and converted to the aza lactose by the action of a galactosyltransferase in the presence of a galactose donor such as for example, UDP-galactose.
- The polymeric composition of the invention may also include αGal oligosaccharide thio sugars, e.g., in which the is oxygen of one or more of the pyran rings of the oligosaccharide is replaced with sulfur to form a tetrahydrothiopyran ring system. The monothiosaccharide may be prepared by known organic chemical techniques from the corresponding monosaccharide and the αGal oligosaccharide derivative thio sugar may be prepared applying enzymatic methods and using the appropriate thio saccharide as the acceptor substrate.
- Those skilled in the art will readily appreciate that libraries of oligosaccharides can be readily generated, and the ability of individual members of the library, alone or displayed on a polymer, to bind to human xenoreactive antibodies can be readily assessed. Moreover, such libraries can be used to identify oligosaccharides which selectively bind xenoreactive antibodies, but which are not substantially immunoreactive with human (or other host) antibodies for, e.g., bacterial carbohydrate epitopes. In such a manner, the xenoantigen(s) of subject polymers can be optimized.
- In certain embodiments, the subject polymers can be derived such that single molecules of polymer simultaneously display two or more different xenoantigens. In other embodiments, the polymeric compositions can be a mixture of different, discrete xenoantigen-polymers to provide a broader spectrum of XNA-neutralizing activity.
- In this regard, McKane et al. (1998) Transplantation 66:626 describe a neoglycoprotein enzyme-linked immunosorbent assay to probe the precise antigenic requirements for the binding of anti-αGal(1,3)Gal epitopes in human sera. Their study revealed antibody heterogeneity amongst the population. In that study, nearly two-thirds of the individuals had IgG that recognized αGal(1,3Gal) di-, tri-, and pentasaccharides (D, T, and P, respectively), termed “DTP phenotype”. The frequency of other phenotypes was—-P, 13%; -TP, 12%; D-P, 8%; and DT-, 1%. In light of the polymorphism in the anti-αGal(1,3) Gal repertoire, the subject method can include a step of determining the anti-αGal phenotype of the patient, and administering a xenoantigen-polymer of the present invention which includes αGal oligosaccharides, e.g., di-, tri-, tetra and pentasaccharides, in a ratio which is determined by the phenotype of the individual to be treated, e.g., wherein the ratio approximates the phenotypic ration of anti-αGal antibodies.
- In other embodiments, the xenoantigen is a protein, or peptidyl portion thereof, which competes with a xenograft for human xenoreactive antibodies. Anti-pig antibodies, for example, have been identified to bind to protein components on the surface of the graft. For instance, HLA-specific antibodies in highly sensitized patients can cause a positive crossmatch against pig lymphocytes. Taylor et al. (1998) Transplantation 65:1634.
- The crosslinking agent used to generate the subject polymers will preferably include at least two functional groups capable of reacting with the scaffold, the antigen, the functional group formed by the covalent linkage of the scaffold with the antigen, or any or all of them. In certain embodiments, the crosslinking agent is sterically or electronically predisposed, or both, to crosslinking as opposed to reacting with two sites within the same molecule (actually not a crosslinking reaction, but rather a cyclization reaction). In certain embodiments, the crosslinking agent is generated in situ from a precursor. In preferred embodiments, the crosslinking agent (48; wherein X represents a leaving group) is generated in situ from N-hydroxysulfosuccinimide (47).
- While not wishing to be bound by any particular theory, in certain embodiments, Applicants believe that the crosslinking agent reacts with the amide linkages formed by the reaction of amines on the scaffold with carboxylates on the antigens; crosslinking agents represented by 48 are particularly effective in these embodiments. In certain embodiments, the average molecular weight of the oligomers is proportional to the reaction time even though all of the functional groups on the scaffolds react with functional groups on the antigens within the first fifteen minutes of the reaction time. The significance of molecular weight is discussed infra. In these embodiments, crosslinking continues after formation of the covalent bonds between the scaffolds and antigens is complete.
- The polymers of the present invention may be synthesized in a single chemical reaction, i.e., by including all necessary reagents in a single reaction vessel, or they may be synthesized in two or more chemical reactions conducted in series. When the polymers of the present invention are synthesized via a series of reactions the intermediate compounds may be purified before being subjected to the next reaction in the series, or they may be used without purification. In certain embodiments, chemical reactions conducted in series may be completed in a single reaction vessel, e.g., by allowing a certain group of reagents to react in the vessel for a desired period, followed by introduction into the vessel of the reagents required for the next reaction in the series. In certain embodiments, the products obtained via these various approaches will comprise the same molecular components, but will differ in their properties or activity in a subject or assay due to differences in their microscopic structures, e.g., the degree of crosslinking, or the identities, locations or ratios of the functional groups involved in the crosslinks. One of ordinary skill in the art will be able to ascertain using no more than ordinary experimentation the reaction protocol and conditions necessary to produce a product of the present invention with properties optimized for a specific assay or subject.
-
- wherein,
- R1, independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
- R5 is H, —OR, NH—Y—R2, or -L-E;
- Y independently for each occurrence is absent, —C(O)—, —C(S)—, —C(NR)—, O, S, or SC;
- B, for one occurrence is —R, and for the other occurrence is —R or from 1-9 saccharide residues;
- R, independently for each occurrence, represents H or a C 1-C6 alkyl or other hydroxyl protecting group, and more preferably represents H for each occurrence;
- R2 is a C 1-C6 alkyl, and more preferably represents CH3;
- X2 represents a linker of 1-10 atoms in length, preferably —O-alkyl, more preferably —O—(CH 2)5—;
- D represents H, or -L-E;
-
- E represents a second PEG subunit of the polymer, wherein, independently for each occurrence of E, the linker group L is covalently attached via R5 or B of a saccharide moiety of the second PEG subunit, or via an amine of the second PEG subunit.
-
- wherein R1, R5, X2 and L are as described above, and
- PEG represents a polyethylene glycol (PEG) subunit of the polymer; and
- X3 represents one or more additional PEG subunits of the polymer, each of which may be derivatived with a saccharide moiety of formula VII,
- wherein the polymer has an average molecular weight in the range of 10,000 daltons to 1,000,000 daltons.
- An exemplary composition of the invention is derived from an octa(amino)polyethylene glycol scaffold to which several αGal(1,3)Gal moieties are linked via a covalent tether, wherein the individual molecules of the scaffold bearing the disaccharide moieties are crosslinked to yield an oligomeric material comprising two or more scaffold-disaccharide molecules.
- In general, the reactions of the polyamino-PEG scaffold with oligosaccharides will be carried out in the pH range of 4 to 7, and most preferably in the pH range of 5-6. The preferred temperature range for the reactions is between 0° C. and 40° C., and more preferably is in the range of 4° C. to 27° C. Examples 23 and 26 highlight the relationship between the pH and reaction temperature and the activity of the composition produced.
- A polar, protic or aprotic solvent may be used. As described in the examples, the reactions can be run in aqueous solutions. Alternatively polar, aprotic solvents such as a nitrogen-dialkyluted carboxymide, lactum, sulfoxide or sulfore, as for example dimethyl formamide, may be used. In those instances, the reactions may be effected without or with the additions of water, for example, up to quantities at which the polymer remains in solution. Non-limiting examples of preparations are discussed in the examples.
- As described in the examples, the molecular weight of the resulting polymer can depend on such factors as reaction time, pH, temperature and reactant concentrations. In those instances wherein the resulting xenopolymer is to be used for depletion of xenoreactive antibodies, larger molecular weight polymers may be used, e.g. polymer compositions having an average molecular weight of 100,000-1,000,000 daltons, though more preferable in the range of 100,000-500,000 daltons, and even more preferably 300,000-500,000 daltons.
- However, when the resulting xenopolymer is to be used at least in part as a tolerogen, e.g. to induce tolerance or anergy to the xenoantigens, it will be preferable to employ polymer formulations at lower molecular weight, e.g. having an average molecular weight of 10,000-300,000 daltons, more preferably having an average molecular weight less than 100,000 daltons, e.g., 25,000-100,000 daltons. Such lower molecular weight polymers, even if not tolerogenic, will have reduced immunogenic activity.
- Moreover, suitable tolerogenic compositions according to this aspect of the invention can be identified according to the following criteria. First, they are preferably non-immunogenic when administered to a primate, including a human. As a practical matter, this can be evaluated by comparing the level of antibodies specific for a candidate polymer in peripheral blood of a primate, including a human, prior to and after administration of the candidate polymer.
- Tolerogenic compositions according to this aspect of the invention may also include pharmaceutically acceptable carriers, diluents, and/or controlled release agents. Certain non-limiting examples of such carriers, diluents and/or controlled release agents include buffered saline, oils, implantable pumps and encapsulated beads.
- Moreover, in certain instances, such as for formulations of the polymer for human therapeutic use, it will be desirable for the composition to be relatively homogenous with respect to polymer size. That is, the polymers can be formulated to have narrow molecular distributions or polydispersity, as defined by the ratio Mw:Mn, where Mw is the weight average molecular weight of the polymer and Mn is the number average molecular weight of the polymer. Thus, in certain preferred embodiments, the subject polymer compositions will have a polydispersity, for the xenopolymer, of 20 or less, more preferably 10 or less, and even more preferably 5 or less.
- In certain embodiments, the subject method for generating the xenopolymers of the present invention is part of an overall method for manufacturing a medicament. Thus, the xenopolymer is formulated with one or more pharmaceutically acceptable excipients, such as described below, in an amount and concentrations to provide at least the efffective dose (ED 50) for depleting xenoreactive antibodies in the patient, inducing tolerance or anergy to the xenoantigen. For instance, the polymer can be formulated such that, for its mode of delivery, a dose of at least the ED50 for inhibitions of HAR can be administered to the patient.
- In preferred embodiments, the factor VIII-polymers have a therapeutic index of at least 10, and more preferably at least 100, 1000, or even 10,000, for the particular mode of administration.
- As part of a process for preparations of a medicament of the present inventions, the polymer can be synthesized and handled under sterile conditions, or can be sterilized prior to formulation. Likewise, contaminants can be removed, including the unreacted reactants and synthesis by-products. The resulting medicament should be sterile, and substantially free of pyrogenic or other toxic contaminants.
- In a first preferred synthetic method, a branched polymer having at least three arms and at least six covalently linked active groups such as amino or hydrazino groups is reacted in the presence of N-(3-dimethlyaminopropyl)-N′-ethylcarbodiimide (EDC) with Galα1-3 Gal having a covalently linked carboxy group. In a second preferred method, the converse of the first method, the branched polymer has covalently linked carboxy groups while the Galα1-3 Gal has a covalently linked amino or hyrazino group.
- In a third preferred method, the branched polymer has at least three arms and at least six active groups such as amino or hydrazino groups, and the αGal(1,3)Gal has a covalently linked aldehyde group. In a fourth preferred method, the converse of the third method, the branched polymer has at least six aldehyde groups while the αGal(1,3)Gal has a covalently linked amino or hydrazino group. In the third and fourth methods, EDC is not required for the reaction to take place.
- In a fifth preferred synthetic method, the branched polymer has at least three arms, with at least three active groups such as amino or hydrazino groups covalently linked to the free ends of the arms, and the αGal(1,3)Gal has a covalently linked carboxy group. In a sixth preferred method, the converse of the fifth method, the branched polymer has at least three covalently linked carboxy groups while the αGal(1,3)Gal has a covalently linked amino or hydrazino group. The fifth and sixth methods are conducted in the presence of EDC and N-hydroxysulfosuccinimide (NHSS). The compositions resulting from the fifth and sixth methods have a covalently linked activation group which is N-hydroxysulfosuccinimide.
- “Mixed arm” syntheses can also be conducted with all sorts of combinations of groups on the ends of the arms, for
instance 7 amino/1 carboxy, or 5/3, or 4/4. Given the guidance of the experimental examples below, one of ordinary skill in the art of chemical synthesis will be able to devise various combinations of reagents that will yield functional compositions within the scope of the present invention. - Various compositions of the invention are designated in FIG. 3 and throughout according to the following nomenclature:
- The letter E denominates a synthesis that used only EDC for coupling.
- The letter N denominates a synthesis that used both EDC and N-hydroxysuccinimide (NHS).
- The letter S denominates a synthesis that used both EDC and N-hydroxysulfosuccinimide (NHSS).
- The letter L denominates a lysine coupled to the ends of branched PEG-amino, and EDC for coupling.
- The letters LS denominate a synthesis that used lysine and NHSS.
- The letters ES denominate a synthesis that used EDC for coupling and a sulfo group coupled to the PEG starting material.
- The first number in the numbering system denominates the number of branches on the PEG, and the second number (10 or 20) denominates the molecular weight of the starting PEG-amine (10,000 or 20,000 Da).
- The product of the
synthesis 410L proved to be 100 times more effective per coupled sugar than the free sugar (IC50=0.5 mg/mL for 410L versus 50 mg/mL for the free sugar). Surprisingly, the compositions which were synthesized using N-hydroxysulfosuccinimide (NHSS; NHS with a sulfo group) achieved 50% inhibition of anti-Gal antibody reactivity at very low concentrations of approximately 10−5 mg/mL to 10−7 mg/mL. Moreover, these compositions were stable for 12 months when stored at a pH in the range of 4 to 7 at temperatures ranging from 1-10° C. - In other exemplary embodiments, the subject xenopolymer is formed by derivatizing an aminodextran with the xenoantigen. In an illustrative process for preparing a xenoantigen-conjugate with an aminodextran, one can begin with a dextran polymer. The dextran is reacted with an oxidizing agent to effect a controlled oxidation of a portion of its carbohydrate rings to generate aldehyde groups. The oxidation is conveniently effected with glycolytic chemical reagents, e.g., NaIO 4, according to conventional procedures. The oxidized dextran is then reacted with a polyamine, such as a diamine. Suitable amines include, e.g., ethylene diamine, propylene diamine or similar polymethylene diamines, diethylene triamine or like polyamines, 1,3-diamino-2-hydroxypropane or other like hydroxylated diamines or polyamines, and the like. Reductive stabilization of the resultant intermediate is effected by reacting the Schiff base intermediate with a reducing agent, e.g., NaBH4, NaBH3CN, or the like. The resultant adduct can be further purified by passage through a conventional sizing column to remove cross-linked dextrans. An estimate of the number of available primary amino groups on the aminodextran can be effected by reaction of a weighed sample with trinitrobenzenesulfonic acid and correlation of the optical density at 420 nm with a standard. Alternatively, the dextran can be derivatized by conventional methods for introducing amine functions, e.g., by reaction with cyanogen bromide, followed by reaction with a diamine.
- Other polyamines which can be utilized in the xenopolymers of the present invention include the non-antigenic amine derived polymers of the Greenwald et al. U.S. Pat. No. 5,730,990, and the non-antigenic branched polymer conjugates of the Martinez et al. U.S. Pat. No. 5,643,575.
- B. Assays For Competitive Inhibition of αGal XNA
- Quantification of circulating anti-αGal antibodies in the serum of an individual and the ability of polymers to remove, bind, and/or neutralize anti-αGal antibodies can be routinely determined applying immunoassays known in the art which may be routinely adapted for such determination. Such immunoassays, include, but are not limited to, competitive and noncompetitive assay systems using techniques such as western blots, radioimmunoassays, ELISA (enzyme linked immunosorbent assay), “sandwich” immunoassays, immunoprecipitation assays, precipitin reactions, gel diffusion precipitin reactions, immunodiffusion assays, agglutination assays (e.g., hemagglutination), complement-fixation assays, immunoradiometric assays, fluorescent immunoassays and protein A immunoassays. These assays may further be applied to determine the dosage of the αGal oligosaccharide polymers of the invention to be administered, as well as to monitor the neutralization and/or removal of anti-αGal antibodies by the αGal oligosaccharide polymers of the invention.
- While assays for measuring circulating anti-αGal antibodies can be accomplished by various immunological methods, ELISA assays have the advantage in that they can be standardized for the immobilized ligand, are reproducibly quantitative, and can be scored for different immunoglobulin isotypes by the use of appropriate secondary detection reagents. ELISA capture ligands for testing human or old world monkey serum antibodies can employ fixed cells, glycolipids, glycoproteins, or oligosaccharides. Such capture ligands include but are not limited to mouse laminin, PK-15 pig kidney cells, immobilized BSA-αGal neoglycoconjugates, and immobilized αGal oligosaccharides. Covalent immobilization of a specific αGal oligosaccharide(s) onto the ELISA immobilized surface, using techniques known in the art, permits the predetermined deposition of known antigenic ligands by covalent chemistry. In addition to determining the ability of the αGal oligosaccharides polymers to bind to and/or neutralize anti-αGal antibodies, such an ELISA is also useful in clinical monitoring of anti-αGal antibodies in blood of patients in advance of, and following xenotransplantation of organs from animals that express the αGal epitope.
- The addition of serial dilutions of sera in the ELISA assays permits the determination of reactive antibody titers, which can serve to quantitate the circulating anti-αGal activity. Specific immunoglobulin isotypes can be monitored by using appropriate reagents, e.g., anti-human IgG or IgM.
- In another embodiment, quantification of anti-αGal antibodies and/or the extent of xenoreactive antibody neutralization or removal upon treatment with the αGal oligosaccharide polymers of the invention is measured using a cell cytotoxicity assay. Such an assay may comprise contacting serum, isolated from a patient using techniques known in the art, with αGal expressing cell monolayers (e.g., pig kidney cells (PK-15), pig aortic endothelial cells, or mouse aortic endothelial cells (MAE). Preferably, these cells are from the same species as the donor of the xenograft, and most preferably from the same tissue-type as the tissue to be transplanted). According to this assay, cytotoxicity is mediated by either endogenous complement, or the sera are heat inactivated (e.g., through heating the sera at 56° C. for 30 minutes) and exogenous complement (e.g., from rabbit or guinea pig) is added. After washing away excess serum, the cells are treated with viable dye mix “live-dead” (calcein AM/ethidium homodimer) which is commercially available in the form of a Live/Dead cytotoxicity kit (Molecular Probes Inc., Eugene, Oreg.) and scored for viability using fluorescence microscopy. Staining with the “live/dead” dye mix allows for clear distinction between live cells, which show cytoplasmic green fluorescence.and dead cells, which show dark cytoplasm land red fluorescent nuclei. The extent of cell lysis that is complement-mediated may be determined by comparing the results observed in the control for which there has been no inactivation of complement, with the lysis observed with complement that has been inactivated through heat treatment.
- The invention also encompasses animal-based model systems, which may include baboon, other old world monkeys, or other animals having serum that contains anti-αGal antibodies. Such animal models may assess the ability of compositions containing an αGal oligosaccharide polymer as an active ingredient to bind to and/or neutralize anti-αGal antibodies, attenuate the rejection of a xenotransplant expressing the αGal epitope and/or to suppress B lymphocytes expressing anti-αGal idiotypes. Generally, this assay may involve exposing animal models to the αGal oligosaccharide polymer, or pharmaceutically acceptable derivative as an active ingredient, at a sufficient concentration and for a time sufficient to elicit the desired effect in the exposed animals. The response of the animals to the exposure may be monitored by assessing the level of anti-αGal reactive antibodies in the serum of the animal, evaluating the appearance of rejections of the xenografted organ, and/or quantitating B lymphocytes expressing anti-αGal idiotypes. Dosages of test agents may be determined by deriving dose-response curves.
- Due to the many similarities between human and baboon immune systems [Neubauer et al., J. Immunogenetics, 8:433-442, 1981; Garver et al., Cytogenetics & Cell Genetics, 27:238-245, 1980; Brodsky et al., Immunogenetics, 155:151-166, 1982; Hammer, C., in Hardy, M. A. (ed.),
Xenograft 25, 115-123 (Elsevier New York, 1989); Stark et al., Transplantation 52(6):1072-1078 (1991); Hammer, C., in Cooper, D. K. C., et al. (eds.), Xenotransplantation, 429-438 (Springer-Verlag 1991)], and because of the large size of baboons, these animals are convenient experimental model recipients of pig organs. These non-human primates also express anti-pig antibodies, and reject pig organs hyperacutely [Lexer et al., J. Heart Transplant, 4:411-418, 1986; Ye, Y., Cooper, D. K. C., in Cooper, D. K. C., et al. (eds.), Xenotransplantation, 389-393 (Springer-Verlang 1991); Cooper et al., J. Heart Transplant, 7:238-246, 1988; Platt et al., Transplantation, 52(2):214-220, 1991]. As human and baboon immunoglobulins are structurally very similar, some of the anti-idiotypic antibodies against human anti-pig antibodies should bind to baboon anti-pig antibodies and inhibit rejection of pig to baboon xenografts. For instance, Geller et al., Transplantation, 55:168-172, 1993, have described an idiotype shared between human and baboon anti-pig antibodies. - Applying these assays, the relative anti-αGal binding activity that a αGal oligosaccharide polymer exhibits against the anti-αGal antibody profile of the serum of an individual may be determined, and the polymer formulation best suited for neutralizing and/or binding the anti-αGal profile of an individual can be determined.
- Other methods for assaying the extent of xenoreactive antibody neutralization and/or removal will be known to the skilled artisan and are within the scope of the invention.
- C. Formulations
- Pharmaceutical compositions comprising the subject xenopolymers, or pharmaceutically acceptable derivatives thereof, can be administered to a patient, preferably a human or old world monkey, by itself, or in pharmaceutical compositions where it is mixed with suitable carriers or excipient(s) at doses to ameliorate symptoms associated with xenograft rejection, prolong xenograft survival in a patient or to direct complement-mediated lytic attack of targeted tissue or cell types.
- Use of pharmaceutically acceptable carriers to formulate the compounds herein disclosed for the practice of the invention into dosages suitable for systemic administration is within the scope of the invention. With proper choice of carrier and suitable manufacturing practice, the compositions of the present invention, in particular, those formulated as solutions, may be administered parenterally, such as by intravenous injection. The compounds can be formulated readily using pharmaceutically acceptable carriers well known in the art into dosages suitable for the form of administration desired.
- The pharmaceutical compositions of the invention may be administered using techniques well known to those in the art. Preferably agents are formulated and administered systemically. Techniques for formulation and administration of the compounds of the invention may be found in “Remington's Pharmaceutical Sciences,” 18th ed., 1990, Mack Publishing Co., Easton, Pa., latest edition. Suitable routes of administration may, for example, include oral, rectal, transmucosal, or intestinal administration; parenteral delivery, including intramuscular, subcutaneous, intramedullary injections, as well as intrathecal, direct intraventricular, intravenous, intraperitoneal, intranasal, or intraocular injections; transdermal, topical, vaginal and the like. The preferred routes of administration are by intravenous infusion, intravenous injection, and intramuscular injection. Dosage forms include but are not limited to tablets, troches, dispersions, suspensions, suppositories, solutions, capsules, gels, syrups, slurries, creams, patches, minipumps and the like. Pharmaceutical compositions for use in accordance with the present invention thus may be formulated in conventional manner using one or more physiologically acceptable carriers comprising excipients and auxiliaries which facilitate processing of the activ6 compounds into preparations which can be used pharmaceutically. Proper formulation is dependent upon the route of administration chosen.
- For injection, the agents of the invention may be formulated in aqueous solutions, preferably in physiologically compatible buffers such as Hanks's solution, Ringer's solution, or physiological saline buffer. For transmucosal administration, penetrants appropriate to the barrier to be permeated are used in the formulation. Such penetrants are generally known in the art.
- For oral administration, the compounds can be formulated readily by combining the active compounds with pharmaceutically acceptable carriers well known in the art. Such carriers enable the compounds of the invention to be formulated as tablets, pills, dragees, capsules, liquids, gels, syrups, slurries, suspensions and the like, for oral ingestion by a patient to be treated. Pharmaceutical preparations for oral use can be obtained in the form of a solid excipient, optionally by grinding a resulting mixture, and processing the mixture of granules, after adding suitable auxiliaries, if desired, to obtain tablets or dragee cores. Suitable excipients are, in particular, fillers such as sugars, including lactose, sucrose, mannitol, or sorbitol; cellulose preparations such as, for example, maize starch, wheat starch, rice starch, potato starch, gelatin, gum tragacanth, methyl cellulose, hydroxypropylmethyl-cellulose, sodium carboxymethylcellulose, and/or polyvinylpyrrolidone (PVP). If desired, disintegrating agents may be added, such as the cross-linked polyvinyl pyrrolidone, agar, or alginic acid or a salt thereof such as sodium alginate.
- Dragee cores are provided with suitable coatings. For this purpose, concentrated sugar solutions may be used, which may optionally contain gum arabic, talc, polyvinyl pyrrolidone, carbopol gel, polyethylene glycol, and/or titanium dioxide, lacquer solutions, and suitable organic solvents or solvent mixtures. Dyestuffs or pigments may be added to the tablets or dragee coatings for identification or to characterize different combinations of active compound doses.
- Pharmaceutical preparations which can be used orally include push-fit capsules made of gelatin, as well as soft, sealed capsules made of gelatin and a plasticizer, such as glycerol or sorbitol. The push-fit capsules can contain the active ingredients in admixture with filler such as lactose, binders such as starches, and/or lubricants such as talc or magnesium stearate and, optionally, stabilizers. In soft capsules, the active compounds may be dissolved or suspended in suitable liquids, such as fatty oils, liquid paraffin, or liquid polyethylene glycols. In addition, stabilizers may be added. All formulations for oral administration should be in dosages suitable for such administration.
- For buccal administration, the compositions may take the form of tablets or lozenges formulated in conventional manner.
- For administration by inhalation, the compounds for use according to the present invention are conveniently delivered in the form of an aerosol spray presentation from pressurized packs or a nebulizer, with the use of a suitable propellant, e.g., dichlorodifluoromethane, trichlorofluoromethane, dichlorotetrafluoroethane, carbon dioxide or other suitable gas. In the case of a pressurized aerosol the dosage unit may be determined by providing a valve to deliver a metered amount. Capsules and cartridges of e.g., gelatin for use in an inhaler or insufflator may be formulated containing a powder mix of the compound and a suitable powder base such as lactose or starch.
- The compounds may be formulated for parenteral administration by injection, e.g., by bolus injection or continuous infusion. Formulations for injection may be presented in unit dosage form, e.g., in ampoules or in multidose containers, with an added preservative.
- The compositions may take such forms as suspensions, solutions or emulsions in oily or aqueous vehicles, and may contain formulatory agents such as suspending, stabilizing and/or dispersing agents.
- Pharmaceutical formulations for parenteral administration include aqueous solutions of the active compounds in water-soluble form. Additionally, suspensions of the active compounds may be prepared as appropriate oily injection suspensions. Suitable lipophilic solvents or vehicles include fatty oils such as sesame oil, or synthetic fatty acid esters, such as ethyl oleate or triglycerides, or liposomes. Aqueous injection suspensions may contain substances which increase the viscosity of the suspension, such as sodium carboxymethyl cellulose, sorbitol, or dextran. Optionally, the suspension may also contain suitable stabilizers or agents which increase the solubility of the compounds to allow for the preparation of highly concentrated solutions.
- Alternatively, the active ingredient may be in powder form for constitution with a suitable vehicle, e.g., sterile pyrogen-free water, before use.
- The compounds may also be formulated in rectal compositions such as suppositories or retention enemas, e.g., containing conventional suppository bases such as cocoa butter or other glycerides.
- In addition to the formulations described previously, the compounds may also be formulated as a depot preparation. Such long acting formulations may be administered by implantation (for example subcutaneously or intramuscularly) or by intramuscular injection. Thus, for example, the compounds may be formulated with suitable polymeric or hydrophobic materials (for example as an emulsion in an acceptable oil) or ion exchange resins, or as sparingly soluble derivatives, for example, as a sparingly soluble salt.
- The pharmaceutical compositions also may comprise suitable solid or gel phase carriers or excipients. Examples of such carriers or excipients include but are not limited to calcium carbonate, calcium phosphate, various sugars, starches, cellulose derivatives, gelatin, and polymers such as polyethylene glycols. Pharmaceutical compositions suitable for use in the present invention include compositions wherein the active ingredients are contained in an effective amount to achieve its intended purpose. Determination of the effective amounts is well within the capability of those skilled in the art, especially in light of the detailed disclosure provided herein.
- D. Illustrative Therapeutic Methods
- A preferred method of neutralizing anti-αGal antibodies involves the administration of the subject αGal oligosaccharide polymers, or pharmaceutically acceptable derivative(s) thereof, in sufficient quantity to block binding of the circulating antibody to the donor endothelium and thereby prevent anti-αGal antibody directed complement-mediated lytic attack of the transplanted tissue.
- It is a primary object of this invention to provide a method and associated compositions for attenuating xenograft rejection or to alleviate trauma caused by anti-αGal antibody directed complement activation by interfering with anti-αGal antibody binding to cell surfaces, in particular donor organ endothelium. Accordingly, the pharmaceutical compositions of the invention may be administered alone, together with, or in seriatim with other therapy regimens for reducing the extent of binding of anti-αGal antibody to donor organ cells or tissue.
- In a specific embodiment, the therapeutic method of the invention is carried out as monotherapy, i.e., as the only agent provided for attenuating xenograft rejection.
- In another embodiment, administration of the αGal oligosaccharide polymer of the invention is combined with other regimens that may accompany neutralization and/or depletion of anti-αGal antibodies using the subject αGal oligosaccharides polymers, including, but not limited to, extracorporeal treatment with column immobilized human antianimal idiotypic antibodies (for example, see U.S. Pat. No. 5,560,911), plasmapheresis (in which all antibodies or specifically, one or more antibody types specific for antigenic epitopes on the surface of donor endothelial cells have been removed from the plasma) and perfusion of blood to be administered to the patient through organs, tissue or cells expressing αGal antigens (such as, for example, hearts, kidneys, erythrocytes, and cell lines derived from pig kidney, pig aortic endothelium, mouse endothelium, etc.).
- Depletion of circulating anti-αGal antibody is a temporary solution, which can overcome the HAR crisis. But the antibody-producing B lymphocytes continue to produce antibody, which can pose a danger of longer-term antibodymediated vascular rejection. These B lymphocytes bear on their membrane surface immunoglobulin with the αGal-binding domain exposed (Geller et al. (1993) Transplantation 55:168172). In certain embodiments, the subject polymer is selected for its ability to induce clonal tolerance to the xenoantigen. In such embodiments, the polymer can also be used in a step for neutralizing and/or depleting circulating XNAs. In other embodiments, the subject polymer can be used principally as a tolerogen, and circulating XNAs can be removed by immunoaphoresis or other regimen for antibody removal.
- E. Conjoint Therapies
- The xenopolymers of the present invention may be administered alone or in combination with other agents useful in attenuating xenograft rejection, including conventional nonspecific immunosuppressive agents, including but not limited to, steroids, cyclosporine, cyclosporine analogs, cyclophosphamide methylprednisone, prednisone, azathioprine, FK-506, 15-deoxyspergualin, and other immunosuppressive agents known in the art. In a further embodiment, the pharmaceutical compositions comprise an antibiotic agent selected from the group consisting of tetracycline, metronidazole, amoxicillin, P lactamases, aminoglycosides, macrolides, quinolones, fluoroquinolones, cephalosporins, erythromycin, ciprofloxacin, and streptomycin. In an additional embodiment, the pharmaceutical composition comprises an anti-inflammatory. Such anti-inflammatories include, but are not limited to, glucocorticoids and the nonsteroidal anti-inflammatories, aminoarylcarboxylic acid derivatives, arylacetic acid derivatives, arylbutyric acid derivatives, arylcarboxylic acids, arylpropionic acid derivatives, pyrazoles, pyrazolones, salicylic acid derivatives, thiazinecarboxamides, c-acetamidocaproic acid, Sadenosylmethionine, 3-amino-4-hydroxybutyric acid, amixetrine, bendazac, benzydamine, bucolome, difenpiramide, ditazol, emorfazone, guaiazulene, nabumetone, nimesulide, orgotein, oxaceprol, paranyline, perisoxal, pifoxime, proquazone, proxazole, and tenidap.
- Combinations may be administered either concomitantly, e.g., as an admixture, separately but simultaneously or concurrently; or sequentially. This includes presentations in which the combined agents are administered together as a therapeutic mixture, and also procedures in which the combined agents are administered separately but simultaneously, e.g., as through separate intravenous lines into the same individual. Administration “in combination” further includes the separate administration of one of the agents given first, followed by the second.
- In certain embodiments, the administration of the subject xenopolymers can be part of a treatment regimen which includes total-body irradiation and splenectomy. See, for example, Cooper et al. (1997) World J Surg 21:901.
- In certain embodiments, the subject therapies include one or more steps of myeloreductive treatment, especially where the xenograft is bone marrow or other hematopoietic stem cell preparations. Such a method may include, e.g., treating the subject, prior to introduction of the donor stem cells, with an cytoreductive agent selected from one or more of alkylating agents (e.g., nitrogen mustards [such as mechloretamine], cyclophosphamide, melphalan and chlorambucil), alkyl sulphonates (e.g., busulphan), nitrosoureas (e.g., carmustine, lomustine, semustine and streptozocine), triazenes (e.g., dacarbazine), antimetabolites (e.g., folic acid analogs such as methotrexate), pyrimidine analogs (e.g. fluorouracil and cytarabine), purine analogs (e.g., fludarabine, idarubicin, cytosine arabinoside, mercaptopurine and thioguanine), vinca alkaloids (e.g., vinblastine, vincristine and vendesine), epipodophyllotoxins (e.g., etoposide and teniposide), antibiotics (e.g., dactinomycin, daunorubicin, doxorubicin, bleomycin, plicamycin and mitomycin), dibromomannitol, deoxyspergualine, dimethyl myleran and thiotepa.
- Preferred myeloreductive non-myeloablative agents are alkylating agents, e.g., cyclophosphamide, or fludarabine or similar substances, however, hematopoietic space creating antibodies or drugs, e.g., inhibitors of cell proliferation, e.g., DSG, or an anti-metabolite, e.g. brequinar, or an anti-T cell antibody, e.g., one or both of an anti-CD4 or anti-CD8 antibody can be used as a myeloreductive non-myeloablative agent.
- Other methods for inhibiting T cell activity that may be suitable for use in the methods described herein include:
- the administration of anti-T cell antibodies, e.g., an ATG preparation, polyclonal or monoclonal antibody directed against CD4, CD8, or CD2 (an anti-CD2 antibody, e.g., the anti-CD2 monoclonal antibody BTI-322 or a humanized version thereof, or an antibody which overlaps or binds the epitope recognized by BTI-322, are particularly useful);
- the administration of an agent, e.g., an antibody, which blocks or otherwise inhibits a pathway, e.g., a costimulatory pathway, of T cell activation (agents, e.g., antibodies, which block the CD28-B7 pathway, e.g., a CTLA4-IgG fusion protein, or agents, e.g., an antibody which blocks the CD40-gp39 pathway, e.g., an anti-gp39 antibody, are particularly suited for use in the method), or generally, by the administration of a treatment which down modulates or otherwise inhibits one or more of the T cell receptor, CD4 co-receptor, CD8 co-receptor or other receptor or co-receptor which promotes T cell activation or maturation;
- the administration of an IL-12 receptor protein (functional antagonist, U.S. Pat. No. 5,831,007);
- the administration of substituted dihydrobenzofurans, spirobenzofuran-2(3H)-cycloalkanes according to U.S. Pat. No. 5,808,109;
- the administeration of anti-asialo antisera; the administration of an immunosuppressive agent, e.g., a macrolide, e.g., cyclosporine, FK506, or rapamycin; and
- the administration of thymic irradiation, or other treatment which creates thymic space.
- In certain embodiments, the myeloreductive treatment includes treating the subject with an immunosuppressant regimen, prior to introduction of the donor stem cells, in an amount sufficient to prevent rejection of the donor stem cells by the host immune system. For example, such immunosuppressant regimens can include, independently for pre- and post-transplantation is both are carried out, a treatment of the subject which inactivates and/or depletes host T-lymphocytes and/or natural killer (NK) cells in the subject. For example, the immunosuppressant regimen includes treatment with T cell-depleting anti-CD4 and/or CD8 antibodies, such as anti-thymocyte globulin (ATG), OKT3, LO-CD2a, or Minnesota anti-lymphoblast globulin (MALG). Preferably, the immunosuprressant regimen, both before and after transplantation, includes administration of ATG.
- In other embodiments, the immunosuppressant regimen includes treatment with one or more of a macrolide immunosuppressant, azathioprine, steroids (e.g., prednisone, methyl prednisolone), or sub-lethal nonmyleoablative irradiation of lymphocyte-containing tissue.
- In certain embodiments, the method may also include inhibiting natural killer cells of the subject preferably prior to introducing the xenograft, e.g., by introducing into the subject an antibody capable of binding to natural killer cells of the subject. One source of anti-NK antibody is anti-human thymocyte polyclonal anti-serum. A second anti-mature T cell antibody can be administered as well, which inhibits T cells as well as NK cells. Anti-T cell antibodies are present, along with anti-NK antibodies, in anti-thymocyte anti-serum. Repeated doses of anti-NK or anti-T cell antibody may be preferable. Monoclonal preparations can be used in the methods of the invention.
- In addition to, or as an alternative to treatment protocols for the receipient, the subject method of administering a xenopolymer can be combined with treatment of the donor and/or donor tissue.
- For instance, the xenograft can be treated with glycolytic enzymes prior to implantation in order to reduce the presence of the αGal epitope. The αGal epitopes can be enzymatically removed by specific endo- or exo-glycosidases and mannosidases. Such glycolytic enzymes and general methods for their use are well known in the art. Examples of glycosidases and mannosidases suitable for use in this aspect of the invention include α- or β-galactosidase, β-N-acetylhexosamimidase, α- or β-mannosidase, endoglycosidase H or F, and peptide-N-glycosidase F. These enzymes are all commercially available from Oxford Glycosystems (Rosedale, N.Y.), and are typically used according to the manufacturer's instructions.
- Antisense RNAs can be used to specifically inhibit gene expression of, e.g., α1,3-GT activity in the donor animal. In another illustrative embodiments, antisense may be used to reduce the human serum reactivity to porcine endothelial cells by antisense-mediated down-regulation of GpIIIa expression (Keams-Jonker et al. (1997) Transplantation 63:588), and tissue from such animals could be used in combinations with, inter alia, the subject xenoploymers.
- Another method to reduce the expression of αGal epitopes would be to perform genetic manipulations referred to in the art as “gene disruption” or “gene knockout”. Gene knockout is a method of genetic manipulation via homologous recombination. These techniques may allow for the use of specially designed DNA molecules (gene knockout constructions) to achieve targeted inactivation (knockout) of a particular gene upon introduction of the construction into a cell.
- In one embodiment, treatment of a xenograft recipient with the subject polymers may be combined with transplantation of an organ or tissue derived from such transgenic “knockout” animals may be described in, e.g., U.S. Pat. No. 5,821,117 entitled “Xenotransplantation Therapies”.
- In other embodiments, the subject polymers may be used as part of a treatment regimen for recipients of xenogenic grafts from transgenic animals engineered to express: human decay-accelerating factor, such as may be isolated from the animals described by Cozzi et al. (1994) Transplant Proc 26:1402; or which express the human complement regulatory protein CD59 and DAF, as taught by Byrne et al. (1996) Transplant Proc 1996 April; 28 (2):759; human α1,2-fucosyltransferase, as might be generated according to Chen et al. (1998) Transplantation 65:832; a combined transgenic animal expressing of α-galactosidase and α1,2-fucosyltransferase, such as might be produced by the methods of Osman et al. (1997) PNAS 94:14677; a combined transgenic animal expressing decay-accelerating factor expression and in which the α1,3-Galactosyltransferase gene is knockout (see, e.g, van Denderen et al. (1997) Transplantation 64:882;
- (iv) Exemplification
- The invention now being generally described will be more readily understood by reference to the following examples which are included merely for purposes of illustration of certain aspects and embodiments of the present invention, and are not intended to limit the invention.
- Synthesis of 410E: 4 Arm 10,000 Molecular Weight Four Amino Groups (αGal(1,3) Gal β1-4 GlcNAc) 4-polyethylene Glycol (PEG) Using N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) for Coupling.
- In a 15 mL plastic centrifuge tube, 300 mg of 4 arm, 10,000 molecular weight, PEG-(NH 2)4 (Shearwater Polymers, Inc., Huntsville, Ala. 35801), 116 mg of EDC (Pierce, Rockford, Ill. 61105) and 53.4 mg of αGal(1,3)Gal β 1-4 GlcNAc (Cytel Corp., San Diego) were dissolved in 3 mL of DI water. The reaction proceeded for one hour after which an additional 116 mg of EDC was added. The reaction proceeded for an additional 3 hours. The reaction mixture was filled into a dialysis tube of molecular weight cut-off of 3,500 and dialyzed five times against 4 liters of DI water. The dialyzed material was freeze-dried to a white powder. A sample of the freeze-dried powder was dissolved in 20 mL of 0.1M sodium borate and transferred to a 50 mL volumetric flask. 100 ul of TNBS (trinitrobenzene sufonic acid) (Pierce, Rockford, Ill. 61105) was added to the volumetric flask, capped and the flask inverted for mixing. Water was added to 50 mL and the solution was mixed by inversion. The sample was incubated for one hour before reading the OD at 421 nm. The amount of free amino groups was calculated by comparing the OD of the sample to a standard curve generated with glycine as standard. Amino group analysis revealed that more than 98% of amino groups were modified.
- Synthesis of 810E: 8 Arm 10,000 Molecular Weight (αGal(1,3)Gal β 1-4 GlcNAc) 8, PEG Using EDC for Coupling.
- In an Eppendorf tube, 100 mg of 8 arm, 10,000 molecular weight PEG-(NH 2)8 (Shearwater Polymers, Inc., Huntsville, Ala. 35801), 80 mg of EDC and 106 mg of αGal(1,3)Gal β 1-4 GlcNAc were dissolved in 1 mL of DI water. The reaction proceeded for one hour after which an additional 80 mg of EDC was added. The reaction proceeded for an additional 3 hours. The reaction mixture was filled into a dialysis tube of molecular weight cut-off 3,500 and dialyzed five times against 4 liters of DI water. The dialyzed material was freeze-dried to a white powder. Amino group analysis revealed that more than 98% of amino groups were modified.
- Synthesis of 820E: 8 Arm 20,000 Molecular Weight (αGal(1,3)Gal β 1-4 GlcNAc) 8, PEG Using EDC for Coupling.
- In a 250 mL plastic screw bottle, 20 grams of 8 arm 20,000 molecular weight PEG-(NH 2)8 (Shearwater Polymers, Inc., Huntsville, Ala. 35801) and 10.6 grams of αGal(1,3)Gal β1-4 GlcNAc were dissolved in 100 mL of DI water by rotating the bottle head over head. To this solution, 8 g of EDC were added and the reaction proceeded for 1.5 hours under rotation after which an additional 8 g of EDC was added. The reaction proceeded for an additional 3.5 hours. The reaction mixture was filled into dialysis tubes of molecular weight cut-off of 3,500 and dialyzed four times against 20 liters of DI water. The dialyzed material was freeze-dried to a white powder. Amino group analysis revealed that more than 98% of amino groups were modified.
- Synthesis of 410N: 4 Arm 10,000 Molecular Weight (αGal(1,3)Gal β 1-4 GlcNAc) 4-PEG Using EDC and N-Hydroxysuccinimide (NHS) Coupling.
- In a 15 mL centrifuge tube, 50 mg of 4 arm 10,000 molecular weight PEG-(NH 2)4, 500 mg of EDC, 26.5 mg NHS (Pierce, Rockford, Ill. 61105) and 39.5 mg of αGal(1,3)Gal β 1-4 GlcNAc were dissolved in 3.125 mL of 0.1 M MES buffer, pH 5.5. The reaction mixture was rotated head over head for 4.5 hours. The reaction mixture was filled into a dialysis tube of molecular weight cut off 3,500 and dialyzed five times against 4 liters of DI water. The dialyzed material was freeze-dried to a white powder. Amino group analysis revealed that more than 98% of amino groups were modified.
- Synthesis of 410S: 4 Arm 10,000 Molecular Weight (αGal(1,3)Gal β 1-4 GlcNAc) 4-PEG Using EDC and N-Hydroxysulfosuccinimide (NHSS) for Coupling.
- In a 500 mL glass beaker with stopper, 12.5 g of 4 arm 10,000 molecular weight PEG-(NH 2)4, 31.25 g of EDC, 3.5 g of NHSS (Pierce, Rockford, Ill. 61105) and 7.5 g of αGal(1,3)Gal β 1-4 GlcNAc were dissolved in 250 mL of 0.1 M MES buffer, pH 5.5. The reaction mixture was shaken on an orbital shaker overnight. The reaction mixture was filled into dialysis tubes of molecular weight cut off 3,500 and dialyzed five times against 20 liters of DI water. The dialyzed material was freeze-dried to a yellowish powder. Amino group analysis revealed that more than 98% of amino groups were modified.
- Synthesis of 810S: 8 Arm 10,000 Molecular Weight (αGal(1,3)Gal β 1-4 GlcNAc) 8-PEG Using EDC and NHSS for Coupling.
- In a 500 mL glass beaker with stopper, 12.5 g of 8 arm 10,000 molecular weight PEG-(NH 2)8, 62.5 g of EDC, 7 g of NHSS and 15 g of αGal(1,3)Gal β 1-4 GlcNAc were dissolved in 250 mL of 0.1 M MES buffer, pH 5.5. The reaction mixture was shaken on an orbital shaker overnight. The reaction mixture was filled into dialysis tubes of molecular weight cut off 3,500 and dialyzed five times against 20 liters of DI water. The dialyzed material was freeze-dried to a yellowish powder. Amino group analysis revealed that more than 98% of amino groups were modified.
- Synthesis of 820S: 8 arm 20,000 molecular weight (αGal(1,3)Gal β 1-4 GlcNAc) 8-PEG Using EDC and NHSS for Coupling.
- In a 500 mL glass beaker with stopper, 12.5 g of 8 arm 20,000 molecular weight PEG-(NH 2)8, 31.25 g of EDC, 3.5 g of NHSS and 7.5 g of αGal(1,3)Gal β 1-4 GlcNAc were dissolved in 250 mL of 0.1 M MES buffer, pH 5.5. The reaction mixture was shaken on an orbital shaker overnight. The reaction mixture was filled into dialysis tubes of molecular weight cut off 3,500 and dialyzed five times against 20 liters of DI water. The dialyzed material was freeze-dried to a yellowish powder. Amino group analysis revealed that more than 98% of amino groups were modified.
- Synthesis of 410LS: 4 Arm 10,000 Molecular Weight Eight Lys-Coupled Amino Groups (αGal(1,3)Gal β 1-4 GlcNAc) 8-PEG Using EDC and NHSS for Coupling.
- PEG-(NH 2)8 was synthesized by covalently linking a lysine residue to each of the amine ends of the 4-arm 10,000 mw PEG. Since a lysine contains 2 amino groups and a carboxy group, the carboxy group was coupled to the amine group of the PEG, thereby doubling the number of amine groups on the PEG.
- In a 15 mL centrifuge tube, 50 mg of 4 arm 10,000 molecular weight PEG-(NH 2)8, 1000 mg of EDC, 100 mg NHS and 79 mg of αGal(1,3)Gal β 1-4 GlcNAc were dissolved in 3.125 mL of 0.1 M MES buffer, pH 5.5. The reaction mixture was rotated head over head for 4.5 hours. The reaction mixture was filled into a dialysis tube of molecular weight cut off 3,500 and dialyzed five times against 4 liters of DI water. The dialyzed material was freeze-dried to a yellowish powder. Amino group analysis revealed that more than 98% of the amino groups were modified.
- Synthesis of 410L: 4 Arm 10,000 Molecular Weight, Eight Amino Groups (αGal(1,3)Gal β 1-4 GlcNAc) 8-PEG Using EDC for Coupling.
- In a 15 mL plastic centrifuge tube, 50 mg of 4 arm 10,000 molecular weight PEG-(NH 2)8, 230 mg of EDC and 105 mg of αGal(1,3)Gal β 1-4 GlcNAc were dissolved in 3 mL of DI water. The reaction proceeded for one hour after which an additional 230 mg of EDC was added. The reaction proceeded for an additional 3 hours. The reaction mixture was filled into a dialysis tube of molecular weight cut-off of 3,500 and was dialyzed five times against 4 liters of DI water. The dialyzed material was freeze-dried to a white powder. Amino group analysis revealed that more than 98% of the amino groups were modified.
- Synthesis of 410SS: 4 Arm 10,000 Molecular Weight, four Amino Groups (αGal(1,3)Gal β 1-4 GlcNAc) 4-sulfo-PEG Using EDC and NHSS for Coupling.
- In a 15 mL centrifuge tube, 50 mg of 4 arm 10,000 molecular weight Sulfo-PEG-(NH 2)4, 500 mg of EDC, 50 mg NHS and 39.5 mg of αGal(1,3)Gal β 1-4 GlcNAc were dissolved in 3.125 mL of 0.1 M MES buffer, pH 5.5. The reaction mixture was rotated head over head for 4.5 hours. The reaction mixture was filled into a dialysis tube of molecular weight cut off 3,500 and dialyzed five times against 4 liters of DI water.
- Synthesis of 410ES: 4 arm 10,000 Molecular Weight, Four Amino Groups (αGal(1,3)Gal β 1-4 GlcNAc) 4-sulfo-PEG Using EDC for Coupling.
- In a 15 mL plastic centrifuge tube, 300 mg of 4 arm 10,000 molecular weight Sulfo-PEG-(NH 2)4, 116 mg of EDC and 53.4 mg of αGal(1,3)Gal β 1-4 GlcNAc were dissolved in 3 mL of DI water. The reaction proceeded for one hour after which an additional 116 mg of EDC was added. The reaction proceeded for an additional 3 hours. The reaction mixture was filled into a dialysis tube of molecular weight cut-off of 3,500 and was dialyzed five times against 4 liters of DI water. The dialyzed solution was freeze-dried to a white powder. Amino group analysis revealed that more than 98% of the amino groups were modified.
- Synthesis of 610S2: Mixed 8 Arm (6 Amino Groups and Two Carboxyl Groups) 10,000 Molecular Weight (αGal(1,3)Gal β 1-4 GlcNAc) 2-(sulfo-NHS)2-PEG.
- In a 15 mL plastic centrifuge tube, 300 mg αGal(1,3)Gal β 1-4 GlcNAc were incubated with 1000 mg of EDC, 100 mg NHSS in 6 mL of 0.1 M MES buffer, pH 5.5 for two hours. 100 mg of mixed 8 arm (6 amino groups and two carboxyl groups) 10,000 molecular weight PEG were added to the solution and the reaction proceeded for three hours, after which 1000 mg EDC was added and the reaction was rotated head over head overnight. The resulting solution was dialyzed five times against DI water and freeze-dried.
- Chemical Analysis of Synthesized Molecules:
- 410S was sent to an outside laboratory for elementary analysis. The sulfur analysis showed the product contained 2.53% sulfur.
- 810S was dissolved in three different buffers and the optical density was measured at 270 nm. At a pH of 3, the OD was 1.4, at
pH 7 the OD was 1.7, and atpH 10 the OD was 4.8, indicating incorporation of sulfo-beta-alanine units. Also, gel filtration analysis using an Aquagel-OH (Polymer Labs, Amhearst, Mass.) gel filtration column, showed all products synthesized with NHSS, to possess a strong adsorption at 270 nm which was associated with the molecular weight of the modified PEG. This excluded the possibility that the adsorption observed stemmed from contamination of the final product from the NHSS used. Elemental analysis of 810S indicated a sulfur content of 2.34%. - Inhibition of Xenoreactive Antibody Activity (IC50).
- Principle of the assay: A competition ELISA was set up in such a way that Gal-molecules linked to human serum albumin and immobilized onto a microtiter plate and Gal-molecules linked to PEG compete for anti-Gal antibodies in baboon serum. The antibodies binding to the Gal-HSA were visualized using secondary antibodies to these antibodies with appropriate color development agents. The color in the case in which no Gal-PEG was added was defined as 100%. The IC50 was defined, for FIG. 3, as the concentration of Gal-PEG needed to inhibit 50% of the color development when trying to detect antibodies immobilized onto the Gal-HSA plate. For FIG. 24, the IC50 is defined in terms of mass per volume, e.g. mg/mL, of polymer.
- Briefly, a 96 well microtiter was coated with 100 ul/well of a 30 ug/mL solution of αGal(1,3)Gal β 1-4 GlcNAc-human serum albumin (Gal-HSA) and incubated for 90 minutes at room temperature. After incubation with Gal-HSA, the plate was blocked with 1% HSA in PBS for 60 minutes.
- While the microtiter plate was coated and blocked, samples were prepared as follows: Fifteen different dilution solutions were prepared for 1 plate. The first dilution solution was 1% HSA in PBS. Dilution solutions 3-5 were obtained by preparing a 2.5 mL stock solution of 10 mM αGal(1,3)Gal β 1-4 GlcNAc in 1% HSA/PBS and then serially diluting 1/10 three times. Dilution solutions 6-15 were obtained by preparing a 2.5 mL stock solution of the first concentration of modified PEG and then serially diluting 1/10 nine times in 1% HSA in PBS.
- The plate was incubated for 90 minutes at 4° C. The plate was washed five times with PBS containing 0.5
% Tween 20 and blot dried. - When assaying for xenoreactive IgM, 100 ul/well of Goat anti-human IgM-biotin antibody, diluted 1/4000 in 1% HSA/PBS, was added.
- When assaying for xenoreactive IgG, 100 ul/well of Goat anti-human IgG-biotin antibody, diluted 1/4000 in 1% HSA/PBS, was added.
- The biotinylated secondary antibody was incubated for one hour at 4° C. and afterwards the plate was washed five times with PBS containing 0.5
% Tween 20 and blot dried. Hereafter, 100 ul/well streptavidin-alkaline phosphatase conjugate was added at a dilution of 1/4000 in 1% HSA/PBS and incubated for 30 minutes at room temperature. The microtiter plate was washed five times with PBS containing 0.5% Tween 20 and blot dried. 150 ul developer per well was added and the plate incubated for 30 minutes at room temperature. The color development was stopped by addition of 40 ul/well of 3 M NaOH. The plate was read in a plate reader at wavelength of 405 nm. - The IC50 data was calculated from the percent inhibition from the dilution of baboon control in 1% HSA/PBS which gave this highest point within the linear range.
- % Inhibition=(([OD of BC in HSA]−[OD of BC in αGal(1,3)Gal β 1-4 GlcNAc or PEG-(αGal(1,3)Gal β 1-4 GlcNAc) x])/OD of BC in HSA)*100
- Results: As shown in FIG. 24, the molecules made using NHSS achieved 50% inhibition of xenoreactive antibody activity at much lower concentrations than those made using EDC alone. FIG. 24 also provides IC50 values for a variety of different polymeric formulations. In terms of experimental protocol, the only significant difference between the experiements producing the data of FIGS. 3 and 24 was to use a new pipette tip for additions of αGal preparations to the wells of the ELISA plate, which should prevent any inadvertant contamination with higher concentrations of the αGal sample. Furthermore, the values reported in FIG. 24 are in mass per volume, rather than molar. According to FIG. 24, the 810S preparation had an IC50 of 5×10 4 mg/mL, compared to the 810E preparation with an IC50 of 0.1 mg/mL. For instance, the 810S molecule achieved 50% inhibition at 1×10−5 M, compared to the 801E molecule which required 1×10−1 M for the same effect.
- Enzyme-Linked Immunospot (ELISPOT) Assays.
- To each well of the first 10 columns of a sterile hydrazine modified polystyrene 96-well plate (Corning Costar, Cambridge, Mass.), 100 μL per well of a solution containing 3.2 mg/mL αGal(1,3)Gal β 1-4 GlcNAc, 3.1 mg/mL EDC and 2.4 mg/mL NHS were added in a darkened cold room and the reaction proceeded for four hours. After careful washing with sterile H 2O, the plates were completely covered with plastic strips and stored at 4° C. until use. Plates were blocked with sterile 400 ul/well of PBS/1% HSA for 2 hours at 37° C. Peripheral blood mononuclear cells were isolated by Ficoll-Hypaque (specific gravity, 1.077 g/mL; Pharmacia Fine Chemicals, Piscatawy, N.J.) density gradients at 400 g for 30 min. at 22° C. Low density mononuclear cells at the interfaces were harvested, washed twice by centrifugation (400 g for 10 min.) and resuspended in serum-free complete culture medium consisting of RPMI 1640 (GIBCO, Grand Island, N.Y.) supplemented with 1% HSA, 2 mM L-glutamine, and 100 U/mL penicillin/100 ug/mL streptomycin (GIBCO, Grand Island, N.Y.). Manual hemacytometer cell counts and viability determinations were performed using trypan blue exclusion dye. Thereafter, serial dilutions of PBMNCs (starting at 5×105 cells) in 100 ul of serum-free complete culture medium were added to each well and incubated for 18-24 hours at 37° C. in 5% CO2. After washing 8 times using PBS/0.1% Tween-20, the plates were incubated with biotinylated isotype specific goat anti-human Abs (biotin goat anti-human IgM Cat#62-7540; biotin goat anti-human IgG (H&L) Cat# 62-7140, Zymed, San Francisco, Calif.)(1:1000) at 37° C. for 4 hours. After washing, streptavidin conjugated alkaline phosphatase (Cat# 43-4322, Zymed, San Francisco, Calif.) (1:1000) was added for 30 min. at 37° C. Finally, after washing an additional 8 times with PBS/0.1% Tween-20, 5-Bromo-4-chloro-3indolyl-phosphate (Cat# B-8503, Sigma, St. Louis, Mo.) dissolved in 1% low-melt sea-plaque agarose (FMC Bioproducts, Cat#50101) was added as gel substrate to each well. Plates were incubated overnight in the dark at room temperature and single Ab-secreting, spot forming cells (ASC) were visualized, analyzed and counted under low magnification using an inverted phase microscope. Data represents total isotype specific anti-gal Ab-secreting cells/106 PBMNCs, calculated from serially diluted cell samples.
- The short-term in vitro effects of TPC on peripheral blood-derived ASCs:
- short-term in vitro effects of TPC on peripheral blood-derived ASCs were assessed by incubating (2 hours at 37° C., with rotation at 6 rpm) heparinized peripheral blood from a normal donor in the presence or absence of 50 ug/mL TPC (410S). After 2 hours, mononuclear cells from both the untreated and the TPC treated blood samples were obtained and assayed for ASCs. TPC alone appeared to have no effect on total cell yield and viability or on the number of detectable ASCs (data not shown). These findings indicate that under these in vitro culture conditions, TPC is not apparently cytotoxic to any particular peripheral blood mononuclear cell population (T cells, B cells and monocytes/macrophages) and has no direct inhibitory or stimulatory effect on anti-Gal ASC.
- Effect of TPC on T Cell-Independent Anti-Gal B Cell Activation:
- Purified CD19+B cells were obtained by positive selection with the use of anti-CD19+ immunomagnetic beads (Pan-B (CD19); Dynal, Lake Success, N.Y.). The B cell population obtained was >95% reactive with the anti-CD20 mAb (CD20-PE, clone L27; Becton Dickinson, San Jose Calif.). Purified CD19+B cells (1×10 5/well) were cultured with formalinized Staphylococcus aureus cells (SAC, Cowan strain, Calbiochem) (1:10,000) plus IL-2 (50 ng/mL), IL-4 (50 ng/mL) (R&D Systems, Minneapolis, Minn.) and anti-CD40 (1 ug/mL, Cat#33070D; Pharmingen, San Diego, Calif.), in 96-well round bottom culture plates (Corning) in 0.2 mL of
RPMI 140 medium supplemented with 10% human AB serum (anti-Gal depleted), 2 mM L-glutamine, 25 mM Hepes, and 100 U/mL penicillin/100 ug/mL streptomycin (GIBCO, Grand Island, N.Y.) at 37° C. in a humidified atmosphere with 5% CO2 for 5 days. To examine the effects of TPC on xenoreactive ASC, B cells were pretreated with TPC (410S; 1 mg/mL) for 30 min. at 37° C. and then added to the cultures after extensive washing. In some experiments, 50 ug/mL of TPC (410S) was added directly to the cultures at initiation and daily thereafter. Following the 5 day culture period, cells were washed extensively, counted, viability determined and the number of ASC determined using the anti-Gal ELISPOT assay. In comparison to nonTPC treated cultures, TPC appeared to have no effect on total cell yield and viability or on the number of detectable ASCs (data not shown). - Effect of TPC on Xenoreactive Antibody Levels in Cynomolgous Monkeys.
-
TPC molecule 410S was prepared as described in Example 5. 410S or PEG control was administered to cynomolgous monkeys in doses of either 50 mg or 250 mg every 3 days for 2 weeks. Blood was drawn for serum before and after each administration. During the subsequent 2 week period, blood was drawn each Monday and Friday. Serum samples were analyzed for xenoreactive IgG (X IgG) and xenoreactive IgM (X IgM). - Results are shown for IgM in FIG. 4 and for IgG in FIG. 5. In the case of anti-Gal IgM levels, both the 50 mg and the 250 mg doses dramatically reduced the level compared to control animals treated with the blocked PEG. After 6 TPC treatments the IgM level in the 250 mg dose was approximately 5% of the initial level and the IgM level in the 50 mg dose was approximately 15% of the initial level. This low level of anti-Gal IgM was maintained even after dosing with TPC had stopped. Indeed, at 15 days post-treatment the level in the 250 mg animals was averaging 15% of the predosing level. In the 50 mg animals the level was aproximately 40% of the initial level. These data strongly suggest that TPC can deplete IgM levels and that this depletion has a long lasting effect. Similar data were seen with the anti-Gal IgG levels although the effect was not as dramatic and there was not as evident a dose-responsive effect. The reasons for this are not clear although it is important to recognize that the most important antibodies in terms of hyperacute rejection are those of the IgM class.
- TPC Treatment with Discordant Xenotransplant in Cynomolgous Monkeys.
- The
TPC molecule 410S was prepared as described above in Example 5. Three monkeys received 250 mg TPC (# 19-97, 18-97, 21-97), and two monkeys received 250 mg control vehicle (#28-97, 23-97) every 3 days for 2 weeks (total 6 doses/animal) via IV infusion. 250 mg/5 mL per vial was supplied as sterile solution to be injected into 50 ML sterile physiological saline in an IV bag and administered IV at a rate of approximately 2-3 mLs/min. Prior to administration and 15-60 minutes after administration was complete, 1-3 mLs of blood was drawn for serum from each monkey at a site distant from the site of administration. On the day of the 6th dose, each monkey underwent a heterotopic heart transplant following drug infusion. A heart from a transgenic pig which expresses human CD59 and human CD55 was placed in the abdominal position as described (Byrne, et al., 1997, Transplantation 63:149-155) and remained in place up to 6 hours before being explanted. - Following the transplant and explant, the monkeys continued infusion of either TPC or control vehicle, as above, once per day for 9 days. Blood was drawn for serum analysis before and after each administration. The monkeys were monitored for at least 15 days after cessation of TPC administration, and blood was drawn 2 times per week for serum analysis. One monkey died 2 days post-transplant.
- Results are shown in FIGS. 6 to 9.
- The control animal (Cyno 23-97, see FIG. 6) experienced a 28-fold increase in IgG XNA levels, and a 25-fold increase in IgM XNA levels post-transplant. Most likely, this was due to stimulation by the pig heart tissue of B cells which produce IgM anti-Gal antibodies to differentiate into IgM-XNA producing plasma cells, and due to stimulation of B cells which produce IgM anti-Gal antibodies to class switch to become IgG XNA-producing B cells and then to differentiate into IgG XNA-producing plasma cells. In contrast, the monkeys which received TPC maintained low levels of xenoreactive antibodies during the 9 days of TPC administration following explant of the pig heart. Following cessation of TPC, XNA levels rose, but not as dramatically as in the non-TPC-treated control.
- In Vivo Effects of TPC Administration on Circulating Anti-Gal ASC:
- To investigate the in vivo response of circulating anti-Gal ASC to TPC administration, we measured the number of ASC per 10 6 peripheral blood mononuclear cells isolated from cynomologous monkeys treated with either TPC (410S) or blocked-PEG as control. Blocked PEG is a 4-arm, 10,000 MW PEG-amine that was reacted with acetic anhydride in order to block the amine-groups of the PEG. Briefly, heparinized peripheral blood samples (2-3 mL) from TPC and PEG treated monkeys were obtained prior to experimentation, after 6 dosages of TPC, 7 days following porcine heart transplantation with daily infusions, 7 days post infusion termination, and 18 days post infusion. Results are shown in FIGS. 10-11. Mononuclear cells were isolated via Ficoll-Hypaque gradient centrifugation and assayed for anti-Gal IgM and IgG ASC using the ELISPOT assay as described in Example 15. Circulating anti-Gal ASC (IgM and IgG) numbers were inhibited 70-99% following 6 dosages of TPC over 18 days; whereas PEG treatment resulted in a modest (9-35%) decrease in IgM ASC with no significant changes in the number of IgG ASC (FIGS. 2&3). At
day 7 post transplantation (porcine transgenic heart), circulating IgM and IgG ASC levels decreased 98.5-100% and 70-99.7%, respectively in monkeys treated daily with TPC. In contrast, the number of IgG ASC in the PEG treated animal increased 14.9-fold with no detectable change in the number of IgM ASC. Seven days after the termination of TPC treatment, 14 days post transplantation, circulating IgG and IgM ASC were 27-84% and 1.3-9.8% of baseline values, respectively. In the PEG treated monkey, IgG ASC were 4.4 above baseline and IgM ASC levels reduced 51%. IgG ASC values from TPC treated monkeys returned to near normal baseline values at 18 days post TPC termination, however, while the number of IgG ASC in the PEG treated monkey remained elevated (5.3-fold over baseline). Taken together, these findings suggest that TPC is highly effective in depleting anti-Gal ASC from the circulation during and for several weeks post treatment. - Effect of TPC on Xenoreactive Antibody Level in Baboons.
- The
TPC molecule 410S was synthesized as described in Example 5 above. Baboons were first immunoapheresed using α-gal-columns in order to remove circulating αGal antibodies, and to set a basis for comparison of the ability of TPC to remove αGal antibodies from the circulation. - Baboon subjects were housed and cared for under the federal guidelines for research institutions where experiments involving animals are conducted. These guidelines are published as National Institutes of Health (NH) Publication #86-23 entitled The Guide for Care and Use of Laboratory Animals.
- The procedures for treating and transplanting the baboons are described in Shu S. Lin et al., Transplant Immunol. 5:212-218, 1997. Descriptions of methods for making αGal-columns are contained in U.S. Pat. Nos. 5,695,759 and 5,651,968, which are herein incorporated by reference. Briefly, each baboon subject was prepared for plasma treatment by induction of anesthesia with ketamine HCl and pentamine, and anesthesia was then maintained during all procedures under inhalation and Forane™ gas. The subject's bloodstream was asceptically connected by intravenous puncture to a plasmapheresis machine which separated the plasma from the cells and platelets. The plasma was asceptically conducted to an α-gal column; effluent from the column was pumped and remixed on-line with the cells and platelets, and reinfused to the subject. This procedure was carried out continuously for 2-3 plasma volumes. The blood flow rate of 75-100 cc/min (with a plasma flow rate of 35-50 cc/min), was well tolerated by the subject. The entire column procedure lasted 1-1.5 hours.
- 250 mg of TPC (410S, as described in Example 5 above) was administered as noted in FIGS. 12 and 13.
- Results:
- Baboon 377-96 (FIG. 14)
-
Immunoapheresis # 1 removed 75% of IgM αGal antibodies from the circulation. Five days later, IgM XNAs rebounded to about 80% of pretreatment levels. A second immunoapheresis procedure brought circulating IgM XNAs to 25% of pretreatment levels. One day later, XNAs rebounded to about 55% of original levels. A third immunoapheresis brought XNA levels to 30% baseline values, and a subsequent infusion of 250 mg TPC post-immunoapheresis resulted in undetectable levels of IgM XNAs. Three days later IgM XNA levels had rebounded to about 15% pretreatment levels. The animal developed an infection at the catheter site and the experiment was terminated. This data suggested that TPC was at least as efficient, and perhaps more efficient at reducing circulating IGM XNA levels compared to immunoapheresis. - Baboon 376-96 (FIG. 15)
-
Immunoapheresis # 1 reduced circulating IgM XNA levels to 40% of pretreatment levels. Two days later, a rebound to about 50% of pretreatment levels was observed, and after a second immunoapheresis plus administration of 250 mg TPC, circulating IgM xXNA levels were undetectable. Three days later, a rebound in IgM XNA to 35% pretreatment levels occurred, and administration of 250 mg TPC brought these levels to about 5%. Six subsequent administrations of 250 mg TPC every 3 or 4 days brought circulating levels of IgM XNA lower each time, and a less dramatic rebound effect was noted. On day 31, a kidney from a transgenic pig expressing human CD59, human CD46 and human DAF was transplanted into the baboon, and a bilateral nephrectomey was performed on the baboon native kidneys. Thus, the baboon was dependent upon the porcine kidneys for physiological function. The transplanted kidney exhibited primary nonfunction and the baboon was euthanized onpost-op day 4. Pathology analysis of the explanted pig kidney revealed no evidence of hyperacute rejection. - Large Scale One-Pot Preparation Using NHSS
- In a one
liter glass beaker 25 grams of 8 arm PEG-NH2, MW 10,000, 30 grams of Trisaccharide Free Acid and 14 grams of NHSS are dissolved by addition of 500 ml of 0.1 M MES buffer, pH 5.5. When all components are dissolved, 125 grams of EDC are added to the solution. The solution is stirred in alevel 2 hood at ambient temperature for a minimum of 12 hours. - The diafiltration apparatus is set up and sterilized according to the manufacturer's instructions. The diafiltration system is drained and rinsed with sterile water until the pH of both the permeate and retentate streams are between pH 5.5 to 7.0. A minimum of 5 buffer exchanges is performed against sterile saline. The carboy connection is clamped off and the air filter line is opened. The diafiltered solution is concentrated to a third of the original volume. The volume is brought up to 750 mL sterile saline and sterile filtered through a 0.2% filter and stored at 4° C.
- Activity of Product as a Function of NHSS Concentration in the One-Pot Synthesis
- In five 15 mL centrifuge tubes, 250 mg PEG-(NH 2)8 with a molecular weight of 20,000, 150 mg of GalGalGlcNAc, 625 mg EDC and 90 mg, 70 mg, 50 mg, 30 mg and 10 mg NHSS were dissolved in 5 mL of 50 mM MES buffer, pH 5.5, respectively, and the reactions carried out overnight. The respective solutions were dialyzed against DI water with four exchanges and lyophilized. The approximate IC50's were measured as described above and are listed below.
NHSS [mg] IC50 [mg/mL] 10 5 × 10−3 mg/ mL 30 1 × 10−3 mg/ mL 50 5 × 10−5 mg/ mL 70 5 × 10−6 mg/ mL 90 2 × 10−6 mg/mL - Activity of Product as a Function of Sugar Concentration in the One-Pot Synthesis
- In five 15 mL centrifuge tubes, 250 mg PEG-(NH 2)8 with a molecular weight of 20,000, 625 mg EDC, 70 mg NHSS, and 210 mg, 150 mg, 125 mg, 100 mg and 75 mg of GalGalGlcNAc were dissolved in 5 mL of 50 mM MES buffer, pH 5.5, respectively, and the reactions carried out overnight. The respective solutions were dialyzed against DI water with four exchanges and lyophilized. The approximate IC50s were measured as described above and are listed below.
Sugar [mg] IC50 [mg/mL] 210 5 × 10−6 mg/ mL 150 5 × 10−6 mg/mL 125 4 × 10−5 mg/ mL 100 8 × 10−4 mg/ mL 75 1 × 10−3 mg/mL - Activity of Product as a Function of pH in the One-Pot Synthesis
- In six 15 mL centrifuge tubes, 250 mg PEG-(NH 2)8 with a molecular weight of 20,000, 625 mg EDC, 70 mg NHSS, and 150 mg of GalGalGlcNAc were dissolved in 5 mL of 50 mM MES buffer,
pH 5, 5.5 and 6, and 0.1 MMOPS buffer pH 7 and 7.5, respectively, and additionally in DI water. The reactions were carried out overnight. The respective solutions were dialyzed against DI water with four exchanges and lyophilized. The approximate IC50's were measured as described above and are listed below.pH IC50 [mg/mL] 5 5 × 10−6 mg/mL 5.5 5 × 10−6 mg/ mL 6 5 × 10−6 mg/ mL 7 9 × 10−4 mg/mL 7.5 6 × 10−3 mg/ mL DI water 1 × 10−5 mg/mL - Activity of Product as a Function of EDC Concentration in the One-Pot Synthesis
- In seven 15 mL centrifuge tubes, 250 mg PEG-(NH 2)8 with a molecular weight of 20,000, 70 mg NHSS, 150 mg of GalGalGlcNAc and 100 mg, 200 mg, 300 mg, 400 mg, 500 mg, 625 mg and 800 mg EDC, were dissolved in 5 mL of 50 mM MES buffer, pH 5.5, respectively. The reactions were carried out overnight. The respective solutions were dialyzed against DI water with four exchanges and lyophilized. The approximate ICSO's were measured as described above and are listed below.
EDC [mg] IC50 [mg/mL] 100 9 × 10−6 mg/ mL 200 9 × 10−6 mg/mL 300 7 × 10−6 mg/mL 400 5 × 10−6 mg/ mL 500 5 × 10−6 mg/mL 625 5 × 10−6 mg/mL 800 5 × 10−6 mg/mL - Activity of Product as a Function of the Temperature of the One-Pot Synthesis
- In three 50 mL centrifuge tubes, 500 mg PEG-(NH 2)8 with a molecular weight of 20,000, 300 mg of GalGalGlcNAc, 1250 mg EDC and 140 mg NHSS were dissolved in 10 mL of 100 mM MES buffer, pH 5.5. The reactions were carried out overnight at 4° C., room temperature and 37° C. The respective solutions were dialyzed against DI water with four exchanges and lyophilized. The approximate IC50s were measured as described above and are listed below.
Temperature IC50 [mg/mL] 4° C. 2 × 10−6 mg/ mL RT 5 × 10−6 mg/ mL 37° C. 5 × 10−4 mg/mL - Activity of Product as a Function of pH in the Two-Pot Synthesis
- In six 15 mL centrifuge tubes, 250
mg 820E, 625 mg EDC and 70 mg NHSS were dissolved in 5 mL of 50 mM MES buffer,pH 5, 5.5, 6 and 6.5, and 0.1 MMOPS buffer pH 7 and 7.5, respectively. The reactions were carried out overnight. The respective solutions were dialyzed against DI water with four exchanges and lyophilized. The approximate IC50s were measured as described above and are listed below.pH IC50 [mg/mL] 5 5 × 10−5 mg/mL 5.5 5 × 10−5 mg/ mL 6 8 × 10−5 mg/mL 6.5 5 × 10−4 mg/ mL 7 5 × 10−4 mg/mL 7.5 8 × 10−3 mg/mL - Activity of Product as a Function of EDC Concentration in the Two-Pot Synthesis
- In three 15 mL centrifuge tubes, 250
mg EDC [mg] IC50 [mg/mL] 100 8 × 10−3 mg/ mL 200 4 × 10−4 mg/mL 375 8 × 10−4 mg/mL - Activity of Product as a Function of 820E Concentration in the Two-Pot Synthesis
- In three 15 mL centrifuge tubes, 70 mg NHSS, 625 mg EDC and 250 mg, 200 mg and 125
mg 820E were dissolved in 5 mL of 50 mM MES buffer, pH 5.5, respectively. The reactions were carried out overnight. The respective solutions were dialyzed against DI water with four exchanges and lyophilized. The approximate IC50's were measured as described above and are listed below.820E [mg/mL] IC50 [mg/mL] 25 8 × 10−3 mg/ mL 40 4 × 10−5 mg/ mL 50 5 × 10−5 mg/mL - Reaction Kinetics of the One-Pot Synthesis
- In order to follow the formation of 810S, one gram of 8 arm PEG-NH 2, MW 10,000, 5 grams of EDC, 0.56 grams of NHSS and 1.2 grams of the trisaccharide free acid were dissolved in 20 mL of 0.1 M MES buffer, pH 5.5. 2 mL samples were taken at 5 minutes, 30 minutes, one hour, 2 hours, 2.5 hours, three hours, four hours, five hours and overnight. The reaction was stopped by addition of 1 M Tris, pH 8.5. The respective solutions were dialyzed against DI water with four exchanges and lyophilized. The approximate IC50s were measured as described above and are listed below.
Time [min] IC50 [mg/mL] 5 2 × 10−2 mg/ mL 30 3 × 10−3 mg/ mL 60 6 × 10−4 mg/ mL 120 8 × 10−5 mg/ mL 150 6 × 10−5 mg/ mL 180 5 × 10−5 mg/mL 240 3 × 10−5 mg/mL 300 2 × 10−5 mg/mL 960 5 × 10−6 mg/mL - Activity of Various Fractions of 810S
- 810 S was fractionated by diafiltration through successively higher molecular weight cut-off membranes. 50 grams of 810 S (lot#808003) was
first diafiltered 6 times against sterile water to remove salt through a nominally 3,000 MW cut-off membrane. The retentate was then diafiltered six times against sterile water successively through nominally 30,000 MW, 100,000 MW, 300,000 MW and 500,000 MW cut-off membranes. The permeates and the final retentate were lyophilized. The dry powders were weighed and a mass balance established as listed below. The approximate IC50s were measured as described above and are listed below.Fraction IC50 [mg/mL] Mass Balance [%] Original 810 S 5 × 10−6 mg/ mL 100 3K > X < 30K permeate 8 × 10−3 mg/mL 1.1 30K > X < 100K permeate 6 × 10−4 mg/mL 16.7 100K > X < 300K permeate 4 × 10−4 mg/mL 37.2 300K > X < 500K permeate 7 × 10−7 mg/ mL 10 >500K retentate 7 × 10−7 mg/ mL 35 - Synthesis and Activity of a Composition Comprising a Dendridic Polyamine Bearing Sixteen Amines
- 200 mg of a dendritic polyamidoamine with 16 amines, onto each end is coupled a PEG-amine of
molecular weight 2000 was dissolved in 0.1 MES buffer, pH 5.5, and 250 mg GalGalGlcNAc was added and the pH readjusted to pH 5.5. 500 mg EDC was added and the reaction was rotated head over head overnight. The solution was dialyzed three times against 4 liters of DI water and lyophilized. An IC50 Elisa as described above was performed and the approximate IC50 was determined to be 1 mg/mL. - Synthesis and Activity of a Composition Comprising a Dendridic Polyamine Bearing Thirty-two Amines
- 200 mg of a dendritic polyamidoamine with 32 amines, onto each end is coupled a PEG-amine of
molecular weight 2000 was dissolved in 0.1 MES buffer, pH 5.5, and 250 mg GalGalGlcNAc was added and the pH readjusted to pH 5.5. 500 mg EDC was added and the reaction was rotated head over head overnight. The solution was dialyzed three times against 4 liters of DI water and lyophilized. An IC50 Elisa as described above was performed and the approximate IC50 was determined to be 1 mg/mL. - Synthesis and Activity of a Composition Comprising a Dextran-Amine (M 1˜10,000)
- 200 mg of a 10,000 molecular weight dextran-amine (Molecular Probes, Oregon) was dissolved in 0.1 MES buffer, pH 5.5, and 250 mg GalGalGlcNAc was added and the pH readjusted to pH 5.5. 500 mg EDC was added and the reaction was rotated head over head overnight. The solution was dialyzed three times against 4 liters of DI water and lyophilized. An IC 50 Elisa as described above was performed and the approximate IC50 was determined to be 10−3 mg/mL.
- Synthesis and Activity of a Composition Comprising a Dextran-Amine (M 1˜40,000)
- 200 mg of a 40,000 molecular weight dextran-amine (Molecular Probes, Oregon) was dissolved in 0.1 MES buffer, pH 5.5, and 250 mg GalGalGlcNAc was added and the pH readjusted to pH 5.5. 500 mg EDC was added and the reaction was rotated head over head overnight. The solution was dialyzed three times against 4 liters of DI water and lyophilized. An IC 50 Elisa as described above was performed and the approximate IC50 was determined to be 5×10−6 mg/mL.
- Synthesis and Activity of a Composition Comprising a Dextran-Amine (M 1˜70,000)
- 200 mg of a 70,000 molecular weight dextran-amine (Molecular Probes) was dissolved in 0.1 MES buffer, pH 5.5, and 250 mg GalGalGlcNAc was added and the pH readjusted to pH 5.5. 500 mg EDC was added and the reaction was rotated head over head overnight. The solution was dialyzed three times against 4 liters of DI water and lyophilized. An IC 50 Elisa as described above was performed and the approximate IC50 was determined to be 10−5 mg/mL.
- Synthesis and Activity of a Composition Comprising a Poly(lysine)-Amine (M 1˜30,000)
- 200 mg of a 30,000 molecular weight poly-lysine (Sigma) was dissolved in 0.1 MES buffer, pH 5.5, and 500 mg GalGalGlcNAc was added and the pH readjusted to pH 5.5. 1400 mg EDC was added and the reaction was rotated head over head overnight. The solution was dialyzed four times against 4 liters of DI water and lyophilized. An IC 50 Elisa as described above was performed and the approximate IC50 was determined to be 10−5 mg/mL.
- Synthesis of 810S: 8 Arm 10,000 Molecular Weight (αGal(1,3)Gal α 1-4 GlcNAc) 8-PEG Using EDC and NHSS for Coupling.
- 62.5 g of EDC, 7 g of NHSS and 15 g of αGal(1,3)Gal β 1-4 GlcNAc were dissolved in 50 mL of 0.1 M MES buffer, pH 5.5. The resulting solution was then transferred into a 500 mL glass beaker with stopper, in which 12.5 g of 8 arm 10,000 molecular weight PEG-(NH 2)8 was dissolved in 100 mL of 0.1 M MES buffer, pH 5.5. The solution was brought up to 250 mL by addition of 0.1 M MES buffer, pH 5.5. The reaction mixture was shaken on an orbital shaker overnight. The reaction mixture was filled into dialysis tubes of molecular weight cut off 3,500 and dialyzed five times against 20 liters of DI water. The dialyzed material was freeze-dried to a yellowish powder. Amino group analysis revealed that more than 98% of amino groups were modified.
- Comparison of 810S with Immunoapheresis
- A series of experiments were initiated to directly compare the effects of 810S vs. immunoapheresis on circulating α-gal antibodies and peripheral α-gal antibody secreting cells (ASCs) in baboons.
- A. Experimental Design:
- A total of 6 baboons were used. Three baboons (designated 51-98, 48-98 and 47-98) underwent immunoapheresis (IP) using α-gal columns, with plasma from 3 blood volumes being passed over the columns every 3 days for a total of 6 treatments. Three other baboons (designated 46-98, 52-98, 50-98) received IV infusions of 810S at 50 mg/kg every 3 days for a total of 6 treatments. No immunosuppression or exposure to pig organs was included in the protocol.
- In both groups, serum samples and blood were collected to measure α-gal antibody levels and ASCs during the treatment period and during a follow-up period out to approximately 150-180 days.
- B. Results:
- Each IP treatment removed circulating IgM and IgG XNAs to 10-20% of initial levels, with a rebound of 50% of initial levels occurring in between each treatment. This is slightly different from what is usually observed, although our experience is with animals under immunosuppression. Typically, after plasma from 3 blood volumes passes over an α-Gal column, XNAs are undetectable in the post-treatment serum. A rebound of XNAs to 20-60% of the initial levels is usually observed after the 1st treatment, and this rebound effect generally diminishes with each IP treatment.
- Immediately after the final IP treatment, IgG and IgM XNA levels were at 7-17% and 3-15% of initial baseline levels, respectively. See FIGS. 26 and 31- 33. Two weeks after the end of IP treatment (day 30), circulating IgG XNA levels for 2 of the baboons were at approximately 60% of initial levels and remained relatively constant out to day 145, while in the 3rd animal the XNAs had returned to approx. 90% initial levels by
day 30 and reached about 1.5×baseline levels by day 145. See FIGS. 27 and 31-33. Total IgM and IgG levels in all 3 animals remained relatively constant throughout the experiment. See FIGS. 31-33. - After the 1st treatment with 8108, circulating IgG and IgM XNA levels in all 3 baboons dropped to between 1-13% of initial levels. See FIGS. 26 and 28- 30. After the final 810S treatment, IgG XNA levels were 1-9% of baseline values, and IgM XNA levels were 1-3% of baseline values. See FIGS. 27-30. By
day 30, IgG XNA levels had reached 20-33% of baseline values, and IgM XNA levels were 8-18% of baseline values. See FIGS. 26 and 27. - FIGS. 34 and 35 show the average fold-change in IgG and IgM response, respectively, for each of the IP and 810S treated animals.
- To investigate whether 810S was still in baboon serum by
day 30, serum was mixed with human serum and analyzed by ELISA for competition with human XNA binding to GAL-HSA. Results indicatedday 30 baboon serum does not compete with human XNA binding to GAL, and therefore demonstrates that 810S is not still in circulation. - On day 184, IgG XNA levels in 2 810S-treated baboons were still at 30-38% of baseline values, whereas in one baboon IgG XNA levels had returned to baseline. The IgM XNA levels in this 3rd baboon reached 60% of baseline levels by day 184, while IgM XNA levels in the other 2 baboons reached 24-39% of baseline values. Total IgM and IgG levels in all 3 animals remained relatively constant throughout the experiment.
- Baboon blood was also analyzed periodically for IgG and IgM XNA ASCs. As expected, IP had minimal effect on ASCs. By the second experiment with 810S IgM and IgG ASC levels were reduced to 20-40% of initial levels, by the fourth treatment, IgM and IgG ASCs were at about 10% initial levels, and after the sixth treatment they were approximately 3-6% of starting levels. On
day - C. Summary:
- Analysis of XNA levels in baboons treated with IP indicates a reduction in XNA levels after each treatment, with rebounds observed in between treatments, and a gradual return of antibody when treatment is stopped. IgG XNA levels in 2 animals only returned to about 60% of initial values, which is unusual compared to what is typically observed. IgG XNAs in the 3rd animal, and IgM XNAs in all 3 animals did return to baseline values, with on animal overshooting this level slightly. In comparison, XNA levels are immediately reduced upon infusion of 810S, and the levels remain depressed during the entire course of the treatment. In 810S-treated baboons, XNAs show a much slower return after 810S treatment is discontinued compared to baboons treated with IP. Compare IP and 810S plots on FIGS. 34 and 35.
- Analysis of circulating IgM and IgG ASCs demonstrates, as expected, no effect when animals undergo IP. However, an effect on the levels of these cells due to 810S infusion was clearly observed. ASC levels dropped soon after initiation of 810S treatment, and three weeks after 810S was discontinued, ASCs had not yet returned to initial levels.
- IP physically removes circulating antibodies, and their return to the circulation is expected due to re-synthesis. While the mode of action of 810S is less clear, it is apparent from in vitro ELISA experiments that 810S can bind XNAs and prevent them from binding αGal epitopes. The sustained reduction on XNA levels several weeks after 810S treatment is discontinued, and the downregulation of αGal ASCs, implies that 810S has an immunoregulatory function, e.g., is tolerogenic, as well.
- (v) Incorporation by Reference
- All of the above-cited references and publications are hereby incorporated by reference.
- (vi) Equivalents
- Those skilled in the art will recognize, or be able to ascertain using no more than routine experimentation, many equivalents of the specific embodiments of the invention described herein. Such equivalents are intended to be encompassed by the following claims.
Claims (95)
1. A polymer, synthesized by a cross-coupling reaction of
(i) polymer scaffold units comprising a plurality of nucleophilic or electrophilic functional groups, and
(ii) xenoantigens comprising a functional group capable of reacting with the functional groups presented on the polymer scaffold unit,
which reaction is carried out in the presence of a crosslinking reagent under conditions wherein polymer scaffold units are coupled with xenoantigens, and separate polymer scaffold units are cross-linked to one another.
2. The polymer of claim 1 , wherein the functional groups for the polymer scaffold units and xenoantigens are selected from the group consisting of amine and carboxylic acid, alcohol and alkyl halide or sulfonate, thiol and alkyl halide or sulfonate, phosphine and alkyl halide or sulfonate, phosphite and alkyl halide or sulfonate, and aldehyde or ketone and amine.
3. The polymer of claim 1 , wherein the functional groups produce an amide, ether, thioether, or phosphate linkage between the polymer scaffold unit and xenoantigen upon cross-coupling.
4. The polymer of claim 1 , wherein the functional groups produce an amide linkage between the polymer scaffold unit and xenoantigen upon cross-coupling.
5. The polymer of claim 1 , wherein the cross-coupling is carried out in the presence of an activating agent that activates the functional groups of the polymer scaffold units and xenoantigens for forming a covalent bond there between.
6. The polymer of claim 5 , wherein the activating group is selected from the group consisting of dehydrating agents, Bronsted acid, Bronsted base, Lewis acid, Lewis base, acyl halide, and phosphoryl halide.
7. The polymer of claim 5 , wherein the activating agent is a diimide
8. The polymer of claim 7 , wherein the activating agent is dicyclohexyldiimide [DCC] or ethyl-3-(dimethylamino)propyldiimide [EDC].
9. The polymer of claim 1 , wherein the polymer scaffold units are pharmacologically-acceptable, non-immunogenic molecules.
10. The polymer of claim 9 , wherein the polymer scaffold units include polyethylene glycol bearing nucleophilic or electrophilic functional groups.
11. The polymer of claim 10 , wherein the nucleophilic or electrophilic functional groups on the polymer scaffold units are selected from the group consisting of amines, alcohols, thiols, selenols, phosphines, aldehydes, ketones, acid chlorides, acids, esters, alkyl halides, and alkyl sulfonates.
12. The polymer of claim 11 , wherein the functional group on the polymer scaffold unit is an amino or carboxylate moiety, and the functional group of the xenoantigen reacts therewith to form an amide linkage.
13. The polymer of claim 12 , wherein the polymer scaffold unit is octa(amino)polyethylene glycol.
14. The polymer of claim 1 , wherein the polymer scaffold units have a molecular weight in the range of 1,000 Da to 100,000 Da.
15. The polymer of claim 1 , wherein the xenoantigen is selected from the group consisting of a carbohydrate, a peptide, a glycopeptide and a lipid.
16. The polymer of claim 1 , 4, 7, 10, 12 or 14, wherein the xenoantigen is an oligosaccharide.
17. The polymer of claim 16 , wherein the xenoantigen includes an epitope which is cross-reactive with an xenoreactive antibody against α-galactosyl moieties.
18. The polymer of claim 17 , wherein the xenoantigen includes an oligosacchride having an αGal(1,3)Gal moiety represented by the general formula:
wherein:
R, independently for each occurrence, represents H or a C1-C6 alkyl or other hydroxyl protecting group;
R′ is absent or represents an oligosacchride consisting of 1-8 saccharide residues; and
X represents a bond or linker moiety linking the oligosacchride moiety to the polymer backbone.
19. The polymer of claim 17 , wherein the xenoantigen includes an oligosacchride having represented by one of the general formula:
wherein,
R1, independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
R, independently for each occurrence, represents H or a C1-C6 alkyl or other hydroxyl protecting group;
R2 is a C1-C6 alkyl;
Y, independently for each ocurrence, is absent, or —C(S)—, —S(°)2, —C(O)-A-;
X represents a bond or linker moiety linking the oligosacchride moiety to the polymer backbone;
Z is O or S; and
A is —NH—, —NH—(C1-C6 alkyl)-, —NH—(C1-C6 alkenyl)-, -O—, —O—(C1-C6 alkyl)-, —O- (C1-C6 alkenyl)-, —S—, —S—(C1-C6 alkyl)-, —S—(C1-C6 alkenyl)-.
20. The polymer of claim 17 , wherein the xenoantigen includes one or more of:
αGal(1,3) βGal; αGal(1,3)βGal(1,4)βGlcNAc; or αGal(1,3)βGal(1,4)βGlc moieties.
21. The polymer of claim 16 , 17 or 18, wherein the xenoantigen includes di-, tri-, tetra- and penta-oligosacchrides.
22. The polymer of claim 16 , wherein the xenoantigen includes one or more of: αGal(1,2)Gal, αGal(1,4)Gal, βGal(1,3)GalNAc or 3-O-sulphated galactose (SO4-3Gal).
23. The polymer of claim 1 or 16, which polymer includes two or more different xenoantigens.
24. The polymer of claim 1 , wherein the crosslinking agent comprises two functional groups capable of reacting with the polymer scaffold units, the xenoantigen, the covalent linkage of the scaffold with the xenoantigen, or a combination thereof.
25. The polymer of claim 23 , wherein the crosslinking agent is derived from N-hydroxysulfosuccinimide.
26. The polymer of claim 1 , wherein the cross-coupling reaction is carried out in the pH range of 4 to 7.
27. The polymer of claim 1 , wherein the cross-coupling reaction is carried out in the temperature range of 0° C. and 40° C.
28. The polymer of claim 1 , 25 or 26, wherein the cross-coupling reaction is carried out a temperature, pH, reactant concentration and for a time sufficient to yield a polymer having a nominal molecular weight of 100,000-500,000 daltons.
29. The polymer of claim 1 , 25 or 26, wherein the cross-coupling reaction is carried out a temperature, pH, reactant concentration and for a time sufficient to yield a polymer having a nominal molecular weight less than 100,000 daltons.
30. The polymer of claim 29 , which polymer induces B cell anergy for the xenoantigen.
31. The polymer of claim 1 , 27 or 28, having a polydispersity of 20 or less.
32. The polymer of claim 1 or 28, wherein the polymer is a non-immunogenic polymer.
33. The polymer of claim 1 or 28, wherein the polymer is tolerogenic for the xenoantigens.
34. The polymer of claim 1 , which polymer is formulated with a pharmaceutical excipient.
35. The polymer of claim 1 or 34, which polymer is formulated as a sterile formulation.
36. The use of the polymer of claim 1 in the manufacture of a medicament for the prevention of discordant graft rejection, said medicament administered to a patient which is to receive, or has received, a discordant tissue graft, and in an amount sufficient to reduce the severity of rejection of the graft.
37. The medicament of claim 36 , having a therapeutic index for preventing discordant graft rejection of at least 10.
38. A preparation of the polymer according to claim 1 , which polymer is homogenous with respect to the xenoantigen displayed thereon.
39. A preparation of the polymer according to claim 1 , which polymer has two or more different xenoantigens displayed thereon.
40. A kit comprising two or more polymers according to claim 1 , each polymer isolated from the other and homogenous with respect to the xenoantigen displayed thereon but different from at least one other polymer of the kit.
41. A polymer, comprising a polymer backbone of polyethylene glycol subunits, at least a portion of which have attached thereto a saccharide moiety represented in the general formula (VII):
wherein,
R1, independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
R5 is H, —OR, NH—Y—R2, or -L-E;
Y independently for each occurrence is absent, —C(O)—, —C(S)—, —C(NR)—, O, S, or Se;
B, for one occurrence is —R, and for the other occurrence is —R or from 1-9 saccharide residues;
R, independently for each occurrence, represents H or a C1-C6 alkyl or other hydroxyl protecting group;
R2 is a C1-C6 alkyl;
X2 represents a linker of 1-10 atoms in the length;
D represents H, or -L-E;
L represents a linker of 1-20 atoms in length; and
E represents a second PEG subunit of the polymer, wherein, independently for each occurrence of E, the linker group L is covalently attached via R5 or B of a saccharide moiety of the second PEG subunit, or via an amine of the second PEG subunit.
42. A polymer, represented by the general formula
wherein
R1, independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
R5 is H, —OR, NH—Y—R2, or -L-E;
Y independently for each occurrence is absent, —C(O)—, —C(S)—, —C(NR)—, O, S, or Se;
B, for one occurrence is —R, and for the other occurrence is —R or from 1-9 saccharide residues;
R, independently for each occurrence, represents H or a C1-C6 alkyl or other hydroxyl protecting group;
R2 is a C1-C6 alkyl;
X2 represents a linker of 1-10 atoms in the length D represents H, or -L-E;
L represents a linker a linker of 1-20 atoms in length;
E represents another polyethylene glycol subunit of the polymer having a saccharide moiety VII attached thereto, wherein, independently for each occurrence of E, the linker group L is covalently attached via R5 or B in the saccharide moiety.
PEG represents a polyethylene glycol subunit; and
X3 represents one or more additional polyethylene glycol subunit which may be derivatived with a α saccharide moiety of formula VII,
wherein the polymer has an average molecular weight in the range of 10,000 daltons to 1,000,000 daltons.
44. A composition comprising: a non-immunogenic pharmacologically acceptable carrier; and multiple αGal(1,3)Gal moieties covalently linked to said carrier.
45. A composition useful for reducing plasma levels of anti-αGal(1,3)Gal) antibodies in a primate subject, said composition comprising:
a non-immunogenic, cross-linked polymer; and
multiple αGal(1,3)Gal moieties covalently linked to said carrier.
46. The composition of claim 44 or 45, wherein said carrier is poly(amino)polyethylene glycol.
47. The composition of claim 45 , wherein the αGal(1,3)Gal moieties are represented by the general formula:
wherein:
R, independently for each occurrence, represents H or a C1-C6 alkyl or other hydroxyl protecting group;
R′ is absent or represents an oligosacchride of 1-8 saccharide residues; and
X represents a bond or linker moiety linking the oligosacchride moiety to the carrier.
48. The composition of claim 45 , wherein the αGal(1,3)Gal moiety is selected from the group consisting of αGal(1,3)αGal moieties; αGal(1,3)βGal(1,4)PGlcNAc moieties; and αGal(1,3)βGal(1,4)βGlc moieties.
49. The composition of any of claims 45, wherein the αGal(1,3)Gal moiety is a di-, tri-, tetra- or penta-oligosacchride.
50. The composition of claim 45 , wherein the carrier includes multiple polymer scaffold units crosslinked therebetween.
51. The composition of claim 50 , wherein the polymer scaffold units are crosslinked by a reagent derived from N-hydroxysulfosuccinimide.
52. A method for synthesizing a composition useful for reducing plasma levels of anti-(αGal(1,3)Gal) antibodies in a primate subject, said method comprising reacting in a solution at a pH range of 4 to 7 the following reagents:
a) a branched polymer having at least three arms, and at least six active groups covalently linked to the free ends of said arms, said active groups being selected from the group consisting of amino groups and hydrazino groups;
b) an αGal(1,3)Gal-containing moiety having a carboxy group covalently linked thereto; and
c) N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide.
53. A method for synthesizing a composition useful for reducing plasma levels of anti-(αGal(1,3)Gal) antibodies in a primate subject, said method comprising reacting in a solution at a pH range of 4 to 7 the following reagents:
a) a branched polymer having at least three arms, and at least six carboxy groups covalently linked to the free ends of said arms;
b) an αGal(1,3)Gal-containing moiety having an amino group or hydrazino group covalently linked thereto; and
c) N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide.
54. A method for synthesizing a composition useful for reducing plasma levels of anti-(αGal(1,3)Gal) antibodies in a primate subject, said method comprising reacting in a solution at a pH range of 4 to 7 the following reagents:
a) a branched polymer having at least three arms, and at least six active groups covalently linked to the free ends of said arms, said active groups being selected from the group consisting of amino groups and hydrazino groups; and
b) an αGal(1,3)Gal-containing moiety having an aldehyde group covalently linked thereto.
55. A method for synthesizing a composition useful for reducing plasma levels of anti-(αGal(1,3)Gal) antibodies in a primate subject, said method comprising reacting in a solution at a pH range of 4 to 7 the following reagents:
a) a branched polymer having at least three arms, and at least six aldehyde groups covalently linked to the free ends of said arms; and
b) an αGal(1,3)Gal-containing moiety having an amino group or hydrazino group covalently linked thereto.
56. A method for synthesizing a composition useful for reducing plasma levels of anti-(αGal(1,3)Gal) antibodies in a primate subject, said method comprising reacting in a solution at a pH range of 4 to 7 the following reagents:
a) a branched polymer having at least three arms, and at least three active groups covalently linked to the free ends of said arms, said active groups being selected from the group consisting of amino groups and hydrazino groups;
b) an αGal(1,3)Gal-containing moiety having a carboxy group covalently linked thereto;
c) N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide; and
d) N-hydroxysulfosuccinimide.
57. A method for synthesizing a composition useful for reducing plasma levels of anti-(αGal(1,3)Gal) antibodies in a primate subject, said method comprising reacting in a solution at a pH range of 4 to 7 the following reagents:
a) a branched polymer having at least three arms, and at least three carboxy groups covalently linked to the free ends of said arms;
b) an αGal(1,3)Gal-containing moiety having an amino group or hydrazino group covalently linked thereto;
c) N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide; and
d) N-hydroxysulfosuccinimide.
58. The method of claim 52 , 53, 54, 55, 56, or 57, wherein said αGal(1,3)Gal moiety is reacted at a one-fold to ten-fold molar excess to said arms of said branched polymer.
59. A composition produced by the method of claim 52 , 53, 54, 55, 56 or 57.
60. A method for formulating a composition useful for reducing plasma levels of anti-xenograft antibodies in a subject, said method comprising
A. generating a polymer by a cross-coupling reaction of
(i) polymer scaffold units comprising a plurality of nucleophilic or electrophilic functional groups, and
(ii) xenoantigens comprising a functional group capable of reacting with the functional groups presented on the polymer scaffold unit,
which cross-coupling reaction is carried out in the presence of a crosslinking reagent under conditions wherein polymer scaffold units are coupled with xenoantigens, and polymer scaffold units of the polymer are cross-linked to one another; and
B. formulating the polymer as a sterile pharmaceutical prepartion.
61. The method of claim 60 , wherein the cross-coupling reaction includes a diimide activating agent for activating the coupling of the xenoantigens and polymer scaffold units.
62. The method of claim 60 , wherein the polymer scaffold units are pharmacologically-acceptable, non-immunogenic polymers.
63. The method of claim 60 , wherein the polymer scaffold units are polyethylene glycol.
64. The method of claim 60 , wherein the xenoantigen is an oligosaccharide.
65. The method of claim 64 , wherein the xenoantigen includes an epitope which is cross-reactive with an xenoreactive antibody against α-galactosyl moieties.
66. The method of claim 65 , wherein the xenoantigen includes an oligosacchrides having a αGal(1,3)Gal moiety represented by the general formula:
wherein:
R, independently for each occurrence, represents H or a C1-C6 alkyl or other hydroxyl protecting group;
R′ is absent or represents an oligosacchride of 1-8 saccharide residues; and
X represents a bond or linker moiety linking the oligosacchride moiety to the polymer backbone.
67. The method of claim 65 , wherein the xenoantigen includes an oligosacchride having represented by one of the general formula:
wherein,
R1, independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
R, independently for each occurrence, represents H or a C1-C6 alkyl or other hydroxyl protecting group;
R2 is a C1-C6 alkyl;
Y, independently for each ocurrence, is absent, or —C(S)—, —S(O)2—, —C(O)-A-;
X represents a bond or linker moiety linking the oligosacchride moiety to the polymer backbone;
Z is O or S; and
A is —NH—, —NH—(C1-C6 alkyl)-, —NH—(C1-C6 alkenyl)-, —O—, —O—(C1-C6 alkyl)-, —O—(C1-C6 alkenyl)-, —S—, —S—(C1-C6 alkyl)-, —S—(C1-C6 alkenyl)-.
68. The method of claim 60 , wherein the crosslinking agent is derived from N-hydroxysulfosuccinimide.
69. The method of claim 60 , wherein the cross-coupling reaction is carried out a temperature, pH, reactant concentration and for a time sufficient to yield a polymer having an average molecular weight of 100,000-500,000 daltons.
70. The method of claim 60 , wherein the cross-coupling reaction is carried out a temperature, pH, reactant concentration and for a time sufficient to yield a polymer having an average molecular weight of having an average molecular weight less than 100,000 daltons.
71. A method for attenuating rejection of tissues or cells from a donor animal of one species transplanted to a recipient animal of another species, comprising administering to the recipient animal an amount of a polymer reactive with xenoreactive antibodies of the recipient animal effective for delaying or lessening the severity of a graft rejection response, wherein the polymer has epitopes cross-reactive with an xenoantigen of the graft, and rejection of the tissue is mediated at least in part by the expression of the xenoantigen on the tissue.
72. The method of claim 71 , wherein the polymer is administered to the animal as a pre-treatment regimen before the animal is transplanted with the tissues or cells.
73. The method of claim 71 or 72, wherein the polymer is administered to the animal after the animal has been transplanted with the tissues or cells.
74. The method of claim 73 , wherein the polymer is administered in an amount sufficient to suppress hyperacute rejection of the transplanted tissue or cells.
75. The method of claim 60 , wherein the polymer is a polyethylene glycol polymer having a multitude epitopes which are cross-reactive with an xenoreactive antibody against α-galactosyl moieties.
76. The method of claim 75 , wherein the epitopes comprise oligosacchrides having a αGal(1,3)Gal moiety represented by the general formula:
wherein:
R, independently for each occurrence, represents H or a C1-C6 alkyl or other hydroxyl protecting group;
R′ is absent or represents an oligosacchride of 1-8 saccharide residues; and
X represents a bond or linker moiety linking the oligosacchride moiety to the polymer backbone.
77. The method of claim 75 , wherein the epitopes comprise an oligosacchride having represented by one of the general formula:
wherein,
R1, independently for each ocurrence, is H, —OR, —SR or —N(H)—Y—R;
R, independently for each occurrence, represents H or a C1-C6 alkyl or other hydroxyl protecting group;
R2 is a C1-C6 alkyl;
Y, independently for each ocurrence, is absent, or —C(S)—, —S(O)2—, —C(O)-A-;
X represents a bond or linker moiety linking the oligosacchride moiety to the polymer backbone;
Z is O or S; and
A is —NH—, —NH—(C1-C6 alkyl)-, —NH—(C1-C6 alkenyl)-, —O—, —O—(C1-C6 alkyl)-, —O—(C1-C6 alkenyl)-, —S—, —S—(C1-C6 alkyl)-, —S—(C1-C6 alkenyl)-.
78. The method of claim 75 , wherein the recipient animal does not possess an endogenous UDP galactose:β-D-galactosyl-1,4-N-acetyl-D-glucosminide α(1,3) galactosyltransferase (α1,3-GT) activity, or produce or display the αGal(1,3)Gal xenoantigen on its cells, tissues or organs.
79. The method of claim 78 , wherein the recipient animal is a human or old world primate.
80. The method of claim 75 , wherein the donor animal is a swine.
81. The method of claim 71 , wherein the transplanted tissue is a kidney, heart, lung or liver.
82. The method of claim 71 , wherein the transplanted tissue bone marrow or other preparation of hematopoietic cells.
83. A method for reducing plasma levels of anti-(αGal(1,3)Gal) antibodies in a primate subject, said method comprising administering to said subject a composition comprising:
a non-immunogenic pharmacologically acceptable carrier; and
multiple αGal(1,3)Gal moieties covalently linked to said carrier.
84. The method of claim 82 wherein said composition further comprises at least one crosslinking group covalently linked to said composition.
85. The method of claim 84 wherein said activation group is derived from N-hydroxysulfosuccinimide.
86. The method of any one of claims 83-85 wherein said carrier is polyethylene glycol.
87. A complex comprising one or more B-cells associated with a composition comprising:
a non-immunogenic pharmacologically acceptable carrier; and
multiple αGal(1,3)Gal moieties covalently linked to said carrier.
88. A complex comprising one or more antibodies associated with a composition comprising:
a non-immunogenic pharmacologically acceptable carrier; and
multiple αGal(1,3)Gal moieties covalently linked to said carrier.
89. A kit for detecting and quantifying anti-(αGal(1,3)Gal) antibody-secreting cells in the blood of a subject comprising:
a multi-well plate to which αGal(1,3)Gal is covalently coupled;
blocking solution comprising a buffer and human serum albumin;
serum-free complete culture medium;
anti-human immunoglobulin antibodies; and
means for labeling said anti-human immunoglobulin antibodies bound to said plate.
90. A method for detecting and quantifying anti-(αGal(1,3)Gal) antibody-secreting cells in the blood of a subject comprising:
providing peripheral blood mononuclear cells isolated from a blood sample of said subject;
providing a multi-well plate to which αGal(1,3)Gal is covalently coupled;
incubating said peripheral blood mononuclear cells on said plate for a sufficient time and under appropriate conditions to allow anti-(αGal(1,3)Gal) antibody-secreting cells and their antibodies to bind specifically to said αGal(1,3)Gal coated on said plate;
washing said plate to remove non-binding cells;
incubating said anti-(αGal(1,3)Gal) antibody-secreting cells and
their antibodies bound to said αGal(1,3)Gal coated on said plate with anti-human immunoglobulin antibodies;
washing said plate to remove non-binding anti-human immunoglobulin antibodies;
applying a means to label anti-human immunoglobulin antibodies bound to said plate; and
quantifying single anti-(αGal(1,3)Gal) antibody-secreting, spot forming cells indicated by spots formed on said plate by the binding of said labelled anti-human immunoglobulin antibodies.
91. The polymer of claim 1 , 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 or 15, wherein said polymer is synthesized in two or more chemical reactions conducted in series.
92. The method of claim 52 , 53, 54, 55, 56, 57, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, or 70, wherein the composition is synthesized in two or more chemical reactions conducted in series.
93. A polymer comprising: a non-immunogenic pharmacologically acceptable polymer;
and multiple epitopes covalently linked to said carrier, which epitopes are specifically cross-reactive with xenoreactive antibodies.
94. The polymer of claim 93 , which polymer is selected from the group consisting of straight chain polymers and branched polymers.
95. The polymer of claim 93 , wherein the epitopes are specifically cross-reactive with human xenoreactive antibodies for tissue from a non-human mammal.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/402,845 US20040141944A1 (en) | 1998-04-15 | 2003-03-28 | Inhibition of xenoreactive antibodies |
| US11/932,737 US20080124396A1 (en) | 1998-04-15 | 2007-10-31 | Inhibition of Xenoreactive Antibodies |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US6052598A | 1998-04-15 | 1998-04-15 | |
| US09/292,153 US6572867B1 (en) | 1998-04-15 | 1999-04-15 | Inhibition of xenoreactive antibodies |
| US10/402,845 US20040141944A1 (en) | 1998-04-15 | 2003-03-28 | Inhibition of xenoreactive antibodies |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/292,153 Division US6572867B1 (en) | 1998-04-15 | 1999-04-15 | Inhibition of xenoreactive antibodies |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/932,737 Division US20080124396A1 (en) | 1998-04-15 | 2007-10-31 | Inhibition of Xenoreactive Antibodies |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20040141944A1 true US20040141944A1 (en) | 2004-07-22 |
Family
ID=22030043
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/292,153 Expired - Fee Related US6572867B1 (en) | 1998-04-15 | 1999-04-15 | Inhibition of xenoreactive antibodies |
| US10/402,845 Abandoned US20040141944A1 (en) | 1998-04-15 | 2003-03-28 | Inhibition of xenoreactive antibodies |
| US11/932,737 Abandoned US20080124396A1 (en) | 1998-04-15 | 2007-10-31 | Inhibition of Xenoreactive Antibodies |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/292,153 Expired - Fee Related US6572867B1 (en) | 1998-04-15 | 1999-04-15 | Inhibition of xenoreactive antibodies |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/932,737 Abandoned US20080124396A1 (en) | 1998-04-15 | 2007-10-31 | Inhibition of Xenoreactive Antibodies |
Country Status (10)
| Country | Link |
|---|---|
| US (3) | US6572867B1 (en) |
| EP (1) | EP1087791B1 (en) |
| JP (1) | JP2002511431A (en) |
| AT (1) | ATE304865T1 (en) |
| AU (1) | AU761831B2 (en) |
| CA (1) | CA2326618A1 (en) |
| DE (2) | DE69927369T2 (en) |
| ES (1) | ES2249890T3 (en) |
| HK (1) | HK1036223A1 (en) |
| WO (1) | WO1999052561A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9420770B2 (en) | 2009-12-01 | 2016-08-23 | Indiana University Research & Technology Corporation | Methods of modulating thrombocytopenia and modified transgenic pigs |
| US9642899B2 (en) | 2010-05-06 | 2017-05-09 | Mayo Foundation For Medical Education And Research | Implantation of a cardiac xenograft from a B4GALNT2KO and GTKO transgenic pig to reduce immunogenicity |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6399578B1 (en) * | 1998-12-09 | 2002-06-04 | La Jolla Pharmaceutical Company | Conjugates comprising galactose α1,3 galactosyl epitopes and methods of using same |
| CA2353620A1 (en) * | 1998-12-09 | 2000-06-15 | La Jolla Pharmaceutical Company | Methods and formulations for reducing circulating antibodies |
| CA2396966A1 (en) * | 2000-01-13 | 2001-07-19 | John Papageorgiou | Methods for synthesis of .alpha.-d-gal (1.fwdarw.3) gal-containing oligosaccharides |
| US7270961B2 (en) * | 2002-04-16 | 2007-09-18 | Hui Sunny Chang | In vitro assay for quantitating secreted antibodies in lymphocyte supernatant for evaluation of vaccine or antigen induced specific antibody secretion from ex vivo circulating antibody-secreting lymphocytes |
| WO2006044577A1 (en) * | 2004-10-13 | 2006-04-27 | Ilypsa, Inc. | Pharmaceutical compositions comprising a toxin-binding oligosaccharide and a polymeric particle |
| US20060078534A1 (en) * | 2004-10-13 | 2006-04-13 | Dominique Charmot | Toxin binding compositions |
| FR2886298A1 (en) * | 2005-05-24 | 2006-12-01 | Ifremer | ANTIBODY OR FRAGMENT OF ANTIBODIES COUPLED WITH AN IMMUNOGENIC AGENT |
| WO2008101177A2 (en) * | 2007-02-16 | 2008-08-21 | University Of Virginia Patent Foundation | Ige antibodies to chimeric or humanized igg therapeutic monoclonal antibodies as a screening test for anaphylaxis |
| EP2095829A1 (en) * | 2008-02-27 | 2009-09-02 | LEK Pharmaceuticals D.D. | Selenium containing modifying agents and conjugates |
| US9891219B2 (en) * | 2008-10-10 | 2018-02-13 | Mayo Foundation For Medical Education And Research | Methods for treating neuromyelitis optica (NMO) by administration of eculizumab to an individual that is aquaporin-4 (AQP4)-IgG autoantibody positive |
| US20130149331A1 (en) * | 2011-05-03 | 2013-06-13 | Peng George Wang | Rhamnose and forssman conjugated immunogenic agents |
| US9533214B2 (en) | 2012-09-25 | 2017-01-03 | Igt | Gaming system and method for providing plays of multiple games |
| ES2865148T3 (en) | 2014-03-13 | 2021-10-15 | Univ Basel | Carbohydrate ligands that bind to IgM antibodies against myelin-associated glycoprotein |
| EP2987503A1 (en) * | 2014-08-22 | 2016-02-24 | Institut d'Investigació Biomèdica de Bellvitge (IDIBELL) | Methods and reagents for prevention and/or treatment of infection |
| WO2016065046A1 (en) | 2014-10-22 | 2016-04-28 | Indiana University Research & Technology Corporation | Triple transgenic pigs suitable for xenograft |
| US11091591B2 (en) | 2015-09-16 | 2021-08-17 | Universität Basel | Carbohydrate ligands that bind to antibodies against glycoepitopes of glycosphingolipids |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5126131A (en) * | 1983-01-24 | 1992-06-30 | The Johns Hopkins University | Therapeutic suppression of specific immune responses by administration of antigen-competitive conjugates. |
| US5191066A (en) * | 1990-12-07 | 1993-03-02 | Abbott Laboratories | Site-specific conjugation of immunoglobulins and detectable labels |
| US5219564A (en) * | 1990-07-06 | 1993-06-15 | Enzon, Inc. | Poly(alkylene oxide) amino acid copolymers and drug carriers and charged copolymers based thereon |
| US5268454A (en) * | 1991-02-08 | 1993-12-07 | La Jolla Pharmaceutical Company | Composition for inducing humoral anergy to an immunogen comprising a t cell epitope-deficient analog of the immunogen conjugated to a nonimmunogenic carrier |
| US5679352A (en) * | 1992-02-03 | 1997-10-21 | Connaught Laboratories Limited | Synthetic Haemophilus influenzae conjugate vaccine |
| US5866135A (en) * | 1994-04-21 | 1999-02-02 | North American Vaccine, Inc. | Group A streptococcal polysaccharide immunogenic compositions and methods |
| US5879675A (en) * | 1994-03-15 | 1999-03-09 | Medical College Of Pennsylvania And Hahnemann University | Compositions and methods for vaccines comprising α-galactosyl epitopes |
| US6676946B2 (en) * | 1997-03-27 | 2004-01-13 | Institut Pasteur | Multiple antigen glycopeptide carbohydrate vaccine comprising the same and use thereof |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SE8001748L (en) * | 1980-03-05 | 1981-09-06 | Kaellenius Gunilla | COMPOSITIONS FOR THERAPEUTICAL OR DIAGNOSTIC APPLICATION AS PROCEDURES FOR THERAPEUTICS |
| JP3201523B2 (en) | 1991-08-23 | 2001-08-20 | アルバータ リサーチ カウンスル | Methods and compositions for attenuating antibody-mediated xenograft rejection at human receptors |
| US6444655B1 (en) * | 1995-12-21 | 2002-09-03 | Procur Ab | Galactopyranosides and their use |
| CA2279544A1 (en) * | 1997-02-05 | 1998-08-06 | Biotransplant Incorporated | Induction of b cell tolerance |
| CN1185253C (en) * | 1997-04-18 | 2005-01-19 | 诺瓦提斯公司 | Neoglycoproteins |
| US6096725A (en) | 1997-07-02 | 2000-08-01 | Neose Technologies, Inc. | Methods of using αGal oligosaccharides as immune system targeting agents |
-
1999
- 1999-04-15 AU AU35645/99A patent/AU761831B2/en not_active Ceased
- 1999-04-15 DE DE69927369T patent/DE69927369T2/en not_active Expired - Fee Related
- 1999-04-15 US US09/292,153 patent/US6572867B1/en not_active Expired - Fee Related
- 1999-04-15 ES ES99917553T patent/ES2249890T3/en not_active Expired - Lifetime
- 1999-04-15 WO PCT/US1999/008326 patent/WO1999052561A1/en active IP Right Grant
- 1999-04-15 AT AT99917553T patent/ATE304865T1/en not_active IP Right Cessation
- 1999-04-15 CA CA002326618A patent/CA2326618A1/en not_active Abandoned
- 1999-04-15 JP JP2000543171A patent/JP2002511431A/en active Pending
- 1999-04-15 DE DE1087791T patent/DE1087791T1/en active Pending
- 1999-04-15 EP EP99917553A patent/EP1087791B1/en not_active Expired - Lifetime
-
2001
- 2001-09-28 HK HK01106879A patent/HK1036223A1/en not_active IP Right Cessation
-
2003
- 2003-03-28 US US10/402,845 patent/US20040141944A1/en not_active Abandoned
-
2007
- 2007-10-31 US US11/932,737 patent/US20080124396A1/en not_active Abandoned
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5126131A (en) * | 1983-01-24 | 1992-06-30 | The Johns Hopkins University | Therapeutic suppression of specific immune responses by administration of antigen-competitive conjugates. |
| US5219564A (en) * | 1990-07-06 | 1993-06-15 | Enzon, Inc. | Poly(alkylene oxide) amino acid copolymers and drug carriers and charged copolymers based thereon |
| US5191066A (en) * | 1990-12-07 | 1993-03-02 | Abbott Laboratories | Site-specific conjugation of immunoglobulins and detectable labels |
| US5268454A (en) * | 1991-02-08 | 1993-12-07 | La Jolla Pharmaceutical Company | Composition for inducing humoral anergy to an immunogen comprising a t cell epitope-deficient analog of the immunogen conjugated to a nonimmunogenic carrier |
| US5679352A (en) * | 1992-02-03 | 1997-10-21 | Connaught Laboratories Limited | Synthetic Haemophilus influenzae conjugate vaccine |
| US5879675A (en) * | 1994-03-15 | 1999-03-09 | Medical College Of Pennsylvania And Hahnemann University | Compositions and methods for vaccines comprising α-galactosyl epitopes |
| US5866135A (en) * | 1994-04-21 | 1999-02-02 | North American Vaccine, Inc. | Group A streptococcal polysaccharide immunogenic compositions and methods |
| US6676946B2 (en) * | 1997-03-27 | 2004-01-13 | Institut Pasteur | Multiple antigen glycopeptide carbohydrate vaccine comprising the same and use thereof |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9420770B2 (en) | 2009-12-01 | 2016-08-23 | Indiana University Research & Technology Corporation | Methods of modulating thrombocytopenia and modified transgenic pigs |
| US9642899B2 (en) | 2010-05-06 | 2017-05-09 | Mayo Foundation For Medical Education And Research | Implantation of a cardiac xenograft from a B4GALNT2KO and GTKO transgenic pig to reduce immunogenicity |
| US11141470B2 (en) | 2010-05-06 | 2021-10-12 | Mayo Foundation For Medical Education And Research | B4GALNT2 knock out pig |
Also Published As
| Publication number | Publication date |
|---|---|
| AU761831B2 (en) | 2003-06-12 |
| CA2326618A1 (en) | 1999-10-21 |
| HK1036223A1 (en) | 2001-12-28 |
| US20080124396A1 (en) | 2008-05-29 |
| DE1087791T1 (en) | 2002-02-21 |
| ES2249890T3 (en) | 2006-04-01 |
| ATE304865T1 (en) | 2005-10-15 |
| WO1999052561A1 (en) | 1999-10-21 |
| EP1087791B1 (en) | 2005-09-21 |
| EP1087791A1 (en) | 2001-04-04 |
| AU3564599A (en) | 1999-11-01 |
| DE69927369D1 (en) | 2006-02-02 |
| US6572867B1 (en) | 2003-06-03 |
| DE69927369T2 (en) | 2006-06-22 |
| JP2002511431A (en) | 2002-04-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20080124396A1 (en) | Inhibition of Xenoreactive Antibodies | |
| Ohdan et al. | Mixed chimerism induced without lethal conditioning prevents T cell–and anti-Galα1, 3Gal–mediated graft rejection | |
| US6399578B1 (en) | Conjugates comprising galactose α1,3 galactosyl epitopes and methods of using same | |
| US7498023B2 (en) | Blockade of T cell migration into epithelial GVHD target tissues as an approach to achieving anti-tumor effects against lymphohematopoietic malignancies without GVHD | |
| JPH10511989A (en) | Cell regulatory complex of specific binding pair members | |
| Ohdan et al. | Tolerization of Galα1, 3Gal‐reactive B cells in pre‐sensitized α1, 3‐galactosyltransferase‐deficient mice by nonmyeloablative induction of mixed chimerism | |
| OSORIO et al. | PROLONGATION OF IN VIVO MOUSE ISLET ALLOGRAFT SURVIVAL BY MODULATION OF MHC CLASS I ANTIGENs | |
| US20030165475A1 (en) | Non-lethal methods for conditioning a recipient for bone marrow transplantation | |
| Wu et al. | GENETIC CONTROL OF THE HUMORAL IMMUNE RESPONSE TO XENOGRAFTS: I. Functional Characterization of Rat Monoclonal Antibodies to Hamster Heart Xenografts: 1 | |
| Bach et al. | Xenotransplantation: A current perspective | |
| WO1998033528A9 (en) | Induction of b cell tolerance | |
| US20030017152A1 (en) | Non-lethal methods for conditioning a recipient for bone marrow transplantation | |
| JP2012530106A (en) | HLA-Gα1 multimer and pharmaceutical use thereof | |
| Ohdan et al. | B cell tolerance to xenoantigens | |
| US20020168371A1 (en) | Process for reducing antibody response against xenografts | |
| Rodriguez‐Barbosa et al. | Fetal porcine thymus engraftment, survival and CD4 reconstitution in αGal‐KO mice is impaired in the presence of high levels of antibodies against αGal | |
| EP0847280B1 (en) | Compositions and their uses against hyperacute rejection of xenografts and certain diseases | |
| US20040047865A1 (en) | Compositions and their uses | |
| Bersztel | Modulation of the Immune Response in Concordant Xenotransplantation | |
| AU8936501A (en) | Induction of B cell tolerance |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: MAYO FOUNDATION FOR MEDICAL EDUCATION AND RESEARCH Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:BIOSCIENCE 2002 LLC;REEL/FRAME:014066/0540 Effective date: 20031016 Owner name: BIOSCIENCE 2002 LLC, ILLINOIS Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:NEXTRAN, INC.;REEL/FRAME:014070/0682 Effective date: 20030211 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |