US20080160344A1 - Novel soluble compound and organic electroluminescent devices - Google Patents
Novel soluble compound and organic electroluminescent devices Download PDFInfo
- Publication number
- US20080160344A1 US20080160344A1 US12/068,805 US6880508A US2008160344A1 US 20080160344 A1 US20080160344 A1 US 20080160344A1 US 6880508 A US6880508 A US 6880508A US 2008160344 A1 US2008160344 A1 US 2008160344A1
- Authority
- US
- United States
- Prior art keywords
- group
- carbon atoms
- substituted
- unsubstituted
- groups
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 150000001875 compounds Chemical class 0.000 title abstract description 67
- 239000010410 layer Substances 0.000 claims abstract description 89
- 238000000034 method Methods 0.000 claims abstract description 28
- 238000005401 electroluminescence Methods 0.000 claims abstract description 23
- 239000010409 thin film Substances 0.000 claims abstract description 20
- 239000003960 organic solvent Substances 0.000 claims abstract description 10
- 239000002356 single layer Substances 0.000 claims abstract description 4
- -1 anthracendiyl group Chemical group 0.000 claims description 793
- 125000004432 carbon atom Chemical group C* 0.000 claims description 131
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 claims description 45
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 claims description 33
- 125000004122 cyclic group Chemical group 0.000 claims description 27
- 125000000217 alkyl group Chemical group 0.000 claims description 23
- 125000003118 aryl group Chemical group 0.000 claims description 19
- 239000002904 solvent Substances 0.000 claims description 14
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 12
- 125000003545 alkoxy group Chemical group 0.000 claims description 10
- 125000003710 aryl alkyl group Chemical group 0.000 claims description 9
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims description 9
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 9
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 9
- 125000004430 oxygen atom Chemical group O* 0.000 claims description 8
- 125000001424 substituent group Chemical group 0.000 claims description 8
- 229910052717 sulfur Inorganic materials 0.000 claims description 8
- 125000004434 sulfur atom Chemical group 0.000 claims description 8
- 125000004665 trialkylsilyl group Chemical group 0.000 claims description 8
- SCYULBFZEHDVBN-UHFFFAOYSA-N 1,1-Dichloroethane Chemical compound CC(Cl)Cl SCYULBFZEHDVBN-UHFFFAOYSA-N 0.000 claims description 7
- 125000003277 amino group Chemical group 0.000 claims description 6
- 125000003342 alkenyl group Chemical group 0.000 claims description 5
- 125000004453 alkoxycarbonyl group Chemical group 0.000 claims description 5
- 125000006615 aromatic heterocyclic group Chemical group 0.000 claims description 5
- 125000002029 aromatic hydrocarbon group Chemical group 0.000 claims description 5
- 125000004104 aryloxy group Chemical group 0.000 claims description 5
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 5
- 125000005843 halogen group Chemical group 0.000 claims description 5
- 125000001624 naphthyl group Chemical group 0.000 claims description 5
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 5
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 claims description 4
- 125000003647 acryloyl group Chemical group O=C([*])C([H])=C([H])[H] 0.000 claims description 4
- 125000002252 acyl group Chemical group 0.000 claims description 4
- 125000003282 alkyl amino group Chemical group 0.000 claims description 4
- 125000005103 alkyl silyl group Chemical group 0.000 claims description 4
- 125000004414 alkyl thio group Chemical group 0.000 claims description 4
- 125000002529 biphenylenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C12)* 0.000 claims description 4
- 125000000609 carbazolyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3NC12)* 0.000 claims description 4
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 4
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 claims description 4
- 238000004132 cross linking Methods 0.000 claims description 4
- 125000004663 dialkyl amino group Chemical group 0.000 claims description 4
- 125000003983 fluorenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3CC12)* 0.000 claims description 4
- 125000004446 heteroarylalkyl group Chemical group 0.000 claims description 4
- 125000000623 heterocyclic group Chemical group 0.000 claims description 4
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 4
- 229910052757 nitrogen Inorganic materials 0.000 claims description 4
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 4
- 125000000843 phenylene group Chemical group C1(=C(C=CC=C1)*)* 0.000 claims description 4
- 229920000570 polyether Polymers 0.000 claims description 4
- 125000005504 styryl group Chemical group 0.000 claims description 4
- 239000008096 xylene Substances 0.000 claims description 4
- UGUHFDPGDQDVGX-UHFFFAOYSA-N 1,2,3-thiadiazole Chemical group C1=CSN=N1 UGUHFDPGDQDVGX-UHFFFAOYSA-N 0.000 claims description 3
- VXQBJTKSVGFQOL-UHFFFAOYSA-N 2-(2-butoxyethoxy)ethyl acetate Chemical compound CCCCOCCOCCOC(C)=O VXQBJTKSVGFQOL-UHFFFAOYSA-N 0.000 claims description 3
- FPZWZCWUIYYYBU-UHFFFAOYSA-N 2-(2-ethoxyethoxy)ethyl acetate Chemical compound CCOCCOCCOC(C)=O FPZWZCWUIYYYBU-UHFFFAOYSA-N 0.000 claims description 3
- FCNCGHJSNVOIKE-UHFFFAOYSA-N 9,10-diphenylanthracene Chemical group C1=CC=CC=C1C(C1=CC=CC=C11)=C(C=CC=C2)C2=C1C1=CC=CC=C1 FCNCGHJSNVOIKE-UHFFFAOYSA-N 0.000 claims description 3
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 claims description 3
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical group C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 claims description 3
- 125000004062 acenaphthenyl group Chemical group C1(CC2=CC=CC3=CC=CC1=C23)* 0.000 claims description 3
- 125000005577 anthracene group Chemical group 0.000 claims description 3
- 150000001298 alcohols Chemical class 0.000 claims description 2
- 125000001792 phenanthrenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C=CC12)* 0.000 claims 1
- 125000001425 triazolyl group Chemical group 0.000 claims 1
- 230000001747 exhibiting effect Effects 0.000 abstract description 6
- 239000000243 solution Substances 0.000 description 39
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 30
- 238000006243 chemical reaction Methods 0.000 description 25
- 230000015572 biosynthetic process Effects 0.000 description 24
- 238000003786 synthesis reaction Methods 0.000 description 24
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 21
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 21
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 20
- 239000011369 resultant mixture Substances 0.000 description 20
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 16
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 16
- 239000000463 material Substances 0.000 description 16
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 15
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 15
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 14
- 230000001603 reducing effect Effects 0.000 description 14
- 239000007787 solid Substances 0.000 description 14
- 229910052783 alkali metal Inorganic materials 0.000 description 13
- 239000002019 doping agent Substances 0.000 description 11
- 239000010408 film Substances 0.000 description 11
- 239000012044 organic layer Substances 0.000 description 11
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 10
- 239000007864 aqueous solution Substances 0.000 description 10
- 229910052786 argon Inorganic materials 0.000 description 10
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 10
- 239000000843 powder Substances 0.000 description 10
- 150000001340 alkali metals Chemical class 0.000 description 9
- 239000000047 product Substances 0.000 description 9
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 8
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 8
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 8
- 238000000605 extraction Methods 0.000 description 8
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 8
- 235000019341 magnesium sulphate Nutrition 0.000 description 8
- 229920006395 saturated elastomer Polymers 0.000 description 8
- 239000000758 substrate Substances 0.000 description 8
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 8
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 7
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 7
- 239000000203 mixture Substances 0.000 description 7
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 7
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 7
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 7
- 239000011780 sodium chloride Substances 0.000 description 7
- 239000000126 substance Substances 0.000 description 7
- 125000006083 1-bromoethyl group Chemical group 0.000 description 6
- 125000001478 1-chloroethyl group Chemical group [H]C([H])([H])C([H])(Cl)* 0.000 description 6
- 125000004066 1-hydroxyethyl group Chemical group [H]OC([H])([*])C([H])([H])[H] 0.000 description 6
- 238000005160 1H NMR spectroscopy Methods 0.000 description 6
- 125000000022 2-aminoethyl group Chemical group [H]C([*])([H])C([H])([H])N([H])[H] 0.000 description 6
- 125000005999 2-bromoethyl group Chemical group 0.000 description 6
- 125000001340 2-chloroethyl group Chemical group [H]C([H])(Cl)C([H])([H])* 0.000 description 6
- 125000001731 2-cyanoethyl group Chemical group [H]C([H])(*)C([H])([H])C#N 0.000 description 6
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 6
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 6
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 6
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 6
- 125000004202 aminomethyl group Chemical group [H]N([H])C([H])([H])* 0.000 description 6
- 125000005997 bromomethyl group Chemical group 0.000 description 6
- 229910052792 caesium Inorganic materials 0.000 description 6
- 125000004218 chloromethyl group Chemical group [H]C([H])(Cl)* 0.000 description 6
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 6
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 6
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 6
- 125000003136 n-heptyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 6
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 6
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 6
- 238000004528 spin coating Methods 0.000 description 6
- 238000003756 stirring Methods 0.000 description 6
- 150000001342 alkaline earth metals Chemical class 0.000 description 5
- 229910052782 aluminium Inorganic materials 0.000 description 5
- 238000001914 filtration Methods 0.000 description 5
- 238000010898 silica gel chromatography Methods 0.000 description 5
- RJSOJVMJGWGVKB-UHFFFAOYSA-N 10-[3,5-bis(4-pentylphenyl)phenyl]-9-[4-(2,2-diphenylethenyl)phenyl]-1-phenylanthracene Chemical compound C1=CC(CCCCC)=CC=C1C1=CC(C=2C=CC(CCCCC)=CC=2)=CC(C=2C3=CC=CC(=C3C(C=3C=CC(C=C(C=4C=CC=CC=4)C=4C=CC=CC=4)=CC=3)=C3C=CC=CC3=2)C=2C=CC=CC=2)=C1 RJSOJVMJGWGVKB-UHFFFAOYSA-N 0.000 description 4
- YEJRWHAVMIAJKC-UHFFFAOYSA-N 4-Butyrolactone Chemical compound O=C1CCCO1 YEJRWHAVMIAJKC-UHFFFAOYSA-N 0.000 description 4
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 4
- 239000007818 Grignard reagent Substances 0.000 description 4
- MZRVEZGGRBJDDB-UHFFFAOYSA-N N-Butyllithium Chemical compound [Li]CCCC MZRVEZGGRBJDDB-UHFFFAOYSA-N 0.000 description 4
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 4
- 150000001454 anthracenes Chemical class 0.000 description 4
- 239000013078 crystal Substances 0.000 description 4
- 238000010828 elution Methods 0.000 description 4
- 239000011521 glass Substances 0.000 description 4
- 150000004795 grignard reagents Chemical class 0.000 description 4
- 150000004820 halides Chemical class 0.000 description 4
- 239000005457 ice water Substances 0.000 description 4
- 229910052749 magnesium Inorganic materials 0.000 description 4
- 239000011777 magnesium Substances 0.000 description 4
- 229910052761 rare earth metal Inorganic materials 0.000 description 4
- 150000002910 rare earth metals Chemical class 0.000 description 4
- 239000011541 reaction mixture Substances 0.000 description 4
- 229910052708 sodium Inorganic materials 0.000 description 4
- 239000011734 sodium Substances 0.000 description 4
- HUCFDUOIMSRYAA-UHFFFAOYSA-N 1-bromo-4-(2,2-diphenylethenyl)benzene Chemical group C1=CC(Br)=CC=C1C=C(C=1C=CC=CC=1)C1=CC=CC=C1 HUCFDUOIMSRYAA-UHFFFAOYSA-N 0.000 description 3
- 125000001637 1-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C(*)=C([H])C([H])=C([H])C2=C1[H] 0.000 description 3
- 125000002941 2-furyl group Chemical group O1C([*])=C([H])C([H])=C1[H] 0.000 description 3
- 125000001622 2-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C(*)C([H])=C([H])C2=C1[H] 0.000 description 3
- 125000000389 2-pyrrolyl group Chemical group [H]N1C([*])=C([H])C([H])=C1[H] 0.000 description 3
- 125000000175 2-thienyl group Chemical group S1C([*])=C([H])C([H])=C1[H] 0.000 description 3
- 125000003682 3-furyl group Chemical group O1C([H])=C([*])C([H])=C1[H] 0.000 description 3
- 125000001397 3-pyrrolyl group Chemical group [H]N1C([H])=C([*])C([H])=C1[H] 0.000 description 3
- 125000001541 3-thienyl group Chemical group S1C([H])=C([*])C([H])=C1[H] 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 3
- PCLIMKBDDGJMGD-UHFFFAOYSA-N N-bromosuccinimide Chemical compound BrN1C(=O)CCC1=O PCLIMKBDDGJMGD-UHFFFAOYSA-N 0.000 description 3
- 239000007983 Tris buffer Substances 0.000 description 3
- 229960000583 acetic acid Drugs 0.000 description 3
- 229910001508 alkali metal halide Inorganic materials 0.000 description 3
- 150000008045 alkali metal halides Chemical class 0.000 description 3
- 229910001615 alkaline earth metal halide Inorganic materials 0.000 description 3
- 125000002078 anthracen-1-yl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C3C([*])=C([H])C([H])=C([H])C3=C([H])C2=C1[H] 0.000 description 3
- 125000000748 anthracen-2-yl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C3C([H])=C([*])C([H])=C([H])C3=C([H])C2=C1[H] 0.000 description 3
- 125000004429 atom Chemical group 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
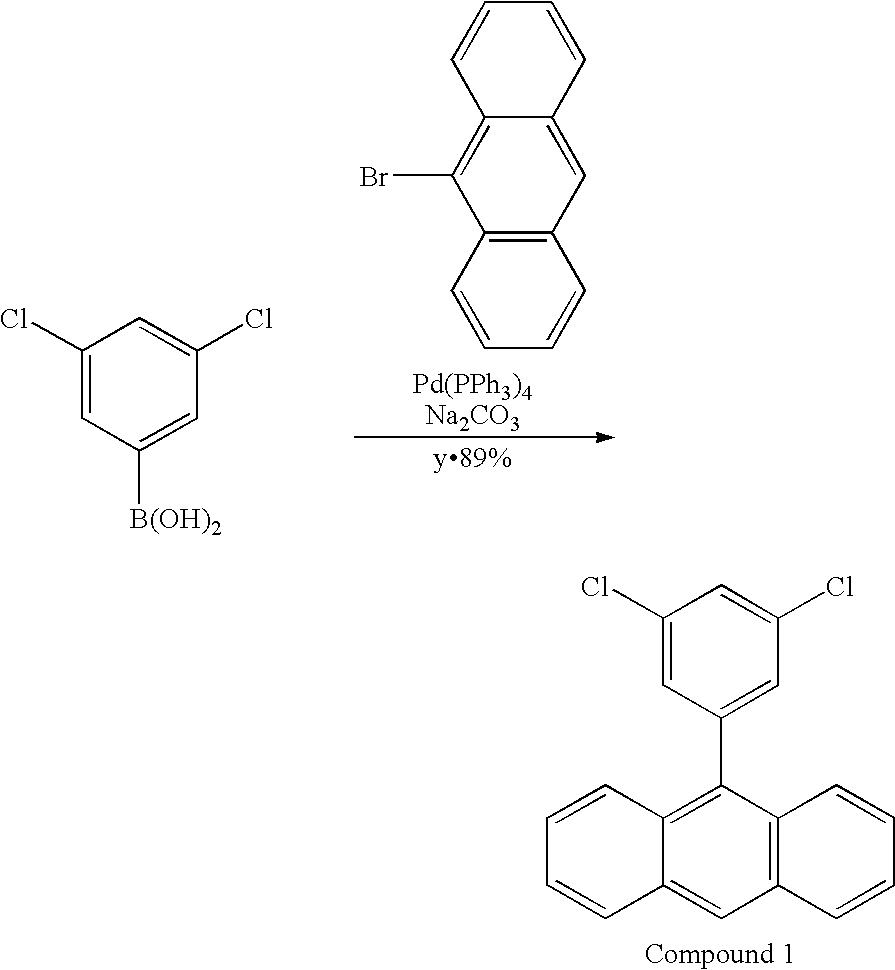
- 229940125904 compound 1 Drugs 0.000 description 3
- 238000004821 distillation Methods 0.000 description 3
- 239000000284 extract Substances 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 150000002484 inorganic compounds Chemical class 0.000 description 3
- 229910010272 inorganic material Inorganic materials 0.000 description 3
- 239000011810 insulating material Substances 0.000 description 3
- 229910052744 lithium Inorganic materials 0.000 description 3
- 125000000040 m-tolyl group Chemical group [H]C1=C([H])C(*)=C([H])C(=C1[H])C([H])([H])[H] 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 125000003261 o-tolyl group Chemical group [H]C1=C([H])C(*)=C(C([H])=C1[H])C([H])([H])[H] 0.000 description 3
- 125000001037 p-tolyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)C([H])([H])[H] 0.000 description 3
- 229910052700 potassium Inorganic materials 0.000 description 3
- NLKNQRATVPKPDG-UHFFFAOYSA-M potassium iodide Chemical compound [K+].[I-] NLKNQRATVPKPDG-UHFFFAOYSA-M 0.000 description 3
- 239000002244 precipitate Substances 0.000 description 3
- 238000010992 reflux Methods 0.000 description 3
- 229910052701 rubidium Inorganic materials 0.000 description 3
- 239000004065 semiconductor Substances 0.000 description 3
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 3
- 235000017557 sodium bicarbonate Nutrition 0.000 description 3
- 238000005019 vapor deposition process Methods 0.000 description 3
- LLKIMPGBYPXOIN-UHFFFAOYSA-N 2,6-bis(2-ethylhexoxy)anthracene Chemical compound C1=C(OCC(CC)CCCC)C=CC2=CC3=CC(OCC(CC)CCCC)=CC=C3C=C21 LLKIMPGBYPXOIN-UHFFFAOYSA-N 0.000 description 2
- QGEXFGIPDGFMTD-UHFFFAOYSA-N 2,6-bis(2-ethylhexoxy)anthracene-9,10-dione Chemical compound CCCCC(CC)COC1=CC=C2C(=O)C3=CC(OCC(CC)CCCC)=CC=C3C(=O)C2=C1 QGEXFGIPDGFMTD-UHFFFAOYSA-N 0.000 description 2
- FCGSOWRQUPAARO-UHFFFAOYSA-N 4-tert-butyl-2-(4-tert-butylbenzoyl)benzoic acid Chemical compound C1=CC(C(C)(C)C)=CC=C1C(=O)C1=CC(C(C)(C)C)=CC=C1C(O)=O FCGSOWRQUPAARO-UHFFFAOYSA-N 0.000 description 2
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 2
- GGFNGYJKGAOMML-UHFFFAOYSA-N 9,10-bis[4-(2,2-diphenylethenyl)phenyl]-2,6-bis(2-ethylhexoxy)anthracene Chemical compound C=12C=C(OCC(CC)CCCC)C=CC2=C(C=2C=CC(C=C(C=3C=CC=CC=3)C=3C=CC=CC=3)=CC=2)C2=CC(OCC(CC)CCCC)=CC=C2C=1C(C=C1)=CC=C1C=C(C=1C=CC=CC=1)C1=CC=CC=C1 GGFNGYJKGAOMML-UHFFFAOYSA-N 0.000 description 2
- OEYAQHPNRYVRMG-UHFFFAOYSA-N 9,10-bis[4-(2,2-diphenylethenyl)phenyl]-2-(2-phenylpropan-2-yl)anthracene-9,10-diol Chemical compound C=1C=C(C(C2=CC=CC=C2C2(C=3C=CC(C=C(C=4C=CC=CC=4)C=4C=CC=CC=4)=CC=3)O)(O)C=3C=CC(C=C(C=4C=CC=CC=4)C=4C=CC=CC=4)=CC=3)C2=CC=1C(C)(C)C1=CC=CC=C1 OEYAQHPNRYVRMG-UHFFFAOYSA-N 0.000 description 2
- ZWGIGJCGPXGYRS-UHFFFAOYSA-N 9,10-dibromo-2,6-bis(2-ethylhexoxy)anthracene Chemical compound C1=C(OCC(CC)CCCC)C=CC2=C(Br)C3=CC(OCC(CC)CCCC)=CC=C3C(Br)=C21 ZWGIGJCGPXGYRS-UHFFFAOYSA-N 0.000 description 2
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 2
- 238000005481 NMR spectroscopy Methods 0.000 description 2
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 2
- NFHFRUOZVGFOOS-UHFFFAOYSA-N Pd(PPh3)4 Substances [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 2
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 2
- 0 [1*]C/C(=C/c1ccc(Cc2ccc(/C=C(\C[3*])C[4*])cc2)cc1)C[2*].[5*]C.[6*]C.[7*]C.[8*]C Chemical compound [1*]C/C(=C/c1ccc(Cc2ccc(/C=C(\C[3*])C[4*])cc2)cc1)C[2*].[5*]C.[6*]C.[7*]C.[8*]C 0.000 description 2
- VSCWAEJMTAWNJL-UHFFFAOYSA-K aluminium trichloride Chemical compound Cl[Al](Cl)Cl VSCWAEJMTAWNJL-UHFFFAOYSA-K 0.000 description 2
- MWPLVEDNUUSJAV-UHFFFAOYSA-N anthracene Natural products C1=CC=CC2=CC3=CC=CC=C3C=C21 MWPLVEDNUUSJAV-UHFFFAOYSA-N 0.000 description 2
- APAJFZPFBHMFQR-UHFFFAOYSA-N anthraflavic acid Chemical compound OC1=CC=C2C(=O)C3=CC(O)=CC=C3C(=O)C2=C1 APAJFZPFBHMFQR-UHFFFAOYSA-N 0.000 description 2
- SIPUZPBQZHNSDW-UHFFFAOYSA-N bis(2-methylpropyl)aluminum Chemical compound CC(C)C[Al]CC(C)C SIPUZPBQZHNSDW-UHFFFAOYSA-N 0.000 description 2
- YNHIGQDRGKUECZ-UHFFFAOYSA-L bis(triphenylphosphine)palladium(ii) dichloride Chemical compound [Cl-].[Cl-].[Pd+2].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 YNHIGQDRGKUECZ-UHFFFAOYSA-L 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 238000004440 column chromatography Methods 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
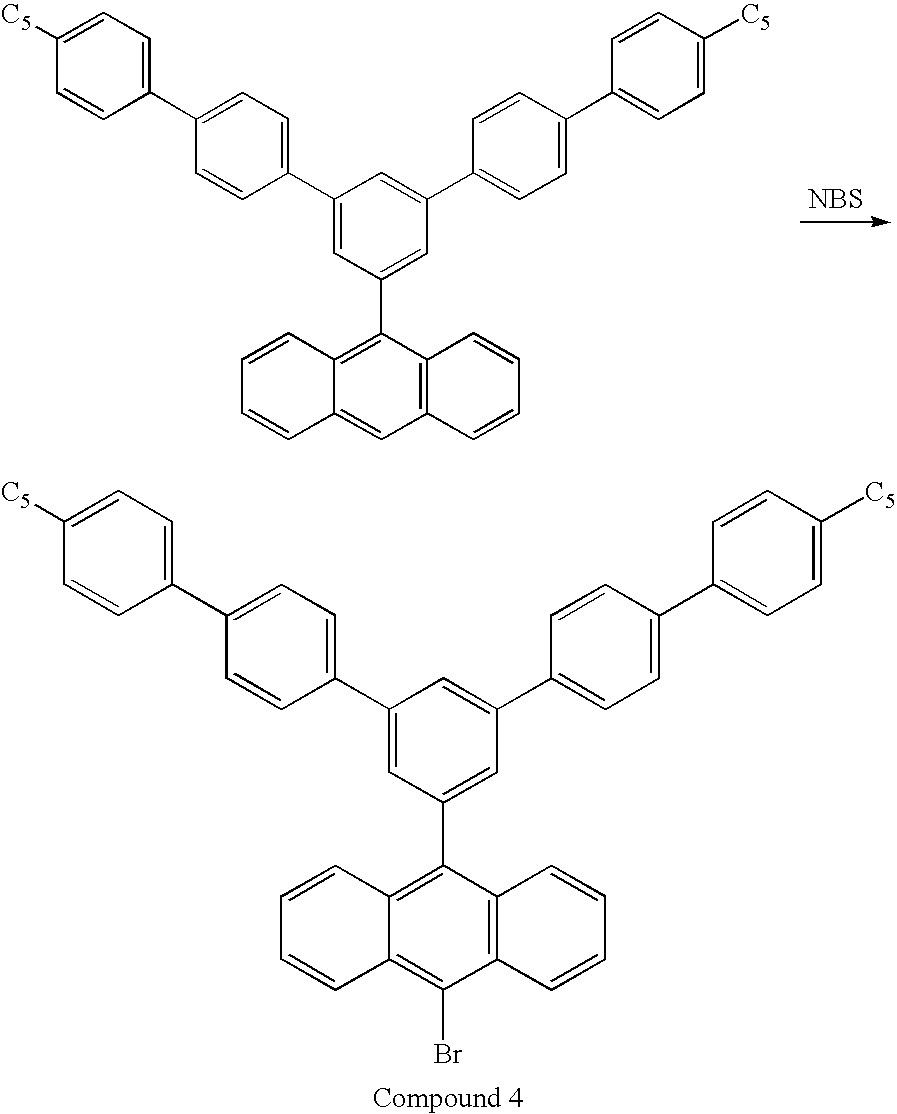
- 229940125782 compound 2 Drugs 0.000 description 2
- 229940126214 compound 3 Drugs 0.000 description 2
- 238000009833 condensation Methods 0.000 description 2
- 230000005494 condensation Effects 0.000 description 2
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 2
- 238000007598 dipping method Methods 0.000 description 2
- 125000004185 ester group Chemical group 0.000 description 2
- 150000002222 fluorine compounds Chemical class 0.000 description 2
- 229910052738 indium Inorganic materials 0.000 description 2
- 229910052740 iodine Inorganic materials 0.000 description 2
- 239000011630 iodine Substances 0.000 description 2
- 239000001989 lithium alloy Substances 0.000 description 2
- KWGKDLIKAYFUFQ-UHFFFAOYSA-M lithium chloride Chemical compound [Li+].[Cl-] KWGKDLIKAYFUFQ-UHFFFAOYSA-M 0.000 description 2
- 238000001451 molecular beam epitaxy Methods 0.000 description 2
- 150000004767 nitrides Chemical class 0.000 description 2
- 150000004866 oxadiazoles Chemical class 0.000 description 2
- 229960003540 oxyquinoline Drugs 0.000 description 2
- 150000002987 phenanthrenes Chemical group 0.000 description 2
- 125000005561 phenanthryl group Chemical group 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 2
- 125000001725 pyrenyl group Chemical group 0.000 description 2
- MCJGNVYPOGVAJF-UHFFFAOYSA-N quinolin-8-ol Chemical compound C1=CN=C2C(O)=CC=CC2=C1 MCJGNVYPOGVAJF-UHFFFAOYSA-N 0.000 description 2
- 238000005215 recombination Methods 0.000 description 2
- 230000006798 recombination Effects 0.000 description 2
- 239000012047 saturated solution Substances 0.000 description 2
- 238000001275 scanning Auger electron spectroscopy Methods 0.000 description 2
- 239000000741 silica gel Substances 0.000 description 2
- 229910002027 silica gel Inorganic materials 0.000 description 2
- 239000012279 sodium borohydride Substances 0.000 description 2
- 229910000033 sodium borohydride Inorganic materials 0.000 description 2
- 229910000029 sodium carbonate Inorganic materials 0.000 description 2
- YTZKOQUCBOVLHL-UHFFFAOYSA-N tert-butylbenzene Chemical compound CC(C)(C)C1=CC=CC=C1 YTZKOQUCBOVLHL-UHFFFAOYSA-N 0.000 description 2
- VZGDMQKNWNREIO-UHFFFAOYSA-N tetrachloromethane Chemical compound ClC(Cl)(Cl)Cl VZGDMQKNWNREIO-UHFFFAOYSA-N 0.000 description 2
- CXWXQJXEFPUFDZ-UHFFFAOYSA-N tetralin Chemical compound C1=CC=C2CCCCC2=C1 CXWXQJXEFPUFDZ-UHFFFAOYSA-N 0.000 description 2
- 150000003852 triazoles Chemical group 0.000 description 2
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 2
- 238000007740 vapor deposition Methods 0.000 description 2
- DKYRKAIKWFHQHM-UHFFFAOYSA-N (3,5-dichlorophenyl)boronic acid Chemical compound OB(O)C1=CC(Cl)=CC(Cl)=C1 DKYRKAIKWFHQHM-UHFFFAOYSA-N 0.000 description 1
- KZPYGQFFRCFCPP-UHFFFAOYSA-N 1,1'-bis(diphenylphosphino)ferrocene Chemical compound [Fe+2].C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1 KZPYGQFFRCFCPP-UHFFFAOYSA-N 0.000 description 1
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 description 1
- CDJVRDWEOHIONR-UHFFFAOYSA-N 1,2-ditert-butylanthracene Chemical compound C1=CC=CC2=CC3=C(C(C)(C)C)C(C(C)(C)C)=CC=C3C=C21 CDJVRDWEOHIONR-UHFFFAOYSA-N 0.000 description 1
- QBUAKMLKDPMPNM-UHFFFAOYSA-N 1,2-ditert-butylanthracene-9,10-dione Chemical compound C1=CC=C2C(=O)C3=C(C(C)(C)C)C(C(C)(C)C)=CC=C3C(=O)C2=C1 QBUAKMLKDPMPNM-UHFFFAOYSA-N 0.000 description 1
- YJTKZCDBKVTVBY-UHFFFAOYSA-N 1,3-Diphenylbenzene Chemical group C1=CC=CC=C1C1=CC=CC(C=2C=CC=CC=2)=C1 YJTKZCDBKVTVBY-UHFFFAOYSA-N 0.000 description 1
- KLCLIOISYBHYDZ-UHFFFAOYSA-N 1,4,4-triphenylbuta-1,3-dienylbenzene Chemical class C=1C=CC=CC=1C(C=1C=CC=CC=1)=CC=C(C=1C=CC=CC=1)C1=CC=CC=C1 KLCLIOISYBHYDZ-UHFFFAOYSA-N 0.000 description 1
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 1
- VXJTWTJULLKDPY-UHFFFAOYSA-N 1-bromo-4-(4-pentylphenyl)benzene Chemical group C1=CC(CCCCC)=CC=C1C1=CC=C(Br)C=C1 VXJTWTJULLKDPY-UHFFFAOYSA-N 0.000 description 1
- 125000004973 1-butenyl group Chemical group C(=CCC)* 0.000 description 1
- XRJRPXQYFXKDQO-UHFFFAOYSA-N 1-n,4-n-diphenyl-1-n,4-n-bis[4-(2-phenylethenyl)phenyl]naphthalene-1,4-diamine Chemical compound C=1C=CC=CC=1C=CC(C=C1)=CC=C1N(C=1C2=CC=CC=C2C(N(C=2C=CC=CC=2)C=2C=CC(C=CC=3C=CC=CC=3)=CC=2)=CC=1)C1=CC=CC=C1 XRJRPXQYFXKDQO-UHFFFAOYSA-N 0.000 description 1
- WWOBNWLAMDMSCT-UHFFFAOYSA-N 1-n,6-n-diphenyl-1-n,6-n-bis[4-(2-phenylethenyl)phenyl]pyrene-1,6-diamine Chemical compound C=1C=CC=CC=1C=CC(C=C1)=CC=C1N(C=1C2=CC=C3C=CC(=C4C=CC(C2=C43)=CC=1)N(C=1C=CC=CC=1)C=1C=CC(C=CC=2C=CC=CC=2)=CC=1)C1=CC=CC=C1 WWOBNWLAMDMSCT-UHFFFAOYSA-N 0.000 description 1
- 125000004343 1-phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 125000001462 1-pyrrolyl group Chemical group [*]N1C([H])=C([H])C([H])=C1[H] 0.000 description 1
- NNMZGTBDLXKEFF-UHFFFAOYSA-N 2,3-dimethyl-1-n,4-n-diphenyl-1-n,4-n-bis[4-(2-phenylethenyl)phenyl]naphthalene-1,4-diamine Chemical compound C=12C=CC=CC2=C(N(C=2C=CC=CC=2)C=2C=CC(C=CC=3C=CC=CC=3)=CC=2)C(C)=C(C)C=1N(C=1C=CC(C=CC=2C=CC=CC=2)=CC=1)C1=CC=CC=C1 NNMZGTBDLXKEFF-UHFFFAOYSA-N 0.000 description 1
- GDNGHUJEIRASIR-UHFFFAOYSA-N 2,7-ditert-butylanthracene-9,10-dione Chemical compound C1=C(C(C)(C)C)C=C2C(=O)C3=CC(C(C)(C)C)=CC=C3C(=O)C2=C1 GDNGHUJEIRASIR-UHFFFAOYSA-N 0.000 description 1
- 125000006280 2-bromobenzyl group Chemical group [H]C1=C([H])C(Br)=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 125000004974 2-butenyl group Chemical group C(C=CC)* 0.000 description 1
- 125000006282 2-chlorobenzyl group Chemical group [H]C1=C([H])C(Cl)=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 125000006290 2-hydroxybenzyl group Chemical group [H]OC1=C(C([H])=C([H])C([H])=C1[H])C([H])([H])* 0.000 description 1
- 125000006481 2-iodobenzyl group Chemical group [H]C1=C([H])C(I)=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 125000000094 2-phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- NZWIYPLSXWYKLH-UHFFFAOYSA-N 3-(bromomethyl)heptane Chemical compound CCCCC(CC)CBr NZWIYPLSXWYKLH-UHFFFAOYSA-N 0.000 description 1
- 125000006279 3-bromobenzyl group Chemical group [H]C1=C([H])C(=C([H])C(Br)=C1[H])C([H])([H])* 0.000 description 1
- 125000004975 3-butenyl group Chemical group C(CC=C)* 0.000 description 1
- 125000003852 3-chlorobenzyl group Chemical group [H]C1=C([H])C(=C([H])C(Cl)=C1[H])C([H])([H])* 0.000 description 1
- 125000006291 3-hydroxybenzyl group Chemical group [H]OC1=C([H])C([H])=C([H])C(=C1[H])C([H])([H])* 0.000 description 1
- 125000006482 3-iodobenzyl group Chemical group [H]C1=C([H])C(=C([H])C(I)=C1[H])C([H])([H])* 0.000 description 1
- KGQKVLGGYJDPTB-UHFFFAOYSA-N 3-n,9-n-diphenyl-3-n,9-n-bis[4-(2-phenylethenyl)phenyl]perylene-3,9-diamine Chemical group C=1C=CC=CC=1C=CC(C=C1)=CC=C1N(C=1C=2C=CC=C3C=4C=CC(=C5C=CC=C(C=45)C(C=23)=CC=1)N(C=1C=CC=CC=1)C=1C=CC(C=CC=2C=CC=CC=2)=CC=1)C1=CC=CC=C1 KGQKVLGGYJDPTB-UHFFFAOYSA-N 0.000 description 1
- BWGRDBSNKQABCB-UHFFFAOYSA-N 4,4-difluoro-N-[3-[3-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-thiophen-2-ylpropyl]cyclohexane-1-carboxamide Chemical compound CC(C)C1=NN=C(C)N1C1CC2CCC(C1)N2CCC(NC(=O)C1CCC(F)(F)CC1)C1=CC=CS1 BWGRDBSNKQABCB-UHFFFAOYSA-N 0.000 description 1
- CMSGUKVDXXTJDQ-UHFFFAOYSA-N 4-(2-naphthalen-1-ylethylamino)-4-oxobutanoic acid Chemical compound C1=CC=C2C(CCNC(=O)CCC(=O)O)=CC=CC2=C1 CMSGUKVDXXTJDQ-UHFFFAOYSA-N 0.000 description 1
- 125000006281 4-bromobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1Br)C([H])([H])* 0.000 description 1
- 125000006283 4-chlorobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1Cl)C([H])([H])* 0.000 description 1
- 125000003143 4-hydroxybenzyl group Chemical group [H]C([*])([H])C1=C([H])C([H])=C(O[H])C([H])=C1[H] 0.000 description 1
- 125000006483 4-iodobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1I)C([H])([H])* 0.000 description 1
- YLJYVKLZVHWUCT-UHFFFAOYSA-N 5-tert-butyl-2-benzofuran-1,3-dione Chemical compound CC(C)(C)C1=CC=C2C(=O)OC(=O)C2=C1 YLJYVKLZVHWUCT-UHFFFAOYSA-N 0.000 description 1
- SDNCQXHPNAHYLN-UHFFFAOYSA-N 9,10-bis[4-(2,2-diphenylethenyl)phenyl]-2-(2-phenylpropan-2-yl)anthracene Chemical compound C=1C=C2C(C=3C=CC(C=C(C=4C=CC=CC=4)C=4C=CC=CC=4)=CC=3)=C3C=CC=CC3=C(C=3C=CC(C=C(C=4C=CC=CC=4)C=4C=CC=CC=4)=CC=3)C2=CC=1C(C)(C)C1=CC=CC=C1 SDNCQXHPNAHYLN-UHFFFAOYSA-N 0.000 description 1
- ZIRVQSRSPDUEOJ-UHFFFAOYSA-N 9-bromoanthracene Chemical compound C1=CC=C2C(Br)=C(C=CC=C3)C3=CC2=C1 ZIRVQSRSPDUEOJ-UHFFFAOYSA-N 0.000 description 1
- ICCDINIBIQZVHR-UHFFFAOYSA-N 9-n,10-n-diphenyl-9-n,10-n-bis[4-(2-phenylethenyl)phenyl]anthracene-9,10-diamine Chemical compound C=1C=CC=CC=1C=CC(C=C1)=CC=C1N(C=1C2=CC=CC=C2C(N(C=2C=CC=CC=2)C=2C=CC(C=CC=3C=CC=CC=3)=CC=2)=C2C=CC=CC2=1)C1=CC=CC=C1 ICCDINIBIQZVHR-UHFFFAOYSA-N 0.000 description 1
- 229910001316 Ag alloy Inorganic materials 0.000 description 1
- 229910000838 Al alloy Inorganic materials 0.000 description 1
- 229910001148 Al-Li alloy Inorganic materials 0.000 description 1
- HOEQBTKATLANQN-UHFFFAOYSA-N B=NS.BrC1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CCCCC(CC)CBr.CCCCC(CC)COC1=CC=C2C(=C1)C(Br)=C1C=CC(OCC(CC)CCCC)=CC1=C2Br.CCCCC(CC)COC1=CC=C2C(=C1)C(C1=CC=C(C=C(C3=CC=CC=C3)C3=CC=CC=C3)C=C1)=C1C=CC(OCC(CC)CCCC)=CC1=C2C1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CCCCC(CC)COC1=CC=C2C(=O)C3=C(C=CC(OCC(CC)CCCC)=C3)C(=O)C2=C1.CCCCC(CC)COC1=CC=C2C=C3C=C(OCC(CC)CCCC)C=CC3=CC2=C1.O=C1C2=CC(O)=CC=C2C(=O)C2=C1C=CC(O)=C2.[SnH2] Chemical compound B=NS.BrC1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CCCCC(CC)CBr.CCCCC(CC)COC1=CC=C2C(=C1)C(Br)=C1C=CC(OCC(CC)CCCC)=CC1=C2Br.CCCCC(CC)COC1=CC=C2C(=C1)C(C1=CC=C(C=C(C3=CC=CC=C3)C3=CC=CC=C3)C=C1)=C1C=CC(OCC(CC)CCCC)=CC1=C2C1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CCCCC(CC)COC1=CC=C2C(=O)C3=C(C=CC(OCC(CC)CCCC)=C3)C(=O)C2=C1.CCCCC(CC)COC1=CC=C2C=C3C=C(OCC(CC)CCCC)C=CC3=CC2=C1.O=C1C2=CC(O)=CC=C2C(=O)C2=C1C=CC(O)=C2.[SnH2] HOEQBTKATLANQN-UHFFFAOYSA-N 0.000 description 1
- TVDNXOJYOBCIJS-UHFFFAOYSA-N B=NS.CC#CC#CC1=CC=C(C2=CC=C(C3=CC(C4=CC=C(C5=CC=C(C)C=C5)C=C4)=CC(C4=C5C=CC=CC5=C(Br)C5=C4C=CC=C5)=C3)C=C2)C=C1.CC#CC#CC1=CC=C(C2=CC=C(C3=CC(C4=CC=C(C5=CC=C(C)C=C5)C=C4)=CC(C4=C5C=CC=CC5=CC5=C4C=CC=C5)=C3)C=C2)C=C1 Chemical compound B=NS.CC#CC#CC1=CC=C(C2=CC=C(C3=CC(C4=CC=C(C5=CC=C(C)C=C5)C=C4)=CC(C4=C5C=CC=CC5=C(Br)C5=C4C=CC=C5)=C3)C=C2)C=C1.CC#CC#CC1=CC=C(C2=CC=C(C3=CC(C4=CC=C(C5=CC=C(C)C=C5)C=C4)=CC(C4=C5C=CC=CC5=CC5=C4C=CC=C5)=C3)C=C2)C=C1 TVDNXOJYOBCIJS-UHFFFAOYSA-N 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N Benzoic acid Natural products OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- HWPHIEZADLXTNE-UHFFFAOYSA-N BrBr.BrC1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CC(C)(C)C1=CC=C(C(=O)C2=C(C(=O)O)C=CC(C(C)(C)C)=C2)C=C1.CC(C)(C)C1=CC=C2C(=C1)C(Br)=C1C=C(C(C)(C)C)C=CC1=C2Br.CC(C)(C)C1=CC=C2C(=C1)C(Br)=C1C=CC(C(C)(C)C)=CC1=C2Br.CC(C)(C)C1=CC=C2C(=C1)C(C1=CC=C(C=C(C3=CC=CC=C3)C3=CC=CC=C3)C=C1)=C1C=C(C(C)(C)C)C=CC1=C2C1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CC(C)(C)C1=CC=C2C(=C1)C(C1=CC=C(C=C(C3=CC=CC=C3)C3=CC=CC=C3)C=C1)=C1C=CC(C(C)(C)C)=CC1=C2C1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CC(C)(C)C1=CC=C2C(=O)C3=C(C=C(C(C)(C)C)C=C3)C(=O)C2=C1.CC(C)(C)C1=CC=C2C(=O)C3=C(C=CC(C(C)(C)C)=C3)C(=O)C2=C1.CC(C)(C)C1=CC=C2C(=O)OC(=O)C2=C1.CC(C)(C)C1=CC=C2C=C3C=C(C(C)(C)C)C=CC3=CC2=C1.CC(C)(C)C1=CC=C2C=C3C=CC(C(C)(C)C)=CC3=CC2=C1.CC(C)(C)C1=CC=CC=C1.[SnH2] Chemical compound BrBr.BrC1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CC(C)(C)C1=CC=C(C(=O)C2=C(C(=O)O)C=CC(C(C)(C)C)=C2)C=C1.CC(C)(C)C1=CC=C2C(=C1)C(Br)=C1C=C(C(C)(C)C)C=CC1=C2Br.CC(C)(C)C1=CC=C2C(=C1)C(Br)=C1C=CC(C(C)(C)C)=CC1=C2Br.CC(C)(C)C1=CC=C2C(=C1)C(C1=CC=C(C=C(C3=CC=CC=C3)C3=CC=CC=C3)C=C1)=C1C=C(C(C)(C)C)C=CC1=C2C1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CC(C)(C)C1=CC=C2C(=C1)C(C1=CC=C(C=C(C3=CC=CC=C3)C3=CC=CC=C3)C=C1)=C1C=CC(C(C)(C)C)=CC1=C2C1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CC(C)(C)C1=CC=C2C(=O)C3=C(C=C(C(C)(C)C)C=C3)C(=O)C2=C1.CC(C)(C)C1=CC=C2C(=O)C3=C(C=CC(C(C)(C)C)=C3)C(=O)C2=C1.CC(C)(C)C1=CC=C2C(=O)OC(=O)C2=C1.CC(C)(C)C1=CC=C2C=C3C=C(C(C)(C)C)C=CC3=CC2=C1.CC(C)(C)C1=CC=C2C=C3C=CC(C(C)(C)C)=CC3=CC2=C1.CC(C)(C)C1=CC=CC=C1.[SnH2] HWPHIEZADLXTNE-UHFFFAOYSA-N 0.000 description 1
- NETOZUWMNDSLJN-UHFFFAOYSA-N BrC1=C2C=CC=CC2=CC2=C1C=CC=C2.ClC1=CC(Cl)=CC(C2=C3C=CC=CC3=CC3=C2C=CC=C3)=C1.OB(O)C1=CC(Cl)=CC(Cl)=C1 Chemical compound BrC1=C2C=CC=CC2=CC2=C1C=CC=C2.ClC1=CC(Cl)=CC(C2=C3C=CC=CC3=CC3=C2C=CC=C3)=C1.OB(O)C1=CC(Cl)=CC(Cl)=C1 NETOZUWMNDSLJN-UHFFFAOYSA-N 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- KAKZBPTYRLMSJV-UHFFFAOYSA-N Butadiene Chemical group C=CC=C KAKZBPTYRLMSJV-UHFFFAOYSA-N 0.000 description 1
- XRJRPXQYFXKDQO-NBHCHVEOSA-N C(=C/c1ccc(N(c2ccccc2)c2ccc(N(c3ccccc3)c3ccc(/C=C/c4ccccc4)cc3)c3ccccc23)cc1)\c1ccccc1 Chemical compound C(=C/c1ccc(N(c2ccccc2)c2ccc(N(c3ccccc3)c3ccc(/C=C/c4ccccc4)cc3)c3ccccc23)cc1)\c1ccccc1 XRJRPXQYFXKDQO-NBHCHVEOSA-N 0.000 description 1
- AOZCBMQBBJPAEG-UHFFFAOYSA-N C.C.C.C.CN(C)CN(C)CN(C)C Chemical compound C.C.C.C.CN(C)CN(C)CN(C)C AOZCBMQBBJPAEG-UHFFFAOYSA-N 0.000 description 1
- AQGOQEUYICMYAT-UHFFFAOYSA-N C.CC#CC#CC1=CC=C(C2=CC=C(C3=CC(C4=CC=C(C5=CC=C(C)C=C5)C=C4)=CC(C4=C5C=CC=CC5=C(Br)C5=C4C=CC=C5)=C3)C=C2)C=C1.CC#CC#CC1=CC=C(C2=CC=C(C3=CC(C4=CC=C(C5=CC=C(C)C=C5)C=C4)=CC(C4=C5C=CC=CC5=C(C5=CC=C(C=C(C6=CC=CC=C6)C6=CC=CC=C6)C=C5)C5=C4C=CC=C5)=C3)C=C2)C=C1 Chemical compound C.CC#CC#CC1=CC=C(C2=CC=C(C3=CC(C4=CC=C(C5=CC=C(C)C=C5)C=C4)=CC(C4=C5C=CC=CC5=C(Br)C5=C4C=CC=C5)=C3)C=C2)C=C1.CC#CC#CC1=CC=C(C2=CC=C(C3=CC(C4=CC=C(C5=CC=C(C)C=C5)C=C4)=CC(C4=C5C=CC=CC5=C(C5=CC=C(C=C(C6=CC=CC=C6)C6=CC=CC=C6)C=C5)C5=C4C=CC=C5)=C3)C=C2)C=C1 AQGOQEUYICMYAT-UHFFFAOYSA-N 0.000 description 1
- SEKYUTKQFDQLAP-UHFFFAOYSA-N CC#CC#CC1=CC=C(C2=CC=C(B(O)O)C=C2)C=C1.CC#CC#CC1=CC=C(C2=CC=C(Br)C=C2)C=C1 Chemical compound CC#CC#CC1=CC=C(C2=CC=C(B(O)O)C=C2)C=C1.CC#CC#CC1=CC=C(C2=CC=C(Br)C=C2)C=C1 SEKYUTKQFDQLAP-UHFFFAOYSA-N 0.000 description 1
- WRSLWFNLACGGFH-UHFFFAOYSA-N CC#CC#CC1=CC=C(C2=CC=C(B(O)O)C=C2)C=C1.CC#CC#CC1=CC=C(C2=CC=C(C3=CC(C4=CC=C(C5=CC=C(C)C=C5)C=C4)=CC(C4=C5C=CC=CC5=CC5=C4C=CC=C5)=C3)C=C2)C=C1.ClC1=CC(Cl)=CC(C2=C3C=CC=CC3=CC3=C2C=CC=C3)=C1 Chemical compound CC#CC#CC1=CC=C(C2=CC=C(B(O)O)C=C2)C=C1.CC#CC#CC1=CC=C(C2=CC=C(C3=CC(C4=CC=C(C5=CC=C(C)C=C5)C=C4)=CC(C4=C5C=CC=CC5=CC5=C4C=CC=C5)=C3)C=C2)C=C1.ClC1=CC(Cl)=CC(C2=C3C=CC=CC3=CC3=C2C=CC=C3)=C1 WRSLWFNLACGGFH-UHFFFAOYSA-N 0.000 description 1
- DUKXPGBHBVSZRQ-UHFFFAOYSA-N CC#CC#CC1=CC=C(C2=CC=C(C3=CC(C4=CC=C(C5=CC=C(C)C=C5)C=C4)=CC(C4=C5C=CC=CC5=C(C5=CC=C(C=C(C6=CC=CC=C6)C6=CC=CC=C6)C=C5)C5=C4C=CC=C5)=C3)C=C2)C=C1 Chemical compound CC#CC#CC1=CC=C(C2=CC=C(C3=CC(C4=CC=C(C5=CC=C(C)C=C5)C=C4)=CC(C4=C5C=CC=CC5=C(C5=CC=C(C=C(C6=CC=CC=C6)C6=CC=CC=C6)C=C5)C5=C4C=CC=C5)=C3)C=C2)C=C1 DUKXPGBHBVSZRQ-UHFFFAOYSA-N 0.000 description 1
- YMHNPAOZAZISBE-UHFFFAOYSA-N CC(C)(C)C1=CC=C2C(=C1)C(C1=CC=C(C=C(C3=CC=CC=C3)C3=CC=CC=C3)C=C1)=C1C=C(C(C)(C)C)C=CC1=C2C1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CC(C)(C)C1=CC=C2C(=C1)C(C1=CC=C(C=C(C3=CC=CC=C3)C3=CC=CC=C3)C=C1)=C1C=CC(C(C)(C)C)=CC1=C2C1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CCCCC(CC)COC1=CC=C2C(=C1)C(C1=CC=C(C=C(C3=CC=CC=C3)C3=CC=CC=C3)C=C1)=C1C=CC(OCC(CC)CCCC)=CC1=C2C1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1 Chemical compound CC(C)(C)C1=CC=C2C(=C1)C(C1=CC=C(C=C(C3=CC=CC=C3)C3=CC=CC=C3)C=C1)=C1C=C(C(C)(C)C)C=CC1=C2C1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CC(C)(C)C1=CC=C2C(=C1)C(C1=CC=C(C=C(C3=CC=CC=C3)C3=CC=CC=C3)C=C1)=C1C=CC(C(C)(C)C)=CC1=C2C1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CCCCC(CC)COC1=CC=C2C(=C1)C(C1=CC=C(C=C(C3=CC=CC=C3)C3=CC=CC=C3)C=C1)=C1C=CC(OCC(CC)CCCC)=CC1=C2C1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1 YMHNPAOZAZISBE-UHFFFAOYSA-N 0.000 description 1
- AVOYNVQZHXWDOE-UHFFFAOYSA-N CC(C)(C1=CC=CC=C1)C1=CC2=C(C=C1)C(C1=CC=C(C=C(C3=CC=CC=C3)C3=CC=CC=C3)C=C1)=C1C=CC=CC1=C2C1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CCCCCCC1(C)c2cc(C)ccc2-c2ccc(-c3ccc4c(c3)C(C)(C)c3cc(-c5ccc6c(c5)C(C)(CCCCCC)c5cc(C=C(c7ccccc7)c7ccccc7)ccc5-6)ccc3-4)cc21.CCCCCCCC1(CCCCCC)c2ccccc2-c2ccc(-c3c4ccccc4c(-c4ccc(C=C(c5ccccc5)c5ccccc5)cc4)c4ccccc34)cc21.CCCCCCc1cc(C=C(c2ccccc2)c2ccccc2)cc2ccc(-c3c4ccccc4c(-c4ccc5cc(C=C(c6ccccc6)c6ccccc6)cc(CCCCC)c5c4)c4ccccc34)cc12 Chemical compound CC(C)(C1=CC=CC=C1)C1=CC2=C(C=C1)C(C1=CC=C(C=C(C3=CC=CC=C3)C3=CC=CC=C3)C=C1)=C1C=CC=CC1=C2C1=CC=C(C=C(C2=CC=CC=C2)C2=CC=CC=C2)C=C1.CCCCCCC1(C)c2cc(C)ccc2-c2ccc(-c3ccc4c(c3)C(C)(C)c3cc(-c5ccc6c(c5)C(C)(CCCCCC)c5cc(C=C(c7ccccc7)c7ccccc7)ccc5-6)ccc3-4)cc21.CCCCCCCC1(CCCCCC)c2ccccc2-c2ccc(-c3c4ccccc4c(-c4ccc(C=C(c5ccccc5)c5ccccc5)cc4)c4ccccc34)cc21.CCCCCCc1cc(C=C(c2ccccc2)c2ccccc2)cc2ccc(-c3c4ccccc4c(-c4ccc5cc(C=C(c6ccccc6)c6ccccc6)cc(CCCCC)c5c4)c4ccccc34)cc12 AVOYNVQZHXWDOE-UHFFFAOYSA-N 0.000 description 1
- LKRYQJJLYMFTIH-UHFFFAOYSA-N CCCC(CC)CC1(CCOCCOCCOC)c2cc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)ccc2-c2ccc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)cc21.CCCCC(CC)COc1ccc2c(-c3ccc(-c4ccccc4)cc3)c3ccccc3c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c2c1.CCCCCCCC1(CCCCCC)c2cc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)ccc2-c2ccc(-c3ccc(-c4ccccc4)c(-c4ccccc4)c3)cc21.CCCCCCc1ccc2c(-c3ccc(-c4ccccc4)cc3)c3ccccc3c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c2c1.COCCOCCOCCC1(C)c2cc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)ccc2-c2ccc(-c3ccc(-c4ccccc4)cc3)cc21 Chemical compound CCCC(CC)CC1(CCOCCOCCOC)c2cc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)ccc2-c2ccc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)cc21.CCCCC(CC)COc1ccc2c(-c3ccc(-c4ccccc4)cc3)c3ccccc3c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c2c1.CCCCCCCC1(CCCCCC)c2cc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)ccc2-c2ccc(-c3ccc(-c4ccccc4)c(-c4ccccc4)c3)cc21.CCCCCCc1ccc2c(-c3ccc(-c4ccccc4)cc3)c3ccccc3c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c2c1.COCCOCCOCCC1(C)c2cc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)ccc2-c2ccc(-c3ccc(-c4ccccc4)cc3)cc21 LKRYQJJLYMFTIH-UHFFFAOYSA-N 0.000 description 1
- GFADKDJYCDDAFJ-UHFFFAOYSA-N CCCCC(C)c1ccc2c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c3ccccc3c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c2c1.CCCCCCC[Si](C)(C)c1ccc2c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c3ccccc3c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c2c1.CCCCCN(CCCCC)c1ccc2c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c3ccccc3c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c2c1 Chemical compound CCCCC(C)c1ccc2c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c3ccccc3c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c2c1.CCCCCCC[Si](C)(C)c1ccc2c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c3ccccc3c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c2c1.CCCCCN(CCCCC)c1ccc2c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c3ccccc3c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c2c1 GFADKDJYCDDAFJ-UHFFFAOYSA-N 0.000 description 1
- ZCTVCTGQWHFRJW-UHFFFAOYSA-N CCCCC(CC)COCc1ccc2c(-c3ccc(-c4ccc(C=C(c5ccccc5)c5ccccc5)cc4)cc3)c3cc(OCC(CC)CCCC)ccc3c(-c3ccccc3)c2c1.CCCCC(CC)COc1cc(-c2ccc(C=C(c3ccccc3)c3ccccc3)cc2)c(C)cc1-c1c2ccccc2c(-c2cc(C)c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)cc2OCC(CC)CCCC)c2ccccc12.CCCCCCCc1cc(-c2ccc3c(c2)C(C)(C)c2cc(-c4ccc(C=C(c5ccccc5)c5ccccc5)c(CCCCCCC)c4)ccc2-3)ccc1C=CC(c1ccccc1)c1ccccc1 Chemical compound CCCCC(CC)COCc1ccc2c(-c3ccc(-c4ccc(C=C(c5ccccc5)c5ccccc5)cc4)cc3)c3cc(OCC(CC)CCCC)ccc3c(-c3ccccc3)c2c1.CCCCC(CC)COc1cc(-c2ccc(C=C(c3ccccc3)c3ccccc3)cc2)c(C)cc1-c1c2ccccc2c(-c2cc(C)c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)cc2OCC(CC)CCCC)c2ccccc12.CCCCCCCc1cc(-c2ccc3c(c2)C(C)(C)c2cc(-c4ccc(C=C(c5ccccc5)c5ccccc5)c(CCCCCCC)c4)ccc2-3)ccc1C=CC(c1ccccc1)c1ccccc1 ZCTVCTGQWHFRJW-UHFFFAOYSA-N 0.000 description 1
- GFQNDROYPIMHON-UHFFFAOYSA-N CCCCC(CC)COc1cc(-c2ccc(C=C(c3ccccc3)c3ccccc3)cc2)c(OC)cc1-c1ccc(C=C(c2ccccc2)c2ccccc2)cc1 Chemical compound CCCCC(CC)COc1cc(-c2ccc(C=C(c3ccccc3)c3ccccc3)cc2)c(OC)cc1-c1ccc(C=C(c2ccccc2)c2ccccc2)cc1 GFQNDROYPIMHON-UHFFFAOYSA-N 0.000 description 1
- HPDNZZCXIUNGPW-UHFFFAOYSA-N CCCCCCC1(C)c2cc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)ccc2-c2ccc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)cc21.CCCCCCCC1(CCCCCC)c2cc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)ccc2-c2ccc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)cc21.N#CCCCCCCCc1ccc2c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c3ccccc3c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c2c1.[C-]#[N+]C(C)(C)CCCCCC1(CCCCCCC)c2cc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)ccc2-c2ccc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)cc21 Chemical compound CCCCCCC1(C)c2cc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)ccc2-c2ccc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)cc21.CCCCCCCC1(CCCCCC)c2cc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)ccc2-c2ccc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)cc21.N#CCCCCCCCc1ccc2c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c3ccccc3c(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c2c1.[C-]#[N+]C(C)(C)CCCCCC1(CCCCCCC)c2cc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)ccc2-c2ccc(-c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)cc21 HPDNZZCXIUNGPW-UHFFFAOYSA-N 0.000 description 1
- CTBYLZZOVAZWLL-UHFFFAOYSA-N CCc1cccc(N(c2ccc(-c3cc(-c4ccc(N(c5cccc(CC)c5)c5cccc(CC)c5)cc4)cc(-c4ccc(N(c5cccc(CC)c5)c5cccc(CC)c5)cc4)c3)cc2)c2cccc(CC)c2)c1 Chemical compound CCc1cccc(N(c2ccc(-c3cc(-c4ccc(N(c5cccc(CC)c5)c5cccc(CC)c5)cc4)cc(-c4ccc(N(c5cccc(CC)c5)c5cccc(CC)c5)cc4)c3)cc2)c2cccc(CC)c2)c1 CTBYLZZOVAZWLL-UHFFFAOYSA-N 0.000 description 1
- GETQZCLCWQTVFV-UHFFFAOYSA-N CN(C)C Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 229910000846 In alloy Inorganic materials 0.000 description 1
- 229910000733 Li alloy Inorganic materials 0.000 description 1
- FUJCRWPEOMXPAD-UHFFFAOYSA-N Li2O Inorganic materials [Li+].[Li+].[O-2] FUJCRWPEOMXPAD-UHFFFAOYSA-N 0.000 description 1
- LFZAGIJXANFPFN-UHFFFAOYSA-N N-[3-[4-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)piperidin-1-yl]-1-thiophen-2-ylpropyl]acetamide Chemical compound C(C)(C)C1=NN=C(N1C1CCN(CC1)CCC(C=1SC=CC=1)NC(C)=O)C LFZAGIJXANFPFN-UHFFFAOYSA-N 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 235000002597 Solanum melongena Nutrition 0.000 description 1
- 244000061458 Solanum melongena Species 0.000 description 1
- 229910052769 Ytterbium Inorganic materials 0.000 description 1
- XCBIWGCZRNSFDL-UHFFFAOYSA-N [4-(2,2-diphenylethenyl)phenoxy]boronic acid Chemical compound C1=CC(OB(O)O)=CC=C1C=C(C=1C=CC=CC=1)C1=CC=CC=C1 XCBIWGCZRNSFDL-UHFFFAOYSA-N 0.000 description 1
- JFBZPFYRPYOZCQ-UHFFFAOYSA-N [Li].[Al] Chemical compound [Li].[Al] JFBZPFYRPYOZCQ-UHFFFAOYSA-N 0.000 description 1
- JHYLKGDXMUDNEO-UHFFFAOYSA-N [Mg].[In] Chemical compound [Mg].[In] JHYLKGDXMUDNEO-UHFFFAOYSA-N 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- SNAAJJQQZSMGQD-UHFFFAOYSA-N aluminum magnesium Chemical compound [Mg].[Al] SNAAJJQQZSMGQD-UHFFFAOYSA-N 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 125000005427 anthranyl group Chemical group 0.000 description 1
- PYKYMHQGRFAEBM-UHFFFAOYSA-N anthraquinone Natural products CCC(=O)c1c(O)c2C(=O)C3C(C=CC=C3O)C(=O)c2cc1CC(=O)OC PYKYMHQGRFAEBM-UHFFFAOYSA-N 0.000 description 1
- 125000005428 anthryl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C3C(*)=C([H])C([H])=C([H])C3=C([H])C2=C1[H] 0.000 description 1
- 229910052787 antimony Inorganic materials 0.000 description 1
- 125000000732 arylene group Chemical group 0.000 description 1
- 229910052788 barium Inorganic materials 0.000 description 1
- 229910001632 barium fluoride Inorganic materials 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- JZKFIPKXQBZXMW-UHFFFAOYSA-L beryllium difluoride Chemical compound F[Be]F JZKFIPKXQBZXMW-UHFFFAOYSA-L 0.000 description 1
- 229910001633 beryllium fluoride Inorganic materials 0.000 description 1
- 125000006267 biphenyl group Chemical group 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- XZCJVWCMJYNSQO-UHFFFAOYSA-N butyl pbd Chemical compound C1=CC(C(C)(C)C)=CC=C1C1=NN=C(C=2C=CC(=CC=2)C=2C=CC=CC=2)O1 XZCJVWCMJYNSQO-UHFFFAOYSA-N 0.000 description 1
- 229910052793 cadmium Inorganic materials 0.000 description 1
- WUKWITHWXAAZEY-UHFFFAOYSA-L calcium difluoride Chemical compound [F-].[F-].[Ca+2] WUKWITHWXAAZEY-UHFFFAOYSA-L 0.000 description 1
- 229910001634 calcium fluoride Inorganic materials 0.000 description 1
- 125000005566 carbazolylene group Chemical group 0.000 description 1
- 235000011089 carbon dioxide Nutrition 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- 239000013522 chelant Substances 0.000 description 1
- DKSKZHBNJNTXCG-UHFFFAOYSA-N chlorobenzene;1,2,3,4-tetrahydronaphthalene Chemical compound ClC1=CC=CC=C1.C1=CC=C2CCCCC2=C1 DKSKZHBNJNTXCG-UHFFFAOYSA-N 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- ZYGHJZDHTFUPRJ-UHFFFAOYSA-N coumarin Chemical class C1=CC=C2OC(=O)C=CC2=C1 ZYGHJZDHTFUPRJ-UHFFFAOYSA-N 0.000 description 1
- 239000012043 crude product Substances 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 238000010908 decantation Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000003795 desorption Methods 0.000 description 1
- XUCJHNOBJLKZNU-UHFFFAOYSA-M dilithium;hydroxide Chemical compound [Li+].[Li+].[OH-] XUCJHNOBJLKZNU-UHFFFAOYSA-M 0.000 description 1
- VDQVEACBQKUUSU-UHFFFAOYSA-M disodium;sulfanide Chemical compound [Na+].[Na+].[SH-] VDQVEACBQKUUSU-UHFFFAOYSA-M 0.000 description 1
- 230000005684 electric field Effects 0.000 description 1
- 125000003914 fluoranthenyl group Chemical group C1(=CC=C2C=CC=C3C4=CC=CC=C4C1=C23)* 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 229910052733 gallium Inorganic materials 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 125000004836 hexamethylene group Chemical group [H]C([H])([*:2])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[*:1] 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 1
- AMGQUBHHOARCQH-UHFFFAOYSA-N indium;oxotin Chemical compound [In].[Sn]=O AMGQUBHHOARCQH-UHFFFAOYSA-N 0.000 description 1
- 125000001041 indolyl group Chemical group 0.000 description 1
- 238000000622 liquid--liquid extraction Methods 0.000 description 1
- 125000003564 m-cyanobenzyl group Chemical group [H]C1=C([H])C(=C([H])C(C#N)=C1[H])C([H])([H])* 0.000 description 1
- 229910001635 magnesium fluoride Inorganic materials 0.000 description 1
- SJCKRGFTWFGHGZ-UHFFFAOYSA-N magnesium silver Chemical compound [Mg].[Ag] SJCKRGFTWFGHGZ-UHFFFAOYSA-N 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 150000002736 metal compounds Chemical class 0.000 description 1
- 125000005394 methallyl group Chemical group 0.000 description 1
- 239000012046 mixed solvent Substances 0.000 description 1
- DCZNSJVFOQPSRV-UHFFFAOYSA-N n,n-diphenyl-4-[4-(n-phenylanilino)phenyl]aniline Chemical class C1=CC=CC=C1N(C=1C=CC(=CC=1)C=1C=CC(=CC=1)N(C=1C=CC=CC=1)C=1C=CC=CC=1)C1=CC=CC=C1 DCZNSJVFOQPSRV-UHFFFAOYSA-N 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004957 naphthylene group Chemical group 0.000 description 1
- 125000006504 o-cyanobenzyl group Chemical group [H]C1=C([H])C(C#N)=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 239000011368 organic material Substances 0.000 description 1
- 125000005565 oxadiazolylene group Chemical group 0.000 description 1
- 125000006505 p-cyanobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1C#N)C([H])([H])* 0.000 description 1
- 125000006503 p-nitrobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1[N+]([O-])=O)C([H])([H])* 0.000 description 1
- AZQWKYJCGOJGHM-UHFFFAOYSA-N para-benzoquinone Natural products O=C1C=CC(=O)C=C1 AZQWKYJCGOJGHM-UHFFFAOYSA-N 0.000 description 1
- 125000004817 pentamethylene group Chemical group [H]C([H])([*:2])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[*:1] 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 125000005562 phenanthrylene group Chemical group 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229920000137 polyphosphoric acid Polymers 0.000 description 1
- 229910000027 potassium carbonate Inorganic materials 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 125000005548 pyrenylene group Chemical group 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- 125000005551 pyridylene group Chemical group 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- LISFMEBWQUVKPJ-UHFFFAOYSA-N quinolin-2-ol Chemical compound C1=CC=C2NC(=O)C=CC2=C1 LISFMEBWQUVKPJ-UHFFFAOYSA-N 0.000 description 1
- ZXZKYYHTWHJHFT-UHFFFAOYSA-N quinoline-2,8-diol Chemical compound C1=CC(=O)NC2=C1C=CC=C2O ZXZKYYHTWHJHFT-UHFFFAOYSA-N 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 125000003808 silyl group Chemical group [H][Si]([H])([H])[*] 0.000 description 1
- KOUDKOMXLMXFKX-UHFFFAOYSA-N sodium oxido(oxo)phosphanium hydrate Chemical compound O.[Na+].[O-][PH+]=O KOUDKOMXLMXFKX-UHFFFAOYSA-N 0.000 description 1
- VPQBLCVGUWPDHV-UHFFFAOYSA-N sodium selenide Chemical compound [Na+].[Na+].[Se-2] VPQBLCVGUWPDHV-UHFFFAOYSA-N 0.000 description 1
- 229910052979 sodium sulfide Inorganic materials 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 229910052712 strontium Inorganic materials 0.000 description 1
- 229910001637 strontium fluoride Inorganic materials 0.000 description 1
- FVRNDBHWWSPNOM-UHFFFAOYSA-L strontium fluoride Chemical compound [F-].[F-].[Sr+2] FVRNDBHWWSPNOM-UHFFFAOYSA-L 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 229940042055 systemic antimycotics triazole derivative Drugs 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- 125000006836 terphenylene group Chemical group 0.000 description 1
- 125000001935 tetracenyl group Chemical group C1(=CC=CC2=CC3=CC4=CC=CC=C4C=C3C=C12)* 0.000 description 1
- 125000000383 tetramethylene group Chemical group [H]C([H])([*:1])C([H])([H])C([H])([H])C([H])([H])[*:2] 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- 125000005730 thiophenylene group Chemical group 0.000 description 1
- 229910001887 tin oxide Inorganic materials 0.000 description 1
- QHGNHLZPVBIIPX-UHFFFAOYSA-N tin(ii) oxide Chemical class [Sn]=O QHGNHLZPVBIIPX-UHFFFAOYSA-N 0.000 description 1
- 238000011282 treatment Methods 0.000 description 1
- TVIVIEFSHFOWTE-UHFFFAOYSA-K tri(quinolin-8-yloxy)alumane Chemical compound [Al+3].C1=CN=C2C([O-])=CC=CC2=C1.C1=CN=C2C([O-])=CC=CC2=C1.C1=CN=C2C([O-])=CC=CC2=C1 TVIVIEFSHFOWTE-UHFFFAOYSA-K 0.000 description 1
- 125000002306 tributylsilyl group Chemical group C(CCC)[Si](CCCC)(CCCC)* 0.000 description 1
- 125000000026 trimethylsilyl group Chemical group [H]C([H])([H])[Si]([*])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 229910000404 tripotassium phosphate Inorganic materials 0.000 description 1
- 235000019798 tripotassium phosphate Nutrition 0.000 description 1
- NHDIQVFFNDKAQU-UHFFFAOYSA-N tripropan-2-yl borate Chemical compound CC(C)OB(OC(C)C)OC(C)C NHDIQVFFNDKAQU-UHFFFAOYSA-N 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
Classifications
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05B—ELECTRIC HEATING; ELECTRIC LIGHT SOURCES NOT OTHERWISE PROVIDED FOR; CIRCUIT ARRANGEMENTS FOR ELECTRIC LIGHT SOURCES, IN GENERAL
- H05B33/00—Electroluminescent light sources
- H05B33/12—Light sources with substantially two-dimensional radiating surfaces
- H05B33/14—Light sources with substantially two-dimensional radiating surfaces characterised by the chemical or physical composition or the arrangement of the electroluminescent material, or by the simultaneous addition of the electroluminescent material in or onto the light source
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C13/00—Cyclic hydrocarbons containing rings other than, or in addition to, six-membered aromatic rings
- C07C13/28—Polycyclic hydrocarbons or acyclic hydrocarbon derivatives thereof
- C07C13/32—Polycyclic hydrocarbons or acyclic hydrocarbon derivatives thereof with condensed rings
- C07C13/54—Polycyclic hydrocarbons or acyclic hydrocarbon derivatives thereof with condensed rings with three condensed rings
- C07C13/547—Polycyclic hydrocarbons or acyclic hydrocarbon derivatives thereof with condensed rings with three condensed rings at least one ring not being six-membered, the other rings being at the most six-membered
- C07C13/567—Polycyclic hydrocarbons or acyclic hydrocarbon derivatives thereof with condensed rings with three condensed rings at least one ring not being six-membered, the other rings being at the most six-membered with a fluorene or hydrogenated fluorene ring system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C15/00—Cyclic hydrocarbons containing only six-membered aromatic rings as cyclic parts
- C07C15/40—Cyclic hydrocarbons containing only six-membered aromatic rings as cyclic parts substituted by unsaturated carbon radicals
- C07C15/50—Cyclic hydrocarbons containing only six-membered aromatic rings as cyclic parts substituted by unsaturated carbon radicals polycyclic non-condensed
- C07C15/52—Cyclic hydrocarbons containing only six-membered aromatic rings as cyclic parts substituted by unsaturated carbon radicals polycyclic non-condensed containing a group with formula
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C15/00—Cyclic hydrocarbons containing only six-membered aromatic rings as cyclic parts
- C07C15/40—Cyclic hydrocarbons containing only six-membered aromatic rings as cyclic parts substituted by unsaturated carbon radicals
- C07C15/56—Cyclic hydrocarbons containing only six-membered aromatic rings as cyclic parts substituted by unsaturated carbon radicals polycyclic condensed
- C07C15/58—Cyclic hydrocarbons containing only six-membered aromatic rings as cyclic parts substituted by unsaturated carbon radicals polycyclic condensed containing two rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C15/00—Cyclic hydrocarbons containing only six-membered aromatic rings as cyclic parts
- C07C15/40—Cyclic hydrocarbons containing only six-membered aromatic rings as cyclic parts substituted by unsaturated carbon radicals
- C07C15/56—Cyclic hydrocarbons containing only six-membered aromatic rings as cyclic parts substituted by unsaturated carbon radicals polycyclic condensed
- C07C15/60—Cyclic hydrocarbons containing only six-membered aromatic rings as cyclic parts substituted by unsaturated carbon radicals polycyclic condensed containing three rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C211/00—Compounds containing amino groups bound to a carbon skeleton
- C07C211/43—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton
- C07C211/57—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings being part of condensed ring systems of the carbon skeleton
- C07C211/61—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings being part of condensed ring systems of the carbon skeleton with at least one of the condensed ring systems formed by three or more rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C43/00—Ethers; Compounds having groups, groups or groups
- C07C43/02—Ethers
- C07C43/03—Ethers having all ether-oxygen atoms bound to acyclic carbon atoms
- C07C43/14—Unsaturated ethers
- C07C43/164—Unsaturated ethers containing six-membered aromatic rings
- C07C43/168—Unsaturated ethers containing six-membered aromatic rings containing six-membered aromatic rings and other rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C43/00—Ethers; Compounds having groups, groups or groups
- C07C43/02—Ethers
- C07C43/20—Ethers having an ether-oxygen atom bound to a carbon atom of a six-membered aromatic ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C43/00—Ethers; Compounds having groups, groups or groups
- C07C43/02—Ethers
- C07C43/20—Ethers having an ether-oxygen atom bound to a carbon atom of a six-membered aromatic ring
- C07C43/215—Ethers having an ether-oxygen atom bound to a carbon atom of a six-membered aromatic ring having unsaturation outside the six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G61/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G61/02—Macromolecular compounds containing only carbon atoms in the main chain of the macromolecule, e.g. polyxylylenes
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/111—Organic polymers or oligomers comprising aromatic, heteroaromatic, or aryl chains, e.g. polyaniline, polyphenylene or polyphenylene vinylene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
- H10K85/626—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene containing more than one polycyclic condensed aromatic rings, e.g. bis-anthracene
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2603/00—Systems containing at least three condensed rings
- C07C2603/02—Ortho- or ortho- and peri-condensed systems
- C07C2603/04—Ortho- or ortho- and peri-condensed systems containing three rings
- C07C2603/06—Ortho- or ortho- and peri-condensed systems containing three rings containing at least one ring with less than six ring members
- C07C2603/10—Ortho- or ortho- and peri-condensed systems containing three rings containing at least one ring with less than six ring members containing five-membered rings
- C07C2603/12—Ortho- or ortho- and peri-condensed systems containing three rings containing at least one ring with less than six ring members containing five-membered rings only one five-membered ring
- C07C2603/18—Fluorenes; Hydrogenated fluorenes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2603/00—Systems containing at least three condensed rings
- C07C2603/02—Ortho- or ortho- and peri-condensed systems
- C07C2603/04—Ortho- or ortho- and peri-condensed systems containing three rings
- C07C2603/22—Ortho- or ortho- and peri-condensed systems containing three rings containing only six-membered rings
- C07C2603/24—Anthracenes; Hydrogenated anthracenes
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1007—Non-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1011—Condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1014—Carbocyclic compounds bridged by heteroatoms, e.g. N, P, Si or B
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K2102/00—Constructional details relating to the organic devices covered by this subclass
- H10K2102/10—Transparent electrodes, e.g. using graphene
- H10K2102/101—Transparent electrodes, e.g. using graphene comprising transparent conductive oxides [TCO]
- H10K2102/103—Transparent electrodes, e.g. using graphene comprising transparent conductive oxides [TCO] comprising indium oxides, e.g. ITO
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K71/00—Manufacture or treatment specially adapted for the organic devices covered by this subclass
- H10K71/10—Deposition of organic active material
- H10K71/12—Deposition of organic active material using liquid deposition, e.g. spin coating
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/321—Metal complexes comprising a group IIIA element, e.g. Tris (8-hydroxyquinoline) gallium [Gaq3]
- H10K85/324—Metal complexes comprising a group IIIA element, e.g. Tris (8-hydroxyquinoline) gallium [Gaq3] comprising aluminium, e.g. Alq3
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
- H10K85/633—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine comprising polycyclic condensed aromatic hydrocarbons as substituents on the nitrogen atom
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S428/00—Stock material or miscellaneous articles
- Y10S428/917—Electroluminescent
Definitions
- the present invention relates to a novel soluble compound and organic electroluminescence devices and, more particularly, to a novel soluble compound which can be formed into an organic thin film layer in accordance with a wet process and enables producing electroluminescence devices exhibiting a great efficiency of light emission easily and an electroluminescence device utilizing the compound.
- An organic electroluminescence (electroluminescence will be referred to as EL, hereinafter) is a spontaneous light emitting device which utilizes the principle that a fluorescent substance emits light by energy of recombination of holes injected from an anode and electrons injected from a cathode when an electric field is applied.
- the efficiency of hole injection into the light emitting layer can be increased, that the efficiency of forming excitons which are formed by blocking and recombining electrons injected from the cathode can be increased, and that excitons formed within the light emitting layer can be enclosed.
- a two-layered structure having a hole transporting (injecting) layer and an electron transporting and light emitting layer and a three-layered structure having a hole transporting (injecting) layer, a light emitting layer and an electron transporting (injecting) layer are well known.
- the structure of the device and the process for forming the device have been studied.
- chelate complexes such as tris(8-quinolinolato)aluminum, coumarine derivatives, tetraphenyl-butadiene derivatives, bisstyrylarylene derivatives and oxadiazole derivatives are known. It is reported that light in the visible region ranging from blue light to red light can be obtained by using these light emitting materials, and development of a device exhibiting color images is expected (For example, Japanese Patent Application Laid-Open Nos. Heisei 8 (1996)-239655, Heisei 7 (1995)-138561 and Heisei 3 (1991)-200289).
- the present invention has been made to overcome the above drawbacks and has an object of providing a novel soluble compound which can be formed into an organic thin film layer in accordance with a wet process and enables producing electroluminescence devices exhibiting a great efficiency of light emission easily and an electroluminescence device utilizing the compound.
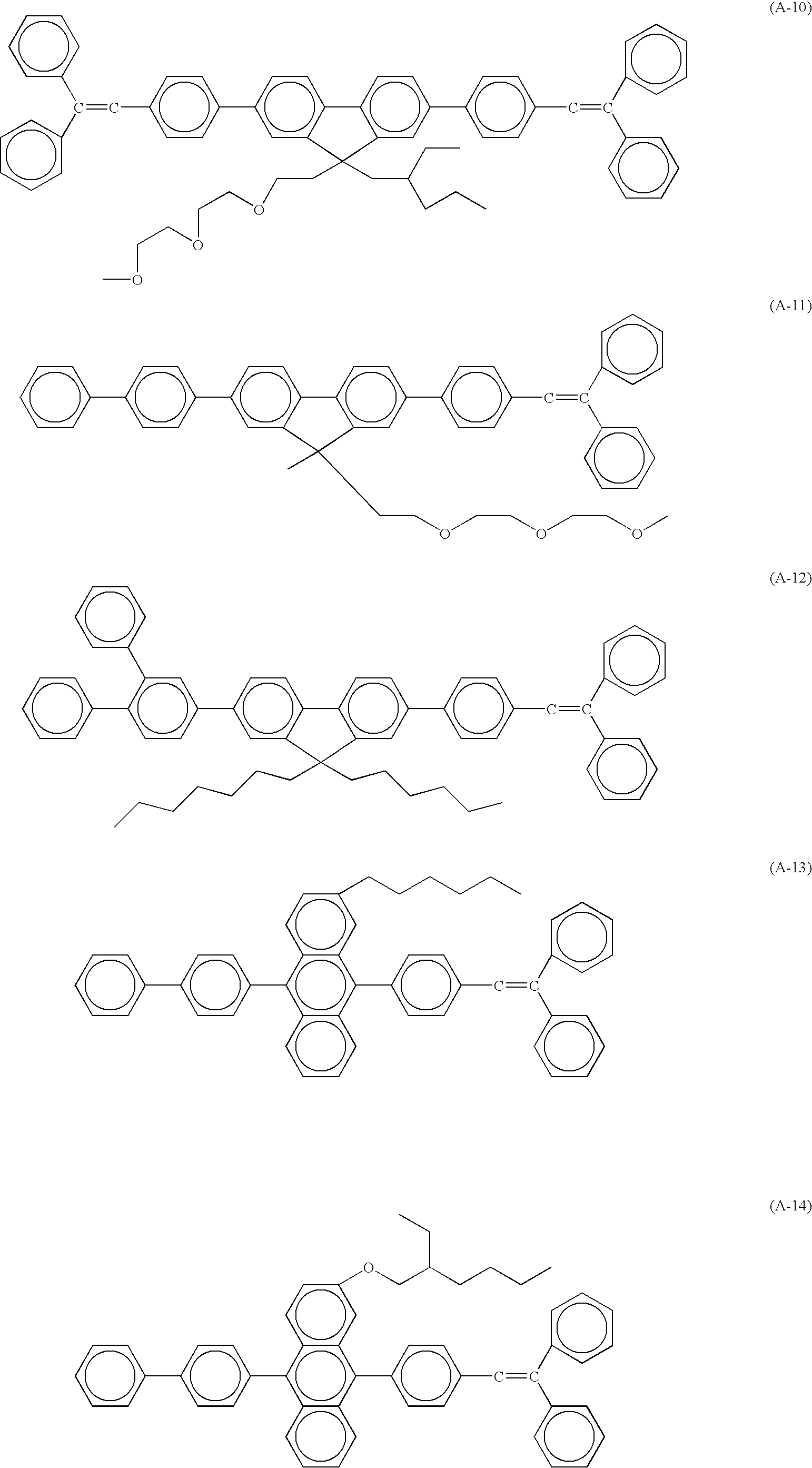
- the present invention provides a novel soluble compound which is a distyrylarylene derivative represented by general formula (1) and has a solubility (20° C.) of 0.5% by weight or greater in an organic solvent, general formula (1) being:
- Ar 1 , Ar 2 , Ar 4 and Ar 5 each independently represent a substituted or unsubstituted phenylene group, a substituted or unsubstituted naphthalene group, a substituted or unsubstituted anthracene group, a substituted or unsubstituted diphenylanthracene group, a substituted or unsubstituted phenanthrene group, a substituted or unsubstituted acenaphthene group, a substituted or unsubstituted biphenylene group, a substituted or unsubstituted fluorene group, a substituted or unsubstituted carbazolyl group, a substituted or unsubstituted thiophene group, a substituted or unsubstituted triazole group or a substituted or unsubstituted thiadiazole group;
- R 1 to R 4 each independently represent hydrogen atom, an alkyl group having 1 to 20 carbon atoms, an alkoxyl group having 1 to 20 carbon atoms, an aryl group having 6 to 20 carbon atoms, a trialkylsilyl group having 3 to 20 carbon atoms or cyano group;
- Ar 3 represents a substituted or unsubstituted anthracendiyl group or a substituted or unsubstituted fluorendiyl group;
- R 5 to R 8 each independently represent hydrogen atom, a halogen atom, hydroxyl group, a substituted or unsubstituted amino group, nitro group, cyano group, a substituted or unsubstituted alkyl group having 1 to 30 carbon atoms, a substituted or unsubstituted alkenyl group having 2 to 30 carbon atoms, a substituted or unsubstituted cycloalkyl group having 5 to 30 carbon atoms, a substituted or unsubstituted alkoxyl group having 1 to 30 carbon atoms, a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, a substituted or unsubstituted aromatic heterocyclic group having 2 to 30 carbon atoms, a substituted or unsubstituted aralkyl group having 7 to 30 carbon atoms, a substituted or unsubstituted aryloxyl group having 6 to 30 carbon atoms, a substituted
- p 0 or 1
- q 0 or 1
- m 0 or an integer of 1 to 3
- n represents an integer of 1 to 3.
- the present invention also provides an organic electroluminescence device which comprises a cathode, an anode and an organic thin film layer comprising a single layer or a plurality of layers and disposed between the cathode and the anode, wherein at least one layer in the organic thin film layer comprises a novel soluble compound described above.
- the soluble compound is a compound which has a soluble substituent, is represented by general formula (1) shown above and has a solubility (20° C.) of 0.5% by weight or greater in an organic solvent. It is sufficient that at least one organic solvent which dissolves 0.5% by weight or more of the novel soluble compound at 20° C. exists.
- the organic solvent is at least one solvent selected from toluene, xylene, N-methylpyrrolidone, ⁇ -butyrolactone, 1,3-dimethyl-2-imidazoline, carbitol acetate, butylcarbitol acetate, dichloromethane, dichloroethane, chlorobenzene tetralin and alcohols having 1 to 10 carbon atoms, and more preferably dichloroethane, toluene, xylene or tetraline.
- solvents selected from toluene, xylene, N-methylpyrrolidone, ⁇ -butyrolactone, 1,3-dimethyl-2-imidazoline, carbitol acetate, butylcarbitol acetate, dichloromethane, dichloroethane, chlorobenzene tetralin and alcohols having 1 to 10 carbon atoms, and more preferably dichloroethane, toluene,
- Ar 1 , Ar 2 , Ar 4 and Ar 5 each independently represent a substituted or unsubstituted phenylene group, a substituted or unsubstituted naphthalene group, a substituted or unsubstituted anthracene group, a substituted or unsubstituted diphenylanthracene group, a substituted or unsubstituted phenanthrene group, a substituted or unsubstituted acenaphthene group, a substituted or unsubstituted biphenylene group, a substituted or unsubstituted fluorene group, a substituted or unsubstituted carbazolyl group, a substituted or unsubstituted thiophene group, a substituted or unsubstituted triazole group or a substituted or unsubstituted thiadiazole group.
- R 1 to R 4 each independently represent hydrogen atom, an alkyl group having 1 to 20 carbon atoms, an alkoxyl group having 1 to 20 carbon atoms, an aryl group having 6 to 20 carbon atoms, a trialkylsilyl group having 3 to 20 carbon atoms or cyano group.
- alkyl group having 1 to 20 carbon atoms examples include methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, n-pentyl group, n-hexyl group, n-heptyl group, n-octyl group, hydroxymethyl group, 1-hydroxyethyl group, 2-hydroxyethyl group, 2-hydroxyisobutyl group, 1,2-dihydroxyethyl group, 1,3-dihydroxyisopropyl group, 2,3-dihydroxy-t-butyl group, 1,2,3-trihydroxypropyl group, chloromethyl group, 1-chloroethyl group, 2-chloroethyl group, 2-chloroisobutyl group, 1,2-dichloroethyl group, 1,3-dichloroisopropyl group, 2,3-dichloro
- the alkoxyl group having 1 to 20 carbon atoms is a group represented by —OY.
- Examples of the group represented by Y include methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, n-pentyl group, n-hexyl group, n-heptyl group, n-octyl group, hydroxymethyl group, 1-hydroxyethyl group, 2-hydroxyethyl group, 2-hydroxyisobutyl group, 1,2-dihydroxyethyl group, 1,3-dihydroxyisopropyl group, 2,3-dihydroxy-t-butyl group, 1,2,3-trihydroxypropyl group, chloromethyl group, 1-chloroethyl group, 2-chloroethyl group, 2-chloroisobutyl group, 1,2-dichloroethyl group,
- Examples of the aryl group having 6 to 20 carbon atoms include phenyl group, naphthyl group, anthryl group, phenanthryl group, naphthacenyl group and pyrenyl group.
- Examples of the substituent to the aryl group include halogen atoms, hydroxyl group, substituted and unsubstituted amino groups described above, nitro group, cyano group, substituted and unsubstituted alkyl groups described above, substituted and unsubstituted alkenyl groups described above, substituted and unsubstituted cycloalkyl groups described above, substituted and unsubstituted alkoxyl groups described above, substituted and unsubstituted aromatic hydrocarbon groups described above, substituted and unsubstituted aromatic heterocyclic groups described above, substituted and unsubstituted aralkyl groups described above, substituted and unsubstituted aryloxyl groups described above, substituted and unsubstituted al
- trialkylsilyl group having 3 to 20 carbon atoms in examples include trimethylsilyl group, triethylsilyl group, tripropylsilyl group, tributylsilyl group, tripentyl silyl group and trihexylsilyl group.
- Ar 3 represents a substituted or unsubstituted anthracendiyl group or a substituted or unsubstituted fluorendiyl group.
- Ar 3 represents:
- Ar 3 represents anthracendiyl group substituted with at least two substituted or unsubstituted t-butyl groups.
- R 5 to R 8 each independently represent hydrogen atom, a halogen atom, hydroxyl group, a substituted or unsubstituted amino group, nitro group, cyano group, a substituted or unsubstituted alkyl group having 1 to 30 carbon atoms, a substituted or unsubstituted alkenyl group having 2 to 30 carbon atoms, a substituted or unsubstituted cycloalkyl group having 5 to 30 carbon atoms, a substituted or unsubstituted alkoxyl group having 1 to 30 carbon atoms, a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, a substituted or unsubstituted aromatic heterocyclic group having 2 to 30 carbon atoms, a substituted or unsubstituted aralkyl group having 7 to 30 carbon atoms, a substituted or unsubstituted aryloxyl group having 6 to 30 carbon atom
- the amino group is represented by —NX 1 X 2 .
- X 1 and X 2 each independently represent hydrogen atom, methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, n-pentyl group, n-hexyl group, n-heptyl group, n-octyl group, hydroxymethyl group, 1-hydroxyethyl group, 2-hydroxyethyl group, 2-hydroxyisobutyl group, 1,2-dihydroxyethyl group, 1,3-dihydroxy-isopropyl group, 2,3-dihydroxy-t-butyl group, 1,2,3-trihydroxypropyl group, chloromethyl group, 1-chloroethyl group, 2-chloroethyl group, 2-chloroisobutyl group, 1,2-dichloroethyl group, 1,3-
- alkyl group having 1 to 30 carbon atoms examples include methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, n-pentyl group, n-hexyl group, n-heptyl group, n-octyl group, hydroxymethyl group, 1-hydroxyethyl group, 2-hydroxyethyl group, 2-hydroxyisobutyl group, 1,2-dihydroxyethyl group, 1,3-dihydroxyisopropyl group, 2,3-dihydroxy-t-butyl group, 1,2,3-trihydroxypropyl group, chloromethyl group, 1-chloroethyl group, 2-chloroethyl group, 2-chloroisobutyl group, 1,2-dichloroethyl group, 1,3-dichloroisopropyl group, 2,3-dichloro
- alkenyl group having 2 to 30 carbon atoms examples include vinyl group, allyl group, 1-butenyl group, 2-butenyl group, 3-butenyl group, 1,3-butadienyl group, 1-methylvinyl group, styryl group, 2,2-diphenylvinyl group, 1,2-diphenylvinyl group, 1-methylallyl group, 1,1-dimethylallyl group, 2-methylallyl group, 1-phenylallyl group, 2-phenylallyl group, 3-phenylallyl group, 3,3-diphenylallyl group, 1,2-dimethylallyl group, 1-phenyl-1-butenyl group and 3-phenyl-1-butenyl group.
- Examples of the cycloalkyl group having 5 to 30 carbon atoms include cyclopropyl group, cyclobutyl group, cyclopentyl group, cyclohexyl group and 4-methylcyclohexyl group.
- the alkoxyl group having 1 to 30 carbon atoms is represented by —OY.
- Examples of the group represented by Y include methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, n-pentyl group, n-hexyl group, n-heptyl group, n-octyl group, hydroxymethyl group, 1-hydroxyethyl group, 2-hydroxyethyl group, 2-hydroxyisobutyl group, 1,2-dihydroxyethyl group, 1,3-dihydroxy-isopropyl group, 2,3-dihydroxy-t-butyl group, 1,2,3-trihydroxypropyl group, chloromethyl group, 1-chloroethyl group, 2-chloroethyl group, 2-chloroisobutyl group, 1,2-dichloroethyl group, 1,3
- aromatic hydrocarbon group having 6 to 30 carbon atoms examples include phenyl group, 1-naphthyl group, 2-naphthyl group, 1-anthryl group, 2-anthryl group, 9-anthryl group, 1-phenanthryl group, 2-phenanthryl group, 3-phenanthryl group, 4-phenanthryl group, 9-phenanthryl group, 1-naphthacenyl group, 2-naphthacenyl group, 9-naphthacenyl group, 1-pyrenyl group, 2-pyrenyl group, 4-pyrenyl group, 2-biphenylyl group, 3-biphenylyl group, 4-biphenylyl group, p-terphenyl-4-yl group, p-terphenyl-3-yl group, p-terphenyl-2-yl group, m-terphenyl-4-yl group, m-terphenyl-3-yl group, m-terphenyl-2-
- aromatic heterocyclic group having 2 to 30 carbon atoms examples include 1-pyrrolyl group, 2-pyrrolyl group, 3-pyrrolyl group, pyradinyl group, 2-pyridinyl group, 3-pyridinyl group, 4-pyridinyl group, 1-indolyl group, 2-indolyl group, 3-indolyl group, 4-indolyl group, 5-indolyl group, 6-indolyl group, 7-indolyl group, 1-isoindolyl group, 2-isoindolyl group, 3-isoindolyl group, 4-isoindolyl group, 5-isoindolyl group, 6-isoindolyl group, 7-isoindolyl group, 2-furyl group, 3-furyl group, 2-benzofuranyl group, 3-benzofuranyl group, 4-benzofuranyl group, 5-benzofuranyl group, 6-benzofuranyl group, 7-benzofurany
- Examples of the aralkyl group having 7 to 30 carbon atoms include benzyl group, 1-phenylethyl group, 2-phenylethyl group, 1-phenyl-isopropyl group, 2-phenylisopropyl group, phenyl-t-butyl group, ⁇ -naphthylmethyl group, 1- ⁇ -naphthylethyl group, 2- ⁇ -naphthylethyl group, 1- ⁇ -naphthylisopropyl group, 2- ⁇ -naphthylisopropyl group, ⁇ -naphthylmethyl group, 1- ⁇ -naphthylethyl group, 2- ⁇ -naphthylethyl group, 1- ⁇ -naphthylisopropyl group, 2- ⁇ -naphthylisopropyl group, 1-pyrrolylmethyl group, 2-(1-pyrrolyl)ethyl group,
- the aryloxyl group having 6 to 30 carbon atoms is represented by —OZ.
- Examples of the group represented by Z include phenyl group, 1-naphthyl group, 2-naphthyl group, 1-anthryl group, 2-anthryl group, 9-anthryl group, 1-phenanthryl group, 2-phenanthryl group, 3-phenanthryl group, 4-phenanthryl group, 9-phenanthryl group, 1-naphthacenyl group, 2-naphthacenyl group, 9-naphthacenyl group, 1-pyrenyl group, 2-pyrenyl group, 4-pyrenyl group, 2-biphenylyl group, 3-biphenylyl group, 4-biphenylyl group, p-terphenyl-4-yl group, p-terphenyl-3-yl group, p-terphenyl-2-yl group, m-terphenyl-4-yl group, m-
- the alkoxycarbonyl group having 2 to 30 carbon atoms is represented by —COOY.
- Examples of the group represented by Y include methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, n-pentyl group, n-hexyl group, n-heptyl group, n-octyl group, hydroxymethyl group, 1-hydroxyethyl group, 2-hydroxyethyl group, 2-hydroxyisobutyl group, 1,2-dihydroxyethyl group, 1,3-dihydroxy-isopropyl group, 2,3-dihydroxy-t-butyl group, 1,2,3-trihydroxypropyl group, chloromethyl group, 1-chloroethyl group, 2-chloroethyl group, 2-chloroisobutyl group, 1,2-dichloroethyl group
- divalent group forming the ring examples include tetramethylene group, pentamethylene group, hexamethylene group, diphenylmethane-2,2′-diyl group, diphenylethane-3,3′-diyl group and diphenylpropane-4,4′-diyl group.
- R 5 to R 8 represents:
- p represents 0 or 1
- q represents 0 or 1
- m represents 0 or an integer of 1 to 3
- n represents an integer of 1 to 3.
- distyrylarylene derivative represented by general formula (1) of the present invention will be shown in the following.
- the distyrylarylene derivative of the present invention is not limited to the compounds shown as the examples.
- C 5 means n-pentyl group.
- the compounds shown in the following as the examples are all dissolved into 1,2-dichloroethane at 20° C. in an amount of 0.5% by weight or more.
- the organic EL device of the present invention comprises a cathode, an anode and an organic thin film layer comprising a single layer or a plurality of layers and disposed between the cathode and the anode, and at least one layer in the organic thin film layer comprises the novel soluble compound described above.
- the electron transporting layer comprises a novel soluble compound described above. It is also preferable that, in the organic electroluminescence device which comprises a cathode, an anode and at least a light emitting layer and a hole transporting layer which are disposed between the cathode and the anode, the hole transporting layer comprises a novel soluble compound described above.
- the light emitting layer comprises an arylamine compound or a distyrylarylene derivative.
- arylamine compound or the distyrylarylene derivative it is preferable that a compound represented by the following general formula (2) or (3) is used.
- Ar 6 represents an aromatic group having 6 to 40 carbon atoms
- Ar 7 and Ar 8 each independently represent hydrogen atom or an aromatic group having 6 to 40 carbon atoms
- the groups represented by Ar 6 to Ar 8 may be substituted
- a represents an integer of 1 to 6, which is the number of condensation.
- Ar 9 and Ar 15 each represent an aromatic group having 6 to 40 carbon atoms
- Ar 10 to Ar 14 each independently represent hydrogen atom or an aromatic group having 6 to 40 carbon atoms
- the groups represented by Ar 9 to Ar 15 may be substituted
- b to e each represent 0 or 1, which is the number of condensation.
- examples of the aromatic group having 6 to 40 carbon atoms include aryl groups such as phenyl group, naphthyl group, anthranyl group, phenanthryl group, pyrenyl group, coronyl group, biphenyl group, terphenyl group, pyrrolyl group, furanyl group, thiophenyl group, benzothiophenyl group, oxathiazolyl group, diphenylanthranyl group, indolyl group, carbazolyl group, pyridyl group, benzoquinolyl group, fluoranthenyl group and acenaphthofluoranthenyl group; and arylene groups such as phenylene group, naphthylene group, anthranylene group, phenanthrylene group, pyrenylene group, coronylene group, biphenylene group, terphenylene group, pyrrolylene group, furanylene
- the aromatic group having 6 to 40 carbon atoms may be substituted.
- substituents include alkyl groups having 1 to 6 carbon atoms such as ethyl group, methyl group, i-propyl group, n-propyl group, s-butyl group, t-butyl group, pentyl group, hexyl group, cyclopentyl group and cyclohexyl group; alkoxyl groups having 1 to 6 carbon atoms such as ethoxyl group, methoxyl group, i-propoxyl group, n-propoxyl group, s-butoxyl group, t-butoxyl group, pentoxyl group, hexyloxyl group, cyclopentoxyl group and cyclohexyloxyl group; aryl groups having a nucleus having 5 to 40 atoms; amino groups substituted with an aryl group having a nucleus having 5 to 40 atoms; ester groups having an aryl group
- the light emitting layer comprises an aromatic cyclic compound having styryl group.
- aromatic cyclic compound examples include N,N′-diphenyl-N,N′-bis(4-styrylphenyl)-1,4-diamino-naphthalene, N,N′-diphenyl-N,N′-bis(4-styrylphenyl)-1,4-diamino-2,3-dimethylnaphthalene, N,N′-diphenyl-N,N′-bis(4-styrylphenyl)-3,8-diaminopyrene, N,N′-diphenyl-N,N′-bis(4-styrylphenyl)-9,10-diamino-anthracene and N,N′-diphenyl-N,N′-bis(4-styrylphenyl)-3,9-diamino perylene.
- the organic EL device of the present invention has a laminate structure having one or more organic layers laminated between the electrodes.
- Examples of the structure include structures of an anode/a light emitting layer/a cathode, an anode/a hole transporting layer/a light emitting layer/an electron transporting layer/a cathode, an anode/a hole transporting layer/a light emitting layer/a cathode and an anode/a light emitting layer/an electron transporting layer/a cathode.
- the compound described in the present invention may be used in any of the above organic thin film layers and may also be used by doping into other hole transporting materials, light emitting materials and electron transporting materials.
- the electron transporting material used for the electron transporting layer in the organic EL device of the present invention is not particularly limited, and compounds conventionally used as the electron transporting material can be used without particular restrictions.
- examples of such compounds include oxadiazole derivatives and triazole derivatives such as 2-(4-biphenylyl)-5-(4-t-butylphenyl)-1,3,4-oxadiazole and bis ⁇ 2-(4-t-butylphenyl)-1,3,4-oxadiazole ⁇ -m-phenylene and quinolinol-based metal complexes.
- insulating materials and semiconductors are preferable.
- the electron transporting layer is constituted with the insulating material or the semiconductor, leak of electric current can be effectively prevented, and the electron injecting property can be improved. It is preferable that at least one metal compound selected from the group consisting of alkali metal chalcogenides, alkaline earth metal chalcogenides, alkali metal halides and alkaline earth metal halides is used as the insulating material. It is preferable that the electron transporting layer is constituted with the alkali metal chalcogenide or the like material since the electron injecting property can be further improved.
- alkali metal chalcogenide examples include Li 2 O, LiO, Na 2 S, Na 2 Se and NaO.
- Preferable examples of the alkaline earth metal chalcogenide include CaO, BaO, SrO, BeO, BaS and CaSe.
- Examples of the alkali metal halide include LiF, NaF, KF, LiCl, KCl and NaCl.
- Examples of the alkaline earth metal halide include fluorides such as CaF 2 , BaF 2 , SrF 2 , MgF 2 and BeF 2 and halides other than the fluorides.
- the semiconductor constituting the electron transporting layer examples include oxides, nitrides and oxide nitrides containing at least one element selected from Ba, Ca, Sr, Yb, Al, Ga, In, Li, Na, Cd, Mg, Si, Ta, Sb and Zn, which are used singly or as a combination of two or more. It is preferable that the inorganic compound constituting the electron transporting layer is in the form of a fine crystalline or amorphous insulating thin film. When the electron transporting layer is constituted with the above insulating thin film, a more uniform thin film can be formed and defective pixels such as dark spots can be decreased.
- the inorganic compound include the alkali metal chalcogenides, the alkaline earth metal chalcogenides, the alkali metal halides and the alkaline earth metal halides which are described above.
- a region transporting electrons or an interface region between the cathode and a layer of an organic thin film comprises a reducing dopant having a work function of 2.9 eV or smaller.
- the reducing dopant is defined as a substance which can reduce the electron transporting compound. Therefore, various types of substances can be used as long as the substance has the specific reducing property.
- the reducing dopant include at least one alkali metal selected from the group consisting of Na (the work function: 2.36 eV), K (the work function: 2.28 eV), Rb (the work function: 2.16 eV) and Cs (the work function: 1.95 eV) and at least one alkaline earth metal selected from the group consisting of Ca (the work function: 2.9 eV), Sr (the work function: 2.0 to 2.5 eV) and Ba (the work function: 2.52 eV).
- reducing dopants having a work function of 2.9 eV or smaller are preferable.
- the more preferable reducing dopants are at least one alkali metal selected from the group consisting of K, Rb and Cs.
- the still more preferable reducing dopants are Rb and Cs, and the most preferable reducing dopant is Cs.
- These alkali metals have particularly great reducing ability, and the luminance of emitted light and the life of the organic EL device are improved by adding these alkali metals in a relatively small amount into the region of electron injection.
- the reducing dopant having a work function of 2.9 eV or smaller combinations of two or more alkali metals are also preferable, and combinations including Cs such as combinations of Cs and Na, Cs and K, Cs and Rb, and Cs, Na and K are more preferable.
- Cs is include in the combination, the reducing ability can be efficiently exhibited, and the luminance of emitted light and the life of the organic EL device can be improved by adding the combination into the region of electron injection.
- the anode of the organic EL device plays the role of injecting holes into the hole transporting layer or the light emitting layer. It is effective that the anode has a work function of 4.5 eV or greater.
- the material of the anode used in the present invention include indium tin oxide alloys (ITO), tin oxides (NESA), gold, silver, platinum and copper.
- ITO indium tin oxide alloys
- NESA tin oxides
- gold silver
- platinum and copper copper
- the cathode a material having a small work function is preferable so that electrons can be injected into the electron transporting layer or the light emitting layer.
- the material of the cathode is not particularly limited. Examples of the material of the cathode include indium, aluminum, magnesium, magnesium-indium alloys, magnesium-aluminum alloys, aluminum-lithium alloys, aluminum-scandium-lithium alloys and magnesium-silver alloys.
- the process for forming the layers in the organic EL device of the present invention is not particularly limited.
- a conventional process such as the vacuum vapor deposition process and the spin coating process can be used.
- the organic thin film layer comprising the compound represented by the above general formula (1) which is used in the organic EL device of the present invention can be formed in accordance with the vacuum vapor deposition process, the molecular beam epitaxy process (the MBE process) or, using a solution prepared by dissolving the compound into a solvent, in accordance with a conventional coating process such as the dipping process, the spin coating process, the casting process, the bar coating process and the roll coating process.
- a conventional coating process such as the dipping process, the spin coating process, the casting process, the bar coating process and the roll coating process.
- the organic EL device exhibiting a great efficiency of light emission can be obtained in accordance with a wet process such as the spin coating process and the dipping process.
- each layer in the organic thin film layer in the organic EL device of the present invention is not particularly limited.
- an excessively thin layer tends to have defects such as pin holes, and an excessively thick layer requires a high applied voltage to decrease the efficiency. Therefore, a thickness in the range of several nm to 1 ⁇ m is preferable.
- Compound (A1) (9,10-bis[4-(2,2-diphenyl-ethenyl)phenyl]-2,6-di(2-ethylhexyloxy)anthracene) is shown in the following.
- the obtained yellow solid was dissolved into 20 ml of isopropyl alcohol (IPA).
- IPA isopropyl alcohol
- a solution prepared by dissolving 0.65 g (17 mmole) of NaBH 4 into 30 ml of IPA was slowly added dropwise, and the obtained solution was heated under stirring for one night. After the reaction was completed, water was added to the reaction solution. The formed precipitates were separated by filtration and washed with water and ethanol, and 5.5 g of the anthracene compound of the object compound was obtained (the yield: 78%; a yellow powder).
- the organic layer was washed with a saturated solution of sodium hydrogencarbonate and a saturated solution of sodium chloride and dried with magnesium sulfate. After the concentration under a reduced pressure, the obtained dark brown residual product was purified in accordance with the silica gel chromatography (the developing solvent: hexane), and 1.1 g of the dibromo compound of the object compound was obtained (the yield: 30%; a yellow powder).
- the reaction solution was cooled with ice water.
- the formed crystals were separated by filtration and washed with 50 ml of methanol and 50 ml of acetone successively, and 0.56 g of a yellow powder was obtained.
- the obtained yellow powder was identified to be Compound (A1) by the measurements in accordance with NMR, IR and the filed desorption mass spectroscopy (FD-MS) (the yield: 60%).
- Compound (A2) (9,10-bis[4-(2,2-diphenyl-ethenyl)phenyl]-2,6/2,7-di-t-butylanthracene) is shown in the following.
- the reaction solution was cooled with ice water.
- the formed crystals were separated by filtration and washed with 50 ml of methanol and 50 ml of acetone successively, and 0.4 g of a yellow powder was obtained.
- the obtained yellow powder was identified to be Compound (A2) by the measurements in accordance with NMR, IR and FD-MS (the yield: 50%).
- the organic layer was washed with a saturated aqueous solution of sodium chloride (50 ml) and dried with magnesium sulfate. After the solvent was removed by distillation, the obtained product was purified in accordance with the column chromatography (silica gel; hexane+30% dichloromethane), and a light yellow solid was obtained (4.5 g; 82%).
- the obtained product was identified to be Compound (A21) by the measurements in accordance with 1 H-NMR and FD-MS.
- a glass substrate (manufactured by GEOMATEC Company) of 25 mm ⁇ 75 mm ⁇ 1.1 mm thickness having an ITO transparent electrode was cleaned by application of ultrasonic wave in isopropyl alcohol for 5 minutes and then by exposure to ozone generated by ultraviolet light for 30 minutes.
- the glass substrate having the transparent electrode lines which had been cleaned was attached to a substrate holder of a vacuum vapor deposition apparatus.
- a light emitting layer was formed using a dichloroethane solution (1.5% by weight) containing 2 parts by weight of Compound (A1) and 1 part by weight of an arylamine compound shown below in accordance with the spin coating process.
- the formed light emitting layer had a thickness of 120 nm.
- Alq film tris(8-quinolinol)aluminum (Alq film) having a thickness of 10 nm was formed.
- the Alq film worked as the electron injecting layer.
- Li the source of lithium: manufactured by SAES GETTERS Company
- Alq the electron injecting layer
- metallic aluminum was vapor deposited to form a metal cathode, and an organic EL device was prepared. The obtained device emitted bluish green light with a luminance of 150 cd/m 2 under application of a direct voltage of 7 V, and the efficiency of light emission was as excellent as 2.67 lumen/W.
- Organic EL devices were prepared in accordance with the same procedures as those conducted in Example 1 except that compounds shown in Table 1 were used in place of Compound (A1) used in Example 1.
- the luminance of the emitted light, the efficiency of light emission and the color of the emitted light exhibited by the prepared devices under application of a direct voltage of 6 V are shown in Table 1.
- Example 2 to 8 exhibited excellent efficiencies of light emission. This result was obtained since the novel soluble compound comprising the distyrylarylene derivative of the present invention used in the light emitting layer had the specific central group having a soluble substituent.
- Example 2 was used in place of Compound (A1) used in Example 1.
- the obtained device exhibited an efficiency of light emission under application of a direct voltage of 6 V of 0.81 lumen/W, which was markedly smaller than those in Examples.
- a glass substrate (manufactured by GEOMATEC Company) of 25 mm ⁇ 75 mm ⁇ 1.1 mm thickness having an ITO transparent electrode was cleaned by application of ultrasonic wave in isopropyl alcohol for 5 minutes and then by exposure to ozone generated by ultraviolet light for 30 minutes.
- the glass substrate having the transparent electrode lines which had been cleaned was attached to a substrate holder of a vacuum vapor deposition apparatus.
- a light emitting layer was formed using a dichloroethane solution (2% by weight) containing 4 parts by weight of Compound (A1) and 1 part by weight of a styrylamine compound shown below in accordance with the spin coating process.
- the formed light emitting layer had a thickness of 130 nm.
- a film of tris(8-quinolinol)aluminum (Alq film) having a thickness of 10 nm was formed on the formed light emitting layer.
- the Alq film worked as the electron injecting layer.
- Li the source of lithium: manufactured by SAES GETTERS Company
- Alq the electron injecting layer
- metallic aluminum was vapor deposited to form a metal cathode, and an organic EL device was prepared.
- the obtained device emitted bluish green light with a luminance of 250 cd/m 2 under application of a direct voltage of 7 V, and the efficiency of light emission was as excellent as 1.91 lumen/W.
- the organic EL device exhibiting a great efficiency of light emission can be produced easily by utilizing the novel soluble compound of the present invention since the organic thin film layer can be formed in accordance with the wet process.
- the organic electroluminescence device of the present invention is very useful as the inexpensive light source for various electronic instruments.
Abstract
A novel soluble compound which is a distyrylarylene derivative having a soluble substituent and a specific central group and having a solubility of 0.5% by weight or greater at 20° C. in an organic solvent; and an organic electroluminescence device having an organic thin film layer which has a single layer or a plurality of layers, is disposed between a cathode and an anode and has at least one layer containing the novel soluble compound. The organic thin film layer can be formed in accordance with a wet process, and the organic electroluminescence device exhibiting a great efficiency of light emission can be produced easily.
Description
- The present invention relates to a novel soluble compound and organic electroluminescence devices and, more particularly, to a novel soluble compound which can be formed into an organic thin film layer in accordance with a wet process and enables producing electroluminescence devices exhibiting a great efficiency of light emission easily and an electroluminescence device utilizing the compound.
- An organic electroluminescence (electroluminescence will be referred to as EL, hereinafter) is a spontaneous light emitting device which utilizes the principle that a fluorescent substance emits light by energy of recombination of holes injected from an anode and electrons injected from a cathode when an electric field is applied.
- Since an organic EL device of the laminate type driven under a low electric voltage was reported by C. W. Tang of Eastman Kodak Company (C. W. Tang and S. A. Vanslyke, Applied Physics Letters, Volume 51, Pages 913, 1987), many studies have been conducted on organic EL devices using organic materials as the constituting materials. Tang et al. used a laminate structure using tris(8-hydroxyquinolinol)aluminum for the light emitting layer and a triphenyldiamine derivative for the hole transporting layer. Advantages of the laminate structure are that the efficiency of hole injection into the light emitting layer can be increased, that the efficiency of forming excitons which are formed by blocking and recombining electrons injected from the cathode can be increased, and that excitons formed within the light emitting layer can be enclosed. As the structure of the organic EL device, a two-layered structure having a hole transporting (injecting) layer and an electron transporting and light emitting layer and a three-layered structure having a hole transporting (injecting) layer, a light emitting layer and an electron transporting (injecting) layer are well known. To increase the efficiency of recombination of injected holes and electrons in the devices of the laminate type, the structure of the device and the process for forming the device have been studied.
- As the light emitting material, chelate complexes such as tris(8-quinolinolato)aluminum, coumarine derivatives, tetraphenyl-butadiene derivatives, bisstyrylarylene derivatives and oxadiazole derivatives are known. It is reported that light in the visible region ranging from blue light to red light can be obtained by using these light emitting materials, and development of a device exhibiting color images is expected (For example, Japanese Patent Application Laid-Open Nos. Heisei 8 (1996)-239655, Heisei 7 (1995)-138561 and Heisei 3 (1991)-200289).
- Devices using anthracene derivatives as the hole transporting material or the light emitting material are disclosed in Japanese Patent No. 3175816. However, although an excellent device emitting blue light can be made by using the anthracene derivatives, an ink in which the anthracene derivatives are dissolved cannot be prepared since the used compounds are not easily soluble in solvents. The device cannot be produced in accordance with a wet process such as the spin coating process, the printing process and the ink-jet process and is produced in accordance with the vacuum vapor deposition process. Therefore, a compound which can be easily formed into a film in accordance with a wet process, which does not require vacuum, and a device using the compound have been desired.
- On the other hand, in Japanese Patent Application Laid-Open No. 2000-143569, a distyryl compound having a soluble substituent is disclosed. However, this compound has a poor light emitting property since this compound does not have the anthracene nucleus or the fluorene nucleus as the central group, and the improvement has been desired.
- The present invention has been made to overcome the above drawbacks and has an object of providing a novel soluble compound which can be formed into an organic thin film layer in accordance with a wet process and enables producing electroluminescence devices exhibiting a great efficiency of light emission easily and an electroluminescence device utilizing the compound.
- As the result of intensive studies by the present inventors to overcome the above drawbacks, it was found that the above drawbacks could be overcome by using a distyrylarylene derivative which is soluble in organic solvents and has a specific central group as the material of the organic thin film layer. The present invention has been completed based on this knowledge.
- The present invention provides a novel soluble compound which is a distyrylarylene derivative represented by general formula (1) and has a solubility (20° C.) of 0.5% by weight or greater in an organic solvent, general formula (1) being:
- wherein Ar1, Ar2, Ar4 and Ar5 each independently represent a substituted or unsubstituted phenylene group, a substituted or unsubstituted naphthalene group, a substituted or unsubstituted anthracene group, a substituted or unsubstituted diphenylanthracene group, a substituted or unsubstituted phenanthrene group, a substituted or unsubstituted acenaphthene group, a substituted or unsubstituted biphenylene group, a substituted or unsubstituted fluorene group, a substituted or unsubstituted carbazolyl group, a substituted or unsubstituted thiophene group, a substituted or unsubstituted triazole group or a substituted or unsubstituted thiadiazole group;
- R1 to R4 each independently represent hydrogen atom, an alkyl group having 1 to 20 carbon atoms, an alkoxyl group having 1 to 20 carbon atoms, an aryl group having 6 to 20 carbon atoms, a trialkylsilyl group having 3 to 20 carbon atoms or cyano group;
- Ar3 represents a substituted or unsubstituted anthracendiyl group or a substituted or unsubstituted fluorendiyl group;
- R5 to R8 each independently represent hydrogen atom, a halogen atom, hydroxyl group, a substituted or unsubstituted amino group, nitro group, cyano group, a substituted or unsubstituted alkyl group having 1 to 30 carbon atoms, a substituted or unsubstituted alkenyl group having 2 to 30 carbon atoms, a substituted or unsubstituted cycloalkyl group having 5 to 30 carbon atoms, a substituted or unsubstituted alkoxyl group having 1 to 30 carbon atoms, a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, a substituted or unsubstituted aromatic heterocyclic group having 2 to 30 carbon atoms, a substituted or unsubstituted aralkyl group having 7 to 30 carbon atoms, a substituted or unsubstituted aryloxyl group having 6 to 30 carbon atoms, a substituted or unsubstituted alkoxycarbonyl group having 2 to 30 carbon atoms or carboxyl group, groups represented by R6 and R5 or R7 and R8 may be bonded to each other and form a cyclic structure which may have substituents;
- p represents 0 or 1, q represents 0 or 1, m represents 0 or an integer of 1 to 3, and n represents an integer of 1 to 3.
- It is sufficient that at least one organic solvent which dissolves 0.5% by weight or more of the novel soluble compound at 20° C. exists.
- The present invention also provides an organic electroluminescence device which comprises a cathode, an anode and an organic thin film layer comprising a single layer or a plurality of layers and disposed between the cathode and the anode, wherein at least one layer in the organic thin film layer comprises a novel soluble compound described above.
- In the present invention, the soluble compound is a compound which has a soluble substituent, is represented by general formula (1) shown above and has a solubility (20° C.) of 0.5% by weight or greater in an organic solvent. It is sufficient that at least one organic solvent which dissolves 0.5% by weight or more of the novel soluble compound at 20° C. exists.
- It is preferable that the organic solvent is at least one solvent selected from toluene, xylene, N-methylpyrrolidone, γ-butyrolactone, 1,3-dimethyl-2-imidazoline, carbitol acetate, butylcarbitol acetate, dichloromethane, dichloroethane, chlorobenzene tetralin and alcohols having 1 to 10 carbon atoms, and more preferably dichloroethane, toluene, xylene or tetraline.
- In general formula (1) shown above, Ar1, Ar2, Ar4 and Ar5 each independently represent a substituted or unsubstituted phenylene group, a substituted or unsubstituted naphthalene group, a substituted or unsubstituted anthracene group, a substituted or unsubstituted diphenylanthracene group, a substituted or unsubstituted phenanthrene group, a substituted or unsubstituted acenaphthene group, a substituted or unsubstituted biphenylene group, a substituted or unsubstituted fluorene group, a substituted or unsubstituted carbazolyl group, a substituted or unsubstituted thiophene group, a substituted or unsubstituted triazole group or a substituted or unsubstituted thiadiazole group.
- In general formula (1) shown above, R1 to R4 each independently represent hydrogen atom, an alkyl group having 1 to 20 carbon atoms, an alkoxyl group having 1 to 20 carbon atoms, an aryl group having 6 to 20 carbon atoms, a trialkylsilyl group having 3 to 20 carbon atoms or cyano group.
- Examples of the alkyl group having 1 to 20 carbon atoms include methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, n-pentyl group, n-hexyl group, n-heptyl group, n-octyl group, hydroxymethyl group, 1-hydroxyethyl group, 2-hydroxyethyl group, 2-hydroxyisobutyl group, 1,2-dihydroxyethyl group, 1,3-dihydroxyisopropyl group, 2,3-dihydroxy-t-butyl group, 1,2,3-trihydroxypropyl group, chloromethyl group, 1-chloroethyl group, 2-chloroethyl group, 2-chloroisobutyl group, 1,2-dichloroethyl group, 1,3-dichloroisopropyl group, 2,3-dichloro-t-butyl group, 1,2,3-trichloropropyl group, bromomethyl group, 1-bromoethyl group, 2-bromoethyl group, 2-bromoisobutyl group, 1,2-dibromoethyl group, 1,3-dibromoisopropyl group, 2,3-dibromo-t-butyl group, 1,2,3-tribromopropyl group, iodomethyl group, 1-iodoethyl group, 2-iodoethyl group, 2-iodoisobutyl group, 1,2-diiodoethyl group, 1,3-diiodoisopropyl group, 2,3-diiodo-t-butyl group, 1,2,3-triiodopropyl group, aminomethyl group, 1-aminoethyl group, 2-aminoethyl group, 2-aminoisobutyl group, 1,2-diaminoethyl group, 1,3-diaminoisopropyl group, 2,3-diamino-t-butyl group, 1,2,3-triaminopropyl group, cyanomethyl group, 1-cyanoethyl group, 2-cyanoethyl group, 2-cyanoisobutyl group, 1,2-dicyanoethyl group, 1,3-dicyanoisopropyl group, 2,3-dicyano-t-butyl group, 1,2,3-tricyanopropyl group, nitromethyl group, 1-nitroethyl group, 2-nitroethyl group, 2-nitroisobutyl group, 1,2-dinitroethyl group, 1,3-dinitroisopropyl group, 2,3-dinitro-t-butyl group and 1,2,3-trinitropropyl group.
- The alkoxyl group having 1 to 20 carbon atoms is a group represented by —OY. Examples of the group represented by Y include methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, n-pentyl group, n-hexyl group, n-heptyl group, n-octyl group, hydroxymethyl group, 1-hydroxyethyl group, 2-hydroxyethyl group, 2-hydroxyisobutyl group, 1,2-dihydroxyethyl group, 1,3-dihydroxyisopropyl group, 2,3-dihydroxy-t-butyl group, 1,2,3-trihydroxypropyl group, chloromethyl group, 1-chloroethyl group, 2-chloroethyl group, 2-chloroisobutyl group, 1,2-dichloroethyl group, 1,3-dichloroisopropyl group, 2,3-dichloro-t-butyl group, 1,2,3-trichloropropyl group, bromomethyl group, 1-bromoethyl group, 2-bromoethyl group, 2-bromoisobutyl group, 1,2-dibromoethyl group, 1,3-dibromoisopropyl group, 2,3-dibromo-t-butyl group, 1,2,3-tribromopropyl group, iodomethyl group, 1-iodoethyl group, 2-iodoethyl group, 2-iodoisobutyl group, 1,2-diiodoethyl group, 1,3-diiodoisopropyl group, 2,3-diiodo-t-butyl group, 1,2,3-triiodopropyl group, aminomethyl group, 1-aminoethyl group, 2-aminoethyl group, 2-aminoisobutyl group, 1,2-diaminoethyl group, 1,3-diaminoisopropyl group, 2,3-diamino-t-butyl group, 1,2,3-triaminopropyl group, cyanomethyl group, 1-cyanoethyl group, 2-cyanoethyl group, 2-cyanoisobutyl group, 1,2-dicyanoethyl group, 1,3-dicyanoisopropyl group, 2,3-dicyano-t-butyl group, 1,2,3-tricyanopropyl group, nitromethyl group, 1-nitroethyl group, 2-nitroethyl group, 2-nitroisobutyl group, 1,2-dinitroethyl group, 1,3-dinitroisopropyl group, 2,3-dinitro-t-butyl group and 1,2,3-trinitropropyl group.
- Examples of the aryl group having 6 to 20 carbon atoms include phenyl group, naphthyl group, anthryl group, phenanthryl group, naphthacenyl group and pyrenyl group. Examples of the substituent to the aryl group include halogen atoms, hydroxyl group, substituted and unsubstituted amino groups described above, nitro group, cyano group, substituted and unsubstituted alkyl groups described above, substituted and unsubstituted alkenyl groups described above, substituted and unsubstituted cycloalkyl groups described above, substituted and unsubstituted alkoxyl groups described above, substituted and unsubstituted aromatic hydrocarbon groups described above, substituted and unsubstituted aromatic heterocyclic groups described above, substituted and unsubstituted aralkyl groups described above, substituted and unsubstituted aryloxyl groups described above, substituted and unsubstituted alkoxycarbonyl groups described above and carboxyl group.
- Examples of the trialkylsilyl group having 3 to 20 carbon atoms in include trimethylsilyl group, triethylsilyl group, tripropylsilyl group, tributylsilyl group, tripentyl silyl group and trihexylsilyl group.
- In general formula (1) shown above, Ar3 represents a substituted or unsubstituted anthracendiyl group or a substituted or unsubstituted fluorendiyl group.
- It is preferable that Ar3 represents:
-
- anthracendiyl group or fluorendiyl group having at least one group selected from:
- (1) linear and branched alkyl groups having 5 or more carbon atoms and an olefinic unsaturated bond,
- (2) linear, branched and cyclic substituted and unsubstituted alkyl groups having 4 or more carbon atoms,
- (3) linear, branched and cyclic substituted and unsubstituted alkyloxyl groups having 5 or more carbon atoms,
- (4) linear, branched and cyclic substituted and unsubstituted alkylthio groups having 5 or more carbon atoms,
- (5) linear, branched and cyclic substituted and unsubstituted alkylsilyl groups having 5 or more carbon atoms,
- (6) linear, branched and cyclic substituted and unsubstituted dialkylsilyl groups having 5 or more carbon atoms,
- (7) linear, branched and cyclic substituted and unsubstituted trialkylsilyl groups having 5 or more carbon atoms,
- (8) alkylamino groups and dialkylamino groups,
- (9) linear and branched cyano-substituted alkyl groups having 4 or more carbon atoms and 1 or 2 cyano groups, and
- (10) polyethers having 2 to 5 ether oxygen atoms which are separated from each other with an alkyl crosslinking having 1 to 3 carbon atoms, or
- anthracendiyl group or fluorendiyl group having a group selected from aryl groups having 6 to 30 carbon atoms, arylalkyl groups having 7 to 30 carbon atoms, heteroarylalkyl groups having at least one of nitrogen atom, oxygen atom and sulfur atom and 2 to 30 carbon atoms, heterocyclic groups having 2 to 30 carbon atoms, alkanoyl groups having 1 to 20 carbon atoms, cycloalkanoyl groups having 6 to 30 carbon atoms, acryloyl groups having 6 to 30 carbon atoms and heteroaryloxyl groups having at least one of oxygen atom and sulfur atom and 2 to 30 carbon atoms, which are substituted with at least one group selected from aforesaid (1) to (10).
- anthracendiyl group or fluorendiyl group having at least one group selected from:
- It is also preferable that Ar3 represents anthracendiyl group substituted with at least two substituted or unsubstituted t-butyl groups.
- In general formula (1) shown above, R5 to R8 each independently represent hydrogen atom, a halogen atom, hydroxyl group, a substituted or unsubstituted amino group, nitro group, cyano group, a substituted or unsubstituted alkyl group having 1 to 30 carbon atoms, a substituted or unsubstituted alkenyl group having 2 to 30 carbon atoms, a substituted or unsubstituted cycloalkyl group having 5 to 30 carbon atoms, a substituted or unsubstituted alkoxyl group having 1 to 30 carbon atoms, a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, a substituted or unsubstituted aromatic heterocyclic group having 2 to 30 carbon atoms, a substituted or unsubstituted aralkyl group having 7 to 30 carbon atoms, a substituted or unsubstituted aryloxyl group having 6 to 30 carbon atoms, a substituted or unsubstituted alkoxycarbonyl group having 2 to 30 carbon atoms or carboxyl group, groups represented by R6 and R5 or R7 and R8 may be bonded to each other and form a cyclic structure which may have substituents.
- The amino group is represented by —NX1X2. X1 and X2 each independently represent hydrogen atom, methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, n-pentyl group, n-hexyl group, n-heptyl group, n-octyl group, hydroxymethyl group, 1-hydroxyethyl group, 2-hydroxyethyl group, 2-hydroxyisobutyl group, 1,2-dihydroxyethyl group, 1,3-dihydroxy-isopropyl group, 2,3-dihydroxy-t-butyl group, 1,2,3-trihydroxypropyl group, chloromethyl group, 1-chloroethyl group, 2-chloroethyl group, 2-chloroisobutyl group, 1,2-dichloroethyl group, 1,3-dichloroisopropyl group, 2,3-dichloro-t-butyl group, 1,2,3-trichloropropyl group, bromomethyl group, 1-bromoethyl group, 2-bromoethyl group, 2-bromoisobutyl group, 1,2-dibromoethyl group, 1,3-dibromoisopropyl group, 2,3-dibromo-t-butyl group, 1,2,3-tribromopropyl group, iodomethyl group, 1-iodoethyl group, 2-iodoethyl group, 2-iodoisobutyl group, 1,2-diiodoethyl group, 1,3-diiodoisopropyl group, 2,3-diiodo-t-butyl group, 1,2,3-triiodopropyl group, aminomethyl group, 1-aminoethyl group, 2-aminoethyl group, 2-aminoisobutyl group, 1,2-diaminoethyl group, 1,3-diaminoisopropyl group, 2,3-diamino-t-butyl group, 1,2,3-triaminopropyl group, cyanomethyl group, 1-cyanoethyl group, 2-cyanoethyl group, 2-cyanoisobutyl group, 1,2-dicyanoethyl group, 1,3-dicyanoisopropyl group, 2,3-dicyano-t-butyl group, 1,2,3-tricyanopropyl group, nitromethyl group, 1-nitroethyl group, 2-nitroethyl group, 2-nitroisobutyl group, 1,2-dinitroethyl group, 1,3-dinitroisopropyl group, 2,3-dinitro-t-butyl group, 1,2,3-trinitropropyl group, phenyl group, 1-naphthyl group, 2-naphthyl group, 1-anthryl group, 2-anthryl group, 9-anthryl group, 1-phenanthryl group, 2-phenanthryl group, 3-phenanthryl group, 4-phenanthryl group, 9-phenanthryl group, 1-naphthacenyl group, 2-naphthacenyl group, 9-naphthacenyl group, 4-styrylphenyl group, 1-pyrenyl group, 2-pyrenyl group, 4-pyrenyl group, 2-biphenylyl group, 3-biphenylyl group, 4-biphenylyl group, p-terphenyl-4-yl group, p-terphenyl-3-yl group, p-terphenyl-2-yl group, m-terphenyl-4-yl group, m-terphenyl-3-yl group, m-terphenyl-2-yl group, o-tolyl group, m-tolyl group, p-tolyl group, p-t-butylphenyl group, p-(2-phenylpropyl)phenyl group, 3-methyl-2-naphthyl group, 4-methyl-1-naphthyl group, 4-methyl-1-anthryl group, 4′-methyl-biphenylyl group, 4″-t-butyl-p-terphenyl-4-yl group, 2-pyrrolyl group, 3-pyrrolyl group, pyradinyl group, 2-pyridinyl group, 3-pyridinyl group, 4-pyridinyl group, 2-indolyl group, 3-indolyl group, 4-indolyl group, 5-indolyl group, 6-indolyl group, 7-indolyl group, 1-isoindolyl group, 3-isoindolyl group, 4-isoindolyl group, 5-isoindolyl group, 6-isoindolyl group, 7-isoindolyl group, 2-furyl group, 3-furyl group, 2-benzofuranyl group, 3-benzofuranyl group, 4-benzofuranyl group, 5-benzofuranyl group, 6-benzofuranyl group, 7-benzofuranyl group, 1-isobenzofuranyl group, 3-isobenzofuranyl group, 4-isobenzofuranyl group, 5-isobenzofuranyl group, 6-isobenzofuranyl group, 7-isobenzofuranyl group, 2-quinolyl group, 3-quinolyl group, 4-quinolyl group, 5-quinolyl group, 6-quinolyl group, 7-quinolyl group, 8-quinolyl group, 1-isoquinolyl group, 3-isoquinolyl group, 4-isoquinolyl group, 5-isoquinolyl group, 6-isoquinolyl group, 7-isoquinolyl group, 8-isoquinolyl group, 2-quinoxanyl group, 5-quinoxanyl group, 6-quinoxanyl group, 1-carbazolyl group, 2-carbazolyl group, 3-carbazolyl group, 4-carbazolyl group, 1-phenanthridinyl group, 2-phenanthridinyl group, 3-phenanthridinyl group, 4-phenanthridinyl group, 6-phenanthridinyl group, 7-phenanthridinyl group, 8-phenanthridinyl group, 9-phenanthridinyl group, 10-phenanthridinyl group, 1-acridinyl group, 2-acridinyl group, 3-acridinyl group, 4-acridinyl group, 9-acridinyl group, 1,7-phenanthrolin-2-yl group, 1,7-phenanthrolin-3-yl group, 1,7-phenanthrolin-4-yl group, 1,7-phenanthrolin-5-yl group, 1,7-phenanthrolin-6-yl group, 1,7-phenanthrolin-8-yl group, 1,7-phenanthrolin-9-yl group, 1,7-phenanthrolin-10-yl group, 1,8-phenanthrolin-2-yl group, 1,8-phenanthrolin-3-yl group, 1,8-phenanthrolin-4-yl group, 1,8-phenanthrolin-5-yl group, 1,8-phenanthrolin-6-yl group, 1,8-phenanthrolin-7-yl group, 1,8-phenanthrolin-9-yl group, 1,8-phenanthrolin-10-yl group, 1,9-phenanthrolin-2-yl group, 1,9-phenanthrolin-3-yl group, 1,9-phenanthrolin-4-yl group, 1,9-phenanthrolin-5-yl group, 1,9-phenanthrolin-6-yl group, 1,9-phenanthrolin-7-yl group, 1,9-phenanthrolin-8-yl group, 1,9-phenanthrolin-10-yl group, 1,10-phenanthrolin-2-yl group, 1,10-phenanthrolin-3-yl group, 1,10-phenanthrolin-4-yl group, 1,10-phenanthrolin-5-yl group, 2,9-phenanthrolin-1-yl group, 2,9-phenanthrolin-3-yl group, 2,9-phenanthrolin-4-yl group, 2,9-phenanthrolin-5-yl group, 2,9-phenanthrolin-6-yl group, 2,9-phenanthrolin-7-yl group, 2,9-phenanthrolin-8-yl group, 2,9-phenanthrolin-10-yl group, 2,8-phenanthrolin-1-yl group, 2,8-phenanthrolin-3-yl group, 2,8-phenanthrolin-4-yl group, 2,8-phenanthrolin-5-yl group, 2,8-phenanthrolin-6-yl group, 2,8-phenanthrolin-7-yl group, 2,8-phenanthrolin-9-yl group, 2,8-phenanthrolin-10-yl group, 2,7-phenanthrolin-1-yl group, 2,7-phenanthrolin-3-yl group, 2,7-phenanthrolin-4-yl group, 2,7-phenanthrolin-5-yl group, 2,7-phenanthrolin-6-yl group, 2,7-phenanthrolin-8-yl group, 2,7-phenanthrolin-9-yl group, 2,7-phenanthrolin-10-yl group, 1-phenazinyl group, 2-phenazinyl group, 1-phenothiazinyl group, 2-phenothiazinyl group, 3-phenothiazinyl group, 4-phenothiazinyl group, 1-phenoxazinyl group, 2-phenoxazinyl group, 3-phenoxazinyl group, 4-phenoxazinyl group, 2-oxazolyl group, 4-oxazolyl group, 5-oxazolyl group, 2-oxadiazolyl group, 5-oxadiazolyl group, 3-furazanyl group, 2-thienyl group, 3-thienyl group, 2-methylpyrrol-1-yl group, 2-methylpyrrol-3-yl group, 2-methylpyrrol-4-yl group, 2-methylpyrrol-5-yl group, 3-methylpyrrol-1-yl group, 3-methyl-pyrrol-2-yl group, 3-methylpyrrol-4-yl group, 3-methylpyrrol-5-yl group, 2-t-butylpyrrol-4-yl group, 3-(2-phenylpropyl)pyrrol-1-yl group, 2-methyl-1-indolyl group, 4-methyl-1-indolyl group, 2-methyl-3-indolyl group, 4-methyl-3-indolyl group, 2-t-butyl-1-indolyl group, 4-t-butyl-1-indolyl group, 2-t-butyl-3-indolyl group or 4-t-butyl-3-indolyl group.
- Examples of the alkyl group having 1 to 30 carbon atoms include methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, n-pentyl group, n-hexyl group, n-heptyl group, n-octyl group, hydroxymethyl group, 1-hydroxyethyl group, 2-hydroxyethyl group, 2-hydroxyisobutyl group, 1,2-dihydroxyethyl group, 1,3-dihydroxyisopropyl group, 2,3-dihydroxy-t-butyl group, 1,2,3-trihydroxypropyl group, chloromethyl group, 1-chloroethyl group, 2-chloroethyl group, 2-chloroisobutyl group, 1,2-dichloroethyl group, 1,3-dichloroisopropyl group, 2,3-dichloro-t-butyl group, 1,2,3-trichloropropyl group, bromomethyl group, 1-bromoethyl group, 2-bromoethyl group, 2-bromoisobutyl group, 1,2-dibromoethyl group, 1,3-dibromoisopropyl group, 2,3-dibromo-t-butyl group, 1,2,3-tribromopropyl group, iodomethyl group, 1-iodoethyl group, 2-iodoethyl group, 2-iodoisobutyl group, 1,2-diiodoethyl group, 1,3-diiodoisopropyl group, 2,3-diiodo-t-butyl group, 1,2,3-triiodopropyl group, aminomethyl group, 1-aminoethyl group, 2-aminoethyl group, 2-aminoisobutyl group, 1,2-diaminoethyl group, 1,3-diaminoisopropyl group, 2,3-diamino-t-butyl group, 1,2,3-triaminopropyl group, cyanomethyl group, 1-cyanoethyl group, 2-cyanoethyl group, 2-cyanoisobutyl group, 1,2-dicyanoethyl group, 1,3-dicyanoisopropyl group, 2,3-dicyano-t-butyl group, 1,2,3-tricyanopropyl group, nitromethyl group, 1-nitroethyl group, 2-nitroethyl group, 2-nitroisobutyl group, 1,2-dinitroethyl group, 1,3-dinitroisopropyl group, 2,3-dinitro-t-butyl group and 1,2,3-trinitropropyl group.
- Examples of the alkenyl group having 2 to 30 carbon atoms include vinyl group, allyl group, 1-butenyl group, 2-butenyl group, 3-butenyl group, 1,3-butadienyl group, 1-methylvinyl group, styryl group, 2,2-diphenylvinyl group, 1,2-diphenylvinyl group, 1-methylallyl group, 1,1-dimethylallyl group, 2-methylallyl group, 1-phenylallyl group, 2-phenylallyl group, 3-phenylallyl group, 3,3-diphenylallyl group, 1,2-dimethylallyl group, 1-phenyl-1-butenyl group and 3-phenyl-1-butenyl group.
- Examples of the cycloalkyl group having 5 to 30 carbon atoms include cyclopropyl group, cyclobutyl group, cyclopentyl group, cyclohexyl group and 4-methylcyclohexyl group.
- The alkoxyl group having 1 to 30 carbon atoms is represented by —OY. Examples of the group represented by Y include methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, n-pentyl group, n-hexyl group, n-heptyl group, n-octyl group, hydroxymethyl group, 1-hydroxyethyl group, 2-hydroxyethyl group, 2-hydroxyisobutyl group, 1,2-dihydroxyethyl group, 1,3-dihydroxy-isopropyl group, 2,3-dihydroxy-t-butyl group, 1,2,3-trihydroxypropyl group, chloromethyl group, 1-chloroethyl group, 2-chloroethyl group, 2-chloroisobutyl group, 1,2-dichloroethyl group, 1,3-dichloroisopropyl group, 2,3-dichloro-t-butyl group, 1,2,3-trichloropropyl group, bromomethyl group, 1-bromoethyl group, 2-bromoethyl group, 2-bromoisobutyl group, 1,2-dibromoethyl group, 1,3-dibromoisopropyl group, 2,3-dibromo-t-butyl group, 1,2,3-tribromopropyl group, iodomethyl group, 1-iodoethyl group, 2-iodoethyl group, 2-iodoisobutyl group, 1,2-diiodoethyl group, 1,3-diiodoisopropyl group, 2,3-diiodo-t-butyl group, 1,2,3-triiodopropyl group, aminomethyl group, 1-aminoethyl group, 2-aminoethyl group, 2-aminoisobutyl group, 1,2-diaminoethyl group, 1,3-diaminoisopropyl group, 2,3-diamino-t-butyl group, 1,2,3-triaminopropyl group, cyanomethyl group, 1-cyanoethyl group, 2-cyanoethyl group, 2-cyanoisobutyl group, 1,2-dicyanoethyl group, 1,3-dicyanoisopropyl group, 2,3-dicyano-t-butyl group, 1,2,3-tricyanopropyl group, nitromethyl group, 1-nitroethyl group, 2-nitroethyl group, 2-nitroisobutyl group, 1,2-dinitroethyl group, 1,3-dinitroisopropyl group, 2,3-dinitro-t-butyl group and 1,2,3-trinitropropyl group.
- Examples of the aromatic hydrocarbon group having 6 to 30 carbon atoms include phenyl group, 1-naphthyl group, 2-naphthyl group, 1-anthryl group, 2-anthryl group, 9-anthryl group, 1-phenanthryl group, 2-phenanthryl group, 3-phenanthryl group, 4-phenanthryl group, 9-phenanthryl group, 1-naphthacenyl group, 2-naphthacenyl group, 9-naphthacenyl group, 1-pyrenyl group, 2-pyrenyl group, 4-pyrenyl group, 2-biphenylyl group, 3-biphenylyl group, 4-biphenylyl group, p-terphenyl-4-yl group, p-terphenyl-3-yl group, p-terphenyl-2-yl group, m-terphenyl-4-yl group, m-terphenyl-3-yl group, m-terphenyl-2-yl group, o-tolyl group, m-tolyl group, p-tolyl group, p-t-butylphenyl group, p-(2-phenylpropyl)phenyl group, 3-methyl-2-naphthyl group, 4-methyl-1-naphthyl group, 4-methyl-1-anthryl group, 4′-methylbiphenylyl group and 4″-t-butyl-p-terphenyl-4-yl group.
- Examples of the aromatic heterocyclic group having 2 to 30 carbon atoms include 1-pyrrolyl group, 2-pyrrolyl group, 3-pyrrolyl group, pyradinyl group, 2-pyridinyl group, 3-pyridinyl group, 4-pyridinyl group, 1-indolyl group, 2-indolyl group, 3-indolyl group, 4-indolyl group, 5-indolyl group, 6-indolyl group, 7-indolyl group, 1-isoindolyl group, 2-isoindolyl group, 3-isoindolyl group, 4-isoindolyl group, 5-isoindolyl group, 6-isoindolyl group, 7-isoindolyl group, 2-furyl group, 3-furyl group, 2-benzofuranyl group, 3-benzofuranyl group, 4-benzofuranyl group, 5-benzofuranyl group, 6-benzofuranyl group, 7-benzofuranyl group, 1-isobenzofuranyl group, 3-isobenzofuranyl group, 4-isobenzofuranyl group, 5-isobenzofuranyl group, 6-isobenzofuranyl group, 7-isobenzofuranyl group, 2-quinolyl group, 3-quinolyl group, 4-quinolyl group, 5-quinolyl group, 6-quinolyl group, 7-quinolyl group, 8-quinolyl group, 1-isoquinolyl group, 3-isoquinolyl group, 4-isoquinolyl group, 5-isoquinolyl group, 6-isoquinolyl group, 7-isoquinolyl group, 8-isoquinolyl group, 2-quinoxanyl group, 5-quinoxanyl group, 6-quinoxanyl group, 1-carbazolyl group, 2-carbazolyl group, 3-carbazolyl group, 4-carbazolyl group, 9-carbazolyl group, 1-phenanthridinyl group, 2-phenanthridinyl group, 3-phenanthridinyl group, 4-phenanthridinyl group, 6-phenanthridinyl group, 7-phenanthridinyl group, 8-phenanthridinyl group, 9-phenanthridinyl group, 10-phenanthridinyl group, 1-acridinyl group, 2-acridinyl group, 3-acridinyl group, 4-acridinyl group, 9-acridinyl group, 1,7-phenanthrolin-2-yl group, 1,7-phenanthrolin-3-yl group, 1,7-phenanthrolin-4-yl group, 1,7-phenanthrolin-5-yl group, 1,7-phenanthrolin-6-yl group, 1,7-phenanthrolin-8-yl group, 1,7-phenanthrolin-9-yl group, 1,7-phenanthrolin-10-yl group, 1,8-phenanthrolin-2-yl group, 1,8-phenanthrolin-3-yl group, 1,8-phenanthrolin-4-yl group, 1,8-phenanthrolin-5-yl group, 1,8-phenanthrolin-6-yl group, 1,8-phenanthrolin-7-yl group, 1,8-phenanthrolin-9-yl group, 1,8-phenanthrolin-10-yl group, 1,9-phenanthrolin-2-yl group, 1,9-phenanthrolin-3-yl group, 1,9-phenanthrolin-4-yl group, 1,9-phenanthrolin-5-yl group, 1,9-phenanthrolin-6-yl group, 1,9-phenanthrolin-7-yl group, 1,9-phenanthrolin-8-yl group, 1,9-phenanthrolin-10-yl group, 1,10-phenanthrolin-2-yl group, 1,10-phenanthrolin-3-yl group, 1,10-phenanthrolin-4-yl group, 1,10-phenanthrolin-5-yl group, 2,9-phenanthrolin-1-yl group, 2,9-phenanthrolin-3-yl group, 2,9-phenanthrolin-4-yl group, 2,9-phenanthrolin-5-yl group, 2,9-phenanthrolin-6-yl group, 2,9-phenanthrolin-7-yl group, 2,9-phenanthrolin-8-yl group, 2,9-phenanthrolin-10-yl group, 2,8-phenanthrolin-1-yl group, 2,8-phenanthrolin-3-yl group, 2,8-phenanthrolin-4-yl group, 2,8-phenanthrolin-5-yl group, 2,8-phenanthrolin-6-yl group, 2,8-phenanthrolin-7-yl group, 2,8-phenanthrolin-9-yl group, 2,8-phenanthrolin-10-yl group, 2,7-phenanthrolin-1-yl group, 2,7-phenanthrolin-3-yl group, 2,7-phenanthrolin-4-yl group, 2,7-phenanthrolin-5-yl group, 2,7-phenanthrolin-6-yl group, 2,7-phenanthrolin-8-yl group, 2,7-phenanthrolin-9-yl group, 2,7-phenanthrolin-10-yl group, 1-phenazinyl group, 2-phenazinyl group, 1-phenothiazinyl group, 2′-phenothiazinyl group, 3-phenothiazinyl group, 4-phenothiazinyl group, 10-phenothiazinyl group, 1-phenoxazinyl group, 2-phenoxazinyl group, 3-phenoxazinyl group, 4-phenoxazinyl group, 10-phenoxazinyl group, 2-oxazolyl group, 4-oxazolyl group, 5-oxazolyl group, 2-oxadiazolyl group, 5-oxadiazolyl group, 3-furazanyl group, 2-thienyl group, 3-thienyl group, 2-methylpyrrol-1-yl group, 2-methylpyrrol-3-yl group, 2-methylpyrrol-4-yl group, 2-methyl-pyrrol-5-yl group, 3-methylpyrrol-1-yl group, 3-methylpyrrol-2-yl group, 3-methylpyrrol-4-yl group, 3-methylpyrrol-5-yl group, 2-t-butylpyrrol-4-yl group, 3-(2-phenylpropyl)pyrrol-1-yl group, 2-methyl-1-indolyl group, 4-methyl-1-indolyl group, 2-methyl-3-indolyl group, 4-methyl-3-indolyl group, 2-t-butyl-1-indolyl group, 4-t-butyl-1-indolyl group, 2-t-butyl-3-indolyl group and 4-t-butyl-3-indolyl group.
- Examples of the aralkyl group having 7 to 30 carbon atoms include benzyl group, 1-phenylethyl group, 2-phenylethyl group, 1-phenyl-isopropyl group, 2-phenylisopropyl group, phenyl-t-butyl group, α-naphthylmethyl group, 1-α-naphthylethyl group, 2-α-naphthylethyl group, 1-α-naphthylisopropyl group, 2-α-naphthylisopropyl group, β-naphthylmethyl group, 1-β-naphthylethyl group, 2-β-naphthylethyl group, 1-β-naphthylisopropyl group, 2-β-naphthylisopropyl group, 1-pyrrolylmethyl group, 2-(1-pyrrolyl)ethyl group, p-methylbenzyl group, m-methylbenzyl group, o-methylbenzyl group, p-chlorobenzyl group, m-chlorobenzyl group, o-chlorobenzyl group, p-bromobenzyl group, m-bromobenzyl group, o-bromobenzyl group, p-iodobenzyl group, m-iodobenzyl group, o-iodobenzyl group, p-hydroxybenzyl group, m-hydroxybenzyl group, o-hydroxybenzyl group, p-aminobenzyl group, m-aminobenzyl group, o-aminobenzyl group, p-nitrobenzyl group, m-nitrobenzyl group, o-nitrobenzyl group, p-cyanobenzyl group, m-cyanobenzyl group, o-cyanobenzyl group, 1-hydroxy-2-phenylisopropyl group and 1-chloro-2-phenylisopropyl group.
- The aryloxyl group having 6 to 30 carbon atoms is represented by —OZ. Examples of the group represented by Z include phenyl group, 1-naphthyl group, 2-naphthyl group, 1-anthryl group, 2-anthryl group, 9-anthryl group, 1-phenanthryl group, 2-phenanthryl group, 3-phenanthryl group, 4-phenanthryl group, 9-phenanthryl group, 1-naphthacenyl group, 2-naphthacenyl group, 9-naphthacenyl group, 1-pyrenyl group, 2-pyrenyl group, 4-pyrenyl group, 2-biphenylyl group, 3-biphenylyl group, 4-biphenylyl group, p-terphenyl-4-yl group, p-terphenyl-3-yl group, p-terphenyl-2-yl group, m-terphenyl-4-yl group, m-terphenyl-3-yl group, m-terphenyl-2-yl group, o-tolyl group, m-tolyl group, p-tolyl group, p-t-butylphenyl group, p-(2-phenylpropyl)phenyl group, 3-methyl-2-naphthyl group, 4-methyl-1-naphthyl group, 4-methyl-1-anthryl group, 4′-methylbiphenylyl group, 4″-t-butyl-p-terphenyl-4-yl group, 2-pyrrolyl group, 3-pyrrolyl group, pyradinyl group, 2-pyridinyl group, 3-pyridinyl group, 4-pyridinyl group, 2-indolyl group, 3-indolyl group, 4-indolyl group, 5-indolyl group, 6-indolyl group, 7-indolyl group, 1-isoindolyl group, 3-isoindolyl group, 4-isoindolyl group, 5-isoindolyl group, 6-isoindolyl group, 7-isoindolyl group, 2-furyl group, 3-furyl group, 2-benzofuranyl group, 3-benzofuranyl group, 4-benzofuranyl group, 5-benzofuranyl group, 6-benzofuranyl group, 7-benzofuranyl group, 1-isobenzofuranyl group, 3-isobenzofuranyl group, 4-isobenzofuranyl group, 5-isobenzofuranyl group, 6-isobenzofuranyl group, 7-isobenzofuranyl group, 2-quinolyl group, 3-quinolyl group, 4-quinolyl group, 5-quinolyl group, 6-quinolyl group, 7-quinolyl group, 8-quinolyl group, 1-isoquinolyl group, 3-isoquinolyl group, 4-isoquinolyl group, 5-isoquinolyl group, 6-isoquinolyl group, 7-isoquinolyl group, 8-isoquinolyl group, 2-quinoxanyl group, 5-quinoxanyl group, 6-quinoxanyl group, 1-carbazolyl group, 2-carbazolyl group, 3-carbazolyl group, 4-carbazolyl group, 1-phenanthridinyl group, 2-phenanthridinyl group, 3-phenanthridinyl group, 4-phenanthridinyl group, 6-phenanthridinyl group, 7-phenanthridinyl group, 8-phenanthridinyl group, 9-phenanthridinyl group, 10-phenanthridinyl group, 1-acridinyl group, 2-acridinyl group, 3-acridinyl group, 4-acridinyl group, 9-acridinyl group, 1,7-phenanthrolin-2-yl group, 1,7-phenanthrolin-3-yl group, 1,7-phenanthrolin-4-yl group, 1,7-phenanthrolin-5-yl group, 1,7-phenanthrolin-6-yl group, 1,7-phenanthrolin-8-yl group, 1,7-phenanthrolin-9-yl group, 1,7-phenanthrolin-10-yl group, 1,8-phenanthrolin-2-yl group, 1,8-phenanthrolin-3-yl group, 1,8-phenanthrolin-4-yl group, 1,8-phenanthrolin-5-yl group, 1,8-phenanthrolin-6-yl group, 1,8-phenanthrolin-7-yl group, 1,8-phenanthrolin-9-yl group, 1,8-phenanthrolin-10-yl group, 1,9-phenanthrolin-2-yl group, 1,9-phenanthrolin-3-yl group, 1,9-phenanthrolin-4-yl group, 1,9-phenanthrolin-5-yl group, 1,9-phenanthrolin-6-yl group, 1,9-phenanthrolin-7-yl group, 1,9-phenanthrolin-8-yl group, 1,9-phenanthrolin-10-yl group, 1,10-phenanthrolin-2-yl group, 1,10-phenanthrolin-3-yl group, 1,10-phenanthrolin-4-yl group, 1,10-phenanthrolin-5-yl group, 2,9-phenanthrolin-1-yl group, 2,9-phenanthrolin-3-yl group, 2,9-phenanthrolin-4-yl group, 2,9-phenanthrolin-5-yl group, 2,9-phenanthrolin-6-yl group, 2,9-phenanthrolin-7-yl group, 2,9-phenanthrolin-8-yl group, 2,9-phenanthrolin-10-yl group, 2,8-phenanthrolin-1-yl group, 2,8-phenanthrolin-3-yl group, 2,8-phenanthrolin-4-yl group, 2,8-phenanthrolin-5-yl group, 2,8-phenanthrolin-6-yl group, 2,8-phenanthrolin-7-yl group, 2,8-phenanthrolin-9-yl group, 2,8-phenanthrolin-10-yl group, 2,7-phenanthrolin-1-yl group, 2,7-phenanthrolin-3-yl group, 2,7-phenanthrolin-4-yl group, 2,7-phenanthrolin-5-yl group, 2,7-phenanthrolin-6-yl group, 2,7-phenanthrolin-8-yl group, 2,7-phenanthrolin-9-yl group, 2,7-phenanthrolin-10-yl group, 1-phenazinyl group, 2-phenazinyl group, 1-phenothiazinyl group, 2-phenothiazinyl group, 3-phenothiazinyl group, 4-phenothiazinyl group, 1-phenoxazinyl group, 2-phenoxazinyl group, 3-phenoxazinyl group, 4-phenoxazinyl group, 2-oxazolyl group, 4-oxazolyl group, 5-oxazolyl group, 2-oxadiazolyl group, 5-oxadiazolyl group, 3-furazanyl group, 2-thienyl group, 3-thienyl group, 2-methylpyrrol-1-yl group, 2-methylpyrrol-3-yl group, 2-methylpyrrol-4-yl group, 2-methylpyrrol-5-yl group, 3-methylpyrrol-1-yl group, 3-methyl-pyrrol-2-yl group, 3-methylpyrrol-4-yl group, 3-methylpyrrol-5-yl group, 2-t-butylpyrrol-4-yl group, 3-(2-phenylpropyl)pyrrol-1-yl group, 2-methyl-1-indolyl group, 4-methyl-1-indolyl group, 2-methyl-3-indolyl group, 4-methyl-3-indolyl group, 2-t-butyl-1-indolyl group, 4-t-butyl-1-indolyl group, 2-t-butyl-3-indolyl group and 4-t-butyl-3-indolyl group.
- The alkoxycarbonyl group having 2 to 30 carbon atoms is represented by —COOY. Examples of the group represented by Y include methyl group, ethyl group, propyl group, isopropyl group, n-butyl group, s-butyl group, isobutyl group, t-butyl group, n-pentyl group, n-hexyl group, n-heptyl group, n-octyl group, hydroxymethyl group, 1-hydroxyethyl group, 2-hydroxyethyl group, 2-hydroxyisobutyl group, 1,2-dihydroxyethyl group, 1,3-dihydroxy-isopropyl group, 2,3-dihydroxy-t-butyl group, 1,2,3-trihydroxypropyl group, chloromethyl group, 1-chloroethyl group, 2-chloroethyl group, 2-chloroisobutyl group, 1,2-dichloroethyl group, 1,3-dichloroisopropyl group, 2,3-dichloro-t-butyl group, 1,2,3-trichloropropyl group, bromomethyl group, 1-bromoethyl group, 2-bromoethyl group, 2-bromoisobutyl group, 1,2-dibromoethyl group, 1,3-dibromoisopropyl group, 2,3-dibromo-t-butyl group, 1,2,3-tribromopropyl group, iodomethyl group, 1-iodoethyl group, 2-iodoethyl group, 2-iodoisobutyl group, 1,2-diiodoethyl group, 1,3-diiodoisopropyl group, 2,3-diiodo-t-butyl group, 1,2,3-triiodopropyl group, aminomethyl group, 1-aminoethyl group, 2-aminoethyl group, 2-aminoisobutyl group, 1,2-diaminoethyl group, 1,3-diaminoisopropyl group, 2,3-diamino-t-butyl group, 1,2,3-triaminopropyl group, cyanomethyl group, 1-cyanoethyl group, 2-cyanoethyl group, 2-cyanoisobutyl group, 1,2-dicyanoethyl group, 1,3-dicyanoisopropyl group, 2,3-dicyano-t-butyl group, 1,2,3-tricyanopropyl group, nitromethyl group, 1-nitroethyl group, 2-nitroethyl group, 2-nitroisobutyl group, 1,2-dinitroethyl group, 1,3-dinitroisopropyl group, 2,3-dinitro-t-butyl group and 1,2,3-trinitropropyl group.
- Examples of the divalent group forming the ring include tetramethylene group, pentamethylene group, hexamethylene group, diphenylmethane-2,2′-diyl group, diphenylethane-3,3′-diyl group and diphenylpropane-4,4′-diyl group.
- It is preferable that at least one of R5 to R8 represents:
-
- at least one group selected from:
- (1) linear and branched alkyl groups having 5 or more carbon atoms and an olefinic unsaturated bond,
- (2) linear, branched and cyclic substituted and unsubstituted alkyl groups having 4 or more carbon atoms,
- (3) linear, branched and cyclic substituted and unsubstituted alkyloxyl groups having 5 or more carbon atoms,
- (4) linear, branched and cyclic substituted and unsubstituted alkylthio groups having 5 or more carbon atoms,
- (5) linear, branched and cyclic substituted and unsubstituted alkylsilyl groups having 5 or more carbon atoms,
- (6) linear, branched and cyclic substituted and unsubstituted dialkylsilyl groups having 5 or more carbon atoms,
- (7) linear, branched and cyclic substituted and unsubstituted trialkylsilyl groups having 5 or more carbon atoms,
- (8) alkylamino groups and dialkylamino groups,
- (9) linear and branched cyano-substituted alkyl groups having 4 or more carbon atoms and 1 or 2 cyano groups, and
- (10) polyethers having 2 to 5 ether oxygen atoms which are separated from each other with an alkyl crosslinking having 1 to 3 carbon atoms, or
- a group selected from aryl groups having 6 to 30 carbon atoms, arylalkyl groups having 7 to 30 carbon atoms, heteroarylalkyl groups having at least one of nitrogen atom, oxygen atom and sulfur atom and 2 to 30 carbon atoms, heterocyclic groups having 2 to 30 carbon atoms, alkanoyl groups having 1 to 20 carbon atoms, cycloalkanoyl groups having 6 to 30 carbon atoms, acryloyl groups having 6 to 30 carbon atoms and heteroaryloxyl groups having at least one of oxygen atom and sulfur atom and 2 to 30 carbon atoms, which are substituted with at least one group selected from aforesaid (1) to (10).
- at least one group selected from:
- In general formula (1) shown above, p represents 0 or 1, q represents 0 or 1, m represents 0 or an integer of 1 to 3, and n represents an integer of 1 to 3.
- Examples of the distyrylarylene derivative represented by general formula (1) of the present invention will be shown in the following. However, the distyrylarylene derivative of the present invention is not limited to the compounds shown as the examples. In the following, C5 means n-pentyl group. The compounds shown in the following as the examples are all dissolved into 1,2-dichloroethane at 20° C. in an amount of 0.5% by weight or more.
- The organic EL device of the present invention comprises a cathode, an anode and an organic thin film layer comprising a single layer or a plurality of layers and disposed between the cathode and the anode, and at least one layer in the organic thin film layer comprises the novel soluble compound described above.
- It is preferable that, in the organic electroluminescence device which comprises a cathode, an anode and at least a light emitting layer and an electron transporting layer which are disposed between the cathode and the anode, the electron transporting layer comprises a novel soluble compound described above. It is also preferable that, in the organic electroluminescence device which comprises a cathode, an anode and at least a light emitting layer and a hole transporting layer which are disposed between the cathode and the anode, the hole transporting layer comprises a novel soluble compound described above.
- It is preferable that the light emitting layer comprises an arylamine compound or a distyrylarylene derivative.
- As the arylamine compound or the distyrylarylene derivative, it is preferable that a compound represented by the following general formula (2) or (3) is used.
- General formula (2) is:
- wherein Ar6 represents an aromatic group having 6 to 40 carbon atoms, Ar7 and Ar8 each independently represent hydrogen atom or an aromatic group having 6 to 40 carbon atoms, the groups represented by Ar6 to Ar8 may be substituted, and a represents an integer of 1 to 6, which is the number of condensation.
- General formula (3) is:
- wherein Ar9 and Ar15 each represent an aromatic group having 6 to 40 carbon atoms, Ar10 to Ar14 each independently represent hydrogen atom or an aromatic group having 6 to 40 carbon atoms, the groups represented by Ar9 to Ar15 may be substituted, and b to e each represent 0 or 1, which is the number of condensation.
- In general formulae (2) and (3) shown above, examples of the aromatic group having 6 to 40 carbon atoms include aryl groups such as phenyl group, naphthyl group, anthranyl group, phenanthryl group, pyrenyl group, coronyl group, biphenyl group, terphenyl group, pyrrolyl group, furanyl group, thiophenyl group, benzothiophenyl group, oxathiazolyl group, diphenylanthranyl group, indolyl group, carbazolyl group, pyridyl group, benzoquinolyl group, fluoranthenyl group and acenaphthofluoranthenyl group; and arylene groups such as phenylene group, naphthylene group, anthranylene group, phenanthrylene group, pyrenylene group, coronylene group, biphenylene group, terphenylene group, pyrrolylene group, furanylene group, thiophenylene group, benzothiopheylene group, oxadiazolylene group, diphenylanthranylene group, indolylene group, carbazolylene group, pyridylene group, benzoquinolylene group, fluoranthenylene group and acenaphtho-fluoranthenylene group. The aromatic group having 6 to 40 carbon atoms may be substituted. Examples of the substituent include alkyl groups having 1 to 6 carbon atoms such as ethyl group, methyl group, i-propyl group, n-propyl group, s-butyl group, t-butyl group, pentyl group, hexyl group, cyclopentyl group and cyclohexyl group; alkoxyl groups having 1 to 6 carbon atoms such as ethoxyl group, methoxyl group, i-propoxyl group, n-propoxyl group, s-butoxyl group, t-butoxyl group, pentoxyl group, hexyloxyl group, cyclopentoxyl group and cyclohexyloxyl group; aryl groups having a nucleus having 5 to 40 atoms; amino groups substituted with an aryl group having a nucleus having 5 to 40 atoms; ester groups having an aryl group having a nucleus having 5 to 40 atoms; ester groups having an alkyl group having 1 to 6 carbon atoms; cyano group; nitro group; and halogen atoms.
- It is preferable that the light emitting layer comprises an aromatic cyclic compound having styryl group. Examples of the aromatic cyclic compound include N,N′-diphenyl-N,N′-bis(4-styrylphenyl)-1,4-diamino-naphthalene, N,N′-diphenyl-N,N′-bis(4-styrylphenyl)-1,4-diamino-2,3-dimethylnaphthalene, N,N′-diphenyl-N,N′-bis(4-styrylphenyl)-3,8-diaminopyrene, N,N′-diphenyl-N,N′-bis(4-styrylphenyl)-9,10-diamino-anthracene and N,N′-diphenyl-N,N′-bis(4-styrylphenyl)-3,9-diamino perylene.
- The organic EL device of the present invention has a laminate structure having one or more organic layers laminated between the electrodes. Examples of the structure include structures of an anode/a light emitting layer/a cathode, an anode/a hole transporting layer/a light emitting layer/an electron transporting layer/a cathode, an anode/a hole transporting layer/a light emitting layer/a cathode and an anode/a light emitting layer/an electron transporting layer/a cathode. The compound described in the present invention may be used in any of the above organic thin film layers and may also be used by doping into other hole transporting materials, light emitting materials and electron transporting materials.
- The electron transporting material used for the electron transporting layer in the organic EL device of the present invention is not particularly limited, and compounds conventionally used as the electron transporting material can be used without particular restrictions. Examples of such compounds include oxadiazole derivatives and triazole derivatives such as 2-(4-biphenylyl)-5-(4-t-butylphenyl)-1,3,4-oxadiazole and bis{2-(4-t-butylphenyl)-1,3,4-oxadiazole}-m-phenylene and quinolinol-based metal complexes. As an inorganic compound constituting the electron transporting layer, insulating materials and semiconductors are preferable. When the electron transporting layer is constituted with the insulating material or the semiconductor, leak of electric current can be effectively prevented, and the electron injecting property can be improved. It is preferable that at least one metal compound selected from the group consisting of alkali metal chalcogenides, alkaline earth metal chalcogenides, alkali metal halides and alkaline earth metal halides is used as the insulating material. It is preferable that the electron transporting layer is constituted with the alkali metal chalcogenide or the like material since the electron injecting property can be further improved.
- Examples of the alkali metal chalcogenide include Li2O, LiO, Na2S, Na2Se and NaO. Preferable examples of the alkaline earth metal chalcogenide include CaO, BaO, SrO, BeO, BaS and CaSe. Examples of the alkali metal halide include LiF, NaF, KF, LiCl, KCl and NaCl. Examples of the alkaline earth metal halide include fluorides such as CaF2, BaF2, SrF2, MgF2 and BeF2 and halides other than the fluorides.
- Examples of the semiconductor constituting the electron transporting layer include oxides, nitrides and oxide nitrides containing at least one element selected from Ba, Ca, Sr, Yb, Al, Ga, In, Li, Na, Cd, Mg, Si, Ta, Sb and Zn, which are used singly or as a combination of two or more. It is preferable that the inorganic compound constituting the electron transporting layer is in the form of a fine crystalline or amorphous insulating thin film. When the electron transporting layer is constituted with the above insulating thin film, a more uniform thin film can be formed and defective pixels such as dark spots can be decreased. Examples of the inorganic compound include the alkali metal chalcogenides, the alkaline earth metal chalcogenides, the alkali metal halides and the alkaline earth metal halides which are described above.
- In the organic EL device of the present invention, it is preferable that a region transporting electrons or an interface region between the cathode and a layer of an organic thin film comprises a reducing dopant having a work function of 2.9 eV or smaller. The reducing dopant is defined as a substance which can reduce the electron transporting compound. Therefore, various types of substances can be used as long as the substance has the specific reducing property. For example, at least one substance selected from the group consisting of alkali metals, alkaline earth metals, rare earth metals, oxides of alkali metals, halides of alkali metals, oxides of alkaline earth metals, halides of alkaline earth metals, oxides of rare earth metals, halides of rare earth metals, organic complexes of alkali metals, organic complexes of alkaline earth metals and organic complexes of rare earth metals, can be used.
- Specific examples of the reducing dopant include at least one alkali metal selected from the group consisting of Na (the work function: 2.36 eV), K (the work function: 2.28 eV), Rb (the work function: 2.16 eV) and Cs (the work function: 1.95 eV) and at least one alkaline earth metal selected from the group consisting of Ca (the work function: 2.9 eV), Sr (the work function: 2.0 to 2.5 eV) and Ba (the work function: 2.52 eV). Among these reducing dopants, reducing dopants having a work function of 2.9 eV or smaller are preferable. The more preferable reducing dopants are at least one alkali metal selected from the group consisting of K, Rb and Cs. The still more preferable reducing dopants are Rb and Cs, and the most preferable reducing dopant is Cs. These alkali metals have particularly great reducing ability, and the luminance of emitted light and the life of the organic EL device are improved by adding these alkali metals in a relatively small amount into the region of electron injection.
- As the reducing dopant having a work function of 2.9 eV or smaller, combinations of two or more alkali metals are also preferable, and combinations including Cs such as combinations of Cs and Na, Cs and K, Cs and Rb, and Cs, Na and K are more preferable. When Cs is include in the combination, the reducing ability can be efficiently exhibited, and the luminance of emitted light and the life of the organic EL device can be improved by adding the combination into the region of electron injection.
- The anode of the organic EL device plays the role of injecting holes into the hole transporting layer or the light emitting layer. It is effective that the anode has a work function of 4.5 eV or greater. Examples of the material of the anode used in the present invention include indium tin oxide alloys (ITO), tin oxides (NESA), gold, silver, platinum and copper. As the cathode, a material having a small work function is preferable so that electrons can be injected into the electron transporting layer or the light emitting layer. The material of the cathode is not particularly limited. Examples of the material of the cathode include indium, aluminum, magnesium, magnesium-indium alloys, magnesium-aluminum alloys, aluminum-lithium alloys, aluminum-scandium-lithium alloys and magnesium-silver alloys.
- The process for forming the layers in the organic EL device of the present invention is not particularly limited. A conventional process such as the vacuum vapor deposition process and the spin coating process can be used.
- The organic thin film layer comprising the compound represented by the above general formula (1) which is used in the organic EL device of the present invention can be formed in accordance with the vacuum vapor deposition process, the molecular beam epitaxy process (the MBE process) or, using a solution prepared by dissolving the compound into a solvent, in accordance with a conventional coating process such as the dipping process, the spin coating process, the casting process, the bar coating process and the roll coating process. In particular, the organic EL device exhibiting a great efficiency of light emission can be obtained in accordance with a wet process such as the spin coating process and the dipping process.
- The thickness of each layer in the organic thin film layer in the organic EL device of the present invention is not particularly limited. In general, an excessively thin layer tends to have defects such as pin holes, and an excessively thick layer requires a high applied voltage to decrease the efficiency. Therefore, a thickness in the range of several nm to 1 μm is preferable.
- The present invention will be described more specifically with reference to examples in the following. However, the present invention is not limited to the examples.
- The route of synthesis of Compound (A1) (9,10-bis[4-(2,2-diphenyl-ethenyl)phenyl]-2,6-di(2-ethylhexyloxy)anthracene) is shown in the following.
- Into a 500 ml flask, 10 g (42 mmole) of 2,6-dihydroxyanthraquinone, 16.5 g (86 mmole) of 2-ethylhexyl bromide, 12 g (87 mmole) of anhydrous potassium carbonate and 200 ml of dimethylformamide (DMF) were placed, and the resultant mixture was heated at 90° C. under stirring for one night. After the reaction was completed, DMF was removed by distillation, and 50 ml of water was added. The reaction solution was treated by extraction with diethyl ether, washed with a saturated aqueous solution of sodium chloride and dried with magnesium sulfate. After the concentration under a reduced pressure, the obtained crude product was recrystallized from methanol, and 12.5 g of the quinone compound of the object compound was obtained (the yield: 65%; a yellow powder).
- Into a 200 ml flask, 7.5 g (16 mmole) of 2,6-di(2-ethylhexyloxy)-anthraquinone, 8 g (67 mmole) of tin and 37.5 ml of acetic acid were placed, and the resultant mixture was heated under the refluxing condition for 2 hours. After the reaction solution was cooled to the room temperature, the uppermost layer was separated by decantation, and the solid components were washed with methylene chloride. The obtained organic layers were combined, washed with water, a saturated aqueous solution of sodium hydrogencarbonate and a saturated aqueous solution of sodium chloride and dried with magnesium sulfate. After the solvent was removed, 7.2 g of a yellow solid was obtained.
- In a 200 ml three-necked flask, the obtained yellow solid was dissolved into 20 ml of isopropyl alcohol (IPA). To the resultant solution, a solution prepared by dissolving 0.65 g (17 mmole) of NaBH4 into 30 ml of IPA was slowly added dropwise, and the obtained solution was heated under stirring for one night. After the reaction was completed, water was added to the reaction solution. The formed precipitates were separated by filtration and washed with water and ethanol, and 5.5 g of the anthracene compound of the object compound was obtained (the yield: 78%; a yellow powder).
- Into a 200 ml three-necked flask, 2.7 g (6 mmole) of 2,6-di(2-ethylhexyloxy)anthracene and 20 ml of DMF were placed and cooled to 0° C. To the obtained suspension, a solution prepared from 2.3 g (12 mmole) of N-bromosuccinimide (NBS) into 5 ml of DMF was slowly added dropwise, and the resultant mixture was stirred at the room temperature for one night. After the reaction was completed, the reaction solution was poured into 100 ml of water, and the resultant mixture was treated by extraction with methylene chloride. The organic layer was washed with a saturated solution of sodium hydrogencarbonate and a saturated solution of sodium chloride and dried with magnesium sulfate. After the concentration under a reduced pressure, the obtained dark brown residual product was purified in accordance with the silica gel chromatography (the developing solvent: hexane), and 1.1 g of the dibromo compound of the object compound was obtained (the yield: 30%; a yellow powder).
- Into a 500 ml three-necked flask equipped with a condenser, 0.16 g (6.6 mmole) of magnesium, a small piece of iodine and 10 ml of tetrahydrofuran (THF) were placed under a stream of argon. After the resultant mixture was stirred at the room temperature for 30 minutes, a solution prepared by dissolving 1 g (3 mmole) of 1-(4-bromophenyl)-2,2-diphenylethylene into 10 ml of THF was added dropwise. After the addition was completed, the resultant mixture was stirred at 60° C. for 1 hours, and a Grignard reagent was prepared.
- Into a 500 ml flask equipped with a condenser, 0.6 g (1 mmole) of 9,10-dibromo-2,6-di(2-ethylhexyloxy)anthracene, 0.04 g (5% by mole) of dichlorobis(triphenylphosphine)palladium, 0.1 ml (1 M; 0.1 mmole) of a toluene solution of diisobutylaluminum hydride and 10 ml of THF were placed under a stream of argon. After the Grignard reagent prepared above was added dropwise to the obtained solution at the room temperature, the resultant mixture was heated under stirring for one night. After the reaction was completed, the reaction solution was cooled with ice water. The formed crystals were separated by filtration and washed with 50 ml of methanol and 50 ml of acetone successively, and 0.56 g of a yellow powder was obtained. The obtained yellow powder was identified to be Compound (A1) by the measurements in accordance with NMR, IR and the filed desorption mass spectroscopy (FD-MS) (the yield: 60%).
- The route of synthesis of Compound (A2) (9,10-bis[4-(2,2-diphenyl-ethenyl)phenyl]-2,6/2,7-di-t-butylanthracene) is shown in the following.
- Into a 500 ml three-necked flask, 36 g (176 mmole) of 4-t-butyl-phthalic anhydride, 27 g (200 mmole) of t-butylbenzene and 100 ml of dichloroethane were placed under a stream of argon and cooled to 0° C. To the obtained mixture, 56 g (420 mmole) of aluminum chloride was slowly added. After the addition was completed, the resultant mixture was stirred at the room temperature for one night. After the reaction was completed, ice was added slowly, and then concentrated hydrochloric acid was added. The formed precipitates were separated by filtration and washed well with water, and 32 g of the benzoic acid compound of the object compound was obtained (the yield: 54%; a white powder).
- Into a 500 ml flask having the egg plant shape equipped with a condenser, 200 ml of polyphosphoric acid was placed and heated at 150° C. Then, 32 g (95 mmole) of 4-t-butyl-2-(4-t-butylbenzoyl)benzoic acid was added in small portions, and the resultant mixture was stirred at the same temperature for 3 hours. After the reaction was completed, the reaction mixture was poured into ice water, and the resultant mixture was treated by liquid-liquid extraction with chloroform. After being dried with magnesium sulfate, the extract was concentrated under a reduced pressure by a rotary evaporator. The obtained crude crystals were recrystallized from hexane, and 21 g of the anthraquinone compound of the object compound was obtained (the yield: 69%; yellow crystals).
- Into a 300 ml flask, 10 g (313 mmole) of di-t-butylanthraquinone, 18 g (151 mmole) of tin and 50 ml of glacial acetic acid were placed, and the resultant mixture was heated under stirring. After the reaction was completed, the reaction solution was poured into ice water, and the resultant mixture was stirred for 30 minutes and treated by extraction with methylene chloride. After being dried with magnesium sulfate, the extract was concentrated under a reduced pressure by a rotary evaporator. The obtained oily solid substance was used for the reaction of the next step without purification.
- In a 500 ml three-necked flask, the oily solid substance obtained above was dissolved into 110 ml of IPA. To the resultant solution, 13 g (333 mmole) of NaBH4 was slowly added, and the obtained mixture was heated under stirring for one night. After the reaction was completed, water was added to the reaction solution. The formed precipitates were separated by filtration and washed with water and ethanol, and 8.8 g of the anthracene compound of the object compound was obtained (the yield: 97%; a yellow powder).
- Into a 300 ml flask, 4 g (13.8 mmole) of di-t-butylanthracene and 150 ml of carbon tetrachloride were placed, and 1.42 ml (27 mmole) of bromine was added dropwise. After the resultant mixture was stirred at the room temperature for one night, the reaction solution was poured into 200 ml of water, and the resultant mixture was treated by extraction with methylene chloride. The organic layer was washed with water, a saturated aqueous solution of sodium hydrogencarbonate and a saturated aqueous solution of sodium chloride and dried with magnesium sulfate. After the organic layer was concentrated under a reduced pressure, the obtained yellow solid was recrystallized from ethanol, and 6 g of the dibromoanthracene compound of the object compound was obtained (the yield: 97%; a yellow powder).
- Into a 500 ml three-necked flask equipped with a condenser, 0.16 g (6.6 mmole) of magnesium, a small piece of iodine and 10 ml of THF were placed under a stream of argon. After the resultant mixture was stirred at the room temperature for 30 minutes, a solution prepared by dissolving 1 g (3 mmole) of 1-(4-bromophenyl)-2,2-diphenylethylene into 10 ml of THF was added dropwise. After the addition was completed, the resultant mixture was stirred at 60° C. for 1 hours, and a Grignard reagent was prepared.
- Into a 500 ml three-necked flask equipped with a condenser, 0.45 g (1 mmole) of 2,6/2,7-di-t-butyl-9,10-dibromoanthracene, 0.04 g (5% by mole) of dichlorobis(triphenylphosphine)palladium, 0.1 ml (1M; 0.1 mmole) of a toluene solution of diisobutylaluminum hydride and 10 ml of THF were placed under a stream of argon. After the Grignard reagent prepared above was added dropwise to the obtained solution at the room temperature, the resultant mixture was heated under stirring for one night. After the reaction was completed, the reaction solution was cooled with ice water. The formed crystals were separated by filtration and washed with 50 ml of methanol and 50 ml of acetone successively, and 0.4 g of a yellow powder was obtained. The obtained yellow powder was identified to be Compound (A2) by the measurements in accordance with NMR, IR and FD-MS (the yield: 50%).
- Under the atmosphere of argon, 4-(2,2-diphenylvinyl)bromobenzene (10 g, 30 mmole, 3 eq) was dissolved into a mixed solvent composed of anhydrous toluene (45 ml) and anhydrous THF (45 ml), and the resultant solution was cooled to −20° C. in a dry ice/methanol bath. To the cooled solution, a hexane solution of n-butyllithium (1.59 mmole/liter, 20 ml, 32 mmole, 1.06 eq) was added, and the obtained solution was stirred at −20° C. for 1 hour. To the resultant solution, 2-(2-phenyl-2-)propyl-anthraquinone (3.5 g, 11 mmole) was added, and the obtained mixture was stirred at the room temperature for 3 hours and then left standing for one night. To the resultant reaction mixture, a saturated aqueous solution of ammonium chloride (50 ml) was added. The organic layer was separated, washed with a saturated aqueous solution of sodium chloride and dried with magnesium sulfate. After the solvent was removed by distillation, the product was purified in accordance with the column chromatography (silica gel; hexane+50% dichloromethane, dichloromethane and finally dichloromethane+3% methanol), and a light yellow amorphous solid was obtained (5.7 g; the yield: 67%).
- 2-(2-Phenyl-2-propyl)-9,10-bis(4-(2,2-diphenylvinyl)phenyl)-9,10-dihydro-9,10-dihydroxyanthracene (5.7 g, 6.7 mmole), potassium iodide (3.3 g, 20 mmole) and sodium phosphinate monohydrate (1.1 g, 10 mmole) were dissolved into acetic acid (50 ml), and the resultant solution was stirred at 100° C. The reaction mixture was diluted with water (50 ml) and treated by extraction with toluene (300 ml). The organic layer was washed with a saturated aqueous solution of sodium chloride (50 ml) and dried with magnesium sulfate. After the solvent was removed by distillation, the obtained product was purified in accordance with the column chromatography (silica gel; hexane+30% dichloromethane), and a light yellow solid was obtained (4.5 g; 82%). The obtained product was identified to be Compound (A21) by the measurements in accordance with 1H-NMR and FD-MS.
-
- Into a three-necked flask, 3,5-dichlorobenzene-1-boronic acid (3.0 g), 9-bromoanthracene (4.47 g) and Pd(PPh3)4 (0.54 g) were placed, and the system was purged with argon. To the resultant mixture, toluene (20 ml) and an aqueous solution (2.4 ml) of sodium carbonate (5.02 g) were added, and the obtained mixture was heated under the refluxing condition for 7 hours. The reaction solution was treated by extraction with toluene, and the extract was concentrated under a reduced pressure. The obtained solid was washed with ethanol, and Compound 1 was obtained (the amount of the product: 4.52 g; the yield: 89%).
- 1H-NMR (CDCl3): δ (ppm) 8.51 (s, 1H), 8.2-8.0 (m, 2H), 7.8-7.0 (m. 9H)
-
- Into a flask purged with argon, 4-bromo-4′-n-pentylbiphenyl (5.0 g) and anhydrous ether (50 ml) were placed and cooled to −20° C. To the cooled solution, a 1.6 M hexane solution (15.2 ml) of n-butyllithium was slowly added dropwise. After 30 minutes, the temperature was elevated to the room temperature, and the mixture was stirred at the room temperature for 1 hour. The obtained reaction solution was added dropwise into an anhydrous ether solution (80 ml) of triisopropyl borate (8.28 g) at −20° C. The temperature was elevated to the room temperature, and the reaction solution was stirred for one night. To the resultant reaction solution, 2N hydrochloric acid was added, and the obtained mixture was stirred for 1 hour. The organic layer was separated and concentrated under a reduced pressure. The obtained solid was purified in accordance with the silica gel column chromatography (the solvent for elution: hexane/ethyl acetate=3/1, 2/1 and 0/1 used in this order), and Compound 2 was obtained (the amount of the product: 1.77 g; the yield: 40%).
- 1H-NMR (CDC3): δ (ppm) 8.3 (d, 1H), 7.9-7.5 (m, 6H), 7.3 (m, 1H), 4.6 (s, 2H), 2.6 (t, 2H), 1.8-1.2 (t, 6H), 0.9 (t, 3H)
-
- Into a three-necked flask purged with argon, Compound 1 (0.75 g), Compound 2 (1.50 g), Ni(dppf)Cl2 (64 mg), tripotassium phosphate (2.84 g) and dioxane (20 ml) were placed, and the resultant mixture was heated under the refluxing condition for 11 hours. To the reaction solution, water (100 ml) was added, and a solid was separated. The separated solid was washed with ethanol and purified in accordance with the silica gel column chromatography (the solvent for elution: hexane/methylene chloride=4/1), and Compound 3 was obtained (the amount of the product: 0.80 g; the yield: 49%).
- 1H-NMR (CDCl3): δ (ppm) 8.6 (s, 1H), 8.2-7.2 (m, 27H), 2.6 (t, 2H), 1.8-1.2 (m, 6H), 0.9 (t, 3H)
-
- Compound 3 (0.80 g) was dissolved into N,N-dimethylformamide (20 ml). N-bromosuccinimide (0.24 g) was added, and the resultant solution was stirred at the room temperature for one day. The obtained solution was treated by extraction by adding water (100 ml) and methylene chloride (100 ml), and the obtained organic layer was washed with 1 N hydrochloric acid (twice each with 50 ml).
- The organic layer was concentrated under a reduced pressure. The obtained solid was purified in accordance with the silica gel column chromatography (the solvent for elution: hexane/methylene chloride=1/1), and Compound 4 was obtained (the amount of the product: 0.98 g; the yield: 110%). Although Compound 4 contained N,N-dimethylformamide, Compound 4 was used for the reaction in the next step without further treatments.
- 1H-NMR (CDCl3): δ (ppm) 8.6 (d, 1H), 8.2-7.2 (m, 27H), 2.7 (t, 2H), 1.8-1.2 (m, 6H), 0.9 (t, 3H)
-
- Into a flask, Compound 4 (0.98 g), 4-(2,2-diphenylethenyl)phenyl-boric acid (0.41 g) and Pd(PPh3)4 (40 mg) were placed under the atmosphere of argon. To the resultant mixture, toluene (10 ml) and an aqueous solution (1.7 ml) of sodium carbonate (0.37 g) were added, and the obtained mixture was heated at 80° C. for 6.5 hours. The reaction mixture was treated by extraction by adding water (100 ml) and methylene chloride (100 ml), and the organic layer was concentrated under a reduced pressure. The obtained solid was purified in accordance with the silica gel column chromatography (the solvent for elution: hexane), and Compound (A22) was obtained (the amount of the product: 0.86 g; the yield: 78%).
- 1H-NMR (CDCl3): δ (ppm) 8.0-7.2 (m, 39H), 2.6 (t, 2H), 1.8-1.2 (m, 6H), 0.9 (t, 3H)
- A glass substrate (manufactured by GEOMATEC Company) of 25 mm×75 mm×1.1 mm thickness having an ITO transparent electrode was cleaned by application of ultrasonic wave in isopropyl alcohol for 5 minutes and then by exposure to ozone generated by ultraviolet light for 30 minutes. The glass substrate having the transparent electrode lines which had been cleaned was attached to a substrate holder of a vacuum vapor deposition apparatus. On the surface of the cleaned substrate at the side having the transparent electrode lines, a light emitting layer was formed using a dichloroethane solution (1.5% by weight) containing 2 parts by weight of Compound (A1) and 1 part by weight of an arylamine compound shown below in accordance with the spin coating process. The formed light emitting layer had a thickness of 120 nm. On the formed light emitting layer, a film of tris(8-quinolinol)aluminum (Alq film) having a thickness of 10 nm was formed. The Alq film worked as the electron injecting layer. Thereafter, Li (the source of lithium: manufactured by SAES GETTERS Company) as the reducing dopant and Alq were binary vapor deposited, and an Alq:Li film was formed as the electron injecting layer (the cathode). On the formed Alq:Li film, metallic aluminum was vapor deposited to form a metal cathode, and an organic EL device was prepared. The obtained device emitted bluish green light with a luminance of 150 cd/m2 under application of a direct voltage of 7 V, and the efficiency of light emission was as excellent as 2.67 lumen/W.
- Organic EL devices were prepared in accordance with the same procedures as those conducted in Example 1 except that compounds shown in Table 1 were used in place of Compound (A1) used in Example 1. The luminance of the emitted light, the efficiency of light emission and the color of the emitted light exhibited by the prepared devices under application of a direct voltage of 6 V are shown in Table 1.
-
TABLE 1 Luminance Efficiency of emitted of light Color of Voltage light emission emitted Compound (V) (cd/m2) (lumen/W) light Example 2 (A2) 6 130 1.94 blue Example 3 (A7) 6 161 2.34 blue Example 4 (A9) 6 95 1.02 blue Example 5 (A10) 6 210 2.56 blue Example 6 (A14) 6 120 1.87 bluish green Example 7 (A17) 6 115 1.13 blue Example 8 (A20) 6 313 2.65 bluish green - As shown in Table 1, the organic EL devices in Example 2 to 8 exhibited excellent efficiencies of light emission. This result was obtained since the novel soluble compound comprising the distyrylarylene derivative of the present invention used in the light emitting layer had the specific central group having a soluble substituent.
- An organic EL devices was prepared in accordance with the same procedures as those conducted in Example 1 except that the following compound described in Japanese Patent Application Laid-Open No. 2000-143589:
- was used in place of Compound (A1) used in Example 1. The obtained device exhibited an efficiency of light emission under application of a direct voltage of 6 V of 0.81 lumen/W, which was markedly smaller than those in Examples.
- In the same procedures as those conducted in Example 1, 4,4″-bis(2,2-diphenylethenyl)-9′,10′-diphenylanthracene was used in place of Compound (A1) and attempted to be dissolved in organic solvents, which were toluene, xylene, N-methylpyrrolidone, γ-butyrolactone, 1,3-dimethyl-2-imidazoline, carbitol acetate, butylcarbitol acetate, dichloromethane, dichloroethane, chlorobenzene and isopropyl alcohol. However, this compound was hardly soluble in these solvents since the solubility was smaller than 0.01% by weight in all of these solvents, and no device could be prepared.
- A glass substrate (manufactured by GEOMATEC Company) of 25 mm×75 mm×1.1 mm thickness having an ITO transparent electrode was cleaned by application of ultrasonic wave in isopropyl alcohol for 5 minutes and then by exposure to ozone generated by ultraviolet light for 30 minutes. The glass substrate having the transparent electrode lines which had been cleaned was attached to a substrate holder of a vacuum vapor deposition apparatus. On the surface of the cleaned substrate at the side having the transparent electrode lines, a light emitting layer was formed using a dichloroethane solution (2% by weight) containing 4 parts by weight of Compound (A1) and 1 part by weight of a styrylamine compound shown below in accordance with the spin coating process. The formed light emitting layer had a thickness of 130 nm. On the formed light emitting layer, a film of tris(8-quinolinol)aluminum (Alq film) having a thickness of 10 nm was formed. The Alq film worked as the electron injecting layer. Thereafter, Li (the source of lithium: manufactured by SAES GETTERS Company) as the reducing dopant and Alq were binary vapor deposited, and an Alq:Li film was formed as the electron injecting layer (the cathode). On the formed Alq:Li film, metallic aluminum was vapor deposited to form a metal cathode, and an organic EL device was prepared. The obtained device emitted bluish green light with a luminance of 250 cd/m2 under application of a direct voltage of 7 V, and the efficiency of light emission was as excellent as 1.91 lumen/W.
- As described above in detail, the organic EL device exhibiting a great efficiency of light emission can be produced easily by utilizing the novel soluble compound of the present invention since the organic thin film layer can be formed in accordance with the wet process.
- Therefore, the organic electroluminescence device of the present invention is very useful as the inexpensive light source for various electronic instruments.
Claims (15)
1. A soluble distyrylarylene derivative represented by general formula (1) and has a solubility (20° C.) of 0.5% by weight or greater in an organic solvent, the general formula (1) being:
wherein Ar1, Ar2, Ar4 and Ar5 each independently represent a substituted or unsubstituted phenylene group, a substituted or unsubstituted naphthalene group, a substituted or unsubstituted anthracene group, a substituted or unsubstituted diphenylanthracene group, a substituted or unsubstituted phenanthrene group, a substituted or unsubstituted acenaphthene group, a substituted or unsubstituted biphenylene group, a substituted or unsubstituted fluorene group, a substituted or unsubstituted carbazolyl group, a substituted or unsubstituted thiophene group, a substituted or unsubstituted triazole group or a substituted or unsubstituted thiadiazole group;
R1 to R4 each independently represent hydrogen atom, an alkyl group having 1 to 20 carbon atoms, an alkoxyl group having 1 to 20 carbon atoms, an aryl group having 6 to 20 carbon atoms, a trialkylsilyl group having 3 to 20 carbon atoms or cyano group;
Ar3 represents a substituted or unsubstituted anthracendiyl group or a substituted;
R5 to R8 each independently represent hydrogen atom, a halogen atom, hydroxyl group, a substituted or unsubstituted amino group, nitro group, cyano group, a substituted or unsubstituted alkyl group having 1 to 30 carbon atoms, a substituted or unsubstituted alkenyl group having 2 to 30 carbon atoms, a substituted or unsubstituted cycloalkyl group having 5 to 30 carbon atoms, a substituted or unsubstituted alkoxyl group having 1 to 30 carbon atoms, a substituted or unsubstituted aromatic hydrocarbon group having 6 to 30 carbon atoms, a substituted or unsubstituted aromatic heterocyclic group having 2 to 30 carbon atoms, a substituted or unsubstituted aralkyl group having 7 to 30 carbon atoms, a substituted or unsubstituted aryloxyl group having 6 to 30 carbon atoms, a substituted or unsubstituted alkoxycarbonyl group having 2 to 30 carbon atoms or carboxyl group, groups represented by R6 and R5 or R7 and R8 may be bonded to each other and form a cyclic structure which may have substituents;
p represents 0 or 1, q represents 0 or 1, m represents 0 or an integer of 1 to 3, and n represents an integer of 1 to 3.
2. A soluble distyrylarylene derivative according to claim 1 , wherein the organic solvent is at least one solvent selected from toluene, xylene, N-methylpyrrolidone, □-butyrolactone, 1,3-dimethyl-2-imidazoline, carbitol acetate, butylcarbitol acetate, dichloromethane, dichloroethane, chloro-benzene and alcohols having 1 to 10 carbon atoms.
3. A soluble distyrylarylene derivative according to claim 1 , wherein Ar3 represents:
anthracendiyl group having at least one group selected from:
(1) linear and branched alkyl groups having 5 or more carbon atoms and an olefinic unsaturated bond,
(2) linear, branched and cyclic substituted and unsubstituted alkyl groups having 4 or more carbon atoms,
(3) linear, branched and cyclic substituted and unsubstituted alkyloxyl groups having 5 or more carbon atoms,
(4) linear, branched and cyclic substituted and unsubstituted alkylthio groups having 5 or more carbon atoms,
(5) linear, branched and cyclic substituted and unsubstituted alkylsilyl groups having 5 or more carbon atoms,
(6) linear, branched and cyclic substituted and unsubstituted dialkylsilyl groups having 5 or more carbon atoms,
(7) linear, branched and cyclic substituted and unsubstituted trialkylsilyl groups having 5 or more carbon atoms,
(8) alkylamino groups and dialkylamino groups,
(9) linear and branched cyano-substituted alkyl groups having 4 or more carbon atoms and 1 or 2 cyano groups, and
(10) polyethers having 2 to 5 ether oxygen atoms which are separated from each other with an alkyl crosslinking having 1 to 3 carbon atoms, or
anthracendiyl group having a group selected from aryl groups having 6 to 30 carbon atoms, arylalkyl groups having 7 to 30 carbon atoms, heteroarylalkyl groups having at least one of nitrogen atom, oxygen atom and sulfur atom and 2 to 30 carbon atoms, heterocyclic groups having 2 to 30 carbon atoms, alkanoyl groups having 1 to 20 carbon atoms, cycloalkanoyl groups having 6 to 30 carbon atoms, acryloyl groups having 6 to 30 carbon atoms and heteroaryloxyl groups having at least one of oxygen atom and sulfur atom and 2 to 30 carbon atoms, which are substituted with at least one group selected from aforesaid (1) to (10).
4. A soluble distyrylarylene derivative according to claim 1 , wherein at least one of R5 to R8 represents:
at least one group selected from:
(1) linear and branched alkyl groups having 5 or more carbon atoms and an olefinic unsaturated bond,
(2) linear, branched and cyclic substituted and unsubstituted alkyl groups having 4 or more carbon atoms,
(3) linear, branched and cyclic substituted and unsubstituted alkyloxyl groups having 5 or more carbon atoms,
(4) linear, branched and cyclic substituted and unsubstituted alkylthio groups having 5 or more carbon atoms,
(5) linear, branched and cyclic substituted and unsubstituted alkylsilyl groups having 5 or more carbon atoms,
(6) linear, branched and cyclic substituted and unsubstituted dialkylsilyl groups having 5 or more carbon atoms,
(7) linear, branched and cyclic substituted and unsubstituted trialkylsilyl groups having 5 or more carbon atoms,
(8) alkylamino groups and dialkylamino groups,
(9) linear and branched cyano-substituted alkyl groups having 4 or more carbon atoms and 1 or 2 cyano groups, and
(10) polyethers having 2 to 5 ether oxygen atoms which are separated from each other with an alkyl crosslinking having 1 to 3 carbon atoms, or
a group selected from aryl groups having 6 to 30 carbon atoms, arylalkyl groups having 7 to 30 carbon atoms, heteroarylalkyl groups having at least one of nitrogen atom, oxygen atom and sulfur atom and 2 to 30 carbon atoms, heterocyclic groups having 2 to 30 carbon atoms, alkanoyl groups having 1 to 20 carbon atoms, cycloalkanoyl groups having 6 to 30 carbon atoms, acryloyl groups having 6 to 30 carbon atoms and heteroaryloxyl groups having at least one of oxygen atom and sulfur atom and 2 to 30 carbon atoms, which are substituted with at least one group selected from aforesaid (1) to (10).
5. A soluble according to claim 1 , wherein Ar3 represents anthracendiyl group substituted with at least two substituted or unsubstituted t-butyl groups.
6. An organic electroluminescence device which comprises a cathode, an anode and an organic thin film layer comprising a single layer or a plurality of layers and disposed between the cathode and the anode, wherein at least one layer in the organic thin film layer comprises a soluble distyrylarylene derivative described in claim 1 .
7. An organic electroluminescence device which comprises a cathode, an anode and at least a light emitting layer and an electron transporting layer which are disposed between the cathode and the anode, wherein the electron transporting layer comprises a soluble distyrylarylene derivative described in claim 1 .
8. An organic electroluminescence device which comprises a cathode, an anode and at least a light emitting layer and a hole transporting layer which are disposed between the cathode and the anode, wherein the hole transporting layer comprises a soluble distyrylarylene derivative described in claim 1 .
9. An organic electroluminescence device according to claim 6 , wherein the organic thin film layer is formed in accordance with a wet process.
10. An organic electroluminescence device according to claim 7 , wherein the light emitting layer is formed in accordance with a wet process.
11. An organic electroluminescence device according to claim 8 , wherein the light emitting layer is formed in accordance with a wet process.
12. An organic electroluminescence device according to claim 7 , wherein the light emitting layer comprises an arylamine compound.
13. An organic electroluminescence device according to claim 8 , wherein the light emitting layer comprises an arylamine compound.
14. An organic electroluminescence device according to claim 7 , wherein the light emitting layer comprises an aromatic cyclic compound having styryl group.
15. An organic electroluminescence device according to claim 8 , wherein the light emitting layer comprises an aromatic cyclic compound having styryl group.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/068,805 US20080160344A1 (en) | 2001-10-31 | 2008-02-12 | Novel soluble compound and organic electroluminescent devices |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2001334324 | 2001-10-31 | ||
| JP2001-334324 | 2001-10-31 | ||
| US10/493,236 US7357991B2 (en) | 2001-10-31 | 2002-10-29 | Soluble compound and organic electroluminescent devices |
| PCT/JP2002/011192 WO2003037836A1 (en) | 2001-10-31 | 2002-10-29 | Novel soluble compound and organic electroluminescent devices |
| US12/068,805 US20080160344A1 (en) | 2001-10-31 | 2008-02-12 | Novel soluble compound and organic electroluminescent devices |
Related Parent Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/493,236 Continuation US7357991B2 (en) | 2001-10-31 | 2002-10-29 | Soluble compound and organic electroluminescent devices |
| PCT/JP2002/011192 Continuation WO2003037836A1 (en) | 2001-10-31 | 2002-10-29 | Novel soluble compound and organic electroluminescent devices |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20080160344A1 true US20080160344A1 (en) | 2008-07-03 |
Family
ID=19149472
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/493,236 Expired - Fee Related US7357991B2 (en) | 2001-10-31 | 2002-10-29 | Soluble compound and organic electroluminescent devices |
| US12/068,805 Abandoned US20080160344A1 (en) | 2001-10-31 | 2008-02-12 | Novel soluble compound and organic electroluminescent devices |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/493,236 Expired - Fee Related US7357991B2 (en) | 2001-10-31 | 2002-10-29 | Soluble compound and organic electroluminescent devices |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US7357991B2 (en) |
| EP (1) | EP1440959A1 (en) |
| JP (1) | JPWO2003037836A1 (en) |
| KR (1) | KR20050040835A (en) |
| CN (1) | CN1575269A (en) |
| TW (1) | TW200300166A (en) |
| WO (1) | WO2003037836A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7670506B1 (en) * | 2004-12-30 | 2010-03-02 | E. I. Du Pont De Nemours And Company | Photoactive compositions for liquid deposition |
| US9312500B2 (en) | 2012-08-31 | 2016-04-12 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device |
Families Citing this family (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4170655B2 (en) * | 2002-04-17 | 2008-10-22 | 出光興産株式会社 | Novel aromatic compound and organic electroluminescence device using the same |
| CN101088181A (en) * | 2004-12-28 | 2007-12-12 | 出光兴产株式会社 | organic electroluminescent element |
| US7838127B1 (en) | 2004-12-29 | 2010-11-23 | E. I. Du Pont De Nemours And Company | Metal quinoline complexes |
| US7230107B1 (en) | 2004-12-29 | 2007-06-12 | E. I. Du Pont De Nemours And Company | Metal quinoline complexes |
| JP2006190759A (en) * | 2005-01-05 | 2006-07-20 | Idemitsu Kosan Co Ltd | Organic electroluminescent element |
| JP5528672B2 (en) * | 2005-02-16 | 2014-06-25 | マサチューセッツ インスティテュート オブ テクノロジー | Light-emitting devices containing semiconductor nanocrystals |
| CN101142275B (en) | 2005-03-14 | 2012-07-11 | 西巴特殊化学品控股有限公司 | Novel polymers |
| KR100827917B1 (en) | 2005-11-18 | 2008-05-07 | 주식회사 엘지화학 | Emitting material and organic light emitting diode using the same |
| WO2007101820A1 (en) * | 2006-03-08 | 2007-09-13 | Ciba Holding Inc. | Palladium catalyzed polymerization reaction |
| EP2046705B1 (en) | 2006-07-28 | 2015-09-16 | Basf Se | Novel polymers |
| US20080097013A1 (en) * | 2006-10-19 | 2008-04-24 | Hitachi Maxell, Ltd. | Pigmented ink composition |
| CN1931803B (en) * | 2006-10-30 | 2010-12-15 | 清华大学 | Organic electroluminescent material and its application |
| WO2008063657A2 (en) * | 2006-11-21 | 2008-05-29 | Qd Vision, Inc. | Light emitting devices and displays with improved performance |
| CN101126022B (en) * | 2007-10-08 | 2010-08-04 | 西安近代化学研究所 | Bi-vinyl anthracenes luminescent compounds |
| EP2107062A1 (en) * | 2008-04-03 | 2009-10-07 | SOLVAY (Société Anonyme) | Naphthyl-substituted anthracene derivatives and their use in organic light-emitting diodes |
| US9525148B2 (en) | 2008-04-03 | 2016-12-20 | Qd Vision, Inc. | Device including quantum dots |
| KR101995370B1 (en) | 2008-04-03 | 2019-07-02 | 삼성 리서치 아메리카 인코포레이티드 | Light-emitting device including quantum dots |
| JP5434027B2 (en) * | 2008-09-24 | 2014-03-05 | 住友化学株式会社 | Organic photoelectric conversion element |
| WO2011161416A2 (en) | 2010-06-25 | 2011-12-29 | Cambridge Display Technology Limited | Organic light-emitting composition, device and method |
| GB2499969A (en) | 2010-06-25 | 2013-09-11 | Cambridge Display Tech Ltd | Composition comprising an organic semiconducting material and a triplet-accepting material |
| KR101599961B1 (en) * | 2012-12-26 | 2016-03-04 | 제일모직 주식회사 | Monomer for hardmask composition and hardmask composition including the monomer and method of forming patterns using the hardmask composition |
| CN108586353B (en) * | 2018-06-15 | 2020-08-18 | 华南理工大学 | Organic luminescent material based on anthracene and derivatives thereof, and preparation method and application thereof |
| CN112239414B (en) * | 2020-09-29 | 2022-03-29 | 华南理工大学 | Blue organic semiconductor material based on 2, 6-di-tert-butyl anthracene and preparation method and application thereof |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6214481B1 (en) * | 1996-10-08 | 2001-04-10 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent device |
| US6395411B1 (en) * | 1998-11-12 | 2002-05-28 | Samsung Display Devices Co., Ltd. | Display device adopting light-emitting compound as color-developing substance |
| US6730419B2 (en) * | 2001-08-13 | 2004-05-04 | Samsung Sdi Co., Ltd. | Blue light emitting compound and organic electroluminescent device employing the same as color developing substance |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3175816B2 (en) | 1995-04-04 | 2001-06-11 | 出光興産株式会社 | Organic electroluminescence device |
| JP2000007604A (en) | 1998-06-18 | 2000-01-11 | Idemitsu Kosan Co Ltd | Distyrylarylene derivative and organic electroluminescence element |
| JP2000191560A (en) * | 1998-12-24 | 2000-07-11 | Idemitsu Kosan Co Ltd | Aromatic hydrocarbon compound and organic electroluminescence element using thereof |
-
2002
- 2002-10-29 JP JP2003540119A patent/JPWO2003037836A1/en not_active Withdrawn
- 2002-10-29 WO PCT/JP2002/011192 patent/WO2003037836A1/en active Application Filing
- 2002-10-29 EP EP02770292A patent/EP1440959A1/en not_active Withdrawn
- 2002-10-29 CN CNA02821384XA patent/CN1575269A/en active Pending
- 2002-10-29 KR KR1020047006399A patent/KR20050040835A/en not_active Application Discontinuation
- 2002-10-29 US US10/493,236 patent/US7357991B2/en not_active Expired - Fee Related
- 2002-10-31 TW TW091132303A patent/TW200300166A/en unknown
-
2008
- 2008-02-12 US US12/068,805 patent/US20080160344A1/en not_active Abandoned
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6214481B1 (en) * | 1996-10-08 | 2001-04-10 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent device |
| US6395411B1 (en) * | 1998-11-12 | 2002-05-28 | Samsung Display Devices Co., Ltd. | Display device adopting light-emitting compound as color-developing substance |
| US6730419B2 (en) * | 2001-08-13 | 2004-05-04 | Samsung Sdi Co., Ltd. | Blue light emitting compound and organic electroluminescent device employing the same as color developing substance |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7670506B1 (en) * | 2004-12-30 | 2010-03-02 | E. I. Du Pont De Nemours And Company | Photoactive compositions for liquid deposition |
| US9312500B2 (en) | 2012-08-31 | 2016-04-12 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device |
Also Published As
| Publication number | Publication date |
|---|---|
| US7357991B2 (en) | 2008-04-15 |
| CN1575269A (en) | 2005-02-02 |
| US20050014017A1 (en) | 2005-01-20 |
| TW200300166A (en) | 2003-05-16 |
| JPWO2003037836A1 (en) | 2005-02-17 |
| EP1440959A1 (en) | 2004-07-28 |
| WO2003037836A1 (en) | 2003-05-08 |
| KR20050040835A (en) | 2005-05-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10217943B2 (en) | Organic electroluminescence device and anthracene derivative | |
| US20080160344A1 (en) | Novel soluble compound and organic electroluminescent devices | |
| US7790892B2 (en) | Aromatic compound and organic electroluminescent element containing the same | |
| US7829206B2 (en) | Benzanthracene derivative and electroluminescence device using the same | |
| US7087322B2 (en) | Organic electroluminescence device | |
| US20050233165A1 (en) | Anthracene derivatives and organic electroluminescent devices made by using the same | |
| US20070285009A1 (en) | Bisanthracene derivative and organic electroluminescence device using the same | |
| EP1894923A1 (en) | Benzothiophene derivative and organic electroluminescence device making use of the same | |
| US20100187511A1 (en) | Aromatic amine derivative and organic electroluminescent device using the same | |
| US20060134458A1 (en) | Aromatic amine derivative and organic electroluminescence element | |
| US20080166594A1 (en) | Benzothiophene derivative and organic electroluminescence device making use of the same | |
| JP2002124385A (en) | Organic electroluminescence element | |
| US20110233534A1 (en) | Aromatic amine derivative and organic electroluminescent element comprising the same | |
| JP2882403B1 (en) | Organic electroluminescence device material and organic electroluminescence device using the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |