US20090215225A1 - Tellurium compounds useful for deposition of tellurium containing materials - Google Patents
Tellurium compounds useful for deposition of tellurium containing materials Download PDFInfo
- Publication number
- US20090215225A1 US20090215225A1 US12/392,009 US39200909A US2009215225A1 US 20090215225 A1 US20090215225 A1 US 20090215225A1 US 39200909 A US39200909 A US 39200909A US 2009215225 A1 US2009215225 A1 US 2009215225A1
- Authority
- US
- United States
- Prior art keywords
- tellurium
- silyl
- alkyl
- aryl
- alkoxy
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 0 [1*]N1C([2*])C([3*])N([4*])[Te]1([5*])[5*] Chemical compound [1*]N1C([2*])C([3*])N([4*])[Te]1([5*])[5*] 0.000 description 61
- KBQGOXLRPBXKJF-UHFFFAOYSA-N CC1S[Te]S1 Chemical compound CC1S[Te]S1 KBQGOXLRPBXKJF-UHFFFAOYSA-N 0.000 description 5
- DNEGGVXUUAVDNH-UHFFFAOYSA-N CC1SCS1 Chemical compound CC1SCS1 DNEGGVXUUAVDNH-UHFFFAOYSA-N 0.000 description 4
- LRCRVMIHDCITCE-UHFFFAOYSA-N CC1S[Te]2(S1)SC(C)S2 Chemical compound CC1S[Te]2(S1)SC(C)S2 LRCRVMIHDCITCE-UHFFFAOYSA-N 0.000 description 4
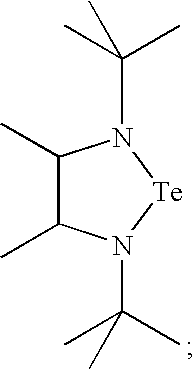
- OODBJMFMAVEECC-UHFFFAOYSA-N CC1C(C)N(C(C)(C)C)CN1C(C)(C)C Chemical compound CC1C(C)N(C(C)(C)C)CN1C(C)(C)C OODBJMFMAVEECC-UHFFFAOYSA-N 0.000 description 3
- URQMQDGJPALMML-UHFFFAOYSA-M CC1C(C)N(C(C)(C)C)[Te]N1C(C)(C)C.I.[LiH].[Li]I.[Li]N(C(C)C(C)N([Li])C(C)(C)C)C(C)(C)C.[Te]=II Chemical compound CC1C(C)N(C(C)(C)C)[Te]N1C(C)(C)C.I.[LiH].[Li]I.[Li]N(C(C)C(C)N([Li])C(C)(C)C)C(C)(C)C.[Te]=II URQMQDGJPALMML-UHFFFAOYSA-M 0.000 description 3
- WFYJFOGJKFUBEG-UHFFFAOYSA-N C.C.C.C.C.CC1SCS1.CC1S[Te]2(S1)SC(C)S2 Chemical compound C.C.C.C.C.CC1SCS1.CC1S[Te]2(S1)SC(C)S2 WFYJFOGJKFUBEG-UHFFFAOYSA-N 0.000 description 1
- NSTLTUVDJKOBEU-UHFFFAOYSA-M C.C.C.CC(C)(C)I(=[Te])=N[Si](C)(C)C.[Li]I.[Li]N(C(C)(C)C)[Si](C)(C)C.[Te]=II Chemical compound C.C.C.CC(C)(C)I(=[Te])=N[Si](C)(C)C.[Li]I.[Li]N(C(C)(C)C)[Si](C)(C)C.[Te]=II NSTLTUVDJKOBEU-UHFFFAOYSA-M 0.000 description 1
- NJVCNTJXZJRNEA-UHFFFAOYSA-M C.C.C.CC(C)(C)N([Te]N(C(C)(C)C)[Si](C)(C)C)[Si](C)(C)C.CC(C)(C)N[Si](C)(C)C.I.[LiH].[LiH].[Li]I.[Li]N(C(C)(C)C)[Si](C)(C)C.[Te]=II Chemical compound C.C.C.CC(C)(C)N([Te]N(C(C)(C)C)[Si](C)(C)C)[Si](C)(C)C.CC(C)(C)N[Si](C)(C)C.I.[LiH].[LiH].[Li]I.[Li]N(C(C)(C)C)[Si](C)(C)C.[Te]=II NJVCNTJXZJRNEA-UHFFFAOYSA-M 0.000 description 1
- HBISMDDERWIVTC-UHFFFAOYSA-N C.C.C[Si](C)(C)N([K])[Si](C)(C)C.C[Si](C)(C)N([Te]N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C.C[Si](C)(C)N[Si](C)(C)C.[KH].[Te]=II Chemical compound C.C.C[Si](C)(C)N([K])[Si](C)(C)C.C[Si](C)(C)N([Te]N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C.C[Si](C)(C)N[Si](C)(C)C.[KH].[Te]=II HBISMDDERWIVTC-UHFFFAOYSA-N 0.000 description 1
- KYWOKEHSLCIONI-UHFFFAOYSA-N C.CC1C(C)N(C(C)(C)C)[Te]N1C(C)(C)C Chemical compound C.CC1C(C)N(C(C)(C)C)[Te]N1C(C)(C)C KYWOKEHSLCIONI-UHFFFAOYSA-N 0.000 description 1
- WNEQSMIJWNYZKW-UHFFFAOYSA-O CC(C1C)N(C(C)(C)C)[TeH+]N1C(C)(C)C Chemical compound CC(C1C)N(C(C)(C)C)[TeH+]N1C(C)(C)C WNEQSMIJWNYZKW-UHFFFAOYSA-O 0.000 description 1
- WNEQSMIJWNYZKW-UHFFFAOYSA-N CC(C1C)N(C(C)(C)C)[Te]N1C(C)(C)C Chemical compound CC(C1C)N(C(C)(C)C)[Te]N1C(C)(C)C WNEQSMIJWNYZKW-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10N—ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10N70/00—Solid-state devices without a potential-jump barrier or surface barrier, and specially adapted for rectifying, amplifying, oscillating or switching
- H10N70/011—Manufacture or treatment of multistable switching devices
- H10N70/021—Formation of the switching material, e.g. layer deposition
- H10N70/023—Formation of the switching material, e.g. layer deposition by chemical vapor deposition, e.g. MOCVD, ALD
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C333/00—Derivatives of thiocarbamic acids, i.e. compounds containing any of the groups, the nitrogen atom not being part of nitro or nitroso groups
- C07C333/14—Dithiocarbamic acids; Derivatives thereof
- C07C333/16—Salts of dithiocarbamic acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C395/00—Compounds containing tellurium
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F7/00—Compounds containing elements of Groups 4 or 14 of the Periodic System
- C07F7/02—Silicon compounds
- C07F7/08—Compounds having one or more C—Si linkages
- C07F7/10—Compounds having one or more C—Si linkages containing nitrogen having a Si-N linkage
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/22—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the deposition of inorganic material, other than metallic material
- C23C16/30—Deposition of compounds, mixtures or solid solutions, e.g. borides, carbides, nitrides
- C23C16/305—Sulfides, selenides, or tellurides
Definitions
- the present invention relates to precursors for use in depositing tellurium-containing films on substrates such as wafers or other microelectronic device substrates, as well as associated processes of making and using such precursors, and source packages of such precursors.
- the present invention relates to tellurium precursors useful for depositing tellurium-containing films on substrates such as wafers or other microelectronic device substrates, as well as associated processes of making and using such precursors, and source packages of such precursors.
- the invention relates to a tellurium precursor selected from among:
- the invention relates to a compound of the formula
- the invention relates to a composition
- a composition comprising:
- Te(IV) organyls having the formula TeR 1 R 2 R 3 R 4 wherein each of R 1 , R 2 , R 3 and R 4 is the same as or different from others, and each is independently selected from H, halogen, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 3 -C 8 cycloalkyl, C 6 -C 10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- a still further aspect of the invention relates to a precursor vapor comprising vapor of a tellurium precursor selected from the group consisting of
- Another aspect of the invention relates to a method of depositing a tellurium-containing film on a substrate, comprising volatilizing a tellurium precursor to form a precursor vapor, and contacting the substrate with the precursor vapor under deposition conditions to form the tellurium-containing film on the substrate, wherein said tellurium precursor is selected from the group consisting of:
- a further aspect of the invention relates to a packaged precursor, comprising a precursor storage and vapor dispensing vessel having disposed therein a tellurium precursor selected from among:
- Yet another aspect of the invention relates to a method for the preparation of a tellurium dialkylamide compound, comprising reacting tellurium dihalide with a metal amide to yield said tellurium dialkylamide compound.
- a further aspect of the invention relates to a method of forming a GST film on a substrate, comprising depositing tellurium on the substrate from vapor of a tellurium precursor selected from among:
- the invention in another aspect relates to a method of making a PCRAM device, comprising forming a GST film on a substrate for fabrication of said device, wherein said forming comprises depositing tellurium on the substrate from vapor of a tellurium precursor selected from among:
- the invention in a further aspect relates to a tellurium compound including at least one ethylenediamide ligand, wherein tellurium is in oxidation state (II) or (IV).
- Another aspect of the invention relates to a tellurium (IV) compound selected from the group consisting of:
- An additional aspect of the invention relates to a tellurium (IV) compound of the formula:
- the invention relates to a tellurium (II) compound of the formula:
- a still further aspect of the invention relates to a method of forming a tellurium or tellurium-containing film on a substrate, comprising volatilizing a tellurium compound as described above, to form a tellurium precursor vapor, and contacting the tellurium precursor vapor with the substrate to deposit tellurium thereon.
- the invention relates to a method of making a tellurium (IV) compound, comprising the following reaction:
- a further aspect of the invention relates to a method of making a tellurium (IV) compound, comprising the following reaction:
- the invention relates to N,N′-di-tert-butylethylenediamide telluriumdichloride.
- a further aspect of the invention relates to NHTe(Cl)NMe 2 .
- the invention relates to a method of making a tellurium (II) compound, comprising one of the following reactions (A)-(C):
- a further aspect of the invention relates to a method of making a tellurium (II) compound, comprising the following reaction:
- a further aspect of the invention relates to a diorgano ditelluride compound comprising organo groups each of which is selected from C 1 -C 12 hydrocarbyl groups, silyl and substituted silyl.
- the invention also pertains to a method of forming a tellurium or tellurium-containing film on a substrate, comprising vaporizing Te 2 (t-Bu) 2 to form a corresponding vapor, and contacting said vapor with said substrate to form said tellurium or tellurium-containing film thereon.
- Still another aspect of the invention relates to a packaged tellurium reagent, comprising a reagent storage and dispensing vessel containing a tellurium reagent of a type as described above.
- the invention further relates to a method of combating pre-reaction of precursors described herein in a vapor deposition process for forming a film on a substrate, wherein the precursors described herein are susceptible to pre-reaction adversely affecting the film.
- the method involves introducing to the process a pre-reaction-combating agent selected from the group consisting of (i) heteroatom (O, N, S) organo Lewis base compounds, (ii) free radical inhibitors, and (iii) deuterium-containing reagents.
- Another aspect of the invention relates to a method of combating pre-reaction of the precursors described in a vapor deposition process in which multiple feed streams are flowed to a deposition locus to form a film on a substrate, wherein at least one of said multiple feed streams includes a precursor susceptible to pre-reaction adversely affecting the film.
- the method involves introducing to at least one of said multiple feed streams or supplied materials therefor, or to the deposition locus, a pre-reaction-combating agent selected from the group consisting of (i) heteroatom (O, N, S) organo Lewis base compounds, (ii) free radical inhibitors, and (iii) deuterium-containing reagents.
- a still further aspect of the invention relates to a composition, comprising a precursor as described herein and a pre-reaction-combating agent for said precursor, said pre-reaction-combating agent being selected from the group consisting of (i) heteroatom (O, N, S) organo Lewis base compounds, (ii) free radical inhibitors, and (iii) deuterium-containing reagents.
- the invention relates to a process wherein the pre-reaction combating reagent is introduced to passivate the surface of a growing film or slow the deposition rate, followed by reactivation using an alternative precursor or co-reactant (for example H 2 , NH 3 , plasma, H 2 O, hydrogen sulfide, hydrogen selenide, diorganotellurides, diorganosulfides, diorganoselenides, etc.).
- an alternative precursor or co-reactant for example H 2 , NH 3 , plasma, H 2 O, hydrogen sulfide, hydrogen selenide, diorganotellurides, diorganosulfides, diorganoselenides, etc.
- Pre-reaction-combating agents can be selected from the group consisting of (i) heteroatom (O, N, S) organo Lewis base compounds, (ii) free radical inhibitors, and (iii) deuterium-containing reagents.
- Another aspect of the invention relates to a vapor phase deposition process for forming a film on a substrate involving cyclic contacting of the substrate with at least one film precursor described herein that is undesirably pre-reactive in the vapor phase.
- the process involves introducing to said film during growth thereof a pre-reaction-combating reagent that is effective to passivate a surface of said film or to slow rate of deposition of said film precursor, and after introducing said pre-reaction-combating reagent, reactivating said film with a different film precursor.
- FIG. 1 is a schematic representation of a material storage and dispensing package containing a precursor of the present invention, in one embodiment thereof.
- FIG. 2 is an ORTEP diagram of the structure of NHTeCl 2 , i.e., N,N′-di-tert-butylethylenediamide telluriumdichloride.
- FIG. 3 is an ORTEP diagram of the structure of Me 2 NHTeCl 2 .
- FIG. 4 is an ORTEP diagram of the structure of NHTe(Cl)NMe 2 .
- FIG. 5 is an ORTEP diagram of the structure of Te 2 (t-Bu) 2 .
- FIG. 6 is a simultaneous thermographic analysis plot of STA TG/DSC data for Te 2 (t-BU) 2 .
- FIG. 7 is a STA of Te[N(SiMe 3 ) 2 ] 2 .
- FIG. 8 is a STA of Te[N(SiMe 3 )(t-Bu)] 2
- FIG. 9 is a schematic representation of a vapor deposition system according to one embodiment of the present invention, wherein suppression of pre-reaction of the precursors is achieved by addition of pre-reaction-combating reagent to one or more feed streams in the vapor deposition system.
- the present invention relates to tellurium precursors useful in film-forming applications, e.g., in chemical vapor deposition and atomic layer deposition applications, to form corresponding tellurium-containing films on substrates, as well as associated processes of making and using such precursors, and packaged forms of such precursors.
- film refers to a layer of deposited material having a thickness below 1000 micrometers, e.g., from such value down to atomic monolayer thickness values.
- film thicknesses of deposited material layers in the practice of the invention may for example be below 100, 10, or 1 micrometers, or in various thin film regimes below 200, 100, or 50 nanometers, depending on the specific application involved.
- the term “thin film” means a layer of a material having a thickness below 1 micrometer.
- a carbon number range e.g., in C 1 -C 12 alkyl
- identification of a carbon number range is intended to include each of the component carbon number moieties within such range, so that each intervening carbon number and any other stated or intervening carbon number value in that stated range, is encompassed, it being further understood that sub-ranges of carbon number within specified carbon number ranges may independently be included in smaller carbon number ranges, within the scope of the invention, and that ranges of carbon numbers specifically excluding a carbon number or numbers are included in the invention, and sub-ranges excluding either or both of carbon number limits of specified ranges are also included in the invention.
- C 1 -C 12 alkyl is intended to include methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl and dodecyl, including straight chain as well as branched groups of such types. It therefore is to be appreciated that identification of a carbon number range, e.g., C 1 -C 12 , as broadly applicable to a substituent moiety, enables, in specific embodiments of the invention, the carbon number range to be further restricted, as a sub-group of moieties having a carbon number range within the broader specification of the substituent moiety.
- the carbon number range C 1 -C 12 alkyl may be more restrictively specified, in particular embodiments of the invention, to encompass sub-ranges such as C 1 -C 4 alkyl, C 2 -C 8 alkyl, C 2 -C 4 alkyl, C 3 -C 5 alkyl, or any other sub-range within the broad carbon number range.
- compositions may be further specified in specific embodiments by provisos or limitations excluding specific substituents, groups, moieties or structures, in relation to various specifications and exemplifications thereof set forth herein.
- the invention contemplates restrictively defined compositions, e.g., a composition wherein R i is C 1 -C 12 alkyl, with the proviso that R i ⁇ C 4 alkyl when R j is silyl.
- the invention relates in one aspect to Te(IV) organyls useful for low temperature (T ⁇ 400° C.) deposition of Te-containing films, e.g., for forming germanium-antimony-tellurium (GST) films such as Ge 2 Sb 2 Te 5 on substrates such as wafers in the production of phase change random access memory devices.
- Te-containing films e.g., for forming germanium-antimony-tellurium (GST) films such as Ge 2 Sb 2 Te 5 on substrates such as wafers in the production of phase change random access memory devices.
- GST germanium-antimony-tellurium
- Te(IV) organyls of the invention are suitable for forming such films by techniques such as atomic layer deposition (ALD) and chemical vapor deposition (CVD).
- Preferred precursors of such type are liquid at room temperature (25° C.) and have high volatility and desirable transport properties for ALD and CVD applications.
- Te(IV) organyls having the formula TeR 1 R 2 R 3 R 4 wherein R 1 , R 2 , R 3 and R 4 are organo substituents, are employed to form Te-containing highly conformal films of superior character by a vapor deposition process such as ALD or CVD.
- Te(IV) organyls are utilized having the formula TeR 1 R 2 R 3 R 4 wherein each of R 1 , R 2 , R 3 and R 4 is the same as or different from others, and each is independently selected from H, halogen (fluorine, bromine, iodine and chlorine), C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 3 -C 8 cycloalkyl, C 6 -C 10 aryl, silyl, substituted silyl (silyl having C 1 -C 6 alkyl substituents and/or C 6 -C 10 aryl substituents), amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl.
- alkyl moiety in such aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl substituents can be C 1 -C 6 alkyl or alkyl moieties of other carbon numbers, as may be useful in a given application of such organyl compounds.
- Tellurium(IV) organyls of the invention useful for the aforementioned film-forming applications can readily be formed by the following generalized reaction:
- M is Li or MgX
- X is halide
- each R independently may be H, halogen (fluorine, bromine, iodine and chlorine), C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 3 -C 8 cycloalkyl, C 6 -C 10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl or acetylalkyl, as above described.
- the Te(IV) compounds of the invention are usefully employed as CVD/ALD precursors for the deposition of Te-containing films, e.g., by liquid delivery techniques in which such compounds are provided in compositions including suitable solvent media.
- suitable solvents for such purpose in specific applications may include, without limitation, alkanes (e.g., hexane, heptane, octane, and pentane), aromatics (e.g., benzene or toluene), and amines (e.g., triethylamine, tert-butylamine).
- the solvent medium in which the Te precursor or precursors are dissolved or suspended may be a single-component solvent or a multi-component solvent composition.
- the precursors when in a liquid state can also be delivered neat using ALD/CVD liquid delivery techniques, in which the liquid is volatilized to form a corresponding precursor vapor, which then is contacted with the substrate on which the tellurium-containing film is to be formed, under appropriate vapor deposition conditions.
- the precursors When the precursors are in a solid state, they may be volatilized for delivery using any suitable solid delivery system, such as the solid delivery and vaporizer unit commercially available under the trademark ProE-Vap from ATMI, Inc. (Danbury, Conn., USA).
- the precursor or precursors (since the invention contemplates use of multiple Te precursors of differing type) are volatilized to form the corresponding precursor vapor which then is contacted with a wafer or other substrate to deposit a tellurium-containing layer thereon.
- the precursor vapor formed from the Te precursor may be mixed with carrier or co-reactant gases in various embodiments, to obtain desired deposition thicknesses, growth rates, etc., as will be apparent to those skilled in the art.
- the present invention in various aspects involves compositions and methods in which tellurium dialkyls and ditellurium dialkyls can be utilized as tellurium source reagents, but in other aspects, non-alkyl tellurium and non-alkyl ditellurium compounds are utilized.
- the invention in a further aspect relates to a synthetic route for the preparation of tellurium amide compounds, e.g., tellurium bis-amides that are useful for low temperature deposition of tellurium amides on substrates.
- tellurium amide compounds e.g., tellurium bis-amides that are useful for low temperature deposition of tellurium amides on substrates.
- the tellurium amide compounds can be formed by reacting tellurium dihalide with two equivalents of a metal amide, according to the following reaction.
- TeI 2 is reacted with LiN(t-Bu)(SiMe 3 ) to form the reaction product Te[N(t-Bu)(SiMe 3 )] 2 .
- t-butyl is tertiary butyl
- Me is methyl
- STA NMR spectroscopy and thermal analysis
- TeI 2 is reacted with KN(SiMe 3 ) 2 to form the following reaction product, Te[N(SiMe 3 ) 2 ] 2 .
- a further aspect of the invention relates to asymmetric tellurium compounds including one alkyl substituent and a second ligand containing a heteroatom, e.g., nitrogen or sulfur.
- the second ligand may be of any suitable type, and in specific embodiments is amidinate, guanidinate, or dithiocarbamate.
- the starting material for the asymmetric tellurium compound is ITeN R 1 R 2 wherein each of R 1 and R 2 is the same as or different from the other, and each is independently selected from H, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 3 -C 8 cycloalkyl, C 6 -C 10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, as above described.
- This starting material can be synthesized by the reaction of TeI 2 with one equivalent of a lithium amide as shown below.
- halide starting materials can be formed according to the following reaction.
- the starting material ITeN R 1 R 2 is extremely sensitive to light and air when isolated as an orange solid product, however, it can be placed into solution with a suitable solvent medium, e.g., a hydrocarbon solvent medium, containing pentane, hexane or toluene, or other hydrocarbon species. Such starting material ITeN R 1 R 2 can be reacted in situ in the hydrocarbon solvent medium with an alkyl lithium reagent to obtain an asymmetric tellurium compound, as shown in the reaction below.
- a suitable solvent medium e.g., a hydrocarbon solvent medium, containing pentane, hexane or toluene, or other hydrocarbon species.
- Such starting material ITeN R 1 R 2 can be reacted in situ in the hydrocarbon solvent medium with an alkyl lithium reagent to obtain an asymmetric tellurium compound, as shown in the reaction below.
- each of R 1 , R 2 , R 3 and R 4 is the same as or different from others, and each is independently selected from H, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 3 -C 8 cycloalkyl, C 6 -C 10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R 4 can in addition be halogen or amide;
- each of R 1 , R 2 , R 3 , R 4 and R 5 is the same as or different from others, and each is independently selected from H, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 3 -C 8 cycloalkyl, C 6 -C 10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R 5 can in addition be halogen or amide; and
- each of R 1 , R 2 and R 3 is the same as or different from others, and each is independently selected from H, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 3 -C 8 cycloalkyl, C 6 -C 10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl and wherein R 3 can in addition be halogen or amide,
- the invention therefore provides asymmetric tellurium compounds including tellurium amides, amidinates, guanidinates and dithiocarbamates of a useful character for ALD or CVD deposition of tellurium or tellurium-containing films, e.g., for fabricating GST devices comprising Ge 2 Sb 2 Te 5 films.
- Another aspect of the invention relates to tellurium compounds with ethylenediamine ligands and tellurium compounds with dithiocarbamate ligands, for use in low temperature deposition applications such as fabrication of the aforementioned GST-based phase change memory devices.
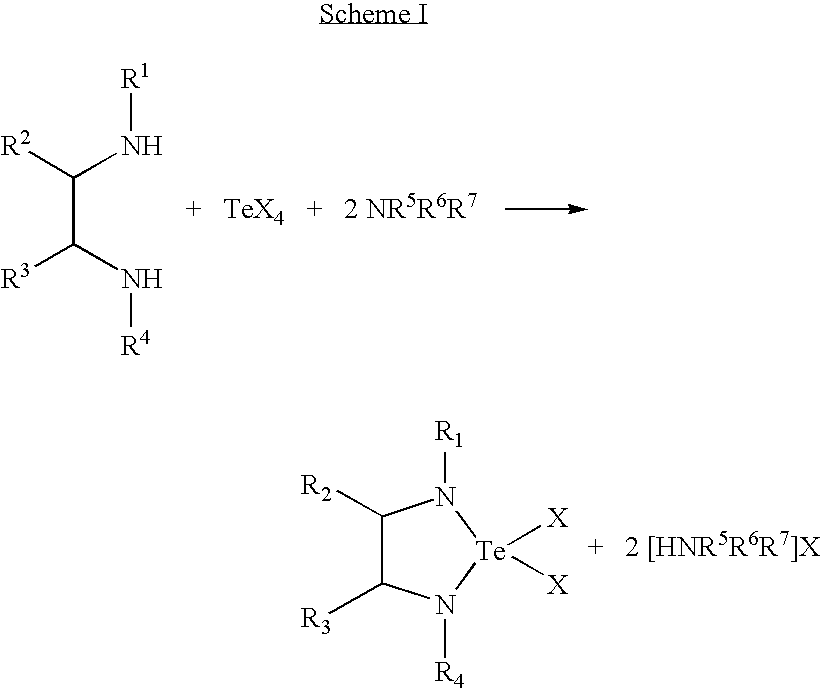
- Tellurium complexes with ethylenediamine type ligands can be synthesized, according to one preferred aspect of the invention, by reacting a lithium salt of the ethylenediamine with a tellurium halide, such as TeX 2 or TeX 4 , wherein X is halogen. From the resulting reaction product, the desired tellurium compounds can be obtained by a salt elimination reaction.
- a lithium salt of the ethylenediamine with a tellurium halide, such as TeX 2 or TeX 4 , wherein X is halogen.
- the following reaction scheme can be employed to produce the tellurium ethylenediamine precursors.
- Tellurium ethylenediamine compounds of such type have high volatility and low decomposition temperatures, and thus are well suited for ALD and CVD applications.
- Tellurium dithiocarbamate precursors of the invention include the following classes (a)-(e):
- each of R 1 , R 2 and R 3 is the same as or different from others, and each is independently selected from H, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 3 -C 8 cycloalkyl, C 6 -C 10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R 3 can in addition be halogen or amide.
- precursors accommodate low temperature deposition applications, having good volatilization and transport properties. They can be delivered in a neat form in the case of precursor compounds in liquid form, or in compositions including suitable solvent media.
- Useful solvents for such purpose in specific applications may include, without limitation, alkanes (e.g., hexane, heptane, octane, and pentane), aromatics (e.g., benzene or toluene), and amines (e.g., triethylamine, tert-butylamine) or mixtures thereof, as above described.
- the precursors when in a solid state can be volatilized for delivery using any suitable solid delivery system, such as the solid delivery and vaporizer unit commercially available under the trademark ProE-Vap from ATMI, Inc. (Danbury, Conn., USA).
- the precursor or precursors are volatilized to form the corresponding precursor vapor which then is contacted with a wafer or other substrate to deposit a tellurium-containing layer thereon, e.g., for forming a GST layer.
- the invention in yet another aspect relates to tellurium compounds with nitrogen donor ligands useful for deposition applications to deposit tellurium or tellurium-containing films on substrates, for applications such as GST phase change random access memory (PRAM) devices.
- PRAM GST phase change random access memory
- This aspect of the invention relates more specifically to Te(II) and Te(IV) precursors having at least one nitrogen-based ligand selected from among amidinates, guanidinates, isoureates and beta-diketoiminates.
- Specific tellurium nitrogen donor ligand precursors of the invention include the following:
- the tellurium compounds of the invention in film formation processes may be used with appropriate co-reactants, e.g., in a continuous deposition mode (CVD) or pulsed/atomic layer deposition mode (ALD), to deposit films of superior character.
- appropriate co-reactants include O 2 and N 2 O for CVD, and more aggressive oxidizers for pulsed deposition, e.g., H 2 O, ozone, and O 2 plasma.
- reducing atmospheres are advantageously used.
- the precursors of the invention can be utilized as low temperature deposition precursors with reducing co-reactants such as hydrogen, H 2 /plasma, amines, imines, hydrazines, silanes, germanes such as GeH 4 , ammonia, alkanes, alkenes and alkynes.
- reducing agents such as H 2 , and NH 3 are preferred, and plasmas of these co-reactants may be used in digital or ALD mode, wherein the co-reactants are separated from the precursor in a pulse train, utilizing general CVD and ALD techniques within the skill of the art, based on the disclosure herein.
- the precursor preferably exhibits self-limiting behavior in one type of atmosphere (e.g., inert or weakly reducing/oxidizing gas environments) and exhibits rapid decomposition to form a desired film in another type of atmosphere (e.g., plasma, strongly reducing/oxidizing environments).
- one type of atmosphere e.g., inert or weakly reducing/oxidizing gas environments
- exhibits rapid decomposition to form a desired film in another type of atmosphere e.g., plasma, strongly reducing/oxidizing environments.
- Liquid delivery formulations can be employed in which precursors that are liquids may be used in neat liquid form, or liquid or solid precursors may be employed in suitable solvents, including for example alkane solvents (e.g., hexane, heptane, octane, and pentane), aryl solvents (e.g., benzene or toluene), amines (e.g., triethylamine, tert-butylamine), imines and hydrazines or mixtures thereof.
- alkane solvents e.g., hexane, heptane, octane, and pentane
- aryl solvents e.g., benzene or toluene
- amines e.g., triethylamine, tert-butylamine
- imines and hydrazines or mixtures thereof e.g., triethylamine, tert
- the thicknesses of metal-containing layers formed using the precursors of the invention can be of any suitable value.
- the thickness of the tellurium-containing layer can be in a range of from 5 nm to 500 nm or more.
- the various tellurium precursor compounds of the invention can be utilized to form GST films in combination any with suitable germanium and antimony precursors, e.g., by CVD and ALD techniques, for applications such as PCRAM device manufacture.
- the process conditions useful for carrying out deposition of Te-containing films can be readily determined within the skill of the art by the simple expedient of selectively varying the delivery and deposition process conditions and characterizing the resulting films, to determine the process conditions envelope most appropriate for a given deposition application.
- Te[N(SiMe 3 ) 2 ] 2 is used as a tellurium precursor for forming tellurium-containing films on substrates, such as GST films, amorphous GeTe films, and SbTe films, by atomic layer deposition (ALD) and chemical vapor deposition (CVD) techniques.
- substrates such as GST films, amorphous GeTe films, and SbTe films
- ALD atomic layer deposition
- CVD chemical vapor deposition
- amorphous GeTe and SbTe are deposited from di-t-butyl tellurium, Te(tBu) 2 , at temperature in a range of from 300° C.-350° C., e.g., 320° C., using bubbler delivery of the telluriuim precursor in an inert carrier gas stream, e.g., N 2 at a flow rate of 20-50 sccm, e.g., 30 sccm.
- the respective germanium and antimony precursors used for such deposition can be of any suitable types, e.g., GeBAMDN, SbTDMA, etc., and such precursors can be delivered for deposition at any suitable volumetric flow rate, e.g., for the aforementioned flow rate of 30 sccm for the illustrative tellurium precursor, Te(tBu) 2 , a flow rate of such Ge or Sb precursor can be on the order of 5 micromoles/minute.
- the resulting amorphous GeTe and SbTe films will have a tellurium content of approximately 40%.
- FIG. 1 is a schematic representation of a material storage and dispensing package 100 containing a tellurium precursor, according to one embodiment of the present invention.
- the material storage and dispensing package 100 includes a vessel 102 that may for example be of generally cylindrical shape as illustrated, defining an interior volume 104 therein.
- the precursor is a solid at ambient temperature conditions, and such precursor may be supported on surfaces of the trays 106 disposed in the interior volume 104 of the vessel, with the trays having flow passage conduits 108 associated therewith, for flow of vapor upwardly in the vessel to the valve head assembly for dispensing, in use of the vessel.
- the solid precursor can be coated on interior surfaces in the interior volume of the vessel, e.g., on the surfaces of the trays 106 and conduits 108 .
- Such coating may be effected by introduction of the precursor into the vessel in a vapor form from which the solid precursor is condensed in a film on the surfaces in the vessel.
- the precursor solid may be dissolved or suspended in a solvent medium and deposited on surfaces in the interior volume of the vessel by solvent evaporation.
- the precursor may be melted and poured onto the surfaces in the interior volume of the vessel.
- the vessel may contain substrate articles or elements that provide additional surface area in the vessel for support of the precursor film thereon.
- the solid precursor may be provided in granular or finely divided form, which is poured into the vessel to be retained on the top supporting surfaces of the respective trays 106 therein.
- a metal foam body may be provided in the interior volume of the vessel, which contains porosity of a specific character adapted for retaining the solid particulate precursor for highly efficient vaporization thereof.
- the vessel 102 has a neck portion 109 to which is joined the valve head assembly 110 .
- the valve head assembly is equipped with a hand wheel 112 in the embodiment shown. In lieu of a hand wheel, the valve head assembly may in turn be coupled or operatively linked to a controller for automated operation.
- the valve head assembly 110 includes a dispensing port 114 , which may be configured for coupling to a fitting or connection element to join flow circuitry to the vessel.
- flow circuitry is schematically represented by arrow A in FIG. 1 , and the flow circuitry may be coupled to a downstream ALD or chemical vapor deposition chamber (not shown in FIG. 1 ).
- the vessel 102 can be heated with a suitable heater, such as a heating jacket, resistance heating elements affixed to the exterior wall surface of the vessel, etc., so that solid precursor in the vessel is at least partially volatilized to provide precursor vapor.
- a suitable heater such as a heating jacket, resistance heating elements affixed to the exterior wall surface of the vessel, etc.
- the input of heat is schematically shown in FIG. 1 by the reference arrow Q.
- the precursor vapor is discharged from the vessel through the valve passages in the valve head assembly 110 when the hand wheel 112 or alternative valve actuator or controller is translated so that the valve is in an open position, whereupon vapor deriving from the precursor is dispensed into the flow circuitry schematically indicated by arrow A.
- the precursor may be provided in a solvent medium, forming a solution or suspension.
- Such precursor-containing solvent composition then may be delivered by liquid delivery and flash vaporized to produce a precursor vapor.
- the precursor vapor is contacted with a substrate under deposition conditions, to deposit the metal on the substrate as a film thereon.
- the precursor is dissolved in an ionic liquid medium, from which precursor vapor is withdrawn from the ionic liquid solution under dispensing conditions.
- the precursor may be stored in an adsorbed state on a suitable solid-phase physical adsorbent storage medium in the interior volume of the vessel.
- the precursor vapor is dispensed from the vessel under dispensing conditions involving desorption of the adsorbed precursor from the solid-phase physical adsorbent storage medium.
- Supply vessels for precursor delivery may be of widely varying type, and may employ vessels such as those commercially available from ATMI, Inc. (Danbury, Conn.) under the trademarks SDS, SAGE, VAC, VACSorb, and ProE-Vap, as may be appropriate in a given storage and dispensing application for a particular precursor of the invention.
- the precursors of the invention thus may be employed to form precursor vapor for contacting with a substrate to deposit a tellurium-containing thin film thereon.
- the invention utilizes the precursors to conduct atomic layer deposition, yielding ALD films of superior conformality that are uniformly coated on the substrate with high step coverage and conformality even on high aspect ratio structures.
- the precursors of the present invention enable a wide variety of microelectronic devices, e.g., semiconductor products, flat panel displays, etc., to be fabricated with tellurium-containing films of superior quality.
- the invention in another aspect relates to a class of tellurium compounds with ethylenediamide-type ligands.
- Such tellurium compounds are useful as precursors for low temperature ALD/CVD of tellurium or tellurium-containing thin films, e.g., for applications such as fabrication of phase change memory devices based on Ge 2 Sb 2 Te 5 (GST).
- This class of compounds includes tellurium (Te) in the oxidation state IV, which is characterized by greater stability than commonly used tellurium precursors in the oxidation state II, thereby affording a beneficial alternative to the commonly used Te(II) precursors which are notoriously unstable with respect to air- and light-sensitivity.
- tellurium compounds are to our knowledge the first examples of tellurium amides in which tellurium is in oxidation state (IV).
- tellurium (IV) amides include, without limitation:
- NHTeCl 2 N,N′-di-tert-butylethylenediamide telluriumdichloride
- NHTeCl 2 2,5-Bis(tert-butyl)-2,5-diaza-1-telluracyclopentane dichloride
- MeNHTeCl 2 N-methyl,N′-tert-butylethylenediamide telluriumdichloride
- NH is an abbreviation for “N-heterocyclic,” and denotes an N-heterocyclic ring system containing tellurium.
- tellurium (IV) compounds are also potentially applicable to the synthesis of tellurium (II) compounds.
- the general synthetic scheme (Scheme I below) is based on reaction of a tellurium (IV) halide with an ethylenediamide type ligand, with the addition of a tertiary amine in order to scavenge the eliminated hydrochloride.
- Derivatives of the ethylenediamide tellurium complexes can be synthesized according to the following reaction scheme (Scheme II), by reacting the corresponding dichloride with a lithium alkyl or lithium amide species.
- One preferred solvent medium comprises an ether type solvent or other somewhat polar solvent in which the tellurium halide is sufficiently soluble.
- Tetrahydrofuran (THF) is one preferred solvent species, while diethyl ether, dimethoxyethane and toluene are also highly advantageous species.
- THF Tetrahydrofuran
- diethyl ether, dimethoxyethane and toluene are also highly advantageous species.
- the choice of a specific solvent medium may be readily empirically determined, based on considerations of solubility, yields and reaction times for specific desired tellurium precursor products.
- FIG. 2 is an ORTEP diagram of the structure of NHTeCl 2 , i.e., N,N′-di-tert-butylethylenediamide telluriumdichloride. This compound is readily purified to high purity by sublimation, and has been confirmed by X-ray crystal structure analysis as existing in a weakly associated dimer solid state.
- FIG. 3 is an ORTEP diagram of the structure of Me 2 NHTeCl 2 , also confirmed by X-ray crystal structure analysis to exist in a weakly associated dimer solid state.
- FIG. 4 is an ORTEP diagram of the structure of NHTe(Cl)NMe 2 .
- This tellurium source compound can be synthesized by a reaction scheme as described above. Such reaction scheme can be utilized to produce a mono-substituted species under mild reaction conditions, e.g., stirring of the reaction volume at room temperature, while a large excess of amide and harsher conditions, such as several days under reflux conditions, can be utilized to produce the disubstituted compound.
- tellurium precursors have utility in various applications for deposition of Te or Te-containing thin films.
- Corresponding alkyl, silyl or amide derivatives (wherein chloro substituents are replaced by alkyl or amide functional groups) can also be readily synthesized.
- Such alkyl or amide derivatives may be preferred in some thin film deposition applications, due to their higher volatility properties, in relation to corresponding chloro compounds having lower volatility as a result of their dimeric nature.
- the invention therefore contemplates the provision of tellurium compounds in which the tellurium central metal atom is coordinated with ethylenediamine-type ligands, with the tellurium central metal atom being in a (II) or (IV) state.
- the invention further contemplates synthesis of ethylenediamine-type tellurium compounds substituted with alkyl and/or amide substituents.
- the invention contemplates use of the foregoing tellurium compounds for CVD and ALD applications, to deposit tellurium or tellurium-containing thin films.
- the invention relates to dialkyl ditellurides useful for CVD and ALD applications carried out at low temperatures to deposit tellurium or tellurium-containing films on substrates, e.g., Sb 2 Te 3 films for the formation of GST films in phase change memory applications.
- the tellurium precursor employed for forming GST films desirably has the ability to deposit at sufficiently low temperature to achieve amorphous Sb 2 Te 3 films with good step coverage, since crystalline films do not provide the necessary step coverage for phase change memory device applications.
- the invention contemplates dialkyl ditellurides, e.g., di-tert-butyl ditelluride, to address such step coverage issues.
- Dialkyl tellurides are conventionally used to deposit tellurium or tellurium-containing films by CVD or ALD in phase change memory applications, but relatively high temperatures are needed to deposit such films. This deficiency can be overcome by use of dialkyl ditellurides, e.g., di-tert-butyl ditelluride, Te 2 (t-Bu) 2 .
- dialkyl ditellurides e.g., Te 2 (t-Bu) 2
- t-Bu Te 2 (t-Bu) 2
- the invention therefore contemplates the use of these dialkyl ditellurides, e.g., Te 2 (t-Bu) 2 , as precursors for the deposition of tellurium or tellurium-containing films on substrates, using CVD or ALD techniques, in applications such as the manufacture of phase change memory devices.
- FIG. 5 is an ORTEP diagram of the structure of Te 2 (t-Bu) 2 .
- FIG. 6 is a simultaneous thermal analysis (STA) plot of thermogravimetry (TG) and differential scanning calorimetry (DSC) data for Te 2 (t-Bu) 2 .
- STA simultaneous thermal analysis
- the alkyl moieties of the dialkyl ditelluride compounds of the invention can be of any suitable type, e.g., C 1 -C 8 alkyl substituents. Examples include methyl, ethyl, isopropyl, and t-butyl. Preferably, such alkyl substituents include tertiary carbon moieties. Tertiary butyl or tertiary carbons in general are preferred as having high radical stability.
- dialkyl ditelluride compounds of the invention include dimethyl ditelluride, diethyl ditelluride, diisopropyl ditelluride, and di-tertiary-butyl ditelluride.
- ditelluride compounds contemplated by the invention can utilize other ligands, such as C 1 -C 12 hydrocarbyl (aryl, fluoroalkyl, allyl, alkenyl, dienyl), or silyl or substituted silyl ligands.
- ligands such as C 1 -C 12 hydrocarbyl (aryl, fluoroalkyl, allyl, alkenyl, dienyl), or silyl or substituted silyl ligands.
- tellurium precursor compounds of the invention can be used for chemical vapor deposition and/or atomic layer deposition, to form tellurium or tellurium-containing films on substrates, e.g., semiconductor wafers or other microelectronic device base structures.
- Such precursor compounds when present in solid phase can be delivered by solid delivery techniques, wherein the solid precursor is contained in a precursor storage and vapor delivery vessel, which is subjected to heating to volatilize the solid precursor, e.g., by sublimation, so that the precursor vapor can be discharged selectively from the vessel, as needed in the downstream deposition process.
- the precursor when present in solid phase may also be dissolved in solvent medium, as described above, and delivered by liquid delivery techniques to a vaporizer, for volatilization to form a precursor vapor that then is contacted under a vapor deposition conditions with a wafer or other suitable substrate.
- the tellurium precursor can be delivered by liquid delivery techniques from a suitable precursor storage vessel. Bubbler techniques may also be employed.
- Te(II) and Te(IV) compounds can be selected based on the process conditions needed for delivery for contacting with the substrate on which Te or Te-containing films are to be formed. In general, it is desired to carry out deposition by CVD and/or ALD techniques at temperatures below 400° C.
- the deposition of tellurium species in accordance with the invention can be carried out to form GST phase change memory devices, or other tellurium-based microelectronic device structures.
- FIG. 7 is a STA plot of the Te[N(SiMe 3 ) 2 ] 2 product.
- TeCl 4 5.00 g (18.56 mmol) of TeCl 4 are suspended in 400 mL of THF (only partially soluble) in a 500 mL Schlenk flask equipped with a magnetic stirring bar. A solution of 6.40 g (37.12 mmol) of N,N′-di-tert-butylethylenediamine and 7.51 g (74.24 mmol) of triethylamine in 10 mL of THF is prepared. This solution in added to the TeCl 4 suspension and the reaction mixture turns cloudy immediately. A mild exothermic reaction is observed. The reaction mixture is stirred overnight a room temperature, and then filtered through a medium glass-filter frit, leading to yellow solution. The volatiles are removed in a vacuum leaving 5.96 g (16.20 mmol; 87.1% yield) of analytically pure product behind as a pale yellow, microcrystalline solid.
- X-ray analysis quality crystals were obtained by dissolving 0.5 g of product in 6 mL of toluene in a sample vial inside a controlled atmosphere glove-box. The solution is filtered through a PTFE syringe filter and then placed in a ⁇ 25° C. freezer. After 16 hours large, pale yellow, plate like crystals of the title compound were obtained, which were suitable for X-ray analysis.
- the compound can be further purified by sublimation.
- a sublimation device is charged with 5 g of material and the material is sublimed at 200 mTorr pressure and an oil bath temperature of 70° C. for two hours. Typical yields of the sublimation vary between 80-90%.
- the product yielded by the foregoing procedure had the following characteristics: 1H NMR in C 6 D 6 , ppm: 3.133 (s, 4H, N—CH 2 —CH 2 —N); 1.189 (s, 18H, N-t-Bu).
- the volatiles are removed in vacuum and the remaining dark brown solid is extracted with 150 mL of n-pentane and filtered through a medium glass-filter frit, resulting in an orange solution.
- the pentane is removed in vacuum leaving 2.60 g (6.25 mmol; 79.5%) of analytically pure product behind as a yellow-orange solid.
- the compound can be further purified by sublimation at 200 mTorr and an oil bath temperature of 100° C. for two hours, affording 2.05 g (4.93 mmol; 63% yield) of product as a yellow, crystalline solid.
- FIG. 8 is a STA plot of the Te[N(SiMe 3 )(t-Bu)] 2 product.
- X-ray analysis quality crystals were obtained by dissolving 0.5 g of product in 5 mL of toluene in a sample vial inside a controlled atmosphere glove-box. The solution is filtered through a PTFE syringe filter and then placed in a ⁇ 25° C. freezer. After 32 hours pale yellow crystals of the title compound were obtained, that were suitable for X-ray analysis.
- GeTe films can be formed using this germanium precursor, denoted GeBAMDN or GeM for ease of notation, and di-tert-butyltelluride, at temperatures below 300° C. or lower, e.g., below 280° C. or even 260° C. or lower. In general, lower temperatures will result in lower content of tellurium, but the specific deposition rate of the film will also depend on the germanium and tellurium delivery rates in the deposition system.
- Di-tert-butyltelluride and Ge[Pr i NC(n-Bu)NPr i ] 2 were used to deposit GeTe films at the illustrative conditions identified below, with the following film thickness and tellurium concentration results.
- the invention in another aspect involves use of control agents to combat vapor phase pre-reaction of the precursors described herein, that otherwise causes uneven nucleation on the substrate, longer incubation times for deposition reactions, and lower quality product films.
- Such pre-reaction may for example be particularly problematic in applications involving chalcogenide films, related source materials (O, S, Se, Te, Ge, Sb, Bi, etc.), and/or manufacture of phase change memory and thermoelectric devices.
- Pre-reaction may occur when the precursor reagents described herein are introduced to the deposition chamber, as in chemical vapor deposition, and may also occur in atomic layer deposition (ALD) processes, depending on the specific arrangement of ALD cycle steps and the specific reagents involved.
- ALD atomic layer deposition
- the invention therefore contemplates the use of control agents with the precursors described herein, whereby detrimental gas phase pre-reactions are suppressed, mitigated or eliminated, so that deposition reactions are induced/enhanced on the substrate surface, and films of superior character are efficiently formed.
- control agents that can be utilized with precursors of the invention for such purpose include agents selected from the group consisting of (i) heteroatom (O, N, S) organo Lewis base compounds, (ii) free radical inhibitors, and (iii) deuterium-containing reagents.
- a pre-reaction suppressant comprising one or more heteroatom (O, N, S) organo Lewis base compounds such as 1,4-dioxane, thioxane, ethers, polyethers, triethylamine (TEA), triazine, diamines, N,N,N′,N′-tetramethylethylenediamine, N,N,N′-trimethylethylenediamine, amines, imines, and pyridine;
- heteroatom such as 1,4-dioxane, thioxane, ethers, polyethers, triethylamine (TEA), triazine, diamines, N,N,N′,N′-tetramethylethylenediamine, N,N,N′-trimethylethylenediamine, amines, imines, and pyridine
- TAA triethylamine
- a free radical inhibitor such as butylated hydroxy toluene (BHT), hydroquinone, butylated hydro anisole (BHA), diphenylamine, ethyl vanillin, etc.;
- pre-reaction-combating agents described above can be introduced to any of the feed streams to the vapor deposition process in which the film is to be formed.
- pre-reaction-combating agents can be introduced to one or more of precursor feed stream(s), inert carrier gas stream(s) to which chalcogenide precursor(s) or other reagents are subsequently added for flow to the deposition chamber, co-reactant feed stream(s) flowed to the deposition chamber, and/or any other stream(s) that is/are flowed to the deposition chamber and in which the pre-reaction-combating agent(s) is/are useful for reduction or elimination of premature reaction of the precursors that would otherwise occur in the absence of such agent(s).
- the aforementioned suppressants, free radical inhibitors and/or deuterium source reagents in specific embodiments are co-injected with the precursor(s), e.g., metal source reagent(s), to effect at least partial reduction of pre-reaction involving the precursor(s) and reagent(s).
- precursor(s) e.g., metal source reagent(s)
- the pre-reaction-combatting agent can alternatively be added directed to the deposition locus, e.g., the deposition chamber to which the precursor vapor is introduced for contacting with the substrate to deposit the film thereon, to suppress deleterious vapor phase pre-reaction involving the precursor(s) and/or other reagents.
- the suppressant, free radical inhibitor and/or deuterium source can be added to a solution containing the precursor and/or another metal source reagent, and the resulting solution can be utilized for liquid delivery processing, in which the solution is flowed to a vaporizer to form a source vapor for contacting with the substrate to deposit the deposition species thereon.
- the suppressant, free radical inhibitor and/or deuterium source can be added to form a mixture or a solution with the precursor and/or another metal source reagent, depending on the respective phases of the materials involved, and their compatibility/solubility.
- the suppressant, free radical inhibitor and/or deuterium source can be utilized for surface treatment of the substrate prior to contacting of the substrate with the precursor and/or other metal source reagent.
- the invention therefore contemplates various vapor deposition compositions and processes for forming films on substrates, in which pre-reaction of the precursors is at least partially attenuated by one or more pre-reaction-combating agents selected from among heteroatom (O, N, S) organo Lewis base compounds, sometimes herein referred to as suppressor agents, free radical inhibitors, and/or deuterium source reagents.
- pre-reaction-combating agents selected from among heteroatom (O, N, S) organo Lewis base compounds, sometimes herein referred to as suppressor agents, free radical inhibitors, and/or deuterium source reagents.
- the control agent can be used for combating pre-reaction of chalcogenide precursor in a process in which multiple feed streams are flowed to a deposition locus to form a film on a substrate, wherein at least one of the multiple feed streams includes a precursor susceptible to pre-reaction adversely affecting the film, in which the method involves introducing the control agent to at least one of such multiple feed streams or supplied materials therefor, or to the deposition locus.
- the pre-reaction combating reagent alternatively can be introduced to passivate the surface of a growing chalcogenide film or slow the deposition rate, followed by reactivation using an alternative precursor or co-reactant (for example H 2 , NH 3 , plasma, H 2 O, hydrogen sulfide, hydrogen selenide, diorganotellurides, diorganosulfides, diorganoselenides, etc.), thereby carrying out passivation/retardation followed by reactivation steps, e.g., as an alternating repetitive sequence.
- Such sequence of passivation/retardation followed by reactivation can be carried out for as many repetitive cycles as desired, in ALD or ALD-like processes. The steps may be carried out for the entire deposition operation, or during some initial, intermediate or final portion thereof.
- the invention therefore contemplates precursor compositions including the precursor and the pre-reaction-combating reagent.
- pre-reaction-combating reagents within the categories of pre-reaction-combating reagents previously described, viz., (i) heteroatom (O, N, S) organo Lewis base compounds, (ii) free radical inhibitors, and (iii) deuterium-containing reagents, suitable pre-reaction-combating reagents for specific applications may be readily determined within the skill of the art, based on the disclosure herein.
- Heteroatom (O, N, S) organo Lewis base compounds may be of varied type, e.g., containing an oxo (—O—) moiety, a nitrogen ring atom or pendant amino or amide substituent, a sulfur ring atom or pendant sulfide, sulfonate or thio group, as effective to at least partially lessen pre-reaction of the precursor and other organo metal reagents in the process system.
- heteroatom (O, N, S) organo Lewis base compounds having utility in specific applications of the invention include, without limitation, 1,4-dioxane, thioxane, ethers, polyethers, triethylamine, triazine, diamines, N,N,N′,N′-tetramethylethylenediamine, N,N,N′-trimethylethylenediamine, amines, imines, pyridine, and the like.
- the heteroatom organo Lewis base compound in various specific embodiments of the invention may include a guanidinate compound, e.g., (Me 2 N) 2 C ⁇ NH.
- heteroatom organo Lewis base compounds for such purpose includes R 3 N, R 2 NH, RNH 2 , R 2 N(CH 2 ) x NR 2 , R 2 NH(CH 2 ) x NR 2 , R 2 N(CR 2 ) x NR 2 , and cyclic amines —N(CH 2 ) x —, imidazole, thiophene, pyrrole, thiazole, urea, oxazine, pyran, furan, indole, triazole, triazine, thiazoline, oxazole, dithiane, trithiane, crown ethers, 1,4,7-triazacyclononane, 1,5,9-triazacyclododecane, cyclen, succinamide, and substituted derivatives of the foregoing, wherein R can be hydrogen or any suitable organo moieties, e.g., hydrogen, C 1 -C 8 alkyl, C 1 -
- the heteroatom organo Lewis base compounds may be utilized in the precursor composition at any suitable concentration, as may be empirically determined by successive deposition runs in which the heteroatom organo Lewis base compound concentration is varied, and character of the resulting film is assessed, to determine an appropriate concentration.
- the heteroatom organo Lewis base compound may be utilized in the concentration of 1-300% of the amount of precursor. Specific sub-ranges of concentration values within a range of 0.01-3 equivalents of the heteroatom organo Lewis base compound may be established for specific classes of precursors, without undue experimentation, based on the disclosure herein.
- the pre-reaction-combating reagent may additionally or alternatively comprise free radical inhibitors that are effective to lessen the extent of pre-reaction between the precursor and another organo metal reagent.
- free radical inhibitors may be of any suitable type, and may for example include hindered phenols.
- Illustrative free radical inhibitors include, without limitation, free radical scavengers selected from the group consisting of: 2,6-ditert-butyl-4-methyl phenol, 2,2,6,6-tetramethyl-1-piperidinyloxy, 2,6-dimethylphenol, 2-tert-butyl-4-hydroxyanisole, 3-tert-butyl-4-hydroxyanisole, propyl ester 3,4,5-trihydroxy-benzoic acid, 2-(1,1-dimethylethyl)-1,4 benzenediol, diphenylpicrylhydrazyl, 4-tert-butylcatechol, N-methylaniline, 2,6-dimethylaniline, p-methoxydiphenylamine, diphenylamine, N,N′-diphenyl-p-phenylenediamine, p-hydroxydiphenylamine, phenol, octadecyl-3-(3,5-di-tert-butyl-4-hydroxyphenyl)prop
- Useful concentrations of the free radical inhibitor may be in a range of from 0.001 to about 0.10% by weight of the weight of the precursor, in various specific embodiments. More generally, any suitable amount of free radical inhibitor may be employed that is effective to combat the pre-reaction of the precursor in the delivery and deposition operations involved in the film formation process.
- deuterium source compounds afford another approach to suppressing pre-reaction of the chalcogenide precursor.
- Such deuterium source compounds may be of any suitable type, and may for example include deuterated pyridine, deuterated pyrimidine, deuterated indole, deuterated imidazole, deuterated amine and amide compounds, deuterated alkyl reagents, etc., as well as deuterated analogs of the precursors that would otherwise be used as containing hydrogen or protonic substituents.
- Deuterides that may be useful in the general practice of invention as pre-reaction-combating reagents include, without limitation, germanium and antimony compounds of the formulae R x GeD 4-x and R x SbD 3-x wherein R can be hydrogen or any suitable organo moieties, e.g., hydrogen, C 1 -C 8 alkyl, C 1 -C 8 alkoxy, C 1 -C 8 alkene, C 1 -C 8 alkyne, and C 1 -C 8 carboxyl, and wherein x is an integer having a value of from 1 to 6.
- the deuterium source reagent may be utilized at any suitable concentration that is effective to combat pre-reaction of the precursor.
- Illustrative deuterium source reagent concentrations in specific embodiments of the invention can be in a range of 0.01 to about 5% by weight, based on the weight of precursor.
- a deuterium source compound may be added to one or more of the feed streams to the vapor deposition process, and/or one of the precursors or other feed stream components may be deuterated in the first instance.
- concentrations of the pre-reaction-combating agents utilized in the practice of the present invention to at least partially eliminate pre-reaction of the precursors can be widely varied in the general practice of the present invention, depending on the temperatures, pressures, flow rates and specific compositions involved.
- concentration of the pre-reaction-combating reagents of the invention therefore are to be appreciated as being of an illustrative character only, with applicable concentrations being readily determinable within the skill of the art, based on the disclosure herein.
- the specific mode of introduction or addition of the pre-reaction-combating agent to one or more of the feed streams to the deposition process may correspondingly be varied, and may for example employ mass flow controllers, flow control valves, metering injectors, or other flow control or modulating components in the flow circuitry joining the source of the pre-reaction-combating agent with the streams being flowed to the deposition process during normal film-forming operation.
- the process system may additionally include analyzers, monitors, controllers, instrumentation, etc., as may be necessary or appropriate to a given implementation of the invention.
- the pre-reaction-combating agent may be mixed with precursor in the first instance, as a starting reagent material for the process.
- the pre-reaction-combating agent may be mixed in liquid solution with the precursor, for liquid delivery of the resulting precursor solution to a vaporizer employed to generate precursor vapor for contact with the substrate to deposit the film thereon.
- the pre-reaction-combating agent may be added to the deposition locus to provide active gas-phase suppression of pre-reaction of the precursor vapor(s) that would otherwise be susceptible to such deleterious interaction.
- the pre-reaction-combating agent may be used as a preliminary surface treatment following which the precursor and co-reactants (e.g., H 2 , NH 3 , plasma, H 2 O, hydrogen sulfide, hydrogen selenide, diorganotellurides, diorganosulfides, diorganoselenides, etc.) are delivered to the substrate surface to effect deposition on such surface.
- the pre-reaction-combating agent may be introduced into one of more of the flow lines to the deposition process and flow to the substrate in the deposition process chamber, prior to initiation of flow of any precursors. After the requisite period of contacting of the substrate with such pre-reaction-combating agent has been completed, the flow of the pre-reaction-combating agent can be terminated, and normal feeding of flow streams to the deposition chamber can be initiated.
- the pre-reaction-combating agent may be introduced in any of a wide variety of ways to effect diminution of the pre-reaction of the precursor in the deposition system.
- a vapor phase deposition system comprising:
- a vapor deposition chamber adapted to hold at least one substrate for deposition of a film thereon;
- first flow circuitry arranged to deliver said reagents from said chemical reagent supply vessels to the vapor deposition chamber
- second flow circuitry arranged to deliver the pre-reaction-combating agent from the pre-reaction-combating agent supply vessel to the first flow circuitry, to said chemical reagent supply vessels and/or to the vapor deposition chamber.
- FIG. 9 is a schematic representation of a vapor deposition system 100 in one embodiment thereof.
- a pre-reaction-combating agent is contained in a supply vessel 110 .
- the pre-reaction-combating agent can comprise a pre-reaction suppressant, a free radical inhibitor, a deuterium source, or a combination of two or more of such agents and/or types of such agents.
- the pre-reaction-combating agent supply vessel is joined by respective flow lines 112 , 114 and 116 , to germanium, antimony and tellurium reagent supply vessels, labeled “G,” “S” and “T,” respectively.
- the germanium precursor in vessel “G” may be a tetraalkyl or tetraamido germanium compound, such as tetramethyl germanium, tetraethyl germanium, tetraallyl germanium, tetrakis(dimethylamino)germane or other organo germanium compounds.
- precursor “G” may be a germylene compound wherein the lone pair on Ge(II) can react in the gas-phase with chalcogen precursors in the absence of a pre-reaction suppresant.
- the antimony precursor in vessel “S” can be a trialkyl or triamido antimony compound, such as tributyl antimony, triisopropyl antimony, tris(dimethylamino)antimony or other organo antimony compound.
- the tellurium precursor in vessel “T” can be a dialkyl or diamido tellurium compound, such as diisopropyl tellurium, dibutyl tellurium, bis[bis(trimethylsilyl)amino]tellurium or other organo tellurium compound.
- the pre-reaction-combating agent therefore can be added to any of the germanium, antimony and/or tellurium precursors in the respective “G,” “S” and “T” vessels, via the corresponding flow line(s), which for such purpose may have flow control valves or other flow-modulating components therein.
- the germanium, antimony and tellurium precursors are flowed in liquid form in feed lines 118 , 120 and 122 , respectively, to the mixing chamber 124 , and the resulting precursor mixture then is flowed from the mixing chamber 124 in line 126 to vaporizer 128 .
- the liquid precursor mixture and pre-reaction-combating agent are volatilized to form a precursor vapor.
- the precursor vapor then flows in line 130 to the showerhead disperser 134 in vapor deposition chamber 132 , for discharge of precursor mixture onto the wafer substrate 136 mounted on susceptor 138 in the deposition chamber.
- the precursor vapor contacting the wafer substrate 136 serves to deposit the germanium, antimony and tellurium metals on the substrate, to form a thin film of germanium-antimony-tellurium (GST) material, e.g., for manufacture of a phase change random access memory device.
- GST germanium-antimony-tellurium
- the contacted precursor vapor, depleted in metals content, is discharged from the vapor deposition chamber 132 in line 140 , and flows to the effluent abatement unit 142 .
- the discharged effluent vapor is treated, e.g., by scrubbing, catalytic oxidation, electrochemical treatment, or in other manner, to yield a final effluent that is discharged from the abatement unit in line 146 .
- the pre-reaction-combating agent could be introduced directly to the mixing chamber 124 , for blending therein with the respective GST precursors.
- the pre-reaction-combating agent could be introduced into manifold 118 , or other mixing chamber, blender, etc., for combination with the precursor that is being transported to the deposition locus.
- the system shown in FIG. 9 employs liquid delivery of the respective precursors. It will be recognized that if solid-phased precursors are employed, then solid delivery techniques may be employed, in which solid precursor is volatilized, e.g., by sublimation of the solid starting material.
- one or more of the germanium, antimony and tellurium precursors could be supplied in the first instance as a deuterated analog of an organo germanium, antimony or tellurium precursor, in which hydrogen substituents of the organo moiety have been replaced with deuterium.
- the pre-reaction-combating reagents may be employed in the broad practice of the present invention to produce improved films for the manufacture of semiconductor products.
- the pre-reaction-combating reagents described herein may be utilized in various combinations in specific applications, to suppress or eliminate pre-reaction of the precursor(s) and provide superior nucleation and final film properties.
Abstract
Precursors for use in depositing tellurium-containing films on substrates such as wafers or other microelectronic device substrates, as well as associated processes of making and using such precursors, and source packages of such precursors. The precursors are useful for deposition of chalcogenide thin films in the manufacture of nonvolatile Phase Change Memory (PCM), by deposition techniques such as chemical vapor deposition (CVD) and atomic layer deposition (ALD).
Description
- The benefit of priority of U.S. Provisional Patent Application 61/030,980 filed on Feb. 24, 2008 and U.S. Provisional Patent Application No. 61/050,183 filed May 2, 2008 is hereby claimed under the provisions of 35 USC 119. The disclosures of U.S. Provisional Patent Application 61/030,980 and U.S. Provisional Patent Application No. 61/050,183 are hereby incorporated herein by reference in their respective entireties, for all purposes.
- The present invention relates to precursors for use in depositing tellurium-containing films on substrates such as wafers or other microelectronic device substrates, as well as associated processes of making and using such precursors, and source packages of such precursors.
- In the manufacture of microelectronic devices, there is emerging interest in the deposition of Ge2Sb2Te5 chalcogenide thin films for nonvolatile Phase Change Memory (PCM), due to its relatively easy integration pathways with silicon-based integrated circuits. Chemical vapor deposition (CVD) and atomic layer deposition (ALD) processing of these materials are of primary interest as deposition techniques for advanced device applications.
- The anticipated use of high aspect ratio geometries in PCMs and the corresponding requirement to achieve smooth films of proper phase and non-segregated character, require processes that are efficient in forming high-quality tellurium-containing films at low temperatures (<400° C.). Suitable tellurium precursors are required that are compatible with such requirements, and that preferably have high volatility, and are liquids at standard temperature and pressure conditions.
- The present invention relates to tellurium precursors useful for depositing tellurium-containing films on substrates such as wafers or other microelectronic device substrates, as well as associated processes of making and using such precursors, and source packages of such precursors.
- In one aspect, the invention relates to a tellurium precursor selected from among:
- (i) Te(IV) organyls having the formula TeR1R2R3R4 wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- (ii) tellurium bis-amides of the formula Te[NR2]2 wherein each R is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- (iii) asymmetric tellurium compounds including one alkyl substituent and a second ligand containing a heteroatom;
- (iv) tellurium compounds with ethylenediamine ligands;
- (v) tellurium compounds with dithiocarbamate ligands;
- (vi) Te(II) and Te(IV) compounds including at least one nitrogen-based ligand selected from among amidinates, guanidinates, isoureates and beta-diketoiminates; and
- (vii) dialkyl ditellurides wherein alkyl is C1-C8 alkyl.
- In another aspect, the invention relates to a compound of the formula
-
XTeNR1R2 - wherein
- X is halogen; and
- each of R1 and R2 is the same as or different from the other, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl.
- In a further aspect, the invention relates to a composition comprising:
- (a) a tellurium precursor selected from among:
- (i) Te(IV) organyls having the formula TeR1R2R3R4 wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- (ii) tellurium bis-amides of the formula Te[NR2]2 wherein each R is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- (iii) asymmetric tellurium compounds including one alkyl substituent and a second ligand containing a heteroatom;
- (iv) tellurium compounds with ethylenediamine ligands;
- (v) tellurium compounds with dithiocarbamate ligands;
- (vi) Te(II) and Te(IV) compounds including at least one nitrogen-based ligand selected from among amidinates, guanidinates, isoureates and beta-diketoiminates; and
- (vii) dialkyl ditellurides wherein alkyl is C1-C8 alkyl; and
- (b) a solvent medium in which said compound is dissolved.
- A further aspect of the invention relates to a composition comprising
- (a) a compound of the formula:
-
ITeNR1R2 - wherein
- each of R1 and R2 is the same as or different from the other, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl; and
- (b) a solvent medium in which said compound is dissolved.
- A still further aspect of the invention relates to a precursor vapor comprising vapor of a tellurium precursor selected from the group consisting of
- (i) Te(IV) organyls having the formula TeR1R2R3R4 wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- (ii) tellurium bis-amides of the formula Te[NR2]2 wherein each R is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- (iii) asymmetric tellurium compounds including one alkyl substituent and a second ligand containing a heteroatom;
- (iv) tellurium compounds with ethylenediamine ligands;
- (v) tellurium compounds with dithiocarbamate ligands;
- (vi) Te(II) and Te(IV) compounds including at least one nitrogen-based ligand selected from among amidinates, guanidinates, isoureates and beta-diketoiminates; and
- (vii) dialkyl ditellurides wherein alkyl is C1-C8 alkyl.
- Another aspect of the invention relates to a method of depositing a tellurium-containing film on a substrate, comprising volatilizing a tellurium precursor to form a precursor vapor, and contacting the substrate with the precursor vapor under deposition conditions to form the tellurium-containing film on the substrate, wherein said tellurium precursor is selected from the group consisting of:
- (i) Te(IV) organyls having the formula TeR1R2R3R4 wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- (ii) tellurium bis-amides of the formula Te[NR2]2 wherein each R is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- (iii) asymmetric tellurium compounds including one alkyl substituent and a second ligand containing a heteroatom;
- (iv) tellurium compounds with ethylenediamine ligands;
- (v) tellurium compounds with dithiocarbamate ligands;
- (vi) Te(II) and Te(IV) compounds including at least one nitrogen-based ligand selected from among amidinates, guanidinates, isoureates and beta-diketoiminates; and
- (vii) dialkyl ditellurides wherein alkyl is C1-C8 alkyl.
- A further aspect of the invention relates to a packaged precursor, comprising a precursor storage and vapor dispensing vessel having disposed therein a tellurium precursor selected from among:
- (i) Te(IV) organyls having the formula TeR1R2R3R4 wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- (ii) tellurium bis-amides of the formula Te[NR2]2 wherein each R is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- (iii) asymmetric tellurium compounds including one alkyl substituent and a second ligand containing a heteroatom;
- (iv) tellurium compounds with ethylenediamine ligands;
- (v) tellurium compounds with dithiocarbamate ligands;
- (vi) Te(II) and Te(IV) compounds including at least one nitrogen-based ligand selected from among amidinates, guanidinates, isoureates and beta-diketoiminates; and
- (vii) dialkyl ditellurides wherein alkyl is C1-C8 alkyl
- Yet another aspect of the invention relates to a method for the preparation of a tellurium dialkylamide compound, comprising reacting tellurium dihalide with a metal amide to yield said tellurium dialkylamide compound.
- A further aspect of the invention relates to a method of forming a GST film on a substrate, comprising depositing tellurium on the substrate from vapor of a tellurium precursor selected from among:
- (i) Te(IV) organyls having the formula TeR1R2R3R4 wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- (ii) tellurium bis-amides of the formula Te[NR2]2 wherein each R is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- (iii) asymmetric tellurium compounds including one alkyl substituent and a second ligand containing a heteroatom;
- (iv) tellurium compounds with ethylenediamine ligands;
- (v) tellurium compounds with dithiocarbamate ligands;
- (vi) Te(II) and Te(IV) compounds including at least one nitrogen-based ligand selected from among amidinates, guanidinates, isoureates and beta-diketoiminates; and
- (vi) Te(II) and Te(IV) compounds including at least one nitrogen-based ligand selected from among amidinates, guanidinates, isoureates and beta-diketoiminates.
- The invention in another aspect relates to a method of making a PCRAM device, comprising forming a GST film on a substrate for fabrication of said device, wherein said forming comprises depositing tellurium on the substrate from vapor of a tellurium precursor selected from among:
- (i) Te(IV) organyls having the formula TeR1R2R3R4 wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- (ii) tellurium bis-amides of the formula Te[NR2]2 wherein each R is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- (iii) asymmetric tellurium compounds including one alkyl substituent and a second ligand containing a heteroatom;
- (iv) tellurium compounds with ethylenediamine ligands;
- (v) tellurium compounds with dithiocarbamate ligands;
- (vi) Te(II) and Te(IV) compounds including at least one nitrogen-based ligand selected from among amidinates, guanidinates, isoureates and beta-diketoiminates; and
- (vi) Te(II) and Te(IV) compounds including at least one nitrogen-based ligand selected from among amidinates, guanidinates, isoureates and beta-diketoiminates.
- The invention in a further aspect relates to a tellurium compound including at least one ethylenediamide ligand, wherein tellurium is in oxidation state (II) or (IV).
- Another aspect of the invention relates to a tellurium (IV) compound selected from the group consisting of:
- N,N′-di-tert-butylethylenediamide telluriumdichloride;
- 2,5-Bis(tert-butyl)-2,5-diaza-1-telluracyclopentane dichloride;
- N-methyl,N′-tert-butylethylenediamide telluriumdichloride;
- N,N′-di-tert-butyl-2,3-dimethylethylenediamide telluriumdichloride; and
- N,N′-di-tert-butylethylenediamide telluriumchloride dimethylamide.
- An additional aspect of the invention relates to a tellurium (IV) compound of the formula:
- wherein:
- R1, R2, R3, R4 and R5 are the same as or different from one another, and each is independently selected from C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl, acetylalkyl and halogen (chlorine, bromine, iodine or fluorine), and each R5 can additionally and independently be hydrogen or amide.
- In a further aspect, the invention relates to a tellurium (II) compound of the formula:
- wherein:
- R1, R2, R3 and R4 are the same as or different from one another, and each is independently selected from C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl, acetylalkyl and halogen (chlorine, bromine, iodine or fluorine), and R2 and R3 can additionally and independently be hydrogen.
- A still further aspect of the invention relates to a method of forming a tellurium or tellurium-containing film on a substrate, comprising volatilizing a tellurium compound as described above, to form a tellurium precursor vapor, and contacting the tellurium precursor vapor with the substrate to deposit tellurium thereon.
- In another aspect, the invention relates to a method of making a tellurium (IV) compound, comprising the following reaction:
- wherein:
- R1, R2, R3, R4, R5, R6 and R7 are the same as or different from one another, and each is independently selected from C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl, acetylalkyl and halogen (chlorine, bromine, iodine or fluorine), and R2 and R3 can additionally and independently be hydrogen; and
- X is halogen (chlorine, bromine, iodine or fluorine).
- A further aspect of the invention relates to a method of making a tellurium (IV) compound, comprising the following reaction:
- wherein:
- R1, R2, R3, R4 and R5 are the same as or different from one another, and each is independently selected from C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl, acetylalkyl and halogen (chlorine, bromine, iodine or fluorine), and each R5 can additionally and independently be hydrogen or amide;
- X is a halogen (chlorine, bromine, iodine or fluorine); and
- M is lithium, sodium or potassium.
- In an additional aspect, the invention relates to N,N′-di-tert-butylethylenediamide telluriumdichloride.
- A further aspect of the invention relates to NHTe(Cl)NMe2.
- In yet another aspect, the invention relates to a method of making a tellurium (II) compound, comprising one of the following reactions (A)-(C):
- wherein:
- R1, R2, R3, R4, R5, R6 and R7 are the same as or different from one another, and each is independently selected from C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl, acetylalkyl and halogen (chlorine, bromine, iodine or fluorine), and R2 and R3 can additionally and independently be hydrogen; and
- X is halogen (chlorine, bromine, iodine or fluorine);
- wherein:
- M═Li, Na, K;
- X=chlorine, bromine, iodine or fluorine;
- R1, R2, R3, R4 can be the same as or different from one another, and each is independently selected from C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl, acetylalkyl and halogen (chlorine, bromine, iodine or fluorine); and
- wherein:
- M═Li, Na, or K;
- X═Cl, Br, I or F;
- R1, R2, R3, R4 can be the same as or different from one another, and each is independently selected from C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl, acetylalkyl, hydrogen and halogen (chlorine, bromine, iodine or fluorine).
- A further aspect of the invention relates to a method of making a tellurium (II) compound, comprising the following reaction:
- A further aspect of the invention relates to a diorgano ditelluride compound comprising organo groups each of which is selected from C1-C12 hydrocarbyl groups, silyl and substituted silyl.
- The invention also pertains to a method of forming a tellurium or tellurium-containing film on a substrate, comprising vaporizing Te2(t-Bu)2 to form a corresponding vapor, and contacting said vapor with said substrate to form said tellurium or tellurium-containing film thereon.
- Still another aspect of the invention relates to a packaged tellurium reagent, comprising a reagent storage and dispensing vessel containing a tellurium reagent of a type as described above.
- In one aspect, the invention further relates to a method of combating pre-reaction of precursors described herein in a vapor deposition process for forming a film on a substrate, wherein the precursors described herein are susceptible to pre-reaction adversely affecting the film. In this aspect, the method involves introducing to the process a pre-reaction-combating agent selected from the group consisting of (i) heteroatom (O, N, S) organo Lewis base compounds, (ii) free radical inhibitors, and (iii) deuterium-containing reagents.
- Another aspect of the invention relates to a method of combating pre-reaction of the precursors described in a vapor deposition process in which multiple feed streams are flowed to a deposition locus to form a film on a substrate, wherein at least one of said multiple feed streams includes a precursor susceptible to pre-reaction adversely affecting the film. The method involves introducing to at least one of said multiple feed streams or supplied materials therefor, or to the deposition locus, a pre-reaction-combating agent selected from the group consisting of (i) heteroatom (O, N, S) organo Lewis base compounds, (ii) free radical inhibitors, and (iii) deuterium-containing reagents.
- A still further aspect of the invention relates to a composition, comprising a precursor as described herein and a pre-reaction-combating agent for said precursor, said pre-reaction-combating agent being selected from the group consisting of (i) heteroatom (O, N, S) organo Lewis base compounds, (ii) free radical inhibitors, and (iii) deuterium-containing reagents.
- In a further aspect, the invention relates to a method of combating pre-reaction of a vapor phase precursor described herein in contact with a substrate for deposition of a film component thereon. The method involves contacting said substrate, prior to said contact of the vapor phase precursor therewith, with a pre-reaction-combating agent selected from the group consisting of (i) heteroatom (O, N, S) organo Lewis base compounds, (ii) free radical inhibitors, and (iii) deuterium-containing reagents.
- In a further aspect, the invention relates to a process wherein the pre-reaction combating reagent is introduced to passivate the surface of a growing film or slow the deposition rate, followed by reactivation using an alternative precursor or co-reactant (for example H2, NH3, plasma, H2O, hydrogen sulfide, hydrogen selenide, diorganotellurides, diorganosulfides, diorganoselenides, etc.). Such passivation/retardation followed by reactivation thus may be carried out in an alternating repetitive sequence, for as many repetitive cycles as desired, in ALD or ALD-like processes. Pre-reaction-combating agents can be selected from the group consisting of (i) heteroatom (O, N, S) organo Lewis base compounds, (ii) free radical inhibitors, and (iii) deuterium-containing reagents.
- Another aspect of the invention relates to a vapor phase deposition process for forming a film on a substrate involving cyclic contacting of the substrate with at least one film precursor described herein that is undesirably pre-reactive in the vapor phase. The process involves introducing to said film during growth thereof a pre-reaction-combating reagent that is effective to passivate a surface of said film or to slow rate of deposition of said film precursor, and after introducing said pre-reaction-combating reagent, reactivating said film with a different film precursor.
- Other aspects, features and embodiments of the invention will be more fully apparent from the ensuing disclosure and appended claims.
-
FIG. 1 is a schematic representation of a material storage and dispensing package containing a precursor of the present invention, in one embodiment thereof. -
FIG. 2 is an ORTEP diagram of the structure of NHTeCl2, i.e., N,N′-di-tert-butylethylenediamide telluriumdichloride. -
FIG. 3 is an ORTEP diagram of the structure of Me2NHTeCl2. -
FIG. 4 is an ORTEP diagram of the structure of NHTe(Cl)NMe2. -
FIG. 5 is an ORTEP diagram of the structure of Te2(t-Bu)2. -
FIG. 6 is a simultaneous thermographic analysis plot of STA TG/DSC data for Te2(t-BU)2. -
FIG. 7 is a STA of Te[N(SiMe3)2]2. -
FIG. 8 is a STA of Te[N(SiMe3)(t-Bu)]2 -
FIG. 9 is a schematic representation of a vapor deposition system according to one embodiment of the present invention, wherein suppression of pre-reaction of the precursors is achieved by addition of pre-reaction-combating reagent to one or more feed streams in the vapor deposition system. - The present invention relates to tellurium precursors useful in film-forming applications, e.g., in chemical vapor deposition and atomic layer deposition applications, to form corresponding tellurium-containing films on substrates, as well as associated processes of making and using such precursors, and packaged forms of such precursors.
- As used herein, the term “film” refers to a layer of deposited material having a thickness below 1000 micrometers, e.g., from such value down to atomic monolayer thickness values. In various embodiments, film thicknesses of deposited material layers in the practice of the invention may for example be below 100, 10, or 1 micrometers, or in various thin film regimes below 200, 100, or 50 nanometers, depending on the specific application involved. As used herein, the term “thin film” means a layer of a material having a thickness below 1 micrometer.
- As used herein, the singular forms “a”, “and”, and “the” include plural referents unless the context clearly dictates otherwise.
- As used herein, the identification of a carbon number range, e.g., in C1-C12 alkyl, is intended to include each of the component carbon number moieties within such range, so that each intervening carbon number and any other stated or intervening carbon number value in that stated range, is encompassed, it being further understood that sub-ranges of carbon number within specified carbon number ranges may independently be included in smaller carbon number ranges, within the scope of the invention, and that ranges of carbon numbers specifically excluding a carbon number or numbers are included in the invention, and sub-ranges excluding either or both of carbon number limits of specified ranges are also included in the invention. Accordingly, C1-C12 alkyl is intended to include methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl and dodecyl, including straight chain as well as branched groups of such types. It therefore is to be appreciated that identification of a carbon number range, e.g., C1-C12, as broadly applicable to a substituent moiety, enables, in specific embodiments of the invention, the carbon number range to be further restricted, as a sub-group of moieties having a carbon number range within the broader specification of the substituent moiety. By way of example, the carbon number range C1-C12 alkyl, may be more restrictively specified, in particular embodiments of the invention, to encompass sub-ranges such as C1-C4 alkyl, C2-C8 alkyl, C2-C4 alkyl, C3-C5 alkyl, or any other sub-range within the broad carbon number range.
- The precursors of the invention may be further specified in specific embodiments by provisos or limitations excluding specific substituents, groups, moieties or structures, in relation to various specifications and exemplifications thereof set forth herein. Thus, the invention contemplates restrictively defined compositions, e.g., a composition wherein Ri is C1-C12 alkyl, with the proviso that Ri≠C4 alkyl when Rj is silyl.
- The invention relates in one aspect to Te(IV) organyls useful for low temperature (T<400° C.) deposition of Te-containing films, e.g., for forming germanium-antimony-tellurium (GST) films such as Ge2Sb2Te5 on substrates such as wafers in the production of phase change random access memory devices.
- The Te(IV) organyls of the invention are suitable for forming such films by techniques such as atomic layer deposition (ALD) and chemical vapor deposition (CVD). Preferred precursors of such type are liquid at room temperature (25° C.) and have high volatility and desirable transport properties for ALD and CVD applications.
- In accordance with another aspect of the invention, Te(IV) organyls having the formula TeR1R2R3R4 wherein R1, R2, R3 and R4 are organo substituents, are employed to form Te-containing highly conformal films of superior character by a vapor deposition process such as ALD or CVD.
- In a preferred aspect, Te(IV) organyls are utilized having the formula TeR1R2R3R4 wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, halogen (fluorine, bromine, iodine and chlorine), C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl (silyl having C1-C6 alkyl substituents and/or C6-C10 aryl substituents), amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl. The alkyl moiety in such aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl substituents can be C1-C6 alkyl or alkyl moieties of other carbon numbers, as may be useful in a given application of such organyl compounds.
- Tellurium(IV) organyls of the invention useful for the aforementioned film-forming applications can readily be formed by the following generalized reaction:
-
TeCl4+4RM→TeR4+MCl - wherein M is Li or MgX, X is halide, and each R independently may be H, halogen (fluorine, bromine, iodine and chlorine), C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl or acetylalkyl, as above described.
- The Te(IV) compounds of the invention are usefully employed as CVD/ALD precursors for the deposition of Te-containing films, e.g., by liquid delivery techniques in which such compounds are provided in compositions including suitable solvent media. Useful solvents for such purpose in specific applications may include, without limitation, alkanes (e.g., hexane, heptane, octane, and pentane), aromatics (e.g., benzene or toluene), and amines (e.g., triethylamine, tert-butylamine). The solvent medium in which the Te precursor or precursors are dissolved or suspended may be a single-component solvent or a multi-component solvent composition.
- The precursors when in a liquid state can also be delivered neat using ALD/CVD liquid delivery techniques, in which the liquid is volatilized to form a corresponding precursor vapor, which then is contacted with the substrate on which the tellurium-containing film is to be formed, under appropriate vapor deposition conditions.
- When the precursors are in a solid state, they may be volatilized for delivery using any suitable solid delivery system, such as the solid delivery and vaporizer unit commercially available under the trademark ProE-Vap from ATMI, Inc. (Danbury, Conn., USA). The precursor or precursors (since the invention contemplates use of multiple Te precursors of differing type) are volatilized to form the corresponding precursor vapor which then is contacted with a wafer or other substrate to deposit a tellurium-containing layer thereon.
- The precursor vapor formed from the Te precursor may be mixed with carrier or co-reactant gases in various embodiments, to obtain desired deposition thicknesses, growth rates, etc., as will be apparent to those skilled in the art.
- The present invention in various aspects involves compositions and methods in which tellurium dialkyls and ditellurium dialkyls can be utilized as tellurium source reagents, but in other aspects, non-alkyl tellurium and non-alkyl ditellurium compounds are utilized.
- The invention in a further aspect relates to a synthetic route for the preparation of tellurium amide compounds, e.g., tellurium bis-amides that are useful for low temperature deposition of tellurium amides on substrates.
- The tellurium amide compounds can be formed by reacting tellurium dihalide with two equivalents of a metal amide, according to the following reaction.
-
TeX2+2MNR2→Te[NR2]2+2MX - wherein:
- X is halogen, preferably Cl, Br or I,
- M is Li, Na, or K, and
- each R is independently selected from among H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, as above described.
- In one preferred embodiment, TeI2 is reacted with LiN(t-Bu)(SiMe3) to form the reaction product Te[N(t-Bu)(SiMe3)]2.
- wherein t-butyl is tertiary butyl, and Me is methyl.
- This product, Te[N(t-Bu)(SiMe3)]2, has been characterized by NMR spectroscopy and thermal analysis (STA), as a low melting solid (mp=77° C.) that shows good transport properties (T50=184° C.) and low residual mass (<2%). This compound is usefully employed as a precursor for the low temperature deposition of tellurium-containing films.
- In another preferred embodiment, TeI2 is reacted with KN(SiMe3)2 to form the following reaction product, Te[N(SiMe3)2]2.
- A further aspect of the invention relates to asymmetric tellurium compounds including one alkyl substituent and a second ligand containing a heteroatom, e.g., nitrogen or sulfur. The second ligand may be of any suitable type, and in specific embodiments is amidinate, guanidinate, or dithiocarbamate.
- In one embodiment, the starting material for the asymmetric tellurium compound is ITeN R1R2 wherein each of R1 and R2 is the same as or different from the other, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, as above described. This starting material can be synthesized by the reaction of TeI2 with one equivalent of a lithium amide as shown below.
- or, more generally, halide starting materials can be formed according to the following reaction.
-
TeX2+MNR1R2→XTeN R1R2+MX - wherein
- M is Li, Na, or K, preferably Li,
- X is halogen, preferably Cl, Br or I, and
- each of R1 and R2 is the same as or different from the other, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, as above described.
- The starting material ITeN R1R2 is extremely sensitive to light and air when isolated as an orange solid product, however, it can be placed into solution with a suitable solvent medium, e.g., a hydrocarbon solvent medium, containing pentane, hexane or toluene, or other hydrocarbon species. Such starting material ITeN R1R2 can be reacted in situ in the hydrocarbon solvent medium with an alkyl lithium reagent to obtain an asymmetric tellurium compound, as shown in the reaction below.
-
XTeNR1R2+MR3→R3TeNR1R2+MX - wherein
- M is Li, Na, or K, preferably Li,
- X is halogen, preferably Cl, Br or I, and
- each of R1, R2 and R3 is the same as or different from the other, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, as above described, and wherein R3 can in addition be amide or halogen.
- Using the same synthetic approach, other ligands, such as: amidinates
- wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R4 can in addition be halogen or amide;
- guanidinates
- wherein each of R1, R2, R3, R4 and R5 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R5 can in addition be halogen or amide; and
- dithiocarbamates
- wherein each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl and wherein R3 can in addition be halogen or amide,
- can be synthesized as well.
- The invention therefore provides asymmetric tellurium compounds including tellurium amides, amidinates, guanidinates and dithiocarbamates of a useful character for ALD or CVD deposition of tellurium or tellurium-containing films, e.g., for fabricating GST devices comprising Ge2Sb2Te5 films.
- Another aspect of the invention relates to tellurium compounds with ethylenediamine ligands and tellurium compounds with dithiocarbamate ligands, for use in low temperature deposition applications such as fabrication of the aforementioned GST-based phase change memory devices.
- Tellurium complexes with ethylenediamine type ligands can be synthesized, according to one preferred aspect of the invention, by reacting a lithium salt of the ethylenediamine with a tellurium halide, such as TeX2 or TeX4, wherein X is halogen. From the resulting reaction product, the desired tellurium compounds can be obtained by a salt elimination reaction.
- The following reaction scheme therefore may be used for production of such tellurium precursors.
- wherein
- M═Li, Na, or K;
- X═Cl, Br, or I;
- each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl.
- Alternatively, the following reaction scheme can be employed to produce the tellurium ethylenediamine precursors.
- wherein
- M═Li, Na, or K;
- X═Cl, Br, or I;
- each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl.
- A specific synthesis procedure for forming a tellurium precursor with ethylenediamine ligands is set forth below.
- Tellurium ethylenediamine compounds of such type have high volatility and low decomposition temperatures, and thus are well suited for ALD and CVD applications.
- Complexes of tellurium with only dithiocarbamate ligands, or including dithiocarbamate and other co-ligands, constitute a further group of precursors useful for ALD and CVD in accordance with the invention. Tellurium dithiocarbamate precursors of the invention include the following classes (a)-(e):
- wherein
- M is Li, Na, or K, preferably Li,
- X is halogen, preferably Cl, Br or I, and
- each R is the same as or different from the other, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- wherein
- each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substitued silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 and R4 can in addition and independently be halogen or amide;
- wherein
- each of R1 and R2 is the same as or different from the other, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
- wherein:
- each of R1, R2, R3, R4 and R5 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substitued silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein each of R3, R4 and R5 can in addition and independently be amide or halogen; and
- wherein each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 can in addition be halogen or amide.
- These precursors accommodate low temperature deposition applications, having good volatilization and transport properties. They can be delivered in a neat form in the case of precursor compounds in liquid form, or in compositions including suitable solvent media. Useful solvents for such purpose in specific applications may include, without limitation, alkanes (e.g., hexane, heptane, octane, and pentane), aromatics (e.g., benzene or toluene), and amines (e.g., triethylamine, tert-butylamine) or mixtures thereof, as above described.
- The precursors when in a solid state can be volatilized for delivery using any suitable solid delivery system, such as the solid delivery and vaporizer unit commercially available under the trademark ProE-Vap from ATMI, Inc. (Danbury, Conn., USA). The precursor or precursors (since the invention contemplates use of multiple Te precursors of differing type) are volatilized to form the corresponding precursor vapor which then is contacted with a wafer or other substrate to deposit a tellurium-containing layer thereon, e.g., for forming a GST layer.
- The invention in yet another aspect relates to tellurium compounds with nitrogen donor ligands useful for deposition applications to deposit tellurium or tellurium-containing films on substrates, for applications such as GST phase change random access memory (PRAM) devices.
- This aspect of the invention relates more specifically to Te(II) and Te(IV) precursors having at least one nitrogen-based ligand selected from among amidinates, guanidinates, isoureates and beta-diketoiminates.
- Specific tellurium nitrogen donor ligand precursors of the invention include the following:
- (A) Te(II) amidinates, guanidinates, and isoureates of the formula
- wherein:
- each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 can in addition be halogen or amide; and
- Z is independently selected from C1-C6 alkoxy, —NR1R2, H, C1-C6 alkyl, C3-C10 cycloalkyl, and C6-C13 aryl;
- (B) Te(IV) amidinates, guanidinates, and isoureates of the formula
- wherein:
- each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein each R3 can in addition and independently be halogen or amide;
- Z is independently selected from C1-C6 alkoxy, —NR1R2, H, C1-C6 alkyl, C3-C10 cycloalkyl, and C6-C13 aryl; and
- x is an integer having a value of from 0 to 4, inclusive;
- (C) Te(II) beta-diketoiminates of the formula
- wherein
- each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl; and
- (D) Te(IV) beta-diketoiminates of the formula
- wherein
- each of R1, R2, R3, R4, R5 and R6 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C 6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl.
- The tellurium compounds of the invention in film formation processes may be used with appropriate co-reactants, e.g., in a continuous deposition mode (CVD) or pulsed/atomic layer deposition mode (ALD), to deposit films of superior character. For oxides, preferred co-reactants include O2 and N2O for CVD, and more aggressive oxidizers for pulsed deposition, e.g., H2O, ozone, and O2 plasma. For metal-like films, reducing atmospheres are advantageously used.
- The precursors of the invention can be utilized as low temperature deposition precursors with reducing co-reactants such as hydrogen, H2/plasma, amines, imines, hydrazines, silanes, germanes such as GeH4, ammonia, alkanes, alkenes and alkynes. For CVD modes of film formation, reducing agents such as H2, and NH3 are preferred, and plasmas of these co-reactants may be used in digital or ALD mode, wherein the co-reactants are separated from the precursor in a pulse train, utilizing general CVD and ALD techniques within the skill of the art, based on the disclosure herein. More aggressive reducing agents can also be used in a digital or ALD mode since co-reactants can be separated, preventing gas phase reactions. For ALD and conformal coverage in high aspect ratio structures, the precursor preferably exhibits self-limiting behavior in one type of atmosphere (e.g., inert or weakly reducing/oxidizing gas environments) and exhibits rapid decomposition to form a desired film in another type of atmosphere (e.g., plasma, strongly reducing/oxidizing environments).
- Liquid delivery formulations can be employed in which precursors that are liquids may be used in neat liquid form, or liquid or solid precursors may be employed in suitable solvents, including for example alkane solvents (e.g., hexane, heptane, octane, and pentane), aryl solvents (e.g., benzene or toluene), amines (e.g., triethylamine, tert-butylamine), imines and hydrazines or mixtures thereof. The utility of specific solvent compositions for particular Te precursors may be readily empirically determined, to select an appropriate single component or multiple component solvent medium for the liquid delivery vaporization and transport of the specific tellurium precursor that is employed. In the case of solid precursors of the invention, a solid delivery system may be utilized, for example, using the ProE-Vap solid delivery and vaporizer unit (commercially available from ATMI, Inc., Danbury, Conn., USA).
- In general, the thicknesses of metal-containing layers formed using the precursors of the invention can be of any suitable value. In a specific embodiment of the invention, the thickness of the tellurium-containing layer can be in a range of from 5 nm to 500 nm or more.
- The various tellurium precursor compounds of the invention can be utilized to form GST films in combination any with suitable germanium and antimony precursors, e.g., by CVD and ALD techniques, for applications such as PCRAM device manufacture. The process conditions useful for carrying out deposition of Te-containing films can be readily determined within the skill of the art by the simple expedient of selectively varying the delivery and deposition process conditions and characterizing the resulting films, to determine the process conditions envelope most appropriate for a given deposition application.
- In one specific embodiment of the invention, Te[N(SiMe3)2]2 is used as a tellurium precursor for forming tellurium-containing films on substrates, such as GST films, amorphous GeTe films, and SbTe films, by atomic layer deposition (ALD) and chemical vapor deposition (CVD) techniques.
- In another embodiment, amorphous GeTe and SbTe are deposited from di-t-butyl tellurium, Te(tBu)2, at temperature in a range of from 300° C.-350° C., e.g., 320° C., using bubbler delivery of the telluriuim precursor in an inert carrier gas stream, e.g., N2 at a flow rate of 20-50 sccm, e.g., 30 sccm. The respective germanium and antimony precursors used for such deposition can be of any suitable types, e.g., GeBAMDN, SbTDMA, etc., and such precursors can be delivered for deposition at any suitable volumetric flow rate, e.g., for the aforementioned flow rate of 30 sccm for the illustrative tellurium precursor, Te(tBu)2, a flow rate of such Ge or Sb precursor can be on the order of 5 micromoles/minute. The resulting amorphous GeTe and SbTe films will have a tellurium content of approximately 40%.
-
FIG. 1 is a schematic representation of a material storage and dispensingpackage 100 containing a tellurium precursor, according to one embodiment of the present invention. - The material storage and dispensing
package 100 includes avessel 102 that may for example be of generally cylindrical shape as illustrated, defining aninterior volume 104 therein. In this specific embodiment, the precursor is a solid at ambient temperature conditions, and such precursor may be supported on surfaces of thetrays 106 disposed in theinterior volume 104 of the vessel, with the trays havingflow passage conduits 108 associated therewith, for flow of vapor upwardly in the vessel to the valve head assembly for dispensing, in use of the vessel. - The solid precursor can be coated on interior surfaces in the interior volume of the vessel, e.g., on the surfaces of the
trays 106 andconduits 108. Such coating may be effected by introduction of the precursor into the vessel in a vapor form from which the solid precursor is condensed in a film on the surfaces in the vessel. Alternatively, the precursor solid may be dissolved or suspended in a solvent medium and deposited on surfaces in the interior volume of the vessel by solvent evaporation. In yet another method the precursor may be melted and poured onto the surfaces in the interior volume of the vessel. For such purpose, the vessel may contain substrate articles or elements that provide additional surface area in the vessel for support of the precursor film thereon. - As a still further alternative, the solid precursor may be provided in granular or finely divided form, which is poured into the vessel to be retained on the top supporting surfaces of the
respective trays 106 therein. As a further alternative, a metal foam body may be provided in the interior volume of the vessel, which contains porosity of a specific character adapted for retaining the solid particulate precursor for highly efficient vaporization thereof. - The
vessel 102 has aneck portion 109 to which is joined thevalve head assembly 110. The valve head assembly is equipped with ahand wheel 112 in the embodiment shown. In lieu of a hand wheel, the valve head assembly may in turn be coupled or operatively linked to a controller for automated operation. Thevalve head assembly 110 includes a dispensingport 114, which may be configured for coupling to a fitting or connection element to join flow circuitry to the vessel. Such flow circuitry is schematically represented by arrow A inFIG. 1 , and the flow circuitry may be coupled to a downstream ALD or chemical vapor deposition chamber (not shown inFIG. 1 ). - In use, the
vessel 102 can be heated with a suitable heater, such as a heating jacket, resistance heating elements affixed to the exterior wall surface of the vessel, etc., so that solid precursor in the vessel is at least partially volatilized to provide precursor vapor. The input of heat is schematically shown inFIG. 1 by the reference arrow Q. The precursor vapor is discharged from the vessel through the valve passages in thevalve head assembly 110 when thehand wheel 112 or alternative valve actuator or controller is translated so that the valve is in an open position, whereupon vapor deriving from the precursor is dispensed into the flow circuitry schematically indicated by arrow A. - In lieu of solid delivery of the precursor, the precursor may be provided in a solvent medium, forming a solution or suspension. Such precursor-containing solvent composition then may be delivered by liquid delivery and flash vaporized to produce a precursor vapor. The precursor vapor is contacted with a substrate under deposition conditions, to deposit the metal on the substrate as a film thereon.
- In one embodiment, the precursor is dissolved in an ionic liquid medium, from which precursor vapor is withdrawn from the ionic liquid solution under dispensing conditions.
- As a still further alternative, the precursor may be stored in an adsorbed state on a suitable solid-phase physical adsorbent storage medium in the interior volume of the vessel. In use, the precursor vapor is dispensed from the vessel under dispensing conditions involving desorption of the adsorbed precursor from the solid-phase physical adsorbent storage medium.
- Supply vessels for precursor delivery may be of widely varying type, and may employ vessels such as those commercially available from ATMI, Inc. (Danbury, Conn.) under the trademarks SDS, SAGE, VAC, VACSorb, and ProE-Vap, as may be appropriate in a given storage and dispensing application for a particular precursor of the invention.
- The precursors of the invention thus may be employed to form precursor vapor for contacting with a substrate to deposit a tellurium-containing thin film thereon.
- In a preferred aspect, the invention utilizes the precursors to conduct atomic layer deposition, yielding ALD films of superior conformality that are uniformly coated on the substrate with high step coverage and conformality even on high aspect ratio structures.
- Accordingly, the precursors of the present invention enable a wide variety of microelectronic devices, e.g., semiconductor products, flat panel displays, etc., to be fabricated with tellurium-containing films of superior quality.
- The invention in another aspect relates to a class of tellurium compounds with ethylenediamide-type ligands. Such tellurium compounds are useful as precursors for low temperature ALD/CVD of tellurium or tellurium-containing thin films, e.g., for applications such as fabrication of phase change memory devices based on Ge2Sb2Te5 (GST). This class of compounds includes tellurium (Te) in the oxidation state IV, which is characterized by greater stability than commonly used tellurium precursors in the oxidation state II, thereby affording a beneficial alternative to the commonly used Te(II) precursors which are notoriously unstable with respect to air- and light-sensitivity.
- These tellurium compounds are to our knowledge the first examples of tellurium amides in which tellurium is in oxidation state (IV). Examples of such tellurium (IV) amides include, without limitation:
-
Formula Compound NHTeCl2 N,N′-di-tert-butylethylenediamide telluriumdichloride NHTeCl2 2,5-Bis(tert-butyl)-2,5-diaza-1-telluracyclopentane dichloride MeNHTeCl2 N-methyl,N′-tert-butylethylenediamide telluriumdichloride DMNHTeCl2 N,N′-di-tert-butyl-2,3-dimethylethylenediamide telluriumdichloride NHTe(Cl)NMe2 N,N′-di-tert-butylethylenediamide telluriumchloride dimethylamide
wherein the term NH is an abbreviation for “N-heterocyclic,” and denotes an N-heterocyclic ring system containing tellurium. - The general synthetic concept for such tellurium (IV) compounds, described more fully below, is also potentially applicable to the synthesis of tellurium (II) compounds. The general synthetic scheme (Scheme I below) is based on reaction of a tellurium (IV) halide with an ethylenediamide type ligand, with the addition of a tertiary amine in order to scavenge the eliminated hydrochloride.
- wherein:
- R1, R2, R3, R4, R5, R6 and R7 are the same as or different from one another, and each is independently selected from C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl, acetylalkyl and halogen (chlorine, bromine, iodine or fluorine), and R2 and R3 can additionally and independently be hydrogen; and
- X is halogen (chlorine, bromine, iodine or fluorine).
- Derivatives of the ethylenediamide tellurium complexes can be synthesized according to the following reaction scheme (Scheme II), by reacting the corresponding dichloride with a lithium alkyl or lithium amide species.
- wherein:
- R1, R2, R3, R4 and R5 are the same as or different from one another, and each is independently selected from C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl, acetylalkyl and halogen (chlorine, bromine, iodine or fluorine), and each R5 can additionally and independently be hydrogen or amide;
- X is a halogen selected from chloride, bromide and iodide; and
- M is lithium, sodium or potassium.
- The foregoing synthesis reactions can be carried out in any suitable solvent medium. One preferred solvent medium comprises an ether type solvent or other somewhat polar solvent in which the tellurium halide is sufficiently soluble. Tetrahydrofuran (THF) is one preferred solvent species, while diethyl ether, dimethoxyethane and toluene are also highly advantageous species. The choice of a specific solvent medium may be readily empirically determined, based on considerations of solubility, yields and reaction times for specific desired tellurium precursor products.
- As one example of the tellurium (IV) amide compounds of the invention,
FIG. 2 is an ORTEP diagram of the structure of NHTeCl2, i.e., N,N′-di-tert-butylethylenediamide telluriumdichloride. This compound is readily purified to high purity by sublimation, and has been confirmed by X-ray crystal structure analysis as existing in a weakly associated dimer solid state. As another example of such tellurium (IV) amide compounds,FIG. 3 is an ORTEP diagram of the structure of Me2NHTeCl2, also confirmed by X-ray crystal structure analysis to exist in a weakly associated dimer solid state. -
FIG. 4 is an ORTEP diagram of the structure of NHTe(Cl)NMe2. This tellurium source compound can be synthesized by a reaction scheme as described above. Such reaction scheme can be utilized to produce a mono-substituted species under mild reaction conditions, e.g., stirring of the reaction volume at room temperature, while a large excess of amide and harsher conditions, such as several days under reflux conditions, can be utilized to produce the disubstituted compound. - Corresponding compounds of tellurium (II) can be synthesized by the following related reaction scheme (Scheme III) conducted in a corresponding solvent medium:
- wherein:
- R1, R2, R3, R4, R5, R6 and R7 are the same as or different from one another, and each is independently selected from C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl, acetylalkyl and halogen (chlorine, bromine, iodine or fluorine), and R2 and R3 can additionally and independently be hydrogen; and
- X is halogen (chlorine, bromine, iodine or fluorine).
- Another synthesis for such tellurium (II) compounds is set out below in Scheme IIIA:
- wherein:
- M═Li, Na, K;
- X=chlorine, bromine, iodine or fluorine;
- R1, R2, R3, R4 can be the same as or different from one another, and each is independently selected from C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl, acetylalkyl and halogen (chlorine, bromine, iodine or fluorine).
- A still further synthesis of such tellurium (II) compounds is set out in Scheme IIIB below:
- wherein:
- M═Li, Na, or K;
- X═Cl, Br, I or F;
- R1, R2, R3, R4 can be the same as or different from one another, and each is independently selected from C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl, acetylalkyl, hydrogen and halogen (chlorine, bromine, iodine or fluorine).
- As a specific example of such tellurium (II) compounds, a synthesis of N,N′-di-tert-butyl butylenediamide tellurium is a set out below utilizing a corresponding lithiated precursor and diiodotellurium as reactants, in an ether solvent medium.
- The foregoing tellurium precursors have utility in various applications for deposition of Te or Te-containing thin films. Corresponding alkyl, silyl or amide derivatives (wherein chloro substituents are replaced by alkyl or amide functional groups) can also be readily synthesized. Such alkyl or amide derivatives may be preferred in some thin film deposition applications, due to their higher volatility properties, in relation to corresponding chloro compounds having lower volatility as a result of their dimeric nature.
- The invention therefore contemplates the provision of tellurium compounds in which the tellurium central metal atom is coordinated with ethylenediamine-type ligands, with the tellurium central metal atom being in a (II) or (IV) state. The invention further contemplates synthesis of ethylenediamine-type tellurium compounds substituted with alkyl and/or amide substituents. In addition, the invention contemplates use of the foregoing tellurium compounds for CVD and ALD applications, to deposit tellurium or tellurium-containing thin films.
- In another aspect, the invention relates to dialkyl ditellurides useful for CVD and ALD applications carried out at low temperatures to deposit tellurium or tellurium-containing films on substrates, e.g., Sb2Te3 films for the formation of GST films in phase change memory applications.
- For phase change memory applications, the tellurium precursor employed for forming GST films desirably has the ability to deposit at sufficiently low temperature to achieve amorphous Sb2Te3 films with good step coverage, since crystalline films do not provide the necessary step coverage for phase change memory device applications. The invention contemplates dialkyl ditellurides, e.g., di-tert-butyl ditelluride, to address such step coverage issues.
- Dialkyl tellurides are conventionally used to deposit tellurium or tellurium-containing films by CVD or ALD in phase change memory applications, but relatively high temperatures are needed to deposit such films. This deficiency can be overcome by use of dialkyl ditellurides, e.g., di-tert-butyl ditelluride, Te2(t-Bu)2.
- The reason for the lower deposition temperature achievable by this class of precursors is a relatively weak tellurium-tellurium bond. An examination of the X-ray crystal structure of Te2(t-Bu)2 reveals a fairly long Te—Te bond of 2.68 A. The lower deposition temperature is also evident from the simultaneous thermal analysis (STA) of Te2(t-Bu)2, which shows a very low T50 of 146° C. Te2(t-Bu)2 and its synthesis are described in the literature (see, for example, C. H. W. Jones, R. D. Sharma, J. of Organomet. Chem. 1983, 255, 61-70, and R. W. Gedridge Jr., K. T. Higa, R. A. Nissan,
Organometallics 1991, 10, 286-291, and U.S. Pat. No. 5,166,428). - The invention therefore contemplates the use of these dialkyl ditellurides, e.g., Te2(t-Bu)2, as precursors for the deposition of tellurium or tellurium-containing films on substrates, using CVD or ALD techniques, in applications such as the manufacture of phase change memory devices.
-
FIG. 5 is an ORTEP diagram of the structure of Te2(t-Bu)2. -
FIG. 6 is a simultaneous thermal analysis (STA) plot of thermogravimetry (TG) and differential scanning calorimetry (DSC) data for Te2(t-Bu)2. - In general, the alkyl moieties of the dialkyl ditelluride compounds of the invention can be of any suitable type, e.g., C1-C8 alkyl substituents. Examples include methyl, ethyl, isopropyl, and t-butyl. Preferably, such alkyl substituents include tertiary carbon moieties. Tertiary butyl or tertiary carbons in general are preferred as having high radical stability.
- Illustrative examples of dialkyl ditelluride compounds of the invention include dimethyl ditelluride, diethyl ditelluride, diisopropyl ditelluride, and di-tertiary-butyl ditelluride.
- Other ditelluride compounds contemplated by the invention can utilize other ligands, such as C1-C12 hydrocarbyl (aryl, fluoroalkyl, allyl, alkenyl, dienyl), or silyl or substituted silyl ligands.
- The above-described tellurium precursor compounds of the invention can be used for chemical vapor deposition and/or atomic layer deposition, to form tellurium or tellurium-containing films on substrates, e.g., semiconductor wafers or other microelectronic device base structures. Such precursor compounds when present in solid phase can be delivered by solid delivery techniques, wherein the solid precursor is contained in a precursor storage and vapor delivery vessel, which is subjected to heating to volatilize the solid precursor, e.g., by sublimation, so that the precursor vapor can be discharged selectively from the vessel, as needed in the downstream deposition process.
- The precursor when present in solid phase may also be dissolved in solvent medium, as described above, and delivered by liquid delivery techniques to a vaporizer, for volatilization to form a precursor vapor that then is contacted under a vapor deposition conditions with a wafer or other suitable substrate. When present in liquid phase, the tellurium precursor can be delivered by liquid delivery techniques from a suitable precursor storage vessel. Bubbler techniques may also be employed.
- The specific delivery technique employed in the practice of the invention utilizing such Te(II) and Te(IV) compounds can be selected based on the process conditions needed for delivery for contacting with the substrate on which Te or Te-containing films are to be formed. In general, it is desired to carry out deposition by CVD and/or ALD techniques at temperatures below 400° C.
- The deposition of tellurium species in accordance with the invention can be carried out to form GST phase change memory devices, or other tellurium-based microelectronic device structures.
- The features and advantages of the invention are more fully shown by the following non-limiting examples.
- 4.78 g (12.53 mmol) of TeI2 are suspended in 100 mL of THF in a 200 mL Schlenk flask equipped with a magnetic stirring bar. A solution of 5.00 g (25.06 mmol) of K[N(SiMe3)2] in 50 mL of THF is prepared in a 100 mL Schlenk flask. The K[N(SiMe3)2] solution is added to the TeI2 suspension via cannula at 0° C. (with ice-bath cooling). The reaction mixture turns yellow immediately and then brown after ca. 10 minutes. It is stirred another hour at 0° C. and then at ambient temperature overnight. The volatiles are removed in vacuum and the remaining dark brown solid is extracted with 100 mL of n-pentane. It is filtered through a medium glass-filter frit resulting in an orange solution. The pentane is removed in vacuum leaving 4.41 g of the crude product behind as an orange solid. The solid is sublimed at 200 mTorr and an oil bath temperature of 100° C. for two hours, affording 3.37 g (7.51 mmol; 60% yield) of analytical pure product as a yellow, crystalline solid.
- The product yielded by the foregoing procedure had the following characteristics: 1H NMR in C6D6, ppm: 0.338 (s, 18H, SiMe3); melting point: 66° C.
FIG. 7 is a STA plot of the Te[N(SiMe3)2]2 product. - 5.00 g (18.56 mmol) of TeCl4 are suspended in 400 mL of THF (only partially soluble) in a 500 mL Schlenk flask equipped with a magnetic stirring bar. A solution of 6.40 g (37.12 mmol) of N,N′-di-tert-butylethylenediamine and 7.51 g (74.24 mmol) of triethylamine in 10 mL of THF is prepared. This solution in added to the TeCl4 suspension and the reaction mixture turns cloudy immediately. A mild exothermic reaction is observed. The reaction mixture is stirred overnight a room temperature, and then filtered through a medium glass-filter frit, leading to yellow solution. The volatiles are removed in a vacuum leaving 5.96 g (16.20 mmol; 87.1% yield) of analytically pure product behind as a pale yellow, microcrystalline solid.
- X-ray analysis quality crystals were obtained by dissolving 0.5 g of product in 6 mL of toluene in a sample vial inside a controlled atmosphere glove-box. The solution is filtered through a PTFE syringe filter and then placed in a −25° C. freezer. After 16 hours large, pale yellow, plate like crystals of the title compound were obtained, which were suitable for X-ray analysis.
- The compound can be further purified by sublimation. In a typical experiment a sublimation device is charged with 5 g of material and the material is sublimed at 200 mTorr pressure and an oil bath temperature of 70° C. for two hours. Typical yields of the sublimation vary between 80-90%. The product yielded by the foregoing procedure had the following characteristics: 1H NMR in C6D6, ppm: 3.133 (s, 4H, N—CH2—CH2—N); 1.189 (s, 18H, N-t-Bu).
- 3.00 g of (7.87 mmol) TeI2 are suspended in 100 mL of diethyl ether in a 200 mL Schlenk flask equipped with a magnetic stirring bar. A solution of 2.38 g (15.73 mmol) of Li[N(SiMe3)(t-Bu)] in 50 mL of diethyl ether is prepared in a 100 mL Schlenk flask. The Li[N(SiMe3)(t-Bu)] solution is added to the TeI2 suspension via cannula at 0° C. (ice-bath cooling). It is stirred another hour at 0° C. and then at ambient temperature overnight. The volatiles are removed in vacuum and the remaining dark brown solid is extracted with 150 mL of n-pentane and filtered through a medium glass-filter frit, resulting in an orange solution. The pentane is removed in vacuum leaving 2.60 g (6.25 mmol; 79.5%) of analytically pure product behind as a yellow-orange solid. The compound can be further purified by sublimation at 200 mTorr and an oil bath temperature of 100° C. for two hours, affording 2.05 g (4.93 mmol; 63% yield) of product as a yellow, crystalline solid. The product yielded by the foregoing procedure had the following characteristics: 1H NMR in C6D6, ppm: 1.400 (s, 9H, t-Bu); 0.399 (s, 9H, SiMe3); melting point: 77° C.
FIG. 8 is a STA plot of the Te[N(SiMe3)(t-Bu)]2 product. - 2.36 g (8.79 mmol) of TeCl4 are suspended in 100 mL of THF (only partially soluble) in a 200 mL Schlenk flask equipped with a magnetic stirring bar. A solution of 1.77 g (8.79 mmol) of N,N′-di-tert-butyl-2,3-dimethylethylenediamine and 1.78 g (17.58 mmol) of triethylamine in 5 mL of THF is prepared. This solution in added to the TeCl4 suspension and the reaction mixture turns cloudy immediately. A mild exothermic reaction is observed. It is stirred overnight a room temperature. The reaction mixture is filtered through a medium glass-filter frit leading to an amber colored solution. The volatiles are removed in a vacuum leaving 1.93 g (4.85 mmol; 55% yield) of analytically pure product behind as an amber colored, microcrystalline solid.
- X-ray analysis quality crystals were obtained by dissolving 0.5 g of product in 5 mL of toluene in a sample vial inside a controlled atmosphere glove-box. The solution is filtered through a PTFE syringe filter and then placed in a −25° C. freezer. After 32 hours pale yellow crystals of the title compound were obtained, that were suitable for X-ray analysis. The product yielded by the foregoing procedure had the following characteristics: 1H NMR in C6D6, ppm: 2.722 (q, 2H, N(Me)-CH—CH-(Me)N); 1.227 (s, 18H, N-t-Bu); 1.080 (s, 3H, N(Me)-CH—CH-(Me)N); 1.059 (s, 3H, N(Me)-CH—CH-(Me)N).
- Di-tert-butyltelluride and Ge[PriNC(n-Bu)NPri]2, wherein Pri is isopropyl, were utilized as respective tellurium and germanium precursors to form a GeTe film. GeTe films can be formed using this germanium precursor, denoted GeBAMDN or GeM for ease of notation, and di-tert-butyltelluride, at temperatures below 300° C. or lower, e.g., below 280° C. or even 260° C. or lower. In general, lower temperatures will result in lower content of tellurium, but the specific deposition rate of the film will also depend on the germanium and tellurium delivery rates in the deposition system.
- Di-tert-butyltelluride and Ge[PriNC(n-Bu)NPri]2 were used to deposit GeTe films at the illustrative conditions identified below, with the following film thickness and tellurium concentration results.
-
Sam- Temperature, pressure, Flow rates of GeM Film Thickness, ple Film Growth Duration and Te(tBu)2 % Te in film 1 280° C., 8 torr, 8 min 20/160 GeM/Te(tBu) 124 {acute over (Å)}, 7.3 % Te 2 μmole/ min 2 260° C., 8 torr, 16 min 20/160 GeM/Te(tBu) 179 {acute over (Å)}, 12.5 % Te 2 μmole/min - The invention in another aspect involves use of control agents to combat vapor phase pre-reaction of the precursors described herein, that otherwise causes uneven nucleation on the substrate, longer incubation times for deposition reactions, and lower quality product films. Such pre-reaction may for example be particularly problematic in applications involving chalcogenide films, related source materials (O, S, Se, Te, Ge, Sb, Bi, etc.), and/or manufacture of phase change memory and thermoelectric devices.
- Pre-reaction may occur when the precursor reagents described herein are introduced to the deposition chamber, as in chemical vapor deposition, and may also occur in atomic layer deposition (ALD) processes, depending on the specific arrangement of ALD cycle steps and the specific reagents involved.
- The invention therefore contemplates the use of control agents with the precursors described herein, whereby detrimental gas phase pre-reactions are suppressed, mitigated or eliminated, so that deposition reactions are induced/enhanced on the substrate surface, and films of superior character are efficiently formed.
- The control agents that can be utilized with precursors of the invention for such purpose include agents selected from the group consisting of (i) heteroatom (O, N, S) organo Lewis base compounds, (ii) free radical inhibitors, and (iii) deuterium-containing reagents.
- These agents can be utilized to lessen deleterious gas phase pre-reaction I'll precursors by various approaches, including:
- (1) addition to the precursor composition of a pre-reaction suppressant comprising one or more heteroatom (O, N, S) organo Lewis base compounds such as 1,4-dioxane, thioxane, ethers, polyethers, triethylamine (TEA), triazine, diamines, N,N,N′,N′-tetramethylethylenediamine, N,N,N′-trimethylethylenediamine, amines, imines, and pyridine;
- (2) addition to the precursor composition of a free radical inhibitor, such as butylated hydroxy toluene (BHT), hydroquinone, butylated hydro anisole (BHA), diphenylamine, ethyl vanillin, etc.;
- (3) use of modified chalcogenide precursors, in which hydrogen substituents have been replaced with deuterium (D) substituents, to provide deuterated analogs for vapor phase deposition; and
- (4) addition to the precursor composition of a deuterium source, to deuterate the precursor in situ.
- The pre-reaction-combating agents described above (suppressants, free radical inhibitors, deuterium sources and/or deuterated precursors) can be introduced to any of the feed streams to the vapor deposition process in which the film is to be formed. For example, such pre-reaction-combating agents can be introduced to one or more of precursor feed stream(s), inert carrier gas stream(s) to which chalcogenide precursor(s) or other reagents are subsequently added for flow to the deposition chamber, co-reactant feed stream(s) flowed to the deposition chamber, and/or any other stream(s) that is/are flowed to the deposition chamber and in which the pre-reaction-combating agent(s) is/are useful for reduction or elimination of premature reaction of the precursors that would otherwise occur in the absence of such agent(s).
- The aforementioned suppressants, free radical inhibitors and/or deuterium source reagents in specific embodiments are co-injected with the precursor(s), e.g., metal source reagent(s), to effect at least partial reduction of pre-reaction involving the precursor(s) and reagent(s).
- The pre-reaction-combatting agent can alternatively be added directed to the deposition locus, e.g., the deposition chamber to which the precursor vapor is introduced for contacting with the substrate to deposit the film thereon, to suppress deleterious vapor phase pre-reaction involving the precursor(s) and/or other reagents.
- As another approach, in the broad practice of the present invention, the suppressant, free radical inhibitor and/or deuterium source can be added to a solution containing the precursor and/or another metal source reagent, and the resulting solution can be utilized for liquid delivery processing, in which the solution is flowed to a vaporizer to form a source vapor for contacting with the substrate to deposit the deposition species thereon.
- Alternatively, if the precursor and/or another metal source reagent are not in an existing solution, the suppressant, free radical inhibitor and/or deuterium source can be added to form a mixture or a solution with the precursor and/or another metal source reagent, depending on the respective phases of the materials involved, and their compatibility/solubility.
- As a still further approach, the suppressant, free radical inhibitor and/or deuterium source can be utilized for surface treatment of the substrate prior to contacting of the substrate with the precursor and/or other metal source reagent.
- The invention therefore contemplates various vapor deposition compositions and processes for forming films on substrates, in which pre-reaction of the precursors is at least partially attenuated by one or more pre-reaction-combating agents selected from among heteroatom (O, N, S) organo Lewis base compounds, sometimes herein referred to as suppressor agents, free radical inhibitors, and/or deuterium source reagents. Use of previously synthesized deuterated precursors or organometal compounds is also contemplated, as an alternative to in situ deuteration with a deuterium source. By suppressing precursor prereaction with these approaches, product films of superior character can be efficiently formed.
- The control agent can be used for combating pre-reaction of chalcogenide precursor in a process in which multiple feed streams are flowed to a deposition locus to form a film on a substrate, wherein at least one of the multiple feed streams includes a precursor susceptible to pre-reaction adversely affecting the film, in which the method involves introducing the control agent to at least one of such multiple feed streams or supplied materials therefor, or to the deposition locus.
- The pre-reaction combating reagent alternatively can be introduced to passivate the surface of a growing chalcogenide film or slow the deposition rate, followed by reactivation using an alternative precursor or co-reactant (for example H2, NH3, plasma, H2O, hydrogen sulfide, hydrogen selenide, diorganotellurides, diorganosulfides, diorganoselenides, etc.), thereby carrying out passivation/retardation followed by reactivation steps, e.g., as an alternating repetitive sequence. Such sequence of passivation/retardation followed by reactivation can be carried out for as many repetitive cycles as desired, in ALD or ALD-like processes. The steps may be carried out for the entire deposition operation, or during some initial, intermediate or final portion thereof.
- The invention therefore contemplates precursor compositions including the precursor and the pre-reaction-combating reagent. Within the categories of pre-reaction-combating reagents previously described, viz., (i) heteroatom (O, N, S) organo Lewis base compounds, (ii) free radical inhibitors, and (iii) deuterium-containing reagents, suitable pre-reaction-combating reagents for specific applications may be readily determined within the skill of the art, based on the disclosure herein.
- Heteroatom (O, N, S) organo Lewis base compounds may be of varied type, e.g., containing an oxo (—O—) moiety, a nitrogen ring atom or pendant amino or amide substituent, a sulfur ring atom or pendant sulfide, sulfonate or thio group, as effective to at least partially lessen pre-reaction of the precursor and other organo metal reagents in the process system. Illustrative examples of heteroatom (O, N, S) organo Lewis base compounds having utility in specific applications of the invention include, without limitation, 1,4-dioxane, thioxane, ethers, polyethers, triethylamine, triazine, diamines, N,N,N′,N′-tetramethylethylenediamine, N,N,N′-trimethylethylenediamine, amines, imines, pyridine, and the like.
- The heteroatom organo Lewis base compound in various specific embodiments of the invention may include a guanidinate compound, e.g., (Me2N)2C═NH.
- One preferred class of heteroatom organo Lewis base compounds for such purpose includes R3N, R2NH, RNH2, R2N(CH2)xNR2, R2NH(CH2)xNR2, R2N(CR2)xNR2, and cyclic amines —N(CH2)x—, imidazole, thiophene, pyrrole, thiazole, urea, oxazine, pyran, furan, indole, triazole, triazine, thiazoline, oxazole, dithiane, trithiane, crown ethers, 1,4,7-triazacyclononane, 1,5,9-triazacyclododecane, cyclen, succinamide, and substituted derivatives of the foregoing, wherein R can be hydrogen or any suitable organo moieties, e.g., hydrogen, C1-C8 alkyl, C1-C8 alkoxy, C1-C8 alkene, C1-C8 alkyne, and C1-C8 carboxyl, and wherein x is an integer having a value of from 1 to 6.
- The heteroatom organo Lewis base compounds may be utilized in the precursor composition at any suitable concentration, as may be empirically determined by successive deposition runs in which the heteroatom organo Lewis base compound concentration is varied, and character of the resulting film is assessed, to determine an appropriate concentration. In various embodiments, the heteroatom organo Lewis base compound may be utilized in the concentration of 1-300% of the amount of precursor. Specific sub-ranges of concentration values within a range of 0.01-3 equivalents of the heteroatom organo Lewis base compound may be established for specific classes of precursors, without undue experimentation, based on the disclosure herein.
- The pre-reaction-combating reagent may additionally or alternatively comprise free radical inhibitors that are effective to lessen the extent of pre-reaction between the precursor and another organo metal reagent. Such free radical inhibitors may be of any suitable type, and may for example include hindered phenols. Illustrative free radical inhibitors include, without limitation, free radical scavengers selected from the group consisting of: 2,6-ditert-butyl-4-methyl phenol, 2,2,6,6-tetramethyl-1-piperidinyloxy, 2,6-dimethylphenol, 2-tert-butyl-4-hydroxyanisole, 3-tert-butyl-4-hydroxyanisole, propyl ester 3,4,5-trihydroxy-benzoic acid, 2-(1,1-dimethylethyl)-1,4 benzenediol, diphenylpicrylhydrazyl, 4-tert-butylcatechol, N-methylaniline, 2,6-dimethylaniline, p-methoxydiphenylamine, diphenylamine, N,N′-diphenyl-p-phenylenediamine, p-hydroxydiphenylamine, phenol, octadecyl-3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate, tetrakis(methylene(3,5-di-tert-butyl-4-hydroxy-hydrocinnamate)methane, phenothiazines, alkylamidonoisoureas, thiodiethylene bis(3,5,-di-tert-butyl-4-hydroxy-hydrocinnamate, 1,2,-bis (3,5-di-tert-butyl-4-hydroxyhydrocinnamoyl)hydrazine, tris (2-methyl-4-hydroxy-5-tert-butylphenyl)butane, cyclic neopentanetetrayl bis(octadecyl phosphite), 4,4′-thiobis(6-tert-butyl-m-cresol, 2,2′-methylenebis(6-tert-butyl-p-cresol), oxalyl bis(benzylidenehydrazide) and mixtures thereof. Preferred free radical inhibitors include BHT, BHA, diphenylamine, ethyl vanillin, and the like.
- Useful concentrations of the free radical inhibitor may be in a range of from 0.001 to about 0.10% by weight of the weight of the precursor, in various specific embodiments. More generally, any suitable amount of free radical inhibitor may be employed that is effective to combat the pre-reaction of the precursor in the delivery and deposition operations involved in the film formation process.
- The deuterium source compounds afford another approach to suppressing pre-reaction of the chalcogenide precursor. Such deuterium source compounds may be of any suitable type, and may for example include deuterated pyridine, deuterated pyrimidine, deuterated indole, deuterated imidazole, deuterated amine and amide compounds, deuterated alkyl reagents, etc., as well as deuterated analogs of the precursors that would otherwise be used as containing hydrogen or protonic substituents.
- Deuterides that may be useful in the general practice of invention as pre-reaction-combating reagents include, without limitation, germanium and antimony compounds of the formulae RxGeD4-x and RxSbD3-x wherein R can be hydrogen or any suitable organo moieties, e.g., hydrogen, C1-C8 alkyl, C1-C8 alkoxy, C1-C8 alkene, C1-C8 alkyne, and C1-C8 carboxyl, and wherein x is an integer having a value of from 1 to 6.
- The deuterium source reagent may be utilized at any suitable concentration that is effective to combat pre-reaction of the precursor. Illustrative deuterium source reagent concentrations in specific embodiments of the invention can be in a range of 0.01 to about 5% by weight, based on the weight of precursor.
- Thus, a deuterium source compound may be added to one or more of the feed streams to the vapor deposition process, and/or one of the precursors or other feed stream components may be deuterated in the first instance.
- The concentrations of the pre-reaction-combating agents utilized in the practice of the present invention to at least partially eliminate pre-reaction of the precursors can be widely varied in the general practice of the present invention, depending on the temperatures, pressures, flow rates and specific compositions involved. The above-described ranges of concentration of the pre-reaction-combating reagents of the invention therefore are to be appreciated as being of an illustrative character only, with applicable concentrations being readily determinable within the skill of the art, based on the disclosure herein.
- The specific mode of introduction or addition of the pre-reaction-combating agent to one or more of the feed streams to the deposition process may correspondingly be varied, and may for example employ mass flow controllers, flow control valves, metering injectors, or other flow control or modulating components in the flow circuitry joining the source of the pre-reaction-combating agent with the streams being flowed to the deposition process during normal film-forming operation. The process system may additionally include analyzers, monitors, controllers, instrumentation, etc., as may be necessary or appropriate to a given implementation of the invention.
- In lieu of introduction or addition of the pre-reaction-combating agent to one or more of the flow streams to the vapor deposition process, the pre-reaction-combating agent may be mixed with precursor in the first instance, as a starting reagent material for the process. For example, the pre-reaction-combating agent may be mixed in liquid solution with the precursor, for liquid delivery of the resulting precursor solution to a vaporizer employed to generate precursor vapor for contact with the substrate to deposit the film thereon.
- As mentioned, the pre-reaction-combating agent may be added to the deposition locus to provide active gas-phase suppression of pre-reaction of the precursor vapor(s) that would otherwise be susceptible to such deleterious interaction.
- As a still further alternative, the pre-reaction-combating agent may be used as a preliminary surface treatment following which the precursor and co-reactants (e.g., H2, NH3, plasma, H2O, hydrogen sulfide, hydrogen selenide, diorganotellurides, diorganosulfides, diorganoselenides, etc.) are delivered to the substrate surface to effect deposition on such surface. For such purpose, the pre-reaction-combating agent may be introduced into one of more of the flow lines to the deposition process and flow to the substrate in the deposition process chamber, prior to initiation of flow of any precursors. After the requisite period of contacting of the substrate with such pre-reaction-combating agent has been completed, the flow of the pre-reaction-combating agent can be terminated, and normal feeding of flow streams to the deposition chamber can be initiated.
- It will be apparent from the foregoing description that the pre-reaction-combating agent may be introduced in any of a wide variety of ways to effect diminution of the pre-reaction of the precursor in the deposition system.
- In one embodiment of the invention, a vapor phase deposition system is contemplated, comprising:
- a vapor deposition chamber adapted to hold at least one substrate for deposition of a film thereon;
- chemical reagent supply vessels containing reagents for forming the film;
- first flow circuitry arranged to deliver said reagents from said chemical reagent supply vessels to the vapor deposition chamber;
- a pre-reaction-combating agent supply vessel containing a pre-reaction-combating agent;
- second flow circuitry arranged to deliver the pre-reaction-combating agent from the pre-reaction-combating agent supply vessel to the first flow circuitry, to said chemical reagent supply vessels and/or to the vapor deposition chamber.
-
FIG. 9 is a schematic representation of avapor deposition system 100 in one embodiment thereof. - In this illustrative system, a pre-reaction-combating agent is contained in a
supply vessel 110. The pre-reaction-combating agent can comprise a pre-reaction suppressant, a free radical inhibitor, a deuterium source, or a combination of two or more of such agents and/or types of such agents. - The pre-reaction-combating agent supply vessel is joined by
respective flow lines - The pre-reaction-combating agent therefore can be added to any of the germanium, antimony and/or tellurium precursors in the respective “G,” “S” and “T” vessels, via the corresponding flow line(s), which for such purpose may have flow control valves or other flow-modulating components therein.
- In the specific process embodiment shown, the germanium, antimony and tellurium precursors are flowed in liquid form in
feed lines chamber 124, and the resulting precursor mixture then is flowed from the mixingchamber 124 inline 126 tovaporizer 128. In the vaporizer, the liquid precursor mixture and pre-reaction-combating agent are volatilized to form a precursor vapor. The precursor vapor then flows inline 130 to the showerhead disperser 134 invapor deposition chamber 132, for discharge of precursor mixture onto thewafer substrate 136 mounted onsusceptor 138 in the deposition chamber. - The precursor vapor contacting the
wafer substrate 136 serves to deposit the germanium, antimony and tellurium metals on the substrate, to form a thin film of germanium-antimony-tellurium (GST) material, e.g., for manufacture of a phase change random access memory device. - The contacted precursor vapor, depleted in metals content, is discharged from the
vapor deposition chamber 132 inline 140, and flows to theeffluent abatement unit 142. In theeffluent abatement unit 142, the discharged effluent vapor is treated, e.g., by scrubbing, catalytic oxidation, electrochemical treatment, or in other manner, to yield a final effluent that is discharged from the abatement unit inline 146. - It will be appreciated that these schematic representation of the vapor deposition system shown in
FIG. 9 is of an illustrative character, and that numerous other arrangements could be utilized for deployment and use of the pre-reaction-combating agent, including those previously illustratively discussed herein. For example, the pre-reaction-combating agent could be introduced directly to the mixingchamber 124, for blending therein with the respective GST precursors. Alternatively, the pre-reaction-combating agent could be introduced intomanifold 118, or other mixing chamber, blender, etc., for combination with the precursor that is being transported to the deposition locus. - The system shown in
FIG. 9 employs liquid delivery of the respective precursors. It will be recognized that if solid-phased precursors are employed, then solid delivery techniques may be employed, in which solid precursor is volatilized, e.g., by sublimation of the solid starting material. - In lieu of using a deuterating agent as the pre-reaction-combating agent in the
FIG. 9 system, one or more of the germanium, antimony and tellurium precursors could be supplied in the first instance as a deuterated analog of an organo germanium, antimony or tellurium precursor, in which hydrogen substituents of the organo moiety have been replaced with deuterium. - The pre-reaction-combating reagents may be employed in the broad practice of the present invention to produce improved films for the manufacture of semiconductor products. In general, the pre-reaction-combating reagents described herein may be utilized in various combinations in specific applications, to suppress or eliminate pre-reaction of the precursor(s) and provide superior nucleation and final film properties.
- While the invention has been described herein in reference to specific aspects, features and illustrative embodiments of the invention, it will be appreciated that the utility of the invention is not thus limited, but rather extends to and encompasses numerous other variations, modifications and alternative embodiments, as will suggest themselves to those of ordinary skill in the field of the present invention, based on the disclosure herein. Correspondingly, the invention as hereinafter claimed is intended to be broadly construed and interpreted, as including all such variations, modifications and alternative embodiments, within its spirit and scope.
Claims (20)
1. A tellurium precursor composition, including a tellurium precursor selected from the group consisting of:
(i) Te(IV) organyls having the formula TeR1R2R3R4 wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(ii) tellurium bis-amides of the formula Te[NR2]2 wherein each R is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(iii) asymmetric tellurium compounds including one alkyl substituent and a second ligand containing a heteroatom;
(iv) tellurium compounds with ethylenediamine ligands;
(v) tellurium compounds with dithiocarbamate ligands;
(vi) Te(II) and Te(IV) compounds including at least one nitrogen-based ligand selected from among amidinates, guanidinates, isoureates and beta-diketoiminates; and
(vii) dialkyl ditellurides wherein alkyl is C1-C8 alkyl.
2. The tellurium precursor composition of claim 1 , comprising a tellurium precursor is selected from the group consisting of Te[N(t-Bu)(SiMe3)]2 and Te[N(SiMe3)2]2.
3. The tellurium precursor composition of claim 1 , comprising a tellurium precursor selected from the group consisting of:
(a) tellurium precursors of the formula
R3TeNR1R2
R3TeNR1R2
wherein
each of R1, R2 and R3 is the same as or different from the other, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 can in addition be amide or halogen;
(b) tellurium amidinate precursors of the formula
wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R4 in addition can be amide or halogen;
(c) tellurium guanidinates of the formula
wherein each of R1, R2, R3, R4 and R5 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R5 in addition can be amide or halogen;
(d) tellurium dithiocarbamates of the formula
wherein each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 in addition can be amide or halogen;
(e) tellurium precursors of the formula:
in a wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(f) tellurium precursors of the formula:
wherein M is Te, and each R is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(h) tellurium precursors of the formula:
wherein each R may be the same as or different from the others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(i) tellurium precursors of the formula:
wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein each of R3 and R4 can in addition and independently be amide or halogen;
(j) tellurium precursors of the formula:
wherein
each R1 and R2 is the same as or different from the others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(k) tellurium precursors of the formula:
wherein each of R1, R2, R3, R4 and R5 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein each of R3, R4 and R5 can in addition and independently be amide or halogen;
(l) tellurium precursors of the formula:
wherein each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 can in addition be amide or halogen;
(m) tellurium precursors of the formula:
wherein
each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 can in addition be amide or halogen; and
Z is independently selected from C1-C6 alkoxy, —NR1R2, H, C1-C6 alkyl, C3-C10 cycloalkyl, and C6-C13 aryl;
(n) tellurium precursors of the formula:
wherein
each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
Z is independently selected from C1-C6 alkoxy, —NR1R2, H, C1-C6 alkyl, C3-C10 cycloalkyl, and C6-C13 aryl, and wherein each R3 can in addition and independently be amide or halogen; and
x is an integer having a value of from 0 to 4, inclusive;
(o) tellurium precursors of the formula:
wherein
each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(p) tellurium precursors of the formula:
wherein
each of R1, R2, R3, R4, R5 and R6 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl; and
(q) tellurium precursors of the formula:
XTeNR1R2
XTeNR1R2
wherein
X is halogen; and
each of R1 and R2 is the same as or different from the other, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl.
4. The tellurium precursor composition of claim 1 , wherein the tellurium precursor comprises N,N′-di-tert-butylenediamide tellurium dichloride.
5. The tellurium precursor composition of claim 1 , wherein the tellurium precursor comprises Me2NHTeCl2 wherein Me is methyl.
6. The tellurium precursor composition of claim 1 , wherein the tellurium precursor comprises NHTe(Cl)NMe2, wherein Me is methyl.
7. The tellurium precursor composition of claim 1 , wherein the tellurium precursor comprises Te2(t-Bu)2 wherein t-Bu is tertiary butyl.
8. The tellurium precursor composition of claim 1 , further comprising a solvent medium in which the tellurium precursor is dissolved.
9. The tellurium precursor composition of claim 8 , wherein the solvent medium comprises a hydrocarbon solvent including one or more of alkanes, aromatics and amines.
10. The tellurium precursor composition of claim 8 , wherein the solvent medium comprises one or more of hexane, heptane, octane, and pentane, benzene, toluene, triethylamine, and tert-butylamine.
11. The tellurium precursor composition of claim 10 , wherein the tellurium precursor is selected from the group consisting of:
(a) tellurium precursors of the formula
R3TeNR1R2
R3TeNR1R2
wherein
each of R1, R2 and R3 is the same as or different from the other, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 can in addition be amide or halogen;
(b) tellurium amidinate precursors of the formula
wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R4 in addition can be amide or halogen;
(c) tellurium guanidinates of the formula
wherein each of R1, R2, R3, R4 and R5 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R5 in addition can be amide or halogen;
(d) tellurium dithiocarbamates of the formula
wherein each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 in addition can be amide or halogen;
(e) tellurium precursors of the formula:
in a wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(f) tellurium precursors of the formula:
wherein M is Te, and each R is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(h) tellurium precursors of the formula:
wherein each R may be the same as or different from the others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(i) tellurium precursors of the formula:
wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein each of R3 and R4 can in addition and independently be amide or halogen;
(j) tellurium precursors of the formula:
wherein
each R1 and R2 is the same as or different from the others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(k) tellurium precursors of the formula:
wherein each of R1, R2, R3, R4 and R5 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein each of R3, R4 and R5 can in addition and independently be amide or halogen;
(l) tellurium precursors of the formula:
wherein each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 can in addition be amide or halogen;
(m) tellurium precursors of the formula:
wherein
each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 can in addition be amide or halogen; and
Z is independently selected from C1-C6 alkoxy, —NR1R2, H, C1-C6 alkyl, C3-C10 cycloalkyl, and C6-C13 aryl;
(n) tellurium precursors of the formula:
wherein
each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
Z is independently selected from C1-C6 alkoxy, —NR1R2, H, C1-C6 alkyl, C3-C10 cycloalkyl, and C6-C13 aryl, and wherein each R3 can in addition and independently be amide or halogen; and
x is an integer having a value of from 0 to 4, inclusive;
(o) tellurium precursors of the formula:
wherein
each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(p) tellurium precursors of the formula:
wherein
each of R1, R2, R3, R4, R5 and R6 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl; and
(q) tellurium precursors of the formula:
XTeNR1R2
XTeNR1R2
wherein
X is halogen; and
each of R1 and R2 is the same as or different from the other, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl.
12. A precursor composition package, comprising a storage and dispensing vessel holding a tellurium precursor composition of claim 1 .
13. The precursor composition package of claim 12 , wherein the tellurium precursor is selected from the group consisting of:
(a) tellurium precursors of the formula
R3TeNR1R2
R3TeNR1R2
wherein
each of R1, R2 and R3 is the same as or different from the other, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 can in addition be amide or halogen;
(b) tellurium amidinate precursors of the formula
wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R4 in addition can be amide or halogen;
(c) tellurium guanidinates of the formula
wherein each of R1, R2, R3, R4 and R5 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R5 in addition can be amide or halogen;
(d) tellurium dithiocarbamates of the formula
wherein each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 in addition can be amide or halogen;
(e) tellurium precursors of the formula:
in a wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(f) tellurium precursors of the formula:
wherein M is Te, and each R is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(h) tellurium precursors of the formula:
wherein each R may be the same as or different from the others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(i) tellurium precursors of the formula:
wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein each of R3 and R4 can in addition and independently be amide or halogen;
(j) tellurium precursors of the formula:
wherein
each R1 and R2 is the same as or different from the others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(k) tellurium precursors of the formula:
wherein each of R1, R2, R3, R4 and R5 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein each of R3, R4 and R5 can in addition and independently be amide or halogen;
(l) tellurium precursors of the formula:
wherein each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 can in addition be amide or halogen;
(m) tellurium precursors of the formula:
wherein
each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 can in addition be amide or halogen; and
Z is independently selected from C1-C6 alkoxy, —NR1R2, H, C1-C6 alkyl, C3-C10 cycloalkyl, and C6-C13 aryl;
(n) tellurium precursors of the formula:
wherein
each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
Z is independently selected from C1-C6 alkoxy, —NR1R2, H, C1-C6 alkyl, C3-C10 cycloalkyl, and C6-C13 aryl, and wherein each R3 can in addition and independently be amide or halogen; and
x is an integer having a value of from 0 to 4, inclusive;
(o) tellurium precursors of the formula:
wherein
each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(p) tellurium precursors of the formula:
wherein
each of R1, R2, R3, R4, R5 and R6 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl; and
(q) tellurium precursors of the formula:
XTeNR1R2
XTeNR1R2
wherein
X is halogen; and
each of R1 and R2 is the same as or different from the other, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl.
14. The tellurium precursor composition of claim 1 , comprising a storage medium for the tellurium precursor.
15. A method of depositing tellurium on a substrate, comprising volatilizing a tellurium precursor composition to form a tellurium precursor vapor, and contacting the tellurium precursor vapor with the substrate to deposit tellurium thereon, wherein the tellurium precursor composition comprises a tellurium precursor selected from the group consisting of:
(i) Te(IV) organyls having the formula TeR1R2R3R4 wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(ii) tellurium bis-amides of the formula Te[NR2]2 wherein each R is independently selected from H, C1-C6 alkyl, C-C 6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(iii) asymmetric tellurium compounds including one alkyl substituent and a second ligand containing a heteroatom;
(iv) tellurium compounds with ethylenediamine ligands;
(v) tellurium compounds with dithiocarbamate ligands;
(vi) Te(II) and Te(IV) compounds including at least one nitrogen-based ligand selected from among amidinates, guanidinates, isoureates and beta-diketoiminates; and
(vii) dialkyl ditellurides wherein alkyl is C1-C8 alkyl.
16. The method of claim 15 , wherein the tellurium precursor is selected from the group consisting of Te[N(t-Bu)(SiMe3)]2 and Te[N(SiMe3)2]2.
17. The method of claim 15 , wherein the tellurium precursor is selected from the group consisting of:
(a) tellurium precursors of the formula
R3TeNR1R2
R3TeNR1R2
wherein
each of R1, R2 and R3 is the same as or different from the other, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 can in addition be amide or halogen;
(b) tellurium amidinate precursors of the formula
wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R4 in addition can be amide or halogen;
(c) tellurium guanidinates of the formula
wherein each of R1, R2, R3, R4 and R5 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R5 in addition can be amide or halogen;
(d) tellurium dithiocarbamates of the formula
wherein each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 in addition can be amide or halogen;
(e) tellurium precursors of the formula:
in a wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(f) tellurium precursors of the formula:
wherein M is Te, and each R is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(h) tellurium precursors of the formula:
wherein each R may be the same as or different from the others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(i) tellurium precursors of the formula:
wherein each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein each of R3 and R4 can in addition and independently be amide or halogen;
(j) tellurium precursors of the formula:
wherein
each R1 and R2 is the same as or different from the others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(k) tellurium precursors of the formula:
wherein each of R1, R2, R3, R4 and R5 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein each of R3, R4 and R5 can in addition and independently be amide or halogen;
(l) tellurium precursors of the formula:
wherein each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 can in addition be amide or halogen;
(m) tellurium precursors of the formula:
wherein
each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl, and wherein R3 can in addition be amide or halogen; and
Z is independently selected from C1-C6 alkoxy, —NR1R2, H, C1-C6 alkyl, C3-C10 cycloalkyl, and C6-C13 aryl;
(n) tellurium precursors of the formula:
wherein
each of R1, R2 and R3 is the same as or different from others, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
Z is independently selected from C1-C6 alkoxy, —NR1R2, H, C1-C6 alkyl, C3-C10 cycloalkyl, and C6-C13 aryl, and wherein each R3 can in addition and independently be amide or halogen; and
x is an integer having a value of from 0 to 4, inclusive;
(o) tellurium precursors of the formula:
wherein
each of R1, R2, R3 and R4 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl;
(p) tellurium precursors of the formula:
wherein
each of R1, R2, R3, R4, R5 and R6 is the same as or different from others, and each is independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, amide, aminoalkyl, alkylamine, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl; and
(q) tellurium precursors of the formula:
XTeNR1R2
XTeNR1R2
wherein
X is halogen; and
each of R1 and R2 is the same as or different from the other, and each is independently selected from H, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C6-C10 aryl, silyl, substituted silyl, aminoalkyl, alkoxyalkyl, aryloxyalkyl, imidoalkyl and acetylalkyl.
18. The method of claim 15 , wherein the tellurium precursor is selected from the group consisting of N,N′-di-tert-butylenediamide tellurium dichloride; Me2NHTeCl2; NHTe(Cl)NMe2; and Te2(t-Bu)2, wherein Me is methyl and t-Bu is tertiary butyl.
19. The method of claim 15 , wherein said contacting comprises atomic layer deposition.
20. The method of claim 19 , wherein tellurium is deposited to form a GST material on the substrate.
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/392,009 US20090215225A1 (en) | 2008-02-24 | 2009-02-24 | Tellurium compounds useful for deposition of tellurium containing materials |
| US13/911,622 US8796068B2 (en) | 2008-02-24 | 2013-06-06 | Tellurium compounds useful for deposition of tellurium containing materials |
| US14/332,924 US9537095B2 (en) | 2008-02-24 | 2014-07-16 | Tellurium compounds useful for deposition of tellurium containing materials |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US3098008P | 2008-02-24 | 2008-02-24 | |
| US5018308P | 2008-05-02 | 2008-05-02 | |
| US12/392,009 US20090215225A1 (en) | 2008-02-24 | 2009-02-24 | Tellurium compounds useful for deposition of tellurium containing materials |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/911,622 Continuation US8796068B2 (en) | 2008-02-24 | 2013-06-06 | Tellurium compounds useful for deposition of tellurium containing materials |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20090215225A1 true US20090215225A1 (en) | 2009-08-27 |
Family
ID=40998722
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/392,009 Abandoned US20090215225A1 (en) | 2008-02-24 | 2009-02-24 | Tellurium compounds useful for deposition of tellurium containing materials |
| US13/911,622 Active US8796068B2 (en) | 2008-02-24 | 2013-06-06 | Tellurium compounds useful for deposition of tellurium containing materials |
| US14/332,924 Active 2029-04-15 US9537095B2 (en) | 2008-02-24 | 2014-07-16 | Tellurium compounds useful for deposition of tellurium containing materials |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/911,622 Active US8796068B2 (en) | 2008-02-24 | 2013-06-06 | Tellurium compounds useful for deposition of tellurium containing materials |
| US14/332,924 Active 2029-04-15 US9537095B2 (en) | 2008-02-24 | 2014-07-16 | Tellurium compounds useful for deposition of tellurium containing materials |
Country Status (1)
| Country | Link |
|---|---|
| US (3) | US20090215225A1 (en) |
Cited By (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080118636A1 (en) * | 2006-11-21 | 2008-05-22 | Samsung Electronics Co., Ltd | Method of forming phase change layer using a germanium precursor and method of manufacturing phase change memory device using the same |
| US20090097305A1 (en) * | 2007-10-11 | 2009-04-16 | Samsung Electronics Co., Ltd. | Method of forming phase change material layer using ge(ii) source, and method of fabricating phase change memory device |
| US20090112009A1 (en) * | 2007-10-31 | 2009-04-30 | Advanced Technology Materials, Inc. | Amorphous ge/te deposition process |
| US20090256127A1 (en) * | 2008-04-15 | 2009-10-15 | American Air Liquide, Inc. | Compounds for Depositing Tellurium-Containing Films |
| US20100279011A1 (en) * | 2007-10-31 | 2010-11-04 | Advanced Technology Materials, Inc. | Novel bismuth precursors for cvd/ald of thin films |
| US20100291299A1 (en) * | 2007-08-08 | 2010-11-18 | Advanced Technology Materials, Inc. | Strontium and barium precursors for use in chemical vapor deposition, atomic layer deposition and rapid vapor deposition |
| US20100317150A1 (en) * | 2006-11-02 | 2010-12-16 | Advanced Technology Materials, Inc. | Antimony and germanium complexes useful for cvd/ald of metal thin films |
| US20110001107A1 (en) * | 2009-07-02 | 2011-01-06 | Advanced Technology Materials, Inc. | Hollow gst structure with dielectric fill |
| US20110111556A1 (en) * | 2008-05-02 | 2011-05-12 | Advanced Technology Materials, Inc. | Antimony compounds useful for deposition of antimony-containing materials |
| US20110124182A1 (en) * | 2009-11-20 | 2011-05-26 | Advanced Techology Materials, Inc. | System for the delivery of germanium-based precursor |
| US20110180905A1 (en) * | 2008-06-10 | 2011-07-28 | Advanced Technology Materials, Inc. | GeSbTe MATERIAL INCLUDING SUPERFLOW LAYER(S), AND USE OF Ge TO PREVENT INTERACTION OF Te FROM SbXTeY AND GeXTeY RESULTING IN HIGH Te CONTENT AND FILM CRYSTALLINITY |
| US8148197B2 (en) | 2010-07-27 | 2012-04-03 | Micron Technology, Inc. | Methods of forming germanium-antimony-tellurium materials and a method of forming a semiconductor device structure including the same |
| CN102637822A (en) * | 2012-03-14 | 2012-08-15 | 宁波大学 | High-purity chalcogenide phase change alloy target and preparation method for same |
| WO2012166255A1 (en) * | 2011-06-01 | 2012-12-06 | International Business Machines Corporation | Method to selectively grow phase change material inside a via hole |
| US8330136B2 (en) | 2008-12-05 | 2012-12-11 | Advanced Technology Materials, Inc. | High concentration nitrogen-containing germanium telluride based memory devices and processes of making |
| CN103111086A (en) * | 2012-12-26 | 2013-05-22 | 成都市幅原环保科技有限责任公司 | Solvent for leaching oil in drillings and leaching method |
| US8617972B2 (en) | 2009-05-22 | 2013-12-31 | Advanced Technology Materials, Inc. | Low temperature GST process |
| US8679894B2 (en) | 2006-05-12 | 2014-03-25 | Advanced Technology Materials, Inc. | Low temperature deposition of phase change memory materials |
| US8796068B2 (en) | 2008-02-24 | 2014-08-05 | Advanced Technology Materials, Inc. | Tellurium compounds useful for deposition of tellurium containing materials |
| US8834968B2 (en) | 2007-10-11 | 2014-09-16 | Samsung Electronics Co., Ltd. | Method of forming phase change material layer using Ge(II) source, and method of fabricating phase change memory device |
| US8853075B2 (en) | 2008-02-27 | 2014-10-07 | L'Air Liquide Société Anonyme pour l'Etude et l'Exploitation des Procédés Georges Claude | Method for forming a titanium-containing layer on a substrate using an atomic layer deposition (ALD) process |
| US8993441B2 (en) | 2013-03-13 | 2015-03-31 | Samsung Electronics Co., Ltd. | Methods of forming a thin layer and methods of manufacturing a phase change memory device using the same |
| US9012876B2 (en) | 2010-03-26 | 2015-04-21 | Entegris, Inc. | Germanium antimony telluride materials and devices incorporating same |
| US9190609B2 (en) | 2010-05-21 | 2015-11-17 | Entegris, Inc. | Germanium antimony telluride materials and devices incorporating same |
| US9443736B2 (en) | 2012-05-25 | 2016-09-13 | Entegris, Inc. | Silylene compositions and methods of use thereof |
| US9534285B2 (en) | 2006-03-10 | 2017-01-03 | Entegris, Inc. | Precursor compositions for atomic layer deposition and chemical vapor deposition of titanate, lanthanate, and tantalate dielectric films |
| US9640757B2 (en) | 2012-10-30 | 2017-05-02 | Entegris, Inc. | Double self-aligned phase change memory device structure |
| US20180282863A1 (en) * | 2015-10-06 | 2018-10-04 | Entegris, Inc. | Cold sintering of solid precursors |
| US10186570B2 (en) | 2013-02-08 | 2019-01-22 | Entegris, Inc. | ALD processes for low leakage current and low equivalent oxide thickness BiTaO films |
| US10580976B2 (en) | 2018-03-19 | 2020-03-03 | Sandisk Technologies Llc | Three-dimensional phase change memory device having a laterally constricted element and method of making the same |
| US10941487B2 (en) | 2009-10-26 | 2021-03-09 | Asm Ip Holding B.V. | Synthesis and use of precursors for ALD of group VA element containing thin films |
| US11072622B2 (en) | 2008-04-25 | 2021-07-27 | Asm International N.V. | Synthesis and use of precursors for ALD of tellurium and selenium thin films |
| WO2021167776A1 (en) * | 2020-02-20 | 2021-08-26 | Applied Materials, Inc. | Deposition of tellurium-containing thin films |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10174433B2 (en) | 2013-12-05 | 2019-01-08 | Honeywell International Inc. | Stannous methanesulfonate solution with adjusted pH |
| CN106536609B (en) | 2014-07-07 | 2022-04-29 | 霍尼韦尔国际公司 | Thermal interface material with ion scavenger |
| WO2016086410A1 (en) | 2014-12-05 | 2016-06-09 | Honeywell International Inc. | High performance thermal interface materials with low thermal impedance |
| US10312177B2 (en) | 2015-11-17 | 2019-06-04 | Honeywell International Inc. | Thermal interface materials including a coloring agent |
| US10781349B2 (en) | 2016-03-08 | 2020-09-22 | Honeywell International Inc. | Thermal interface material including crosslinker and multiple fillers |
| US10501671B2 (en) | 2016-07-26 | 2019-12-10 | Honeywell International Inc. | Gel-type thermal interface material |
| US11041103B2 (en) | 2017-09-08 | 2021-06-22 | Honeywell International Inc. | Silicone-free thermal gel |
| US10428256B2 (en) | 2017-10-23 | 2019-10-01 | Honeywell International Inc. | Releasable thermal gel |
| US11072706B2 (en) | 2018-02-15 | 2021-07-27 | Honeywell International Inc. | Gel-type thermal interface material |
| US11373921B2 (en) | 2019-04-23 | 2022-06-28 | Honeywell International Inc. | Gel-type thermal interface material with low pre-curing viscosity and elastic properties post-curing |
| WO2023038484A1 (en) * | 2021-09-13 | 2023-03-16 | 솔브레인 주식회사 | Film quality improving agent, thin film formation method using same, and semiconductor substrate manufactured therefrom |
Citations (48)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4960916A (en) * | 1989-09-29 | 1990-10-02 | United States Of America As Represented By The Secretary Of The Navy | Organometallic antimony compounds useful in chemical vapor deposition processes |
| US4975299A (en) * | 1989-11-02 | 1990-12-04 | Eastman Kodak Company | Vapor deposition process for depositing an organo-metallic compound layer on a substrate |
| US5091570A (en) * | 1987-12-04 | 1992-02-25 | Secretary Of State For Defence In Her Britannic Majesty's Gov. Of The U.K. | Method for preparation of dialkyl tellurium and dialkyl selenium |
| US5157136A (en) * | 1991-04-09 | 1992-10-20 | Regents Of The University Of California | Single-source metalloorganic precursors to produce ii/vi materials |
| US5166428A (en) * | 1990-06-08 | 1992-11-24 | The Secretary Of State For Defence In Her Britannic Majesty's Government Of The United Kingdom Of Great Britain And Northern Ireland | Method for preparation of organo-tellurium and selenium compounds |
| US5312983A (en) * | 1991-02-15 | 1994-05-17 | Advanced Technology Materials, Inc. | Organometallic tellurium compounds useful in chemical vapor deposition processes |
| US5442112A (en) * | 1991-10-03 | 1995-08-15 | The Secretary Of State For Defence In Her Britannic Majesty's Government Of The United Kingdom Of Great Britain And Northern Ireland | Preparation of dialkyl tellurium and dialkyl selenium |
| US5596522A (en) * | 1991-01-18 | 1997-01-21 | Energy Conversion Devices, Inc. | Homogeneous compositions of microcrystalline semiconductor material, semiconductor devices and directly overwritable memory elements fabricated therefrom, and arrays fabricated from the memory elements |
| US20040012009A1 (en) * | 2002-02-20 | 2004-01-22 | Stmicroelectronics S.R.L. | Sublithographic contact structure, phase change memory cell with optimized heater shape, and manufacturing method thereof |
| US6750079B2 (en) * | 1999-03-25 | 2004-06-15 | Ovonyx, Inc. | Method for making programmable resistance memory element |
| US20040197945A1 (en) * | 2003-04-05 | 2004-10-07 | Rohm And Haas Electronic Materials L.L.C. | Germanium compounds |
| US6872963B2 (en) * | 2002-08-08 | 2005-03-29 | Ovonyx, Inc. | Programmable resistance memory element with layered memory material |
| US20050267345A1 (en) * | 2001-07-02 | 2005-12-01 | The University Of Texas System, Board Of Regents | Applications of light-emitting nanoparticles |
| US6998289B2 (en) * | 2001-08-31 | 2006-02-14 | Intel Corporation | Multiple layer phase-change memory |
| US20060046521A1 (en) * | 2004-09-01 | 2006-03-02 | Vaartstra Brian A | Deposition methods using heteroleptic precursors |
| US20060049447A1 (en) * | 2004-09-08 | 2006-03-09 | Lee Jung-Hyun | Antimony precursor, phase-change memory device using the antimony precursor, and method of manufacturing the phase-change memory device |
| US7029978B2 (en) * | 2003-08-04 | 2006-04-18 | Intel Corporation | Controlling the location of conduction breakdown in phase change memories |
| US20060138393A1 (en) * | 2004-12-27 | 2006-06-29 | Samsung Electronics Co., Ltd. | Ge precursor, GST thin layer formed using the same, phase-change memory device including the GST thin layer, and method of manufacturing the GST thin layer |
| US20060141155A1 (en) * | 2002-11-15 | 2006-06-29 | Havard University | Atomic layer deposition using metal amidinates |
| US20060172083A1 (en) * | 2005-01-31 | 2006-08-03 | Samsung Electronics Co., Ltd | Method of fabricating a thin film |
| US20060180811A1 (en) * | 2005-02-14 | 2006-08-17 | Samsung Electronics Co., Ltd. | Precursor, thin layer prepared including the precursor, method of preparing the thin layer and phase-change memory device |
| US7115927B2 (en) * | 2003-02-24 | 2006-10-03 | Samsung Electronics Co., Ltd. | Phase changeable memory devices |
| US20070090336A1 (en) * | 2005-07-08 | 2007-04-26 | Elpida Memory, Inc | Semiconductor memory |
| US20070121363A1 (en) * | 2005-11-28 | 2007-05-31 | Macronix International Co., Ltd. | Phase Change Memory Cell and Manufacturing Method |
| EP1806427A2 (en) * | 2006-01-10 | 2007-07-11 | Samsung Electronics Co., Ltd. | Method of forming phase change material thin film, and method of manufacturing phase change memory device using the same |
| US20070246748A1 (en) * | 2006-04-25 | 2007-10-25 | Breitwisch Matthew J | Phase change memory cell with limited switchable volume |
| US20080035961A1 (en) * | 2006-08-14 | 2008-02-14 | Industrial Technology Research Institute | Phase-change memory and fabrication method thereof |
| US20080035906A1 (en) * | 2006-07-13 | 2008-02-14 | Samsung Electronics Co., Ltd. | Germanium compound, semiconductor device fabricated using the same, and methods of forming the same |
| US20080078984A1 (en) * | 2006-09-29 | 2008-04-03 | Samsung Electronics Co., Ltd. | Semiconductor device and method of fabricating the same |
| US7397060B2 (en) * | 2005-11-14 | 2008-07-08 | Macronix International Co., Ltd. | Pipe shaped phase change memory |
| US7402851B2 (en) * | 2003-02-24 | 2008-07-22 | Samsung Electronics Co., Ltd. | Phase changeable memory devices including nitrogen and/or silicon and methods for fabricating the same |
| US20080210163A1 (en) * | 2006-11-21 | 2008-09-04 | David Keith Carlson | Independent Radiant Gas Preheating for Precursor Disassociation Control and Gas Reaction Kinetics in Low Temperature CVD Systems |
| US7425735B2 (en) * | 2003-02-24 | 2008-09-16 | Samsung Electronics Co., Ltd. | Multi-layer phase-changeable memory devices |
| US20080290335A1 (en) * | 2007-05-21 | 2008-11-27 | Industrial Technology Research Institute | Phase change memory device and method for fabricating the same |
| US20090020738A1 (en) * | 2007-07-20 | 2009-01-22 | Thomas Happ | Integrated circuit including force-filled resistivity changing material |
| US20090075420A1 (en) * | 2007-09-18 | 2009-03-19 | Samsung Electronics Co., Ltd. | Method of forming chalcogenide layer including te and method of fabricating phase-change memory device |
| US20090097305A1 (en) * | 2007-10-11 | 2009-04-16 | Samsung Electronics Co., Ltd. | Method of forming phase change material layer using ge(ii) source, and method of fabricating phase change memory device |
| US20090101883A1 (en) * | 2006-10-24 | 2009-04-23 | Macronix International Co., Ltd. | Method for manufacturing a resistor random access memory with a self-aligned air gap insulator |
| US20090112009A1 (en) * | 2007-10-31 | 2009-04-30 | Advanced Technology Materials, Inc. | Amorphous ge/te deposition process |
| US20090124039A1 (en) * | 2006-05-12 | 2009-05-14 | Advanced Technology Materials, Inc. | Low temperature deposition of phase change memory materials |
| US7569417B2 (en) * | 2005-09-03 | 2009-08-04 | Samsung Electronics Co., Ltd. | Method of forming a phase changeable material layer, a method of manufacturing a phase changeable memory unit, and a method of manufacturing a phase changeable semiconductor memory device |
| US20090227066A1 (en) * | 2008-03-06 | 2009-09-10 | International Business Machines Corporation | Method of forming ring electrode |
| US20090305458A1 (en) * | 2006-11-02 | 2009-12-10 | Advanced Technology Materials, Inc. | Antimony and germanium complexes useful for cvd/ald of metal thin films |
| US20090321733A1 (en) * | 2008-06-25 | 2009-12-31 | Julien Gatineau | Metal heterocyclic compounds for deposition of thin films |
| US20100190341A1 (en) * | 2007-07-19 | 2010-07-29 | Ips Ltd. | Apparatus, method for depositing thin film on wafer and method for gap-filling trench using the same |
| US20100270527A1 (en) * | 2009-04-27 | 2010-10-28 | Kabushiki Kaisha Toshiba | Phase-change memory device and method of manufacturing the phase-change memory device |
| US7943502B2 (en) * | 2007-06-15 | 2011-05-17 | Samsung Electronics Co., Ltd. | Method of forming a phase change memory device |
| US20110124182A1 (en) * | 2009-11-20 | 2011-05-26 | Advanced Techology Materials, Inc. | System for the delivery of germanium-based precursor |
Family Cites Families (110)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SU768457A1 (en) | 1976-01-04 | 1980-10-07 | Всесоюзный научно-исследовательский и проектно-конструкторский институт добычи угля гидравлическим способом | Catalyst for removing nitrogen oxides from exhaust gases |
| JPS5838296A (en) | 1981-08-31 | 1983-03-05 | Ichiro Kijima | Novel antimony compound |
| US4948623A (en) | 1987-06-30 | 1990-08-14 | International Business Machines Corporation | Method of chemical vapor deposition of copper, silver, and gold using a cyclopentadienyl/metal complex |
| EP0305144A3 (en) * | 1987-08-24 | 1989-03-08 | Canon Kabushiki Kaisha | Method of forming crystalline compound semiconductor film |
| US4946994A (en) * | 1987-12-21 | 1990-08-07 | The United States Of America As Represented By The Secretary Of The Navy | Preparation of ditertiarybutyltelluride |
| US4962214A (en) | 1988-05-11 | 1990-10-09 | Massachusettes Institute Of Technology | Catalytic enantioselective addition of hydrocarbon equivalents to alpha, beta-unsaturated carbonyl compounds |
| US4927670A (en) | 1988-06-22 | 1990-05-22 | Georgia Tech Research Corporation | Chemical vapor deposition of mixed metal oxide coatings |
| US5453494A (en) | 1990-07-06 | 1995-09-26 | Advanced Technology Materials, Inc. | Metal complex source reagents for MOCVD |
| US5296716A (en) | 1991-01-18 | 1994-03-22 | Energy Conversion Devices, Inc. | Electrically erasable, directly overwritable, multibit single cell memory elements and arrays fabricated therefrom |
| US5359205A (en) | 1991-11-07 | 1994-10-25 | Energy Conversion Devices, Inc. | Electrically erasable memory elements characterized by reduced current and improved thermal stability |
| JP3336034B2 (en) | 1992-05-12 | 2002-10-21 | 同和鉱業株式会社 | Manufacturing method of sputtering target |
| US5997642A (en) | 1996-05-21 | 1999-12-07 | Symetrix Corporation | Method and apparatus for misted deposition of integrated circuit quality thin films |
| US5972743A (en) | 1996-12-03 | 1999-10-26 | Advanced Technology Materials, Inc. | Precursor compositions for ion implantation of antimony and ion implantation process utilizing same |
| CN1253597A (en) | 1997-03-11 | 2000-05-17 | 阿尔·阿尔马格公司 | Process and apparatus for coating metals |
| AU724629B2 (en) | 1997-04-16 | 2000-09-28 | Asahi Kasei Kabushiki Kaisha | Process for producing optical information recording medium and optical information recording medium produced by the process |
| US6146608A (en) | 1997-11-24 | 2000-11-14 | Advanced Technology Materials, Inc. | Stable hydride source compositions for manufacture of semiconductor devices and structures |
| US6005127A (en) | 1997-11-24 | 1999-12-21 | Advanced Technology Materials, Inc. | Antimony/Lewis base adducts for Sb-ion implantation and formation of antimonide films |
| US6787186B1 (en) | 1997-12-18 | 2004-09-07 | Advanced Technology Materials, Inc. | Method of controlled chemical vapor deposition of a metal oxide ceramic layer |
| US7098163B2 (en) | 1998-08-27 | 2006-08-29 | Cabot Corporation | Method of producing membrane electrode assemblies for use in proton exchange membrane and direct methanol fuel cells |
| US6123993A (en) | 1998-09-21 | 2000-09-26 | Advanced Technology Materials, Inc. | Method and apparatus for forming low dielectric constant polymeric films |
| US6086779A (en) | 1999-03-01 | 2000-07-11 | Mcgean-Rohco, Inc. | Copper etching compositions and method for etching copper |
| US6281022B1 (en) | 1999-04-28 | 2001-08-28 | Sharp Laboratories Of America, Inc. | Multi-phase lead germanate film deposition method |
| JP2001067720A (en) | 1999-08-31 | 2001-03-16 | Toray Ind Inc | Optical recording medium |
| US6269979B1 (en) | 1999-10-05 | 2001-08-07 | Charles Dumont | Multi-compartmented mixing dispenser |
| GB0004852D0 (en) | 2000-02-29 | 2000-04-19 | Unilever Plc | Ligand and complex for catalytically bleaching a substrate |
| US20020013487A1 (en) | 2000-04-03 | 2002-01-31 | Norman John Anthony Thomas | Volatile precursors for deposition of metals and metal-containing films |
| US6984591B1 (en) | 2000-04-20 | 2006-01-10 | International Business Machines Corporation | Precursor source mixtures |
| JP4621333B2 (en) | 2000-06-01 | 2011-01-26 | ホーチキ株式会社 | Thin film formation method |
| US20020090815A1 (en) | 2000-10-31 | 2002-07-11 | Atsushi Koike | Method for forming a deposited film by plasma chemical vapor deposition |
| US7087482B2 (en) | 2001-01-19 | 2006-08-08 | Samsung Electronics Co., Ltd. | Method of forming material using atomic layer deposition and method of forming capacitor of semiconductor device using the same |
| JP2002220658A (en) | 2001-01-26 | 2002-08-09 | Ricoh Co Ltd | Sputtering target for optical disk and manufacturing method therefor |
| US7005392B2 (en) | 2001-03-30 | 2006-02-28 | Advanced Technology Materials, Inc. | Source reagent compositions for CVD formation of gate dielectric thin films using amide precursors and method of using same |
| ATE340800T1 (en) | 2001-10-26 | 2006-10-15 | Epichem Ltd | PRECURSOR COMPOUNDS FOR CHEMICAL VAPOR DEPOSITION |
| US6861559B2 (en) | 2002-12-10 | 2005-03-01 | Board Of Trustees Of Michigan State University | Iminoamines and preparation thereof |
| US20040215030A1 (en) | 2003-04-22 | 2004-10-28 | Norman John Anthony Thomas | Precursors for metal containing films |
| US7893419B2 (en) | 2003-08-04 | 2011-02-22 | Intel Corporation | Processing phase change material to improve programming speed |
| US20050082624A1 (en) | 2003-10-20 | 2005-04-21 | Evgeni Gousev | Germanate gate dielectrics for semiconductor devices |
| KR100577909B1 (en) | 2003-11-20 | 2006-05-10 | 주식회사 에버테크 | Universal thin film deposit device |
| US7329593B2 (en) | 2004-02-27 | 2008-02-12 | Asm America, Inc. | Germanium deposition |
| US7005665B2 (en) | 2004-03-18 | 2006-02-28 | International Business Machines Corporation | Phase change memory cell on silicon-on insulator substrate |
| US7312165B2 (en) | 2004-05-05 | 2007-12-25 | Jursich Gregory M | Codeposition of hafnium-germanium oxides on substrates used in or for semiconductor devices |
| US7166732B2 (en) | 2004-06-16 | 2007-01-23 | Advanced Technology Materials, Inc. | Copper (I) compounds useful as deposition precursors of copper thin films |
| WO2006012052A2 (en) | 2004-06-25 | 2006-02-02 | Arkema, Inc. | Amidinate ligand containing chemical vapor deposition precursors |
| US20050287747A1 (en) | 2004-06-29 | 2005-12-29 | International Business Machines Corporation | Doped nitride film, doped oxide film and other doped films |
| KR100639206B1 (en) | 2004-06-30 | 2006-10-30 | 주식회사 하이닉스반도체 | Phase-change memory device and method for manufacturing the same |
| KR100642635B1 (en) | 2004-07-06 | 2006-11-10 | 삼성전자주식회사 | Semiconductor integrated circuit devices having a hybrid dielectric layer and methods of fabricating the same |
| KR100632948B1 (en) | 2004-08-06 | 2006-10-11 | 삼성전자주식회사 | Sputtering method for forming a chalcogen compound and method for fabricating phase-changeable memory device using the same |
| US7300873B2 (en) | 2004-08-13 | 2007-11-27 | Micron Technology, Inc. | Systems and methods for forming metal-containing layers using vapor deposition processes |
| WO2006023501A2 (en) | 2004-08-16 | 2006-03-02 | Aviza Technology, Inc. | Direct liquid injection system and method for forming multi-component dielectric films |
| US7390360B2 (en) | 2004-10-05 | 2008-06-24 | Rohm And Haas Electronic Materials Llc | Organometallic compounds |
| JP2006124262A (en) | 2004-11-01 | 2006-05-18 | Dainippon Printing Co Ltd | InSb NANOPARTICLE |
| JP2006156886A (en) | 2004-12-01 | 2006-06-15 | Renesas Technology Corp | Semiconductor integrated circuit device and manufacturing method therefor |
| KR100640620B1 (en) | 2004-12-27 | 2006-11-02 | 삼성전자주식회사 | NOR type flash memory device having twin bit cell scheme |
| US20080286446A1 (en) | 2005-01-28 | 2008-11-20 | Smuruthi Kamepalli | Seed-Assisted MOCVD Growth of Threshold Switching and Phase-Change Materials |
| US20060172067A1 (en) | 2005-01-28 | 2006-08-03 | Energy Conversion Devices, Inc | Chemical vapor deposition of chalcogenide materials |
| US7399666B2 (en) | 2005-02-15 | 2008-07-15 | Micron Technology, Inc. | Atomic layer deposition of Zr3N4/ZrO2 films as gate dielectrics |
| US7488967B2 (en) | 2005-04-06 | 2009-02-10 | International Business Machines Corporation | Structure for confining the switching current in phase memory (PCM) cells |
| EP1710324B1 (en) | 2005-04-08 | 2008-12-03 | STMicroelectronics S.r.l. | PVD process and chamber for the pulsed deposition of a chalcogenide material layer of a phase change memory device |
| KR100681266B1 (en) | 2005-07-25 | 2007-02-09 | 삼성전자주식회사 | Method of manufacturing a variable resistance and method of manufacturing a phase changeable memory device using the same |
| US7525117B2 (en) | 2005-08-09 | 2009-04-28 | Ovonyx, Inc. | Chalcogenide devices and materials having reduced germanium or telluruim content |
| DE102006038885B4 (en) | 2005-08-24 | 2013-10-10 | Wonik Ips Co., Ltd. | Method for depositing a Ge-Sb-Te thin film |
| KR100675289B1 (en) | 2005-11-14 | 2007-01-29 | 삼성전자주식회사 | Phase changeable memory cell array region and methods of forming the same |
| WO2007067604A2 (en) | 2005-12-06 | 2007-06-14 | Structured Materials Inc. | Method of making undoped, alloyed and doped chalcogenide films by mocvd processes |
| KR101330769B1 (en) | 2005-12-12 | 2013-11-18 | 오보닉스, 아이엔씨. | Chalcogenide devices and materials having reduced germanium or telluruim content |
| US20070154637A1 (en) | 2005-12-19 | 2007-07-05 | Rohm And Haas Electronic Materials Llc | Organometallic composition |
| US7812334B2 (en) | 2006-04-04 | 2010-10-12 | Micron Technology, Inc. | Phase change memory elements using self-aligned phase change material layers and methods of making and using same |
| KR100919692B1 (en) | 2006-04-27 | 2009-10-06 | 재단법인서울대학교산학협력재단 | Phase change random access memory cell and method of manufacturing the same |
| KR101095741B1 (en) | 2006-05-31 | 2011-12-21 | 르네사스 일렉트로닉스 가부시키가이샤 | Semiconductor device |
| US8399056B2 (en) | 2006-06-02 | 2013-03-19 | L'Air Liquide, Société Anonyme pour l'Etude et l'Exploitation des Procédés Georges Claude | Method of forming high-k dielectric films based on novel titanium, zirconium, and hafnium precursors and their use for semiconductor manufacturing |
| CN102993050A (en) | 2006-06-28 | 2013-03-27 | 哈佛学院院长等 | Metal(IV) tetra-amidinate compounds and their use in vapor deposition |
| US7638645B2 (en) | 2006-06-28 | 2009-12-29 | President And Fellows Of Harvard University | Metal (IV) tetra-amidinate compounds and their use in vapor deposition |
| KR100780865B1 (en) | 2006-07-19 | 2007-11-30 | 삼성전자주식회사 | Methods of forming a semiconductor device including a phase-change layer |
| KR100791477B1 (en) | 2006-08-08 | 2008-01-03 | 삼성전자주식회사 | A phase-change memory unit, method of manufacturing the phase-change memory unit, a phase-change memory device having the phase-change memory unit and method of manufacturing the phase-change memory device |
| US20080090400A1 (en) | 2006-10-17 | 2008-04-17 | Cheek Roger W | Self-aligned in-contact phase change memory device |
| KR101263822B1 (en) | 2006-10-20 | 2013-05-13 | 삼성전자주식회사 | Method of manufacturing phase change memory device and method of forming phase change layer applied in the same |
| KR101275799B1 (en) | 2006-11-21 | 2013-06-18 | 삼성전자주식회사 | Method of forming phase change layer using Ge precursor for low temperature deposition and method of manufacturing phase change memory device using the same |
| WO2008069821A1 (en) | 2006-12-05 | 2008-06-12 | Advanced Technology Materials, Inc. | Metal aminotroponiminates, bis-oxazolinates and guanidinates |
| KR100932904B1 (en) | 2006-12-05 | 2009-12-21 | 한국전자통신연구원 | Modem performance analysis device and method, and function test method of modem performance analysis device |
| KR20080055508A (en) | 2006-12-15 | 2008-06-19 | 삼성전자주식회사 | Phase change layer having different crystal lattice in single layer and method of forming the same and phase change memory device comprising means for preventing ti diffusion and method of manufacturing the same |
| US7750173B2 (en) | 2007-01-18 | 2010-07-06 | Advanced Technology Materials, Inc. | Tantalum amido-complexes with chelate ligands useful for CVD and ALD of TaN and Ta205 thin films |
| KR100896180B1 (en) | 2007-01-23 | 2009-05-12 | 삼성전자주식회사 | Phase change Random Access Memory comprising phase change material layer formed by selective growth method and method of manufacturing the same |
| FR2913523B1 (en) | 2007-03-09 | 2009-06-05 | Commissariat Energie Atomique | MULTI-LEVEL DATA STORAGE DEVICE WITH PHASE CHANGE MATERIAL |
| CN103151335B (en) | 2007-04-09 | 2016-09-28 | 哈佛学院院长等 | Cobalt nitride layers and their forming method for copper-connection |
| US20080254218A1 (en) | 2007-04-16 | 2008-10-16 | Air Products And Chemicals, Inc. | Metal Precursor Solutions For Chemical Vapor Deposition |
| US8377341B2 (en) | 2007-04-24 | 2013-02-19 | Air Products And Chemicals, Inc. | Tellurium (Te) precursors for making phase change memory materials |
| US20080272355A1 (en) | 2007-05-04 | 2008-11-06 | Samsung Electronics Co., Ltd. | Phase change memory device and method for forming the same |
| KR20090013419A (en) | 2007-08-01 | 2009-02-05 | 삼성전자주식회사 | Phase change memory devices and methods of forming the same |
| KR101370275B1 (en) | 2007-08-21 | 2014-03-05 | 삼성전자주식회사 | Phase Change Random Access Memory and Manufacturing Method for the Same |
| WO2009034775A1 (en) | 2007-09-13 | 2009-03-19 | Nippon Mining & Metals Co., Ltd. | Method for producing sintered body, sintered body, sputtering target composed of the sintered body, and sputtering target-backing plate assembly |
| US8454928B2 (en) | 2007-09-17 | 2013-06-04 | L'air Liquide Societe Anonyme Pour L'etude Et L'exploitation Des Procedes Georges Claude | Tellurium precursors for GST deposition |
| US20090087561A1 (en) | 2007-09-28 | 2009-04-02 | Advanced Technology Materials, Inc. | Metal and metalloid silylamides, ketimates, tetraalkylguanidinates and dianionic guanidinates useful for cvd/ald of thin films |
| KR20090036771A (en) | 2007-10-10 | 2009-04-15 | 삼성전자주식회사 | Method of fabricating phase change memory device having a doped phase change material layer |
| US7960205B2 (en) | 2007-11-27 | 2011-06-14 | Air Products And Chemicals, Inc. | Tellurium precursors for GST films in an ALD or CVD process |
| US20090162973A1 (en) | 2007-12-21 | 2009-06-25 | Julien Gatineau | Germanium precursors for gst film deposition |
| US8318252B2 (en) | 2008-01-28 | 2012-11-27 | Air Products And Chemicals, Inc. | Antimony precursors for GST films in ALD/CVD processes |
| US20090215225A1 (en) | 2008-02-24 | 2009-08-27 | Advanced Technology Materials, Inc. | Tellurium compounds useful for deposition of tellurium containing materials |
| US7935564B2 (en) | 2008-02-25 | 2011-05-03 | International Business Machines Corporation | Self-converging bottom electrode ring |
| WO2009134989A2 (en) | 2008-05-02 | 2009-11-05 | Advanced Technology Materials, Inc. | Antimony compounds useful for deposition of antimony-containing materials |
| US20090275164A1 (en) | 2008-05-02 | 2009-11-05 | Advanced Technology Materials, Inc. | Bicyclic guanidinates and bridging diamides as cvd/ald precursors |
| US8765223B2 (en) | 2008-05-08 | 2014-07-01 | Air Products And Chemicals, Inc. | Binary and ternary metal chalcogenide materials and method of making and using same |
| US8101237B2 (en) | 2008-05-29 | 2012-01-24 | L'Air Liquide SociétéAnonyme pour I'Etude et I'Exploitation des Procédés Georges Claude | Tellurium precursors for film deposition |
| WO2009152108A2 (en) | 2008-06-10 | 2009-12-17 | Advanced Technology Materials, Inc. | GeSbTe MATERIAL INCLUDING SUPERFLOW LAYER(S), AND USE OF Ge TO PREVENT INTERACTION OF Te FROM SbXTeY AND GeXTeY RESULTING IN HIGH Te CONTENT AND FILM CRISTALLINITY |
| US8168811B2 (en) | 2008-07-22 | 2012-05-01 | Advanced Technology Materials, Inc. | Precursors for CVD/ALD of metal-containing films |
| US8124950B2 (en) | 2008-08-26 | 2012-02-28 | International Business Machines Corporation | Concentric phase change memory element |
| US8330136B2 (en) | 2008-12-05 | 2012-12-11 | Advanced Technology Materials, Inc. | High concentration nitrogen-containing germanium telluride based memory devices and processes of making |
| US8617972B2 (en) | 2009-05-22 | 2013-12-31 | Advanced Technology Materials, Inc. | Low temperature GST process |
| WO2011002705A2 (en) | 2009-07-02 | 2011-01-06 | Advanced Technology Materials, Inc. | Hollow gst structure with dielectric fill |
| JP2011066135A (en) | 2009-09-16 | 2011-03-31 | Elpida Memory Inc | Method for fabricating phase-change memory device |
| US8017433B2 (en) | 2010-02-09 | 2011-09-13 | International Business Machines Corporation | Post deposition method for regrowth of crystalline phase change material |
| KR101706809B1 (en) | 2010-03-26 | 2017-02-15 | 엔테그리스, 아이엔씨. | Germanium antimony telluride materials and devices incorporating same |
-
2009
- 2009-02-24 US US12/392,009 patent/US20090215225A1/en not_active Abandoned
-
2013
- 2013-06-06 US US13/911,622 patent/US8796068B2/en active Active
-
2014
- 2014-07-16 US US14/332,924 patent/US9537095B2/en active Active
Patent Citations (57)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5091570A (en) * | 1987-12-04 | 1992-02-25 | Secretary Of State For Defence In Her Britannic Majesty's Gov. Of The U.K. | Method for preparation of dialkyl tellurium and dialkyl selenium |
| US4960916A (en) * | 1989-09-29 | 1990-10-02 | United States Of America As Represented By The Secretary Of The Navy | Organometallic antimony compounds useful in chemical vapor deposition processes |
| US4975299A (en) * | 1989-11-02 | 1990-12-04 | Eastman Kodak Company | Vapor deposition process for depositing an organo-metallic compound layer on a substrate |
| US5166428A (en) * | 1990-06-08 | 1992-11-24 | The Secretary Of State For Defence In Her Britannic Majesty's Government Of The United Kingdom Of Great Britain And Northern Ireland | Method for preparation of organo-tellurium and selenium compounds |
| US5596522A (en) * | 1991-01-18 | 1997-01-21 | Energy Conversion Devices, Inc. | Homogeneous compositions of microcrystalline semiconductor material, semiconductor devices and directly overwritable memory elements fabricated therefrom, and arrays fabricated from the memory elements |
| US5312983A (en) * | 1991-02-15 | 1994-05-17 | Advanced Technology Materials, Inc. | Organometallic tellurium compounds useful in chemical vapor deposition processes |
| US5157136A (en) * | 1991-04-09 | 1992-10-20 | Regents Of The University Of California | Single-source metalloorganic precursors to produce ii/vi materials |
| US5442112A (en) * | 1991-10-03 | 1995-08-15 | The Secretary Of State For Defence In Her Britannic Majesty's Government Of The United Kingdom Of Great Britain And Northern Ireland | Preparation of dialkyl tellurium and dialkyl selenium |
| US6750079B2 (en) * | 1999-03-25 | 2004-06-15 | Ovonyx, Inc. | Method for making programmable resistance memory element |
| US20050267345A1 (en) * | 2001-07-02 | 2005-12-01 | The University Of Texas System, Board Of Regents | Applications of light-emitting nanoparticles |
| US6998289B2 (en) * | 2001-08-31 | 2006-02-14 | Intel Corporation | Multiple layer phase-change memory |
| US20040012009A1 (en) * | 2002-02-20 | 2004-01-22 | Stmicroelectronics S.R.L. | Sublithographic contact structure, phase change memory cell with optimized heater shape, and manufacturing method thereof |
| US6872963B2 (en) * | 2002-08-08 | 2005-03-29 | Ovonyx, Inc. | Programmable resistance memory element with layered memory material |
| US20060141155A1 (en) * | 2002-11-15 | 2006-06-29 | Havard University | Atomic layer deposition using metal amidinates |
| US20090291208A1 (en) * | 2002-11-15 | 2009-11-26 | Gordon Roy G | Atomic layer deposition using metal amidinates |
| US7115927B2 (en) * | 2003-02-24 | 2006-10-03 | Samsung Electronics Co., Ltd. | Phase changeable memory devices |
| US7615401B2 (en) * | 2003-02-24 | 2009-11-10 | Samsung Electronics Co., Ltd. | Methods of fabricating multi-layer phase-changeable memory devices |
| US7402851B2 (en) * | 2003-02-24 | 2008-07-22 | Samsung Electronics Co., Ltd. | Phase changeable memory devices including nitrogen and/or silicon and methods for fabricating the same |
| US7476917B2 (en) * | 2003-02-24 | 2009-01-13 | Samsung Electronics Co., Ltd. | Phase-changeable memory devices including nitrogen and/or silicon dopants |
| US7462900B2 (en) * | 2003-02-24 | 2008-12-09 | Samsung Electronics Co., Ltd. | Phase changeable memory devices including nitrogen and/or silicon |
| US7704787B2 (en) * | 2003-02-24 | 2010-04-27 | Samsung Electronics Co., Ltd. | Methods for fabricating phase changeable memory devices |
| US7425735B2 (en) * | 2003-02-24 | 2008-09-16 | Samsung Electronics Co., Ltd. | Multi-layer phase-changeable memory devices |
| US20040197945A1 (en) * | 2003-04-05 | 2004-10-07 | Rohm And Haas Electronic Materials L.L.C. | Germanium compounds |
| US7029978B2 (en) * | 2003-08-04 | 2006-04-18 | Intel Corporation | Controlling the location of conduction breakdown in phase change memories |
| US20060046521A1 (en) * | 2004-09-01 | 2006-03-02 | Vaartstra Brian A | Deposition methods using heteroleptic precursors |
| US20060049447A1 (en) * | 2004-09-08 | 2006-03-09 | Lee Jung-Hyun | Antimony precursor, phase-change memory device using the antimony precursor, and method of manufacturing the phase-change memory device |
| US20060138393A1 (en) * | 2004-12-27 | 2006-06-29 | Samsung Electronics Co., Ltd. | Ge precursor, GST thin layer formed using the same, phase-change memory device including the GST thin layer, and method of manufacturing the GST thin layer |
| US20060172083A1 (en) * | 2005-01-31 | 2006-08-03 | Samsung Electronics Co., Ltd | Method of fabricating a thin film |
| US20060180811A1 (en) * | 2005-02-14 | 2006-08-17 | Samsung Electronics Co., Ltd. | Precursor, thin layer prepared including the precursor, method of preparing the thin layer and phase-change memory device |
| US7371429B2 (en) * | 2005-02-14 | 2008-05-13 | Samsung Electronics Co., Ltd. | Precursor, thin layer prepared including the precursor, method of preparing the thin layer and phase-change memory device |
| US7728172B2 (en) * | 2005-02-14 | 2010-06-01 | Samsung Electronics Co., Ltd. | Precursor, thin layer prepared including the precursor, method of preparing the thin layer and phase-change memory device |
| US20070090336A1 (en) * | 2005-07-08 | 2007-04-26 | Elpida Memory, Inc | Semiconductor memory |
| US7569417B2 (en) * | 2005-09-03 | 2009-08-04 | Samsung Electronics Co., Ltd. | Method of forming a phase changeable material layer, a method of manufacturing a phase changeable memory unit, and a method of manufacturing a phase changeable semiconductor memory device |
| US7397060B2 (en) * | 2005-11-14 | 2008-07-08 | Macronix International Co., Ltd. | Pipe shaped phase change memory |
| US20070121363A1 (en) * | 2005-11-28 | 2007-05-31 | Macronix International Co., Ltd. | Phase Change Memory Cell and Manufacturing Method |
| US20070160760A1 (en) * | 2006-01-10 | 2007-07-12 | Samsung Electronics Co., Ltd. | Methods of forming phase change material thin films and methods of manufacturing phase change memory devices using the same |
| EP1806427A2 (en) * | 2006-01-10 | 2007-07-11 | Samsung Electronics Co., Ltd. | Method of forming phase change material thin film, and method of manufacturing phase change memory device using the same |
| US20070246748A1 (en) * | 2006-04-25 | 2007-10-25 | Breitwisch Matthew J | Phase change memory cell with limited switchable volume |
| US8288198B2 (en) * | 2006-05-12 | 2012-10-16 | Advanced Technology Materials, Inc. | Low temperature deposition of phase change memory materials |
| US20090124039A1 (en) * | 2006-05-12 | 2009-05-14 | Advanced Technology Materials, Inc. | Low temperature deposition of phase change memory materials |
| US20080035906A1 (en) * | 2006-07-13 | 2008-02-14 | Samsung Electronics Co., Ltd. | Germanium compound, semiconductor device fabricated using the same, and methods of forming the same |
| US20080035961A1 (en) * | 2006-08-14 | 2008-02-14 | Industrial Technology Research Institute | Phase-change memory and fabrication method thereof |
| US20080078984A1 (en) * | 2006-09-29 | 2008-04-03 | Samsung Electronics Co., Ltd. | Semiconductor device and method of fabricating the same |
| US20090101883A1 (en) * | 2006-10-24 | 2009-04-23 | Macronix International Co., Ltd. | Method for manufacturing a resistor random access memory with a self-aligned air gap insulator |
| US20090305458A1 (en) * | 2006-11-02 | 2009-12-10 | Advanced Technology Materials, Inc. | Antimony and germanium complexes useful for cvd/ald of metal thin films |
| US20080210163A1 (en) * | 2006-11-21 | 2008-09-04 | David Keith Carlson | Independent Radiant Gas Preheating for Precursor Disassociation Control and Gas Reaction Kinetics in Low Temperature CVD Systems |
| US20080290335A1 (en) * | 2007-05-21 | 2008-11-27 | Industrial Technology Research Institute | Phase change memory device and method for fabricating the same |
| US7943502B2 (en) * | 2007-06-15 | 2011-05-17 | Samsung Electronics Co., Ltd. | Method of forming a phase change memory device |
| US20100190341A1 (en) * | 2007-07-19 | 2010-07-29 | Ips Ltd. | Apparatus, method for depositing thin film on wafer and method for gap-filling trench using the same |
| US20090020738A1 (en) * | 2007-07-20 | 2009-01-22 | Thomas Happ | Integrated circuit including force-filled resistivity changing material |
| US20090075420A1 (en) * | 2007-09-18 | 2009-03-19 | Samsung Electronics Co., Ltd. | Method of forming chalcogenide layer including te and method of fabricating phase-change memory device |
| US20090097305A1 (en) * | 2007-10-11 | 2009-04-16 | Samsung Electronics Co., Ltd. | Method of forming phase change material layer using ge(ii) source, and method of fabricating phase change memory device |
| US20090112009A1 (en) * | 2007-10-31 | 2009-04-30 | Advanced Technology Materials, Inc. | Amorphous ge/te deposition process |
| US20090227066A1 (en) * | 2008-03-06 | 2009-09-10 | International Business Machines Corporation | Method of forming ring electrode |
| US20090321733A1 (en) * | 2008-06-25 | 2009-12-31 | Julien Gatineau | Metal heterocyclic compounds for deposition of thin films |
| US20100270527A1 (en) * | 2009-04-27 | 2010-10-28 | Kabushiki Kaisha Toshiba | Phase-change memory device and method of manufacturing the phase-change memory device |
| US20110124182A1 (en) * | 2009-11-20 | 2011-05-26 | Advanced Techology Materials, Inc. | System for the delivery of germanium-based precursor |
Cited By (55)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9534285B2 (en) | 2006-03-10 | 2017-01-03 | Entegris, Inc. | Precursor compositions for atomic layer deposition and chemical vapor deposition of titanate, lanthanate, and tantalate dielectric films |
| US8679894B2 (en) | 2006-05-12 | 2014-03-25 | Advanced Technology Materials, Inc. | Low temperature deposition of phase change memory materials |
| US8008117B2 (en) | 2006-11-02 | 2011-08-30 | Advanced Technology Materials, Inc. | Antimony and germanium complexes useful for CVD/ALD of metal thin films |
| US20100317150A1 (en) * | 2006-11-02 | 2010-12-16 | Advanced Technology Materials, Inc. | Antimony and germanium complexes useful for cvd/ald of metal thin films |
| US8709863B2 (en) | 2006-11-02 | 2014-04-29 | Advanced Technology Materials, Inc. | Antimony and germanium complexes useful for CVD/ALD of metal thin films |
| US8268665B2 (en) | 2006-11-02 | 2012-09-18 | Advanced Technology Materials, Inc. | Antimony and germanium complexes useful for CVD/ALD of metal thin films |
| US9219232B2 (en) | 2006-11-02 | 2015-12-22 | Entegris, Inc. | Antimony and germanium complexes useful for CVD/ALD of metal thin films |
| US8003162B2 (en) * | 2006-11-21 | 2011-08-23 | Samsung Electronics Co., Ltd. | Method of forming phase change layer using a germanium precursor and method of manufacturing phase change memory device using the same |
| US20080118636A1 (en) * | 2006-11-21 | 2008-05-22 | Samsung Electronics Co., Ltd | Method of forming phase change layer using a germanium precursor and method of manufacturing phase change memory device using the same |
| US20100291299A1 (en) * | 2007-08-08 | 2010-11-18 | Advanced Technology Materials, Inc. | Strontium and barium precursors for use in chemical vapor deposition, atomic layer deposition and rapid vapor deposition |
| US8455049B2 (en) | 2007-08-08 | 2013-06-04 | Advanced Technology Materials, Inc. | Strontium precursor for use in chemical vapor deposition, atomic layer deposition and rapid vapor deposition |
| US20090097305A1 (en) * | 2007-10-11 | 2009-04-16 | Samsung Electronics Co., Ltd. | Method of forming phase change material layer using ge(ii) source, and method of fabricating phase change memory device |
| US8142846B2 (en) * | 2007-10-11 | 2012-03-27 | Samsung Electronics Co., Ltd. | Method of forming phase change material layer using Ge(II) source, and method of fabrication phase change memory device |
| US8834968B2 (en) | 2007-10-11 | 2014-09-16 | Samsung Electronics Co., Ltd. | Method of forming phase change material layer using Ge(II) source, and method of fabricating phase change memory device |
| US8852686B2 (en) | 2007-10-11 | 2014-10-07 | Samsung Electronics Co., Ltd. | Method of forming phase change material layer using Ge(II) source, and method of fabricating phase change memory device |
| US20090112009A1 (en) * | 2007-10-31 | 2009-04-30 | Advanced Technology Materials, Inc. | Amorphous ge/te deposition process |
| US8093140B2 (en) | 2007-10-31 | 2012-01-10 | Advanced Technology Materials, Inc. | Amorphous Ge/Te deposition process |
| US20100279011A1 (en) * | 2007-10-31 | 2010-11-04 | Advanced Technology Materials, Inc. | Novel bismuth precursors for cvd/ald of thin films |
| US9537095B2 (en) | 2008-02-24 | 2017-01-03 | Entegris, Inc. | Tellurium compounds useful for deposition of tellurium containing materials |
| US8796068B2 (en) | 2008-02-24 | 2014-08-05 | Advanced Technology Materials, Inc. | Tellurium compounds useful for deposition of tellurium containing materials |
| US8853075B2 (en) | 2008-02-27 | 2014-10-07 | L'Air Liquide Société Anonyme pour l'Etude et l'Exploitation des Procédés Georges Claude | Method for forming a titanium-containing layer on a substrate using an atomic layer deposition (ALD) process |
| US8193388B2 (en) | 2008-04-15 | 2012-06-05 | American Air Liquide, Inc. | Compounds for depositing tellurium-containing films |
| US20090256127A1 (en) * | 2008-04-15 | 2009-10-15 | American Air Liquide, Inc. | Compounds for Depositing Tellurium-Containing Films |
| US11072622B2 (en) | 2008-04-25 | 2021-07-27 | Asm International N.V. | Synthesis and use of precursors for ALD of tellurium and selenium thin films |
| US11814400B2 (en) | 2008-04-25 | 2023-11-14 | Asm International N.V. | Synthesis and use of precursors for ALD of tellurium and selenium thin films |
| US8674127B2 (en) | 2008-05-02 | 2014-03-18 | Advanced Technology Materials, Inc. | Antimony compounds useful for deposition of antimony-containing materials |
| US9034688B2 (en) | 2008-05-02 | 2015-05-19 | Entegris, Inc. | Antimony compounds useful for deposition of antimony-containing materials |
| US20110111556A1 (en) * | 2008-05-02 | 2011-05-12 | Advanced Technology Materials, Inc. | Antimony compounds useful for deposition of antimony-containing materials |
| US20110180905A1 (en) * | 2008-06-10 | 2011-07-28 | Advanced Technology Materials, Inc. | GeSbTe MATERIAL INCLUDING SUPERFLOW LAYER(S), AND USE OF Ge TO PREVENT INTERACTION OF Te FROM SbXTeY AND GeXTeY RESULTING IN HIGH Te CONTENT AND FILM CRYSTALLINITY |
| US8330136B2 (en) | 2008-12-05 | 2012-12-11 | Advanced Technology Materials, Inc. | High concentration nitrogen-containing germanium telluride based memory devices and processes of making |
| US8617972B2 (en) | 2009-05-22 | 2013-12-31 | Advanced Technology Materials, Inc. | Low temperature GST process |
| US9070875B2 (en) | 2009-05-22 | 2015-06-30 | Entegris, Inc. | Low temperature GST process |
| US8410468B2 (en) | 2009-07-02 | 2013-04-02 | Advanced Technology Materials, Inc. | Hollow GST structure with dielectric fill |
| US20110001107A1 (en) * | 2009-07-02 | 2011-01-06 | Advanced Technology Materials, Inc. | Hollow gst structure with dielectric fill |
| US11542600B2 (en) | 2009-10-26 | 2023-01-03 | Asm Ip Holding B.V. | Synthesis and use of precursors for ALD of group VA element containing thin films |
| US10941487B2 (en) | 2009-10-26 | 2021-03-09 | Asm Ip Holding B.V. | Synthesis and use of precursors for ALD of group VA element containing thin films |
| US20110124182A1 (en) * | 2009-11-20 | 2011-05-26 | Advanced Techology Materials, Inc. | System for the delivery of germanium-based precursor |
| US9012876B2 (en) | 2010-03-26 | 2015-04-21 | Entegris, Inc. | Germanium antimony telluride materials and devices incorporating same |
| US9190609B2 (en) | 2010-05-21 | 2015-11-17 | Entegris, Inc. | Germanium antimony telluride materials and devices incorporating same |
| US9065048B2 (en) | 2010-07-27 | 2015-06-23 | Micron Technology, Inc. | Methods of forming germanium-antimony-tellurium materials and chalcogenide materials |
| US8148197B2 (en) | 2010-07-27 | 2012-04-03 | Micron Technology, Inc. | Methods of forming germanium-antimony-tellurium materials and a method of forming a semiconductor device structure including the same |
| US8759146B2 (en) | 2010-07-27 | 2014-06-24 | Micron Technology, Inc. | Methods of forming germanium-antimony-tellurium materials and methods of forming a semiconductor device structure including the same |
| US8623734B2 (en) | 2011-06-01 | 2014-01-07 | International Business Machines Corporation | Method to selectively grow phase change material inside a via hole |
| WO2012166255A1 (en) * | 2011-06-01 | 2012-12-06 | International Business Machines Corporation | Method to selectively grow phase change material inside a via hole |
| CN102637822A (en) * | 2012-03-14 | 2012-08-15 | 宁波大学 | High-purity chalcogenide phase change alloy target and preparation method for same |
| US9443736B2 (en) | 2012-05-25 | 2016-09-13 | Entegris, Inc. | Silylene compositions and methods of use thereof |
| US9640757B2 (en) | 2012-10-30 | 2017-05-02 | Entegris, Inc. | Double self-aligned phase change memory device structure |
| CN103111086A (en) * | 2012-12-26 | 2013-05-22 | 成都市幅原环保科技有限责任公司 | Solvent for leaching oil in drillings and leaching method |
| US10186570B2 (en) | 2013-02-08 | 2019-01-22 | Entegris, Inc. | ALD processes for low leakage current and low equivalent oxide thickness BiTaO films |
| US8993441B2 (en) | 2013-03-13 | 2015-03-31 | Samsung Electronics Co., Ltd. | Methods of forming a thin layer and methods of manufacturing a phase change memory device using the same |
| US20180282863A1 (en) * | 2015-10-06 | 2018-10-04 | Entegris, Inc. | Cold sintering of solid precursors |
| US11035038B2 (en) * | 2015-10-06 | 2021-06-15 | Entegris, Inc. | Cold sintering of solid precursors |
| US10580976B2 (en) | 2018-03-19 | 2020-03-03 | Sandisk Technologies Llc | Three-dimensional phase change memory device having a laterally constricted element and method of making the same |
| WO2021167776A1 (en) * | 2020-02-20 | 2021-08-26 | Applied Materials, Inc. | Deposition of tellurium-containing thin films |
| US11408068B2 (en) | 2020-02-20 | 2022-08-09 | Applied Materials, Inc. | Deposition of tellurium-containing thin films |
Also Published As
| Publication number | Publication date |
|---|---|
| US20130288462A1 (en) | 2013-10-31 |
| US9537095B2 (en) | 2017-01-03 |
| US20140329357A1 (en) | 2014-11-06 |
| US8796068B2 (en) | 2014-08-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9537095B2 (en) | Tellurium compounds useful for deposition of tellurium containing materials | |
| US9034688B2 (en) | Antimony compounds useful for deposition of antimony-containing materials | |
| US20090275164A1 (en) | Bicyclic guanidinates and bridging diamides as cvd/ald precursors | |
| EP2062995B1 (en) | Amorphous Ge/Te deposition process | |
| US20090087561A1 (en) | Metal and metalloid silylamides, ketimates, tetraalkylguanidinates and dianionic guanidinates useful for cvd/ald of thin films | |
| EP2078102B1 (en) | Antimony and germanium complexes useful for cvd/ald of metal thin films | |
| US20100279011A1 (en) | Novel bismuth precursors for cvd/ald of thin films | |
| US9240319B2 (en) | Chalcogenide-containing precursors, methods of making, and methods of using the same for thin film deposition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: ADVANCED TECHNOLOGY MATERIALS, INC., CONNECTICUT Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:STENDER, MATTHIAS;XU, CHONGYING;CHEN, TIANNIU;AND OTHERS;REEL/FRAME:022437/0293;SIGNING DATES FROM 20090302 TO 20090308 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |