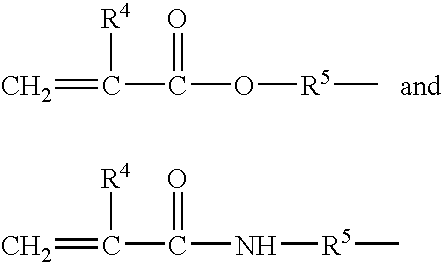US20100310489A1 - Cosmetic makeup and/or care process using a siloxane resin and a film-forming polymer - Google Patents
Cosmetic makeup and/or care process using a siloxane resin and a film-forming polymer Download PDFInfo
- Publication number
- US20100310489A1 US20100310489A1 US12/746,282 US74628208A US2010310489A1 US 20100310489 A1 US20100310489 A1 US 20100310489A1 US 74628208 A US74628208 A US 74628208A US 2010310489 A1 US2010310489 A1 US 2010310489A1
- Authority
- US
- United States
- Prior art keywords
- group
- block
- polymer
- sio
- vinyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 0 [7*]N([8*])C(=O)C(=C)C Chemical compound [7*]N([8*])C(=O)C(=C)C 0.000 description 28
- WJHJGHBFEULRCD-UHFFFAOYSA-N C.C.CO[Si](C)(C)O[Si](C)(C)C Chemical compound C.C.CO[Si](C)(C)O[Si](C)(C)C WJHJGHBFEULRCD-UHFFFAOYSA-N 0.000 description 1
- WXOLFLOUEHDECV-UHFFFAOYSA-N C=C(C)C(=O)OCCC[SiH2]O[Si](C)(C)CC[SiH](C)O[Si](C)(C)C.C=C(C)C(=O)OCCC[SiH2]O[Si](C)(C)CC[SiH](C)O[Si](C)(C)C.C=CC(C)(C)CC[SiH2]C(C)(C)C.C=CC(C)(C)CC[SiH2]C(C)(C)C.C=CC1=CC=C(CC[SiH2]O[Si](C)(C)CC[SiH2]O[Si](C)(C)CC[SiH2]O[Si](C)(C)C)C=C1.O.O.O.[H][Si](C)(C)O[SiH2]CC[Si](C)(C)O[SiH2]C1=CC=C(C=C)C=C1.[H][Si](C)(C)O[SiH](C)CC[Si](C)(C)O[SiH2]CCCOC(=O)C(=C)C Chemical compound C=C(C)C(=O)OCCC[SiH2]O[Si](C)(C)CC[SiH](C)O[Si](C)(C)C.C=C(C)C(=O)OCCC[SiH2]O[Si](C)(C)CC[SiH](C)O[Si](C)(C)C.C=CC(C)(C)CC[SiH2]C(C)(C)C.C=CC(C)(C)CC[SiH2]C(C)(C)C.C=CC1=CC=C(CC[SiH2]O[Si](C)(C)CC[SiH2]O[Si](C)(C)CC[SiH2]O[Si](C)(C)C)C=C1.O.O.O.[H][Si](C)(C)O[SiH2]CC[Si](C)(C)O[SiH2]C1=CC=C(C=C)C=C1.[H][Si](C)(C)O[SiH](C)CC[Si](C)(C)O[SiH2]CCCOC(=O)C(=C)C WXOLFLOUEHDECV-UHFFFAOYSA-N 0.000 description 1
- HUMIELYCWOJCIO-UHFFFAOYSA-N C=C(C)C(=O)OCCC[SiH2]O[Si](C)(C)CC[SiH](C)O[Si](C)(C)C.C=C(C)C(=O)OCCC[SiH2]O[Si](C)(C)CC[SiH](C)O[Si](C)(C)C.C=CC1=CC=C(CC[SiH2]O[Si](C)(C)CC[SiH2]O[Si](C)(C)CC[SiH2]O[Si](C)(C)C)C=C1.O.O.O.[H][Si](C)(C)O[SiH2]CC[Si](C)(C)O[SiH2]C1=CC=C(C=C)C=C1.[H][Si](C)(C)O[SiH](C)CC[Si](C)(C)O[SiH2]CCCOC(=O)C(=C)C Chemical compound C=C(C)C(=O)OCCC[SiH2]O[Si](C)(C)CC[SiH](C)O[Si](C)(C)C.C=C(C)C(=O)OCCC[SiH2]O[Si](C)(C)CC[SiH](C)O[Si](C)(C)C.C=CC1=CC=C(CC[SiH2]O[Si](C)(C)CC[SiH2]O[Si](C)(C)CC[SiH2]O[Si](C)(C)C)C=C1.O.O.O.[H][Si](C)(C)O[SiH2]CC[Si](C)(C)O[SiH2]C1=CC=C(C=C)C=C1.[H][Si](C)(C)O[SiH](C)CC[Si](C)(C)O[SiH2]CCCOC(=O)C(=C)C HUMIELYCWOJCIO-UHFFFAOYSA-N 0.000 description 1
- KZCIUEZIXIKCDA-UHFFFAOYSA-N CC[Si](C)(O[Si](C)(C)C)O[Si](C)(COC)O[Si](C)(C)O[Si](C)(C)C Chemical compound CC[Si](C)(O[Si](C)(C)C)O[Si](C)(COC)O[Si](C)(C)O[Si](C)(C)C KZCIUEZIXIKCDA-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/84—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds obtained by reactions otherwise than those involving only carbon-carbon unsaturated bonds
- A61K8/89—Polysiloxanes
- A61K8/891—Polysiloxanes saturated, e.g. dimethicone, phenyl trimethicone, C24-C28 methicone or stearyl dimethicone
-
- A—HUMAN NECESSITIES
- A45—HAND OR TRAVELLING ARTICLES
- A45D—HAIRDRESSING OR SHAVING EQUIPMENT; EQUIPMENT FOR COSMETICS OR COSMETIC TREATMENTS, e.g. FOR MANICURING OR PEDICURING
- A45D33/00—Containers or accessories specially adapted for handling powdery toiletry or cosmetic substances
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/31—Hydrocarbons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q1/00—Make-up preparations; Body powders; Preparations for removing make-up
- A61Q1/02—Preparations containing skin colorants, e.g. pigments
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q1/00—Make-up preparations; Body powders; Preparations for removing make-up
- A61Q1/02—Preparations containing skin colorants, e.g. pigments
- A61Q1/04—Preparations containing skin colorants, e.g. pigments for lips
- A61Q1/06—Lipsticks
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q1/00—Make-up preparations; Body powders; Preparations for removing make-up
- A61Q1/02—Preparations containing skin colorants, e.g. pigments
- A61Q1/10—Preparations containing skin colorants, e.g. pigments for eyes, e.g. eyeliner, mascara
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/40—Chemical, physico-chemical or functional or structural properties of particular ingredients
- A61K2800/59—Mixtures
- A61K2800/591—Mixtures of compounds not provided for by any of the codes A61K2800/592 - A61K2800/596
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/80—Process related aspects concerning the preparation of the cosmetic composition or the storage or application thereof
- A61K2800/88—Two- or multipart kits
Definitions
- the invention relates to a cosmetic composition for keratin materials, especially the skin, the hair and the nails.
- the invention relates in particular to makeup compositions for keratin materials.
- One of the objects of the patent application is to produce makeup compositions for keratin materials (skin, mucous membranes, fibre, eyelashes and integuments) that allow the application of a total transfer-resistant film with good staying power.
- compositions that have good staying-power properties, to satisfy consumers' expectations. These compositions should also be transfer-resistant, while at the same time offering good comfort properties.
- Formulators are thus in search of starting materials and/or systems for obtaining compositions whose application is characterized by improved staying power and a good level of comfort.
- the term “comfort” means the comfort on application, i.e. a composition that is easy to apply in terms of glidance and of amount applied, without, however, the applied film being too thick and/or tacky.
- the term “comfort” also means the comfort after application, so that the user does not experience any tautness or drying out, in particular.
- polymers are of very diverse chemical nature and are conveyed either in a fatty phase or in an aqueous phase.
- silicone resins especially of MQ type, polyacrylates, latices, etc.
- compositions containing, in a physiologically acceptable medium, a) a siloxane resin comprising the following units:
- R 1 , R 2 and R 3 independently representing an alkyl group containing from 1 to 8 carbon atoms, an aryl group, a carbinol group or an amino group,
- a being between 0.05 and 0.5
- d being between 0.05 and 0.6
- the block ethylenic film-forming polymer comprising at least a first block and at least a second block
- the first block is obtained from at least one acrylate monomer of formula CH 2 ⁇ CH—COOR 2 in which R 2 represents a C 4 to C 12 cycloalkyl group and at least one methacrylate monomer of formula CH 2 ⁇ C(CH 3 )—COOR 2 in which R′ 2 represents a C 4 to C 12 cycloalkyl group
- the second block is obtained from an acrylic acid monomer and from at least one monomer with a glass transition temperature of less than or equal to 20° C.
- Such polymers and the process for preparing them are described, for example, in document EP 1 882 709.
- the siloxane resin comprises the following units:
- R 1 and R 3 independently representing an alkyl group containing from 1 to 8 carbon atoms, R 1 preferably being a methyl group and R 3 preferably being a propyl group,
- a being between 0.05 and 0.5 and preferably between 0.15 and 0.4
- c being greater than zero, preferably between 0.15 and 0.4,
- d being between 0.05 and 0.6, preferably between 0.2 and 0.6 or alternatively between 0.2 and 0.55,
- siloxane resins that may be used according to the invention may be obtained via a process comprising the reaction of:
- the mass ratio A/B is between 95/5 and 15/85.
- the ratio A/B is less than or equal to 70/30.
- compositions according to the invention may be in various forms, especially in the form of a powder, an anhydrous dispersion, a water/oil, water/wax, oil/water, multiple or wax/water emulsion, or a gel.
- compositions according to the invention are found to have very good staying-power and transfer-resistance properties while at the same time maintaining a comfortable deposit, especially when it is applied to the lips.
- the resins that may be used according to the invention are especially those described in patent application WO 2005/075 542, the content of which is incorporated herein by reference.
- the composition according to the invention is liquid.
- the composition according to the invention is solid.
- solid characterizes the state of the composition at room temperature (25° C.) and at atmospheric pressure (760 mmHg).
- the composition according to the invention has, when it is solid, a hardness of between 30 and 300 g, or even from 50 to 200 g.
- the measurement is performed according to the following protocol:
- a sample of the composition under consideration is hot-cast into a stick mould 12.7 mm in diameter.
- the mould is then cooled in a freezer for about one hour.
- the stick of lipstick is then stored at 20° C.
- the hardness of the samples is measured after an interval of 24 hours.
- the hardness of the samples of compositions of the invention is measured using a DFGS2 tensile testing machine sold by the company Indelco-Chatillon.
- the hardness corresponds to the maximum shear force exerted by a rigid tungsten wire 250 ⁇ m in diameter, advancing at a rate of 100 mm/minute.
- the composition according to the invention comprises less than 3% and better still less than 1% by weight of water relative to the total weight of the composition. More preferably, the composition is totally anhydrous.
- anhydrous especially means that water is preferably not deliberately added to the composition, but may be present in trace amount in the various compounds used in the composition.
- the present invention relates to a makeup and/or care process in which the composition as defined previously is applied to keratin materials, and especially to the lips.
- film-forming polymer means a polymer that is capable, by itself or in the presence of an auxiliary film-forming agent, of forming a macroscopically continuous film that adheres to keratin materials, and preferably a cohesive film, better still a film whose cohesion and mechanical properties are such that the said film can be isolated and manipulated individually, for example when the said film is prepared by pouring onto a non-stick surface such as a Teflon-coated or silicone-coated surface.
- the film-forming polymer present in the composition according to the invention is a film-forming block ethylenic polymer (which is preferably essentially linear), which preferably comprises at least a first block and at least a second block with different glass transition temperatures (Tg), the said first and second blocks being linked together via an intermediate block comprising at least one constituent monomer of the first block and at least one constituent monomer of the second block.
- a film-forming block ethylenic polymer which is preferably essentially linear
- Tg glass transition temperatures
- At least one block means one or more blocks.
- block polymer means a polymer comprising at least two different blocks and preferably at least three different blocks.
- the first and second blocks of the block polymer are advantageously mutually incompatible.
- the film-forming polymer present in the composition according to the invention is a block polymer, comprising at least a first block and at least a second block, characterized in that the first block is obtained from at least one acrylate monomer of formula CH 2 ⁇ CH—COOR 2 in which R 2 represents a C 4 to C 12 cycloalkyl group, and from at least one methacrylate monomer of formula CH 2 ⁇ C(CH 3 )—COOR 2 in which R′ 2 represents a C 4 to C 12 cycloalkyl group, and characterized in that the second block is obtained from an acrylic acid monomer and from at least one monomer with a glass transition temperature of less than or equal to 20° C.
- Such polymers and the process for preparing them are described, for example, in document EP 1 882 709.
- the first block and the second block of the polymer of the invention may be advantageously mutually incompatible.
- mutant blocks means that the blend formed from a polymer corresponding to the first block and from a polymer corresponding to the second block is not miscible in the polymerization solvent that is the majority amount by weight of the block polymer, at room temperature (25° C.) and atmospheric pressure (10 5 Pa), for a polymer blend content of greater than or equal to 5% by weight, relative to the total weight of the blend and of the said polymerization solvent, it being understood that:
- the said polymer blend is immiscible in at least one of them.
- this solvent is the majority solvent.
- the said first and second blocks may be advantageously linked together via an intermediate block comprising at least one constituent monomer of the first block and at least one constituent monomer of the second block.
- the intermediate block is a block comprising at least one constituent monomer of the first block and at least one constituent monomer of the second block of the polymer, which enables these blocks to be “compatibilized”.
- the intermediate block comprising at least one constituent monomer of the first block and at least one constituent monomer of the second block of the polymer is a random polymer.
- the intermediate block is derived essentially from constituent monomers of the first block and of the second block.
- the term “essentially” means at least 85%, preferably at least 90%, better still 95% and even better still 100%.
- the intermediate block has a glass transition temperature Tg that is between the glass transition temperatures of the first and second blocks.
- the block polymer according to the invention is advantageously a film-forming block ethylenic polymer.
- ethylenic polymer means a polymer obtained by polymerization of ethylenically unsaturated monomers.
- film-forming polymer means a polymer that is capable of forming, by itself or in the presence of a film-forming auxiliary agent, a continuous film that adheres to a support, especially to keratin materials.
- the polymer used in the composition according to the invention comprises no silicon atoms in its backbone.
- backbone means the main chain of the polymer, as opposed to the pendent side chains.
- the polymer according to the invention is not water-soluble, i.e. the polymer is not soluble in water or in a mixture of water and of linear or branched lower monoalcohols containing from 2 to 5 carbon atoms, for instance ethanol, isopropanol or n-propanol, without pH modification, at an active material content of at least 1% by weight, at room temperature (25° C.).
- the polymer according to the invention is not an elastomer.
- non-elastomeric polymer means a polymer which, when it is subjected to a constraint intended to pull it (for example by 30% relative to its initial length), does not return to a length substantially identical to its initial length when the constraint ceases.
- non-elastomeric polymer denotes a polymer with an instantaneous recovery R i ⁇ 50% and a delayed recovery R 2h ⁇ 70% after having been subjected to a 30% elongation.
- R i is ⁇ 30% and R 2h ⁇ 50%.
- non-elastomeric nature of the polymer is determined according to the following protocol:
- a polymer film is prepared by pouring a solution of the polymer in a Teflon-coated mould, followed by drying for 7 days in an environment conditioned at 23 ⁇ 5° C. and 50 ⁇ 10% relative humidity.
- a film about 100 ⁇ m thick is thus obtained, from which are cut rectangular specimens (for example using a punch) 15 mm wide and 80 mm long.
- This sample is subjected to a tensile stress using a machine sold under the reference Zwick, under the same temperature and humidity conditions as for the drying.
- the specimens are pulled at a speed of 50 mm/min and the distance between the jaws is 50 mm, which corresponds to the initial length (l 0 ) of the specimen.
- the instantaneous recovery R i is determined in the following manner:
- the percentage residual elongation of the specimen ( ⁇ 2h ) is measured after 2 hours (2 hours after returning to zero stress load).
- R 2h ( ⁇ max ⁇ 2h )/( ⁇ max ) ⁇ 100
- a polymer according to one embodiment of the invention has an instantaneous recovery R i of 10% and a delayed recovery R 2h of 30%.
- the polydispersity index of the polymer of the invention is advantageously greater than 2.
- the polydispersity index I of the polymer is equal to the ratio of the weight-average mass Mw to the number-average mass Mn.
- the weight-average molar mass (Mw) and number-average molar mass (Mn) are determined by gel permeation liquid chromatography (THF solvent, calibration curve established with linear polystyrene standards, refractometric detector).
- the weight-average mass (Mw) of the polymer according to the invention is preferably less than or equal to 300 000; it ranges, for example, from 35 000 to 200 000 and better still from 45 000 to 150 000 g/mol.
- the number-average mass (Mn) of the polymer according to the invention is preferably less than or equal to 70 000; it ranges, for example, from 10 000 to 60 000 and better still from 12 000 to 50 000 g/mol.
- the polydispersity index of the polymer according to the invention is advantageously greater than 2, for example ranging from 2 to 9, preferably greater than or equal to 2.5, for example ranging from 2.5 to 8, and better still greater than or equal to 2.8, especially from 2.8 to 6.
- the block polymer of the invention comprises at least a first block and at least a second block.
- the first block is advantageously obtained from at least one acrylate monomer of formula CH 2 ⁇ CH—COOR 2 and from at least one methacrylate monomer of formula CH 2 ⁇ C(CH 3 )—COOR 2 in which R 2 represents a C 4 to C 12 cycloalkyl group.
- the monomers and the proportions thereof are preferably chosen such that the glass transition temperature of the first block is greater than 20° C.
- the second block is advantageously obtained from an acrylic acid monomer and from at least one monomer with a glass transition temperature of less than or equal to 20° C.
- the monomers and the proportions thereof are preferably chosen such that the glass transition temperature of the second block is less than or equal to 20° C.
- the glass transition temperatures indicated for the first and second blocks may be theoretical Tg values determined from the theoretical Tg values of the constituent monomers of each of the blocks, which may be found in a reference manual such as the Polymer Handbook, 3rd Edition, 1989, John Wiley, according to the following relationship, known as Fox's law:
- ⁇ i being the mass fraction of the monomer i in the block under consideration and Tg i being the glass transition temperature of the homopolymer of the monomer i.
- Tg values indicated for the first and second blocks in the present patent application are theoretical Tg values.
- the difference between the glass transition temperatures of the first and second blocks is generally greater than 10° C., preferably greater than 20° C. and better still greater than 30° C.
- the expression: “between . . . and . . . ” is intended to denote a range of values for which the limits mentioned are excluded, and “from . . . to . . . ” and “ranging from . . . to . . . ” are intended to denote a range of values for which the limits are included.
- the first block preferably has a Tg of greater than 20° C., for example a Tg ranging from 20 to 170° C. and preferably greater than or equal to 50° C., for example ranging from 50° C. to 160° C., especially ranging from 90° C. to 130° C.
- the first block is obtained from at least one acrylate monomer of formula CH 2 ⁇ CH—COOR 2 in which R 2 represents a C 4 to C 12 cycloalkyl group, and from at least one methacrylate monomer of formula CH 2 ⁇ C(CH 3 )—COOR′ 2 in which R′ 2 represents a C 4 to C 12 cycloalkyl group.
- the first block may be obtained exclusively from the said acrylate monomer and from the said methacrylate monomer.
- the acrylate monomer and the methacrylate monomer are preferably in mass proportions of between 30/70 and 70/30, preferably between 40/50 and 50/40 and especially of the order of 50/50.
- the proportion of the first block advantageously ranges from 20% to 90%, better still from 30% to 80% and even better still from 60% to 80% by weight of the polymer.
- the first block is obtained by polymerization of isobornyl methacrylate and isobornyl acrylate.
- the first block may also comprise:
- R 7 and R 8 which may be identical or different, each represent a hydrogen atom or a linear or branched C 1 to C 12 alkyl group, such as an n-butyl, t-butyl, isopropyl, isohexyl, isooctyl or isononyl group; or R 7 represents H and R 8 represents a 1,1-dimethyl-3-oxobutyl group, and R′ denotes H or methyl.
- monomers that may be mentioned include N-butylacrylamide, N-t-butylacrylamide, N-isopropylacrylamide, N,N-dimethylacrylamide and N,N-dibutylacrylamide,
- the second block advantageously has a glass transition temperature Tg of less than or equal to 20° C., for example a Tg ranging from ⁇ 100 to 20° C., preferably less than or equal to 15° C., especially ranging from ⁇ 80° C. to 15° C. and better still less than or equal to 10° C., for example ranging from ⁇ 100° C. to 10° C., especially ranging from ⁇ 30° C. to 10° C.
- the second block is obtained from an acrylic acid monomer and from another monomer with a Tg of less than or equal to 20° C.
- the monomer with a Tg of less than or equal to 20° C. is preferably chosen from the following monomers:
- the preferred monomers with a Tg of less than or equal to 20° C. are isobutyl acrylate and 2-ethylhexyl acrylate, or mixtures thereof in all proportions.
- Each of the first and second blocks may contain in minor proportion at least one constituent monomer of the other block.
- the first block may contain at least one constituent monomer of the second block, and vice versa.
- Each of the first and/or second blocks may comprise, in addition to the monomers indicated above, one or more other monomers known as additional monomers, which are different from the main monomers mentioned above.
- This additional monomer is chosen, for example, from:
- R 9 representing a linear or branched C 6 to C 12 alkyl group in which one or more heteroatoms chosen from O, N and S is (are) optionally intercalated, the said alkyl group being substituted with one or more substituents chosen from hydroxyl groups and halogen atoms (Cl, Br, I or F);
- R 10 representing a linear or branched C 1 to C 12 alkyl group substituted with one or more substituents chosen from hydroxyl groups and halogen atoms (Cl, Br, I or F), such as 2-hydroxypropyl acrylate and 2-hydroxyethyl acrylate, or R 10 represents a C 1 to C 12 alkyl-O-POE (polyoxyethylene) with repetition of the oxyethylene unit 5 to 30 times, for example methoxy-POE, or R 10 represents a polyoxyethylenated group comprising from 5 to 30 ethylene oxide units.
- the additional monomer may represent 0.5% to 30% by weight relative to the weight of the polymer. According to one embodiment, the polymer of the invention does not contain any additional monomer.
- the polymer of the invention comprises at least one of the isobornyl acrylate and isobornyl methacrylate monomers in the first block and isobutyl acrylate and acrylic acid monomers in the second block.
- the polymer comprises at least one of the isobornyl acrylate and isobornyl methacrylate monomers in equivalent weight proportion in the first block and isobutyl acrylate and acrylic acid monomers in the second block.
- the polymer comprises at least isobornyl acrylate and isobornyl methacrylate monomers in equivalent weight proportion in the first block, and isobutyl acrylate and acrylic acid monomers in the second block, the first block representing 70% of the weight of the polymer.
- the polymer comprises at least isobornyl acrylate and isobornyl methacrylate monomers in equivalent weight proportion in the first block, and isobutyl acrylate and acrylic acid monomers in the second block, the block with a Tg of greater than 20° C. representing 70% of the weight of the polymer, and acrylic acid representing 5% of the weight of the polymer.
- the film-forming polymer present in the composition according to the invention is a vinyl polymer comprising at least one carbosiloxane dendrimer-based unit.
- the vinyl polymer may especially have a backbone and at least one side chain, which comprises a carbosiloxane dendrimer structure.
- carbosiloxane dendrimer structure in the context of the present invention represents a structure with branched groups of high molecular masses with high regularity in the radial direction starting from the simple backbone.
- Such carbosiloxane dendrimer structures are described in the form of a highly branched siloxane-silylalkylene copolymer in the laid-open Japanese patent application Kokai 9-171 154.
- the vinyl polymer contains carbosiloxane dendrimer-based units that may be represented by the following general formula:
- R 1 represents an aryl group or an alkyl group containing from 1 to 10 carbon atoms
- R 4 represents a hydrogen atom or an alkyl group
- R 5 represents an alkylene group containing from 1 to 10 carbon atoms, such as a methylene group, an ethylene group, a propylene group or a butylene group, the methylene group and the propylene group being preferred
- R 6 represents a hydrogen atom or an alkyl group
- R 7 represents an alkyl group containing from 1 to 10 carbon atoms, such as a methyl group, an ethyl group, a propyl group or a butyl group, the methyl group being preferred
- R 8 represents an alkylene group containing from 1 to 10 carbon atoms, such as a methylene group, an ethylene group, a propylene group or a butylene group, the ethylene group being preferred
- b is an integer from 0 to 4
- c is 0 or 1 such that if c is 0, —(R 8 ) c — represents a bond
- R 1 represents an aryl group or an alkyl group containing from 1 to 10 carbon atoms, in which the alkyl group is preferably represented by a methyl group, an ethyl group, a propyl group, a butyl group, a pentyl group, an isopropyl group, an isobutyl group, a cyclopentyl group or a cyclohexyl group, and in which the aryl group is preferably represented by a phenyl group and a naphthyl group, in which the methyl and phenyl groups are more particularly preferred, and the methyl group is preferred among all.
- the vinyl polymer that contains a carbosiloxane dendrimer structure may be the product of polymerization of
- Y represents an organic group that may be polymerized using radicals
- R 1 represents an aryl group or an alkyl group containing from 1 to 10 carbon atoms
- R 4 represents a hydrogen atom or an alkyl group
- R 5 represents an alkylene group containing from 1 to 10 carbon atoms
- R 6 represents a hydrogen atom or an alkyl group
- R 7 represents an alkyl group containing from 1 to carbon atoms
- R 8 represents an alkylene group containing from 1 to 10 carbon atoms
- b is an integer from 0 to 4
- c is 0 or 1.
- the monomer of vinyl type that is the component (A) in the vinyl polymer is a monomer of vinyl type that contains a radical-polymerizable vinyl group.
- a monomer of vinyl type that contains a radical-polymerizable vinyl group There is no particular limitation as regards the type of such a monomer.
- Multifunctional monomers of vinyl type may also be used.
- the following represent examples of such compounds: trimethylolpropane trimethacrylate, pentaerythrityl trimethacrylate, ethylene glycol dimethacrylate, tetraethylene glycol dimethacrylate, polyethylene glycol dimethacrylate, 1,4-butanediol dimethacrylate, 1,6-hexanediol dimethacrylate, neopentyl glycol dimethacrylate, trimethylolpropane-trioxyethyl methacrylate, tris(2-hydroxyethyl)-isocyanurate dimethacrylate, tris(2-hydroxyethyl)-isocyanurate trimethacrylate, polydimethylsiloxane capped with styryl groups containing divinylbenzene groups on both ends, or similar silicone compounds containing unsaturated groups.
- the carbosiloxane dendrimer which is the component (B), is represented by the following formula:
- radical-polymerizable organic group Y an acryloxymethyl group, a 3-acryloxypropyl group, a methacryloxymethyl group, a 3-methacryloxypropyl group, a 4-vinylphenyl group, a 3-vinylphenyl group, a 4-(2-propenyl)phenyl group, a 3-(2-propenyl)phenyl group, a 2-(4-vinylphenyl)ethyl group, a 2-(3-vinyl-phenyl)ethyl group, a vinyl group, an allyl group, a methallyl group and a 5-hexenyl group.
- R 1 represents an alkyl group or an aryl group containing from 1 to 10 carbon atoms, in which the alkyl group may be a methyl group, an ethyl group, a propyl group, a butyl group, a pentyl group, an isopropyl group, an isobutyl group, a cyclopentyl group or a cyclohexyl group; and the aryl group may be a phenyl group or a naphthyl group.
- the methyl and phenyl groups are particularly preferred, the methyl group being preferred among all.
- X 1 represents a silylalkyl group that is represented by the following formula, when i is equal to 1:
- R 2 represents an alkylene group containing from 2 to 10 carbon atoms, such as an ethylene group, a propylene group, a butylene group, a hexylene group or a similar linear alkylene group; a methylmethylene group, a methylethylene group, a 1-methylpentylene group, a 1,4-dimethylbutylene group or a similar branched alkylene group.
- the ethylene, methylethylene, hexylene, 1-methylpentylene and 1,4-dimethylbutylene groups are preferred among all.
- R 3 represents an alkyl group containing from 1 to 10 carbon atoms, such as methyl, ethyl, propyl, butyl and isopropyl groups.
- R 1 is the same as defined above.
- a i is an integer from 0 to 3
- i is an integer from 1 to 10 that indicates the generation number, which represents the number of repetitions of the silylalkyl group.
- the carbosiloxane dendrimer may be represented by the first general formula shown below, in which Y, R 1 , R 2 and R 3 are the same as defined above, R 12 represents a hydrogen atom or is identical to R 1 ; a 1 is identical to a i .
- the mean total number of groups OR 3 in a molecule is within the range from 0 to 7.
- the carbosiloxane dendrimer may be represented by the second general formula shown below, in which Y, R 1 , R 2 , R 3 and R 12 are the same as defined above; a 1 and a 2 represent the a i of the indicated generation.
- the mean total number of groups OR 3 in a molecule is within the range from 0 to 25.
- the carbosiloxane dendrimer is represented by the third general formula shown below, in which Y, R 2 , R 3 and R 12 are the same as defined above; a 1 , a 2 and a 3 represent the a i of the indicated generation.
- the total mean number of groups OR 3 in a molecule is within the range from 0 to 79.
- a carbosiloxane dendrimer that contains a radical-polymerizable organic group may be represented by the following mean structural formulae:
- the carbosiloxane dendrimer may be manufactured according to the process for manufacturing a branched silalkylene siloxane described in Japanese patent application Hei 9-171 154.
- it may be produced by subjecting an organosilicon compound containing a hydrogen atom linked to a silicon atom, represented by the following general formula:
- the organosilicon compound may be represented by 3-methacryloxypropyltris(dimethyl-siloxy)silane, 3-acryloxypropyltris(dimethylsiloxy)-silane and 4-vinylphenyltris(dimethylsiloxy)silane.
- the organosilicon compound that contains an alkenyl group may be represented by vinyltris(trimethylsiloxy)silane, vinyltris(dimethylphenylsiloxy)silane, and 5-hexenyl-tris(trimethylsiloxy)silane.
- the hydrosilylation reaction is performed in the presence of a chloroplatinic acid, a complex of vinylsiloxane and of platinum, or a similar transition metal catalyst.
- the polymerization ratio between the components (A) and (B), in terms of the weight ratio between (A) and (B), may be within the range from 0/100 to 99.9/0.1 and preferably within the range from 1/99 to 99/1.
- a ratio between the components (A) and (B) of 0/100 means that the compound becomes a homopolymer of component (B).
- the vinyl polymer contains a carbosiloxane dendrimer structure and this polymer may be obtained by copolymerization of the components (A) and (B), or by polymerization of the component (B) alone.
- the polymerization may be a free-radical polymerization or an ionic polymerization, but free-radical polymerization is preferred.
- the polymerization may be performed by bringing about a reaction between the components (A) and (B) in a solution for a period of from 3 to 20 hours in the presence of a radical initiator at a temperature of from 50° C. to 150° C.
- a suitable solvent for this purpose is hexane, octane, decane, cyclohexane or a similar aliphatic hydrocarbon; benzene, toluene, xylene or a similar aromatic hydrocarbon; diethyl ether, dibutyl ether, tetrahydrofuran, dioxane or similar ethers; acetone, methyl ethyl ketone, methyl isobutyl ketone, diisobutyl ketone or similar ketones; methyl acetate, ethyl acetate, butyl acetate, isobutyl acetate or similar esters; methanol, ethanol, isopropanol, butanol or similar alcohols; octamethylcyclotetrasiloxane, decamethylcyclopentasiloxane, hexamethyldisiloxane, octamethyltrisiloxane or a similar
- a radical initiator may be any compound known in the art for standard free-radical polymerization reactions.
- the specific examples of such radical initiators are 2,2′-azobis(isobutyronitrile), 2,2′-azobis(2-methylbutyronitrile), 2,2′-azobis(2,4-dimethylvaleronitrile) or similar compounds of azobis type; benzoyl peroxide, lauroyl peroxide, tert-butyl peroxybenzoate, tert-butyl peroxy-2-ethylhexanoate or a similar organic peroxide.
- These radical initiators may be used alone or in a combination of two or more.
- the radical initiators may be used in an amount of from 0.1 to 5 parts by weight per 100 parts by weight of the components (A) and (B).
- a chain-transfer agent may be added.
- the chain-transfer agent may be 2-mercaptoethanol, butyl mercaptan, n-dodecyl mercaptan, 3-mercaptopropyltrimethoxysilane, a polydimethyl-siloxane containing a mercaptopropyl group or a similar compound of mercapto type; methylene chloride, chloroform, carbon tetrachloride, butyl bromide, 3-chloropropyltrimethoxysilane or a similar halogenated compound.
- the residual unreacted vinyl monomer may be removed under conditions of heating under vacuum.
- the number-average molecular mass of the vinyl polymer containing a carbosiloxane dendrimer may be chosen within the range between 3000 and 2 000 000 and preferably between 5000 and 800 000. It may be a liquid, a gum, a paste, a solid, a powder or any other form.
- the preferred forms are solutions consisting of the dilution of a dispersion or of a powder in solvents.
- the vinyl polymer may be a dispersion of a polymer of vinyl type having a carbosiloxane dendrimer structure in its side molecular chain, in a liquid such as a silicone oil, an organic oil, an alcohol or water.
- the vinyl polymer having a carbosiloxane dendrimer structure in its side molecular chain is the same as that described above.
- the liquid may be a silicone oil, an organic oil, an alcohol or water.
- the silicone oil may be a dimethylpolysiloxane with the two molecular ends capped with trimethylsiloxy groups, a copolymer of methylphenylsiloxane and of dimethylsiloxane having the two molecular ends capped with trimethylsiloxy groups, a copolymer of methyl-3,3,3-trifluoropropylsiloxane and of dimethylsiloxane having the two molecular ends capped with trimethylsiloxy groups, or similar unreactive linear silicone oils, and also hexamethyl-cyclotrisiloxane, octamethylcyclotetrasiloxane, decamethylcyclopentasiloxane, dodecamethylcyclo-hexasiloxane
- the organic oils may be liquid paraffin, isoparaffin, hexyl laurate, isopropyl myristate, myristyl myristate, cetyl myristate, 2-octyldodecyl myristate; isopropyl palmitate, 2-ethylhexyl palmitate, butyl stearate, decyl oleate, 2-octyldodecyl oleate, myristyl lactate, cetyl lactate, lanolin acetate, stearyl alcohol, cetostearyl alcohol, oleyl alcohol, avocado oil, almond oil, olive oil, cocoa oil, jojoba oil, gum oil, sunflower oil, soybean oil, camellia oil, squalane, castor oil, mink oil, cottonseed oil, coconut oil, egg yolk oil, beef tallow, lard, polypropylene glycol monooleate, neopentyl glycol 2-ethyl
- the alcohol may be any type that is suitable for use in combination with a cosmetic product starting material.
- it may be methanol, ethanol, butanol, isopropanol or similar lower alcohols.
- a solution or a dispersion of the alcohol should have a viscosity within the range from 10 to 10 9 mPa at 25° C. To improve the sensory use properties in a cosmetic product, the viscosity should be within the range from 100 to 5 ⁇ 10 8 mPa ⁇ s.
- the solutions and dispersions may be readily prepared by mixing the vinyl polymer having a carbosiloxane dendrimer structure with a silicone oil, an organic oil, an alcohol or water.
- the liquids may be present in the step of polymerization of the polymer of vinyl type having a carbosiloxane dendrimer structure.
- the unreacted residual vinyl monomer should be completely removed by heat treatment of the solution or dispersion under atmospheric pressure or reduced pressure.
- the dispersity of the polymer of vinyl type may be improved by adding a surfactant.
- Such an agent may be hexyl-benzenesulfonic acid, octylbenzenesulfonic acid, decylbenzenesulfonic acid, dodecylbenzenesulfonic acid, cetylbenzenesulfonic acid, myristylbenzenesulfonic acid or anionic surfactants of the sodium salts of these acids; octyltrimethylammonium hydroxide, dodecyltrimethylammonium hydroxide, hexadecyltrimethyl-ammonium hydroxide, octyldimethylbenzylammonium hydroxide, decyldimethylbenzylammonium hydroxide, dioctadecyldimethylammonium hydroxide, beef tallow-trimethylammonium hydroxide, coconut oil-trimethylammonium hydroxide, or a similar cationic surfactant; a polyoxyalkylene alkyl ether,
- the solvents and dispersions may be combined with iron oxide suitable for use with cosmetic products, or a similar pigment, and also zinc oxide, titanium oxide, silicon oxide, mica, talc or similar mineral oxides in powder form.
- a mean particle diameter of the polymer of vinyl type may be within a range of between 0.001 and 100 microns and preferably between 0.01 and 50 microns. The reason for this is that, outside the recommended range, a cosmetic product mixed with the emulsion will not have a nice enough feel on the skin or to the touch, or sufficient spreading properties or a pleasant feel.
- the vinyl polymer contained in the dispersion or the solution may have a concentration in the range between 0.1% and 95% by weight and preferably between 5% and 85% by weight. However, to facilitate the handling and the preparation of the mixture, the range should preferably be between 10% and 75% by weight.
- the vinyl polymer may be one of the polymers described in the examples of patent application EP 0 963 751 or, for example, the product TIB-4-200 sold by Dow Corning.
- the vinyl polymer also comprises at least one organofluorine group.
- polymerized vinyl units constitute the backbone and carbosiloxane dendritic structures and also organofluorine groups are attached to side chains are particularly preferred.
- the organofluorine groups may be obtained by replacing with fluorine atoms all or some of the hydrogen atoms of methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tert-butyl, pentyl, neopentyl, hexyl, cyclohexyl, heptyl, octyl, nonyl, decyl, undecyl, dodecyl, tridecyl, tetradecyl, hexadecyl and octadecyl groups and other alkyl groups of 1 to 20 carbon atoms, and also alkyloxyalkylene groups of 6 to 22 carbon atoms.
- the groups represented by the formula: —(CH 2 ) x —(CF 2 ) y —R 13 are suggested as examples of fluoroalkyl groups obtained by substituting fluorine atoms for hydrogen atoms of alkyl groups.
- the index “x” is 0, 1, 2 or 3 and “y” is an integer from 1 to 20.
- R 13 is an atom or a group chosen from a hydrogen atom, a fluorine atom, —CH(CF 3 ) 2 — and CF(CF 3 ) 2 .
- fluorine-substituted alkyl groups are exemplified by linear or branched polyfluoroalkyl or perfluoroalkyl groups represented by the formulae presented below.
- the groups represented by —CH 2 CH 2 — (CF 2 ) m —CFR 14 —[OCF 2 CF (CF 3 )] n —OC 3 F 7 are suggested as fluoroalkyloxyfluoroalkylene groups obtained by substituting fluorine atoms for hydrogen atoms of alkyloxyalkylene groups.
- the index “m” is 0 or 1
- “n” is 0, 1, 2, 3, 4 or 5
- R 14 is a fluorine atom CF 3 .
- fluoroalkyloxyfluoroalkylene groups are exemplified by the perfluoroalkyloxy-fluoroalkylene groups represented by the formulae presented below:
- the number-average molecular weight of the vinyl polymer used in the present invention may be between 3000 and 2 000 000 and more preferably between 5000 and 800 000.
- This type of fluorinated vinyl polymer may be obtained by addition
- Y is a radical-polymerizable organic group and R 1 and X i are as above, and by subjecting them to a copolymerization.
- the vinyl monomers (A) containing organofluorine groups in the molecule are preferably monomers represented by the general formula: —(CH 2 ) ⁇ CR 15 COOR f .
- R 15 is a hydrogen atom or a methyl group
- R f is an organofluorine group exemplified by the fluoroalkyl and fluoroalkyloxyfluoroalkylene groups described above.
- the compounds represented by the formulae presented below are suggested as specific examples of the component (A). In the formulae presented below “z” is an integer from 1 to 4.
- vinyl polymers represented by the formulae presented below are preferable:
- the vinyl polymers represented by the formulae presented below are particularly preferable.
- the vinyl monomers (B) not containing any organofluorine groups in the molecule may be any monomer containing radical-polymerizable vinyl groups illustrated, for example, by methyl acrylate, methyl methacrylate, ethyl acrylate, ethyl methacrylate, n-propyl acrylate, n-propyl methacrylate, isopropyl acrylate, isopropyl methacrylate, and other lower alkyl acrylates or methacrylates; glycidyl acrylate, glycidyl methacrylate; n-butyl acrylate, n-butyl methacrylate, isobutyl acrylate, isobutyl methacrylate, tert-butyl acrylate, tert-butyl methacrylate, n-hexyl acrylate, n-hexyl acrylate, n-hexyl acrylate, n-hexyl
- vinyl monomers (B) the polyfunctional vinyl monomers illustrated, for example, by trimethylolpropane triacrylate, trimethylolpropane trimethacrylate, pentaerythrityl triacrylate, pentaerythrityl trimethacrylate, ethylene glycol diacrylate, ethylene glycol dimethacrylate, tetraethylene glycol diacrylate, tetraethylene glycol dimethacrylate, polyethylene glycol diacrylate, polyethylene glycol dimethacrylate, 1,4-butanediol diacrylate, 1,4-butanediol dimethacrylate, 1,6-hexanediol diacrylate, 1,6-hexane-diol dimethacrylate, neopentyl glycol diacrylate, neopentyl glycol dimethacrylate, trimethylolpropane-trioxyethyl acrylate, trimethylolpropanetrimethacrylate, trimethylol
- the weight ratio of compound (A) to compound (B) should be within the range from 0.1:99.9 to 100:0 and preferably within the range 1:99 to 100:0.
- the carbosiloxane dendrimer (C) is represented by the general formula (III) indicated above.
- Y is a radical-polymerizable organic group, the type of which is not subject to any special limitations provided that it is an organic group capable of undergoing a radical addition reaction.
- Organic groups containing acryl and methacryl, organic groups containing alkenylaryl, or alkenyl groups of 2 to 10 carbon atoms represented by the general formulae presented below are suggested as specific examples.
- R 4 and R 6 are hydrogen atoms or methyl groups
- R 5 and R 8 are alkylene groups of 1 to 10 carbon atoms
- R 7 is an alkyl group of 1 to 10 carbon atoms.
- the index “b” is an integer from 0 to 4 and “c” is 0 or 1.
- the carbosiloxane dendrimers of this component with a generation number of 3 are represented by the general formula:
- the component (C) is illustrated by carbosiloxane dendrimers represented by formulae of mean composition represented below.
- the carbosiloxane dendrimers of the component (C) may be prepared using the process for preparing siloxane/silylalkylene branched copolymers described in document EP 1 055 674.
- they may be prepared by subjecting organic alkenyl silicones and silicone compounds comprising hydrogen atoms linked to silicon, represented by the general formula:
- the copolymerization ratio of the component (C), in terms of its weight ratio relative to the total weight of compounds (A) and (B) should be within the range from 0.1:99.9 to 99.9:0.1, preferably within the range from 1:99 to 99:1 and even more preferably within the range from 5:95 to 95:5.
- Amino groups may be introduced into the side chains of the vinyl polymer using, included in the component (B), vinyl monomers containing amino groups, such as dimethylaminoethyl acrylate, dimethylaminoethyl methacrylate, diethylaminoethyl acrylate and diethyl-aminoethyl methacrylate, followed by performing a modification with potassium acetate monochloride, ammonium acetate monochloride, the aminomethylpropanol salt of monochloroacetic acid, the triethanolamine salt of monobromoacetic acid, sodium monochloropropionate, and other alkali metal salts of halogenated fatty acids; otherwise, carboxylic acid groups may be introduced into the side chains of the vinyl polymer using, included in the component (B), vinyl monomers containing carboxylic acids, such as acrylic acid, methacrylic acid, itaconic acid, crotonic acid, fumaric acid and maleic acid, and the like, followed by neutral
- the fluorinated vinyl polymer may be one of the polymers described in the examples of patent application WO 03/045337 or, for example, the product TIB-4-100 sold by Dow Corning.
- the vinyl polymer may be present in a content ranging from 0.1% to 70% by weight, relative to the total weight of the composition, preferably ranging from 0.5% to 50% by weight, preferentially ranging from 1% to 40% by weight and more preferably ranging from 5% to 15% by weight.
- the vinyl polymer may be present in the composition in a proportion of at least 3% by weight in the composition, preferably between 5% and 25% by weight, more preferably between 5% and 15% by weight and especially about 10% by weight.
- the film-forming polymer present in the composition according to the invention is a dispersion of homopolymer particles or of acrylic or vinyl radical copolymers dispersed in the liquid fatty phase of the composition.
- the polymer in the form of particles dispersed in the volatile liquid fatty phase is a solid that is insoluble in the liquid fatty phase of the composition even at its softening point, unlike a wax even of polymeric origin, which is itself soluble in the liquid organic phase (or fatty phase) at its melting point.
- composition according to the invention advantageously comprises at least one stable dispersion of generally spherical polymer particles of one or more polymers, in a volatile liquid fatty phase.
- These dispersions may especially be in the form of polymer nanoparticles in stable dispersion in the said liquid organic phase.
- the nanoparticles preferably have a mean size of between 5 and 800 nm and better still between and 500 nm. However, it is possible to obtain polymer particles ranging up to 1 ⁇ m in size.
- the polymer particles in dispersion are insoluble in water-soluble alcohols, for instance ethanol.
- the polymers in dispersion that may be used in the composition of the invention preferably have a molecular weight of about from 2000 to 10 000 000 g/mol and a Tg of from ⁇ 100° C. to 300° C., better still from ⁇ 50° C. to 100° C. and preferably from ⁇ 10° C. to 50° C.
- film-forming polymers preferably having a low Tg, of less than or equal to skin temperature and especially less than or equal to 40° C.
- the polymer used is film-forming, i.e. it is capable of forming an isolable film, by itself or in combination with a plasticizer. It is, however, possible to use a non-film-forming polymer.
- non-film-forming polymer means a polymer that is incapable of forming an isolable film by itself. This polymer can, in combination with a non-volatile compound of the oil type, form a continuous, uniform deposit on the skin and/or the lips.
- film-forming polymers that may be mentioned are acrylic or vinyl free-radical homopolymers or copolymers, preferably with a Tg of less than or equal to 40° C. and especially ranging from ⁇ 10° C. to 30° C., used alone or as a mixture.
- non-film-forming polymers that may be mentioned are optionally crosslinked vinyl or acrylic free-radical homopolymers or copolymers preferably with a Tg of greater than 40° C. and especially ranging from 45° C. to 150° C., used alone or as a mixture.
- free-radical polymer means a polymer obtained by polymerization of unsaturated and especially ethylenic monomers, each monomer being capable of homopolymerizing (unlike polycondensates).
- the free-radical polymers may especially be vinyl polymers or copolymers, especially acrylic polymers.
- the acrylic polymers may result from the polymerization of ethylenically unsaturated monomers containing at least one acid group and/or esters of these acid monomers and/or amides of these acids.
- Monomers bearing an acid group that may be used include ⁇ , ⁇ -ethylenic unsaturated carboxylic acids such as acrylic acid, (meth)acrylic acid, crotonic acid, maleic acid or itaconic acid.
- (Meth)acrylic acid and crotonic acid are preferably used, and more preferably (meth)acrylic acid.
- the acid monomer esters are advantageously chosen from (meth)acrylic acid esters (also known as (meth)acrylates), for instance alkyl (meth)acrylates, in particular of a C 1 -C 20 and preferably C 1 -C 8 alkyl, aryl (meth)acrylates, in particular of a C 6 -C 10 aryl, and hydroxyalkyl (meth)acrylates, in particular of a C 2 -C 6 hydroxyalkyl.
- Alkyl (meth)acrylates that may be mentioned include methyl, ethyl, butyl, isobutyl, 2-ethylhexyl and lauryl (meth)acrylate.
- Hydroxyalkyl (meth)acrylates that may be mentioned include hydroxyethyl (meth)acrylate and 2-hydroxypropyl (meth)acrylate.
- Aryl (meth)acrylates that may be mentioned include benzyl or phenyl acrylate.
- the (meth)acrylic acid esters that are particularly preferred are the alkyl (meth)acrylates.
- Free-radical polymers that are preferably used include copolymers of (meth)acrylic acid and of alkyl (meth)acrylate, especially of a C 1 -C 4 alkyl.
- Methyl acrylates optionally copolymerized with acrylic acid may more preferentially be used.
- Amides of the acid monomers that may be mentioned include (meth)acrylamides, especially N-alkyl(meth)-acrylamides, in particular of a C 2 -C 12 alkyl, such as N-ethylacrylamide, N-t-butylacrylamide and N-octylacrylamide; N-di(C 1 -C 4 )alkyl(meth)acrylamides.
- the acrylic polymers may also result from the polymerization of ethylenically unsaturated monomers containing at least one amine group, in free form or in partially or totally neutralized form, or alternatively in partially or totally quaternized form.
- Such monomers may be, for example, dimethylaminoethyl (meth)acrylate, dimethylaminoethyl(meth)acrylamide, vinylamine, vinylpyridine or diallyldimethylammonium chloride.
- the vinyl polymers may also result from the homopolymerization or copolymerization of at least one monomer chosen from vinyl esters and styrene monomers.
- these monomers may be polymerized with acid monomers and/or esters thereof and/or amides thereof, such as those mentioned previously.
- vinyl esters that may be mentioned include vinyl acetate, vinyl propionate, vinyl neodecanoate, vinyl pivalate, vinyl benzoate and vinyl t-butylbenzoate.
- Styrene monomers that may be mentioned include styrene and ⁇ -methylstyrene.
- the vinyl polymer may be crosslinked with one or more difunctional monomers especially comprising at least two ethylenic unsaturations, such as ethylene glycol di(meth)acrylate or diallyl phthalate.
- the polymer(s) in dispersion in the organic liquid phase may represent, as solids, from 5% to 40%, preferably from 5% to 35% and better still from 8% to 30% of the weight of the composition.
- the composition contains a stabilizer that is solid at room temperature.
- the polymer particles are preferably surface-stabilized by means of a stabilizer that may be a block polymer, a grafted polymer and/or a random polymer, alone or as a mixture.
- the stabilization may take place by any known means, and in particular by direct addition of the block polymer, grafted polymer and/or random polymer during the polymerization.
- the stabilizer is preferably also present in the mixture before polymerization. However, it is also possible to add it continuously, especially when the monomers are also added continuously.
- grafted polymers that may be mentioned are silicone polymers grafted with a hydrocarbon-based chain; hydrocarbon-based polymers grafted with a silicone chain.
- grafted-block or block copolymers comprising at least one block of polyorganosiloxane type and at least one block of a free-radical polymer, for instance grafted copolymers of acrylic/silicone type, may thus be used, which may be used especially when the non-aqueous medium is silicone-based.
- grafted-block or block copolymers comprising at least one block of polyorganosiloxane type and at least one block of a polyether.
- the polyorganosiloxane block may especially be a polydimethylsiloxane or a poly(C 2 -C 18 )alkylmethyl-siloxane;
- the polyether block may be a poly(C 2 -C 10 -alkylene, in particular polyoxyethylene and/or polyoxy-propylene.
- dimethicone copolyols or (C 2 -C 18 )alkyldimethicone copolyols such as those sold under the name Dow Corning 3225C by the company Dow Corning, and lauryl methicones such as those sold under the name Dow Corning Q2-5200 by the company Dow Corning, may be used.
- Grafted-block or block copolymers that may also be mentioned include those comprising at least one block resulting from the polymerization of at least one ethylenic monomer containing one or more optionally conjugated ethylenic bonds, for instance ethylene or dienes such as butadiene and isoprene, and of at least one block of a vinyl polymer and better still a styrene polymer.
- the ethylenic monomer comprises several optionally conjugated ethylenic bonds
- the residual ethylenic unsaturations after the polymerization are generally hydrogenated.
- polystyrene/polyisoprene SI
- polystyrene/polybutadiene SB
- Luvitol HSB Luvitol HSB
- SEP polystyrene/copoly(ethylene-propylene)
- polystyrene/copoly(ethylene-butylene) SEB
- Kraton G1650 SEBS
- Kraton G1651 SEBS
- Kraton G1652 SEBS
- Kraton G1657X SEBS
- Kraton G1701X SEP
- Kraton G1702X SEP
- Kraton G1726X SEW
- Kraton D-1101 SBS
- Kraton D-1102 SBS
- SIS Kraton D-1107
- the polymers are generally known as hydrogenated or non-hydrogenated diene copolymers.
- Gelled Permethyl 99A-750, 99A-753-59 and 99A-753-(mixture of triblock and of star polymer), Versagel 5960 from Penreco (triblock+star polymer); OS129880, OS129881 and OS84383 from Lubrizol (styrene/(meth)acrylate copolymer) may also be used.
- grafted-block or block copolymers comprising at least one block resulting from the polymerization of at least one ethylenic monomer containing one or more ethylenic bonds and of at least one block of an acrylic polymer
- grafted-block or block copolymers comprising at least one block resulting from the polymerization of at least one ethylenic monomer containing one or more ethylenic bonds and of at least one block of a polyether such as a C 2 -C 18 polyalkylene (especially polyethylene and/or polyoxypropylene), mention may be made of polyoxyethylene/polybutadiene or polyoxyethylene/polyisobutylene diblock or triblock copolymers.
- Copolymers based on alkyl acrylates or (meth)acrylates derived from C 1 -C 4 alcohols and on alkyl acrylates or (meth)acrylates derived from C 8 -C 30 alcohols may thus be used. Mention may be made in particular of stearyl (meth)acrylate/methyl (meth)acrylate copolymer.
- the stabilizer is preferably chosen from the group consisting of grafted-block or block copolymers comprising at least one block of polyorganosiloxane type and at least one block of a free-radical polymer or of a polyether or of a polyester, for instance polyoxypropylene and/or polyoxyethylene blocks.
- the stabilizer is preferably chosen from the group formed by:
- Diblock polymers are preferably used as stabilizer.
- a plasticizer may be combined therewith.
- the plasticizer may be chosen from the plasticizers usually used in the field of application and especially from compounds liable to be solvents for the polymer. Coalescers may also be used in order to aid the polymer to form a continuous and homogeneous deposit.
- coalescers or plasticizers that may be used in the invention are especially those mentioned in document FR-A-2 782 917.
- the composition may contain a polymer plasticizer, so as to lower the Tg of the polymer film and to improve the adhesion of the polymer film to its support, in particular to keratin materials.
- the plasticizer especially lowers the glass transition temperature of the polymer by at least 2, 3 or 4° C. and preferably from 5° C. to 20° C. In one preferred embodiment, the plasticizer especially lowers the glass transition temperature of the polymer by at least 2, 3 or 4° C. and preferably from 5° C. to 20° C., when the plasticizer represents not more than 10% by weight of the polymer.
- the compound may be chosen from esters of at least one carboxylic acid comprising 1 to 7 carbon atoms and of a polyol comprising at least four hydroxyl groups.
- the polyol according to the invention may be a saccharide or a saccharide-based polyol, such as erythritol, xylitol or sorbitol.
- the polymer may be a monosaccharide or a polysaccharide comprising 1 to 10 saccharides, preferably from 1 to 4 and more preferably or 2 saccharides.
- the polyol may be chosen from erythritol, xylitol, sorbitol, glucose and sucrose.
- the polyol according to the invention is preferably a disaccharide.
- disaccharides that may be mentioned are sucrose ( ⁇ -D-glucopyranosyl-(1-2)- ⁇ -D-fructofuranose), lactose ( ⁇ -D-galactopyranosyl-(1-4)- ⁇ -D-glucopyranose) and maltose ( ⁇ -D-glucopyranosyl-(1-4)- ⁇ -D-glucopyranose).
- the plasticizer may be formed from a polyol substituted with at least two different monocarboxylic acids, or with at least three different monocarboxylic acids.
- the acid is preferably a monocarboxylic acid chosen in particular from acids comprising 1 to 7 carbon atoms and preferably 1 to 5 carbon atoms, for example acetic acid, n-propanoic acid, isopropanoic acid, n-butanoic acid, isobutanoic acid, tert-butanoic acid, n-pentanoic acid and benzoic acid.
- the ester is sucrose diacetate hexakis(2-methylpropanoate).
- the polymer dispersion may be manufactured as described in document EP-A-749 747.
- a mixture comprising the initial monomers and also a free-radical initiator is prepared. This mixture is dissolved in a solvent referred to hereinbelow in the present description as the “synthesis solvent”.
- the fatty phase is a non-volatile oil
- the polymerization may be performed in an apolar organic solvent (synthesis solvent), followed by adding the non-volatile oil (which should be miscible with the said synthesis solvent) and selectively distilling off the synthesis solvent.
- a synthesis solvent which is such that the initial monomers and the free-radical initiator are soluble therein, and the polymer particles obtained are insoluble therein, so that they precipitate during their formation, is chosen.
- the synthesis solvent may be chosen from alkanes such as heptane, isododecane and cyclohexane.
- the polymerization may be performed directly in the said oil, which thus also acts as synthesis solvent.
- the monomers should also be soluble therein, as should the free-radical initiator, and the polymer obtained should be insoluble therein.
- the monomers are preferably present in the synthesis solvent, before polymerization, in a proportion of 5-201 by weight of the reaction mixture.
- the total amount of monomers may be present in the solvent before the start of the reaction, or part of the monomers may be added gradually as the polymerization reaction proceeds.
- the free-radical initiator may especially be azobisisobutyronitrile or tert-butylperoxy-2-ethyl hexanoate.
- the volatile phase of the composition may be formed from or comprise the synthesis solvent for the dispersed polymer particles.
- compositions according to the invention may contain, besides the film-forming polymers described previously, an additional film-forming or non-film-forming polymer.
- the composition may comprise an aqueous phase and the additional polymer may be present in this aqueous phase.
- the polymer will preferably be a polymer in dispersion or an amphiphilic or associative polymer.
- polymer in dispersion means the water-insoluble polymers present in the form of particles of variable size.
- the polymer may or may not be crosslinked.
- the size of the polymer particles is typically between 25 and 500 nanometres and preferably between 50 and 200 nanometres.
- the following polymers in aqueous dispersion may be used: Ultrasol 2075 from Ganz Chemical, Daitosol 5000 AD from Daito Kasei, Avalure UR 450 from Noveon, DynamX from National Starch, Syntran 5760 from Interpolymer, Acusol OP 301 and from Rohm & Haas, and Neocryl A 1090 from Avecia.
- Neocryl XK-90® The acrylic dispersions sold under the names Neocryl XK-90®, Neocryl A-1070®, Neocryl A-1090®, Neocryl BT-62®, Neocryl A-1079® and Neocryl A-523® by the company Avecia-Neoresins, Dow Latex 432® by the company Dow Chemical, Daitosol 5000 AD® or Daitosol 5000 SJ® by the company Daito Kasey Kogyo; Syntran 5760® by the company Interpolymer, Soltex OPT by the company Rohm & Haas, aqueous dispersions of acrylic or styrene/acrylic polymers sold under the brand name Joncryl® by the company Johnson Polymer, or the aqueous dispersions of polyurethane sold under the names Neorez R-981® and Neorez R-974® by the company Avecia-Neoresins, Avalure UR-405®, Avalure UR-410®, Avalure
- amphiphilic or associative polymers means polymers comprising one or more hydrophilic parts that make them partially water-soluble and one or more hydrophobic parts via which the polymers associate or interact.
- the following associative polymers may be used: Nuvis FX 1100 from Elementis, Aculyn 22, Aculyn 44 and Aculyn 46 from Rohm & Haas, Viscophobe DB 1000 from Amerchol.
- Diblock copolymers formed from a hydrophilic block (polyacrylate or polyethylene glycol) and from a hydrophobic block (polystyrene or polysiloxane) may also be used.
- Polymers that are soluble in an aqueous phase containing monodisperse particles may be avoided, since they may cause aggregation of the monodisperse particles.
- the film-forming polymer may thus be insoluble in such an aqueous phase.
- the composition may comprise an oily phase and the film-forming polymer may be present in this oily phase.
- the polymer may then be in dispersion or in solution.
- Microgels for example KSG
- polymers of the type PS-PA or styrene-based copolymers Kraton, Regalite.
- lipodispersible non-aqueous film-forming polymer dispersions in the form of non-aqueous dispersions of polymer particles in one or more silicone and/or hydrocarbon-based oils, which may be surface-stabilized with at least one stabilizer, especially a block, grafted or random polymer
- free-radical film-forming polymer means a polymer obtained by polymerization of unsaturated and especially ethylenically unsaturated monomers, each monomer being capable of homopolymerizing (unlike polycondensates).
- the film-forming polymers of free-radical type may be, in particular, vinyl polymers or copolymers, in particular acrylic polymers.
- the vinyl film-forming polymers may result from the polymerization of ethylenically unsaturated monomers containing at least one acidic group and/or esters of these acidic monomers and/or amides of these acidic monomers.
- Monomers bearing an acidic group which may be used are ⁇ , ⁇ -ethylenic unsaturated carboxylic acids such as acrylic acid, methacrylic acid, crotonic acid, maleic acid or itaconic acid.
- (Meth)acrylic acid and crotonic acid are preferably used, and more preferably (meth)acrylic acid.
- esters of acidic monomers are advantageously chosen from (meth)acrylic acid esters (also known as (meth)acrylates), especially (meth)acrylates of an alkyl, in particular of a C 1 -C 30 and preferably C 1 -C 20 alkyl, (meth)acrylates of an aryl, in particular of a C 6 -C 10 aryl, and (meth)acrylates of a hydroxyalkyl, in particular of a C 2 -C 6 hydroxyalkyl.
- (meth)acrylic acid esters also known as (meth)acrylates
- alkyl in particular of a C 1 -C 30 and preferably C 1 -C 20 alkyl
- aryl in particular of a C 6 -C 10 aryl
- a hydroxyalkyl in particular of a C 2 -C 6 hydroxyalkyl.
- alkyl (meth)acrylates that may be mentioned are methyl methacrylate, ethyl methacrylate, butyl methacrylate, isobutyl methacrylate, 2-ethylhexyl methacrylate, lauryl methacrylate and cyclohexyl methacrylate.
- hydroxyalkyl (meth)acrylates that may be mentioned are hydroxyethyl acrylate, 2-hydroxypropyl acrylate, hydroxyethyl methacrylate and 2-hydroxypropyl methacrylate.
- aryl (meth)acrylates that may be mentioned are benzyl acrylate and phenyl acrylate.
- the (meth)acrylic acid esters that are particularly preferred are the alkyl (meth)acrylates.
- the alkyl group of the esters may be either fluorinated or perfluorinated, i.e. some or all of the hydrogen atoms of the alkyl group are substituted with fluorine atoms.
- amides of the acid monomers are (meth)acrylamides, and especially N-alkyl(meth)acrylamides, in particular of a C 2 -C 12 alkyl.
- N-alkyl(meth)acrylamides that may be mentioned are N-ethylacrylamide, N-t-butylacrylamide, N-t-octylacrylamide and N-undecylacrylamide.
- the vinyl film-forming polymers may also result from the homopolymerization or copolymerization of monomers chosen from vinyl esters and styrene monomers.
- these monomers may be polymerized with acid monomers and/or esters thereof and/or amides thereof, such as those mentioned above.
- vinyl esters examples include vinyl acetate, vinyl neodecanoate, vinyl pivalate, vinyl benzoate and vinyl t-butylbenzoate.
- Styrene monomers that may be mentioned are styrene and ⁇ -methylstyrene.
- film-forming polycondensates that may be mentioned are polyurethanes, polyesters, polyester-amides, polyamides, epoxyester resins and polyureas.
- the polyurethanes may be chosen from anionic, cationic, nonionic and amphoteric polyurethanes, polyurethane-acrylics, polyurethane-polyvinyl-pyrrolidones, polyester-polyurethanes, polyether-polyurethanes, polyureas and polyurea-polyurethanes, and mixtures thereof.
- the polyesters may be obtained, in a known manner, by polycondensation of dicarboxylic acids with polyols, in particular diols.
- the dicarboxylic acid may be aliphatic, alicyclic or aromatic.
- examples of such acids that may be mentioned are: oxalic acid, malonic acid, dimethylmalonic acid, succinic acid, glutaric acid, adipic acid, pimelic acid, 2,2-dimethylglutaric acid, azelaic acid, suberic acid, sebacic acid, fumaric acid, maleic acid, itaconic acid, phthalic acid, dodecanedioic acid, 1,3-cyclohexanedicarboxylic acid, 1,4-cyclohexanedicarboxylic acid, isophthalic acid, terephthalic acid, 2,5-norbornanedicarboxylic acid, diglycolic acid, thiodipropionic acid, 2,5-naphthalene-dicarboxylic acid or 2,6-naphthalenedicarboxylic acid.
- These dicarboxylic acid monomers may be used alone or as a combination
- the diol may be chosen from aliphatic, alicyclic and aromatic diols.
- the diol used is preferably chosen from: ethylene glycol, diethylene glycol, triethylene glycol, 1,3-propanediol, cyclohexanedimethanol and 4-butanediol.
- Other polyols that may be used are glycerol, pentaerythritol, sorbitol and trimethylol-propane.
- the polyesteramides may be obtained in a manner analogous to that of the polyesters, by polycondensation of diacids with diamines or amino alcohols.
- Diamines that may be used are ethylenediamine, hexamethylenediamine and meta- or para-phenylenediamine.
- An amino alcohol that may be used is monoethanolamine.
- the polyester may also comprise at least one monomer bearing at least one group —SO 3 M, with M representing a hydrogen atom, an ammonium ion NH 4 + or a metal ion such as, for example, an Na + , Li + , K + , Mg 2+ , Ca 2+ , Cu 2+ , Fe 2+ or Fe 3+ ion.
- M representing a hydrogen atom, an ammonium ion NH 4 + or a metal ion such as, for example, an Na + , Li + , K + , Mg 2+ , Ca 2+ , Cu 2+ , Fe 2+ or Fe 3+ ion.
- a difunctional aromatic monomer comprising such a group —SO 3 M may be used in particular.
- the aromatic nucleus of the difunctional aromatic monomer also bearing a group —SO 3 M as described above may be chosen, for example, from benzene, naphthalene, anthracene, biphenyl, oxybiphenyl, sulfonylbiphenyl and methylenebiphenyl nuclei.
- difunctional aromatic monomers also bearing a group —SO 3 M mention may be made of: sulfoisophthalic acid, sulfoterephthalic acid, sulfophthalic acid, 4-sulfonaphthalene-2,7-dicarboxylic acid.
- the film-forming polymer may be a polymer dissolved in a liquid fatty phase comprising organic solvents or oils (the film-forming polymer is thus said to be a liposoluble polymer).
- the liquid fatty phase preferably comprises a volatile oil, optionally mixed with a non-volatile oil.
- liposoluble polymers examples include copolymers of vinyl ester (the vinyl group being directly linked to the oxygen atom of the ester group and the vinyl ester containing a saturated, linear or branched hydrocarbon-based radical of 1 to 19 carbon atoms, linked to the carbonyl of the ester group) and of at least one other monomer which may be a vinyl ester (other than the vinyl ester already present), an ⁇ -olefin (containing from 8 to 28 carbon atoms), an alkyl vinyl ether (in which the alkyl group comprises from 2 to 18 carbon atoms) or an allylic or methallylic ester (containing a saturated, linear or branched hydrocarbon-based radical of 1 to 19 carbon atoms, linked to the carbonyl of the ester group).
- vinyl ester the vinyl group being directly linked to the oxygen atom of the ester group and the vinyl ester containing a saturated, linear or branched hydrocarbon-based radical of 1 to 19 carbon atoms, linked to the carbonyl of the ester
- copolymers may be crosslinked with the aid of crosslinking agents, which may be either of the vinyl type or of the allylic or methallylic type, such as tetraallyloxyethane, divinylbenzene, divinyl octane-dioate, divinyl dodecanedioate and divinyl octadecane-dioate.
- crosslinking agents may be either of the vinyl type or of the allylic or methallylic type, such as tetraallyloxyethane, divinylbenzene, divinyl octane-dioate, divinyl dodecanedioate and divinyl octadecane-dioate.
- copolymers examples include the following copolymers: vinyl acetate/allyl stearate, vinyl acetate/vinyl laurate, vinyl acetate/vinyl stearate, vinyl acetate/octadecene, vinyl acetate/octadecyl vinyl ether, vinyl propionate/allyl laurate, vinyl propionate/vinyl laurate, vinyl stearate/1-octadecene, vinyl acetate/1-dodecene, vinyl stearate/ethyl vinyl ether, vinyl propionate/cetyl vinyl ether, vinyl stearate/allyl acetate, vinyl 2,2-dimethyloctanoate/vinyl laurate, allyl 2,2-dimethylpentanoate/vinyl laurate, vinyl dimethylpropionate/vinyl stearate, allyl dimethylpropionate/vinyl stearate, vinyl propionate/
- liposoluble film-forming polymers examples include copolymers of a vinyl ester and of at least one other monomer that may be a vinyl ester, especially vinyl neodecanoate, vinyl benzoate and vinyl t-butylbenzoate, an ⁇ -olefin, an alkyl vinyl ether or an allylic or methallylic ester.
- liposoluble film-forming polymers examples include liposoluble copolymers, and in particular those resulting from the copolymerization of vinyl esters containing from 9 to 22 carbon atoms or of alkyl acrylates or methacrylates, and alkyl radicals containing from 10 to 20 carbon atoms.
- Such liposoluble copolymers may be chosen from copolymers of polyvinyl stearate, polyvinyl stearate crosslinked with the aid of divinylbenzene, of diallyl ether or of diallyl phthalate, polystearyl (meth)acrylate, polyvinyl laurate and polylauryl (meth)acrylate, it being possible for these poly(meth)acrylates to be crosslinked with the aid of ethylene glycol dimethacrylate or tetraethylene glycol dimethacrylate.
- the liposoluble copolymers defined above are known and are described in particular in patent application FR-A-2 232 303; they may have a weight-average molecular weight ranging from 2000 to 500 000 and preferably from 4000 to 200 000.
- liposoluble film-forming polymers that may be used in the invention, mention may also be made of polyalkylenes and in particular copolymers of C 2 -C 20 alkenes, such as polybutene, alkylcelluloses with a linear or branched, saturated or unsaturated C 1 -C 8 alkyl radical, for instance ethylcellulose and propylcellulose, copolymers of vinylpyrrolidone (VP) and in particular copolymers of vinylpyrrolidone and of C 2 to C 40 and better still C 3 to C 20 alkene.
- polyalkylenes and in particular copolymers of C 2 -C 20 alkenes such as polybutene, alkylcelluloses with a linear or branched, saturated or unsaturated C 1 -C 8 alkyl radical, for instance ethylcellulose and propylcellulose
- VP vinylpyrrolidone
- V vinylpyrrolidone
- VP copolymers which may be used in the invention, mention may be made of the copolymers of VP/vinyl acetate, VP/ethyl methacrylate, butylated polyvinyl-pyrrolidone (PVP), VP/ethyl methacrylate/methacrylic acid, VP/eicosene, VP/hexadecene, VP/triacontene, VP/styrene or VP/acrylic acid/lauryl methacrylate.
- PVP polyvinyl-pyrrolidone
- silicone resins which are generally soluble or swellable in silicone oils, which are crosslinked polyorganosiloxane polymers.
- the nomenclature of silicone resins is known under the name “MDTQ”, the resin being described as a function of the various siloxane monomer units it comprises, each of the letters “MDTQ” characterizing a type of unit.
- polymethyl-silsesquioxane resins examples include those sold by the company Wacker under the reference Resin MK, such as Belsil PMS MK, or by the company Shin-Etsu under the reference KR-220L.
- polypropyl-silsesquioxane resins examples include those sold under the reference DC670 by the company Dow Corning.
- Siloxysilicate resins that may be mentioned include trimethyl siloxysilicate (TMS) resins such as those sold under the reference SR 1000 by the company General Electric or under the reference TMS 803 by the company Wacker. Mention may also be made of the trimethyl siloxysilicate resins sold in a solvent such as cyclomethicone, sold under the name KF-7312J by the company Shin-Etsu, and DC 749 and DC 593 by the company Dow Corning.
- TMS trimethyl siloxysilicate
- a resin according to the invention with a trimethyl siloxysilicate resin or a polypropylsilsesquioxane resin makes it possible to improve the durability of the transfer resistance.
- silicone resin copolymers such as those mentioned above with polydimethylsiloxanes, for instance the pressure-sensitive adhesive copolymers sold by the company Dow Corning under the reference Bio-PSA and described in document U.S. Pat. No. 5,162,410, or the silicone copolymers derived from the reaction of a silicone resin, such as those described above, and of a diorganosiloxane, as described in document WO 2004/073 626.
- the film-forming polymer may be chosen from block or random polymers and/or copolymers especially comprising polyurethanes, polyacrylics, silicones, fluoro polymers, butyl rubbers, ethylene copolymers, natural gums and polyvinyl alcohols, and mixtures thereof.
- the monomers of the block or random copolymers comprising at least one combination of monomers whose resulting polymer has a glass transition temperature of less than room temperature (25° C.) may be chosen especially from butadiene, ethylene, propylene, acrylic, methacrylic, isoprene, isobutene and a silicone, and mixtures thereof.
- the film-forming polymer may also be present in the first and/or second composition in the form of particles dispersed in an aqueous phase or in a non-aqueous solvent phase, which is generally known as a latex or pseudolatex.
- aqueous phase or in a non-aqueous solvent phase
- latex or pseudolatex The techniques for preparing these dispersions are well known to those skilled in the art.
- composition according to the invention may comprise a plasticizer that promotes the formation of a film with the film-forming polymer.
- a plasticizer may be chosen from any compound known to those skilled in the art as being capable of satisfying the desired function.
- film-forming systems that may be used in the compositions according to the invention, mention may be made of systems in which the film is formed in situ at the time of application of the composition or of a mixture of compositions containing two silicone compounds that react together when they are placed in contact.
- Such systems are described especially in patent application WO 2007/071 706, the content of which is incorporated herein by reference.
- Systems of this type are also described in patent applications US 2007/142 575 and US 2007/142 599, the content of which is also incorporated herein by reference.
- compositions according to the invention may contain an elastomer, especially a polyglycerolated silicone elastomer.
- an elastomeric crosslinked organopolysiloxane that may be obtained by a crosslinking addition reaction of a diorganopolysiloxane containing at least one hydrogen bonded to silicon and of polyglycerolated compounds containing ethylenically unsaturated groups, especially in the presence of a platinum catalyst.
- Polyglycerolated silicone elastomers that may be used include those sold under the names KSG-710, KSG-810, KSG-820, KSG-830 and KSG-840 by the company Shin-Etsu.
- compositions according to the invention may also comprise an additional emulsifying silicone elastomer.
- Polyoxyalkylenated silicone elastomers that may be used include those sold under the names KSG-21, KSG-20, KSG-30, KSG-31, KSG-32, KSG-33, KSG-210, KSG-310, KSG-320, KSG-330, KSG-340 and X-226146 by the company Shin-Etsu, and DC9010 and DC9011 by the company Dow Corning.
- these particular elastomers may make it possible to improve the transfer-resistance and comfort (suppleness) properties of the compositions comprising them.
- compositions according to the invention may also comprise a non-emulsifying elastomer.
- Non-emulsifying elastomers are especially described in patent applications JP-A-61-194 009, EP-A-242 219, EP-A-285 886 and EP-A-765 656, the content of which is incorporated by reference.
- Spherical non-emulsifying elastomers that may be used include those sold under the names DC9040, DC9041, DC9509, DC9505 and DC9506 by the company Dow Corning.
- the spherical non-emulsifying silicone elastomer may also be in the form of an elastomeric crosslinked organopolysiloxane powder coated with silicone resin, especially with silsesquioxane resin, as described, for example, in U.S. Pat. No. 5,538,793, the content of which is incorporated by reference.
- elastomers are sold under the names KSP-100, KSP-101, KSP-102, KSP-103, KSP-104 and KSP-105 by the company Shin-Etsu.
- elastomeric crosslinked organopolysiloxanes in the form of spherical powders may be powders of a hybrid silicone functionalized with fluoroalkyl groups, sold especially under the name KSP-200 by the company Shin-Etsu; powders of a hybrid silicone functionalized with phenyl groups, sold especially under the name KSP-300 by the company Shin-Etsu.
- Silicone elastomers bearing a group MQ such as those sold by the company Wacker under the names Belsil RG100, Belsil RPG33 and, preferentially, RG80, may also be used in the compositions according to the invention. These particular elastomers, when they are in combination with the resins according to the invention, may make it possible to improve the transfer-resistance properties of the compositions comprising them.
- composition according to the invention comprises at least one liquid fatty phase comprising at least one oil.
- the oil may be chosen from hydrocarbon-based oils, silicone oils and fluoro oils.
- the oil may be chosen from volatile oils and non-volatile oils, and mixtures thereof.
- hydrocarbon-based oil means an oil formed essentially from, or even consisting of, carbon and hydrogen atoms, and possibly oxygen and nitrogen atoms, and containing no silicon or fluorine atoms; it may contain ester, ether, amine or amide groups.
- silicon oil means an oil containing at least one silicon atom, and especially containing Si—O groups.
- fluoro oil means an oil containing at least one fluorine atom.
- composition according to the invention may comprise at least one volatile oil.
- volatile oil means an oil (or non-aqueous medium) capable of evaporating on contact with the skin in less than one hour, at room temperature and atmospheric pressure.
- the volatile oil is a volatile cosmetic oil, which is liquid at room temperature, especially having a non-zero vapour pressure, at room temperature and atmospheric pressure, in particular having a vapour pressure ranging from 0.13 Pa to 40 000 Pa (10 ⁇ 3 to 300 mmHg), preferably ranging from 1.3 Pa to 13 000 Pa (0.01 to 100 mmHg) and preferentially ranging from 1.3 Pa to 1300 Pa (0.01 to 10 mmHg).
- the volatile oil generally has a boiling point, measured at atmospheric pressure, ranging from 150° C. to 260° C. and preferably ranging from 170° C. to 250° C.
- composition according to the invention may comprise a volatile hydrocarbon-based oil chosen especially from hydrocarbon-based oils with a flash point ranging from 40° C. to 102° C., preferably ranging from 40° C. to 55° C. and preferentially ranging from 40° C. to 50° C.
- Volatile hydrocarbon-based oils that may be mentioned include volatile hydrocarbon-based oils containing from 8 to 16 carbon atoms and mixtures thereof, and especially branched C 8 -C 16 alkanes, for instance C 8 -C 16 isoalkanes (also known as isoparaffins), isododecane, isodecane, isohexadecane and, for example, the oils sold under the trade name Isopar or Permethyl, branched C 8 -C 16 esters, for instance isohexyl neopentanoate, and mixtures thereof.
- volatile hydrocarbon-based oils containing from 8 to 16 carbon atoms and mixtures thereof, and especially branched C 8 -C 16 alkanes, for instance C 8 -C 16 isoalkanes (also known as isoparaffins), isododecane, isodecane, isohexadecane and, for example, the oils sold under the trade name Isopar or Permethyl
- the volatile hydrocarbon-based oil is chosen from volatile hydrocarbon-based oils containing from 8 to 16 carbon atoms, and mixtures thereof, in particular from isododecane, isodecane and isohexadecane, and is especially isododecane.
- linear hydrocarbon-based volatile oils containing from 8 to 16 carbon atoms will advantageously be used.
- Volatile silicone oils that may be mentioned include linear or cyclic silicones containing from 2 to 7 silicon atoms, these silicones optionally comprising alkyl or alkoxy groups containing from 1 to 10 carbon atoms.
- volatile silicone oils that may be used in the invention, mention may be made especially of octamethylcyclotetrasiloxane, decamethylcyclopentasiloxane, dodecamethylcyclohexasiloxane, heptamethyl-hexyltrisiloxane, heptamethyloctyltrisiloxane, octamethyltrisiloxane and decamethyltetrasiloxane, and mixtures thereof.
- the volatile oil may be present in the composition according to the invention in a content ranging from 0.1% to 90% by weight, preferably ranging from 1% to 70% by weight and preferentially ranging from 5% to 50% by weight, relative to the total weight of the composition.
- composition according to the invention may comprise at least one non-volatile oil.
- Non-volatile hydrocarbon-based oils that may be used include liquid paraffin (or petroleum jelly), squalane, hydrogenated polyisobutylene (Parleam oil), perhydrosqualene, mink oil, turtle oil, soybean oil, sweet almond oil, beauty-leaf oil, palm oil, grapeseed oil, sesame seed oil, corn oil, arara oil, rapeseed oil, sunflower oil, cottonseed oil, apricot oil, castor oil, avocado oil, jojoba oil, olive oil or cereal germ oil; linoleic acid, oleic acid, lauric acid or stearic acid esters; fatty esters, especially of C 12 -C 36 , such as isopropyl myristate, isopropyl palmitate, butyl stearate, hexyl laurate, diisopropyl adipate, isononyl isononanoate, 2-ethylhexyl palmitate, 2-hexyldec
- the non-volatile oil may be present in a content ranging from 0.1% to 70% by weight, preferably ranging from 0.5% to 60% by weight and preferentially ranging from 1% to 50% by weight relative to the total weight of the non-volatile liquid fatty phase.
- volatile or non-volatile linear silicone oils will advantageously be used.
- the combination of a resin according to the invention and of a linear silicone oil may especially improve the transfer resistance.
- phenyl silicone oils will advantageously be used.
- the combination of a resin according to the invention and of a phenyl silicone oil may especially improve the gloss and comfort and reduce the tacky sensation.
- composition according to the invention may comprise a structuring agent.
- structuring agent means a compound capable of increasing the viscosity of the composition.
- the structuring agent makes it possible especially to obtain a composition that can have a texture ranging from fluid to solid textures.
- the structuring agent may be present in the composition in a content ranging from 0.05% to 40% by weight, preferably ranging from 0.1% to 30% by weight and preferentially ranging from 0.1% to 25% by weight, relative to the total weight of the composition.
- the structuring agent may be chosen especially from thickeners (oily-medium thickeners; aqueous-medium thickeners), organogelling agents, waxes, pasty compounds and gums.
- the aqueous-medium thickener may be chosen from:
- the oily-medium thickener may be chosen from:
- organogelling agents may be chosen from those described in patent application WO-A-03/105 788, the content of which is incorporated by reference.
- A is a group of formula:
- R′ being a linear or branched C 1 to C 4 alkyl radical and the *s symbolizing the points of attachment of the group A to each of the two nitrogen atoms of the rest of the compound of general formula (I), and
- R 1 being a linear or branched C 1 -C 4 alkyl radical, and the *s symbolizing the points of attachment of the group A to each of the two nitrogen atoms of the rest of the compound of general formula (I), and
- a carbon-based radical especially a linear, branched and/or cyclic, saturated or unsaturated hydrocarbon-based radical (alkyl), containing 1 to 18 carbon atoms, and possibly comprising 1 to 8 heteroatoms chosen from N, O, Si and S; or
- n is between 0 and 100, especially between 1 and 80, or even 2 to 20;
- R 2 to R 6 being, independently of each other, carbon-based radicals, especially linear or branched hydrocarbon-based radicals (alkyl) containing 1 to 12 and especially 1 to 6 carbon atoms, and possibly comprising 1 to 4 heteroatoms, especially O;
- carbon-based radicals especially linear, branched and/or cyclic, saturated or unsaturated hydrocarbon-based radicals (alkyl), containing 1 to 18 carbon atoms, and possibly comprising 1 to 4 heteroatoms chosen from N, O, Si and S;
- n is between 0 and 100, especially between 1 and 80, or even 2 to 20;
- R′ 2 to R′ 6 being, independently of each other, carbon-based radicals, especially linear or branched hydrocarbon-based radicals (alkyl), containing 1 to 12 and especially 1 to 6 carbon atoms, and possibly comprising 1 to 4 heteroatoms, especially O; and
- radicals R and/or R′ are of formula (III), such as those described in patent application FR-A-2 900 819,
- composition may comprise at least one solid fatty substance chosen from waxes, as structuring agent.
- the wax under consideration in the context of the present invention is generally a lipophilic compound that is solid at room temperature (25° C.), with a solid/liquid reversible change of state, having a melting point of greater than or equal to 30° C., which may be up to 200° C. and in particular up to 120° C.
- the waxes that are suitable for the invention may have a melting point of greater than or equal to 45° C. and in particular greater than or equal to 55° C.
- the melting point corresponds to the temperature of the most endothermic peak observed by thermal analysis (DSC) as described in standard ISO 11357-3; 1999.
- the melting point of the wax may be measured using a differential scanning calorimeter (DSC), for example the calorimeter sold under the name MDSC 2920 by the company TA Instruments.
- the measuring protocol is as follows:
- a sample of 5 mg of wax placed in a crucible is subjected to a first temperature rise ranging from ⁇ 20° C. to 100° C., at a heating rate of 10° C./minute, it is then cooled from 100° C. to ⁇ 20° C. at a cooling rate of 10° C./minute and is finally subjected to a second temperature increase ranging from ⁇ 20° C. to 100° C. at a heating rate of 5° C./minute.
- the variation of the difference in power absorbed by the empty crucible and by the crucible containing the sample of wax is measured as a function of the temperature.
- the melting point of the compound is the temperature value corresponding to the top of the peak of the curve representing the variation in the difference in absorbed power as a function of the temperature.
- the waxes that may be used in the compositions according to the invention are chosen from waxes that are solid at room temperature of animal, plant, mineral or synthetic origin, and mixtures thereof.
- waxes that are suitable for the invention, mention may be made especially of hydrocarbon-based waxes, for instance beeswax, lanolin wax, Chinese insect waxes, rice bran wax, carnauba wax, candelilla wax, ouricury wax, esparto grass wax, berry wax, shellac wax, Japan wax and sumach wax; montan wax, orange wax and lemon wax, microcrystalline waxes, paraffins and ozokerite; polyethylene waxes, the waxes obtained by Fischer-Tropsch synthesis and waxy copolymers, and also esters thereof.
- hydrocarbon-based waxes for instance beeswax, lanolin wax, Chinese insect waxes, rice bran wax, carnauba wax, candelilla wax, ouricury wax, esparto grass wax, berry wax, shellac wax, Japan wax and sumach wax; montan wax, orange wax and lemon wax, microcrystalline waxes, paraffins and ozokerite; polyethylene waxe
- waxes obtained by catalytic hydrogenation of animal or plant oils containing linear or branched C 8 -C 32 fatty chains.
- isomerized jojoba oil such as the trans-isomerized partially hydrogenated jojoba oil manufactured or sold by the company Desert Whale under the commercial reference Iso-Jojoba-50®, hydrogenated sunflower oil, hydrogenated castor oil, hydrogenated coconut oil, hydrogenated lanolin oil and bis(1,1,1-trimethylol-propane) tetrastearate sold under the name Hest 2T-4S® by the company Heterene.
- silicone waxes C 30-45 alkyl dimethicone
- fluoro waxes C 30-45 alkyl dimethicone
- waxes obtained by hydrogenation of castor oil esterified with cetyl alcohol sold under the names Phytowax ricin 16L64® and 22L73® by the company Sophim, may also be used. Such waxes are described in patent application FR-A-2 792 190.
- a wax that may be used is a C 20 -C 40 alkyl (hydroxystearyloxy)stearate (the alkyl group containing from 20 to 40 carbon atoms), alone or as a mixture.
- Such a wax is especially sold under the names Kester Wax K 82 P®, Hydroxypolyester K 82 P®, Kester Wax K 80 P® and Kester Wax K 82H® by the company Koster Keunen.
- microwaxes that may be used in the compositions according to the invention, mention may be made especially of carnauba microwaxes, such as the product sold under the name MicroCare 350® by the company Micro Powders, synthetic microwaxes, such as the product sold under the name MicroEase 114S® by the company Micro Powders, microwaxes consisting of a mixture of carnauba wax and polyethylene wax, such as the products sold under the names Micro Care 300® and 310® by the company Micro Powders, microwaxes consisting of a mixture of carnauba wax and of synthetic wax, such as the product sold under the name Micro Care 325® by the company Micro Powders, polyethylene microwaxes, such as the products sold under the names Micropoly 200®, 220®, 220L® and 250S® by the company Micro Powders, and polytetrafluoroethylene microwaxes, such as the products sold under the names Microslip 519® and 519 L® by the company Micro Powders.
- composition according to the invention may have a wax content ranging from 0.1% to 50% by weight and better still from 1% to 30% by weight relative to the total weight of the composition.
- composition according to the invention may comprise at least one pasty compound as structuring agent.
- Pasty fatty substances are considered as solid fatty substances for the purposes of the present invention.
- the term “pasty” means a lipophilic fatty compound that undergoes a reversible solid/liquid change of state and that comprises in the solid state an anisotropic crystal organization, and comprises, at a temperature of 23° C., a liquid fraction and a solid fraction.
- the starting melting point of the pasty compound is less than 23° C.
- the liquid fraction of the pasty compound measured at 23° C. may represent 9% to 97% by weight of the compound. This liquid fraction at 23° C. preferably represents between 15% and 85% and more preferably between 40% and 85% by weight.
- the liquid fraction by weight of the pasty compound at 23° C. is equal to the ratio of the heat of fusion consumed at 23° C. to the heat of fusion of the pasty compound.
- the heat of fusion of the pasty compound is the heat consumed by the compound to change from the solid state to the liquid state.
- the pasty compound is said to be in the solid state when all of its mass is in solid form.
- the pasty compound is said to be in the liquid state when all of its mass is in liquid form.
- the heat of fusion of the pasty compound is equal to the area under the curve of the thermogram obtained using a differential scanning calorimeter (DSC), such as the calorimeter sold under the name MDSC 2920 by the company TA Instrument, with a temperature rise of 5 or 10° C. per minute, according to standard ISO 11357-3:1999.
- DSC differential scanning calorimeter
- the heat of fusion of the pasty compound is the amount of energy required to make the compound change from the solid state to the liquid state. It is expressed in J/g.
- the heat of fusion consumed at 23° C. is the amount of energy absorbed by the sample to change from the solid state to the state that it has at 23° C., constituted of a liquid fraction and a solid fraction.
- the liquid fraction of the pasty compound, measured at 32° C. preferably represents from 30% to 100% by weight of the compound, preferably from 50% to 100% and more preferably from 60% to 100% by weight of the compound.
- the temperature of the end of the melting range of the pasty compound is less than or equal to 32° C.
- the liquid fraction of the pasty compound measured at 32° C. is equal to the ratio of the heat of fusion consumed at 32° C. to the heat of fusion of the pasty compound.
- the heat of fusion consumed at 32° C. is calculated in the same manner as the heat of fusion consumed at 23° C.
- the pasty compound is preferably chosen from synthetic compounds and compounds of plant origin.
- a pasty compound may be obtained by synthesis from starting materials of plant origin.
- esters that are especially preferred are:
- the composition comprises a total content of pasty fatty substances ranging from 0.5% to 50% by weight, preferably from 1% to 40% by weight and better still from 5% to 30% by weight relative to the weight of the composition.
- the gums are generally polydimethylsiloxanes (PDMS) of high molecular weight or cellulose gums or polysaccharides.
- PDMS polydimethylsiloxanes
- composition according to the invention may comprise at least one surfactant.
- the surfactant may be lipophilic and/or hydrophilic, used alone or in combination.
- the surfactant may be chosen from nonionic, anionic, cationic and amphoteric surfactants.
- the nonionic surfactant may be chosen from:
- the C 8 -C 22 alkyl dimethicone copolyol is advantageously a compound of formula (I) below:
- n 10 to 100
- a C 8 -C 22 alkyl dimethicone copolyol that may be mentioned is cetyl dimethicone copolyol, for instance the product sold under the name Abil EM-90 by the company Goldschmidt.
- Dimethicone copolyols that may be used include those corresponding to formula (II) below:
- R 1 , R 2 and R 3 independently of each other, represent a C 1 -C 6 alkyl radical or a radical —(CH 2 ) X —(OCH 2 CH 2 ) y —(OCH 2 CH 2 CH 2 ) z —OR 4 , at least one radical R 1 , R 2 or R 3 not being an alkyl radical; R 4 being a hydrogen, a C 1 -C 2 alkyl radical or a C 2 -C 4 acyl radical;
- A is an integer ranging from 0 to 200;
- B is an integer ranging from 0 to 50; on condition that A and B are not simultaneously equal to zero;
- x is an integer ranging from 1 to 6;
- y is an integer ranging from 1 to 30;
- z is an integer ranging from 0 to 5.
- R 1 ⁇ R 3 methyl radical
- x is an integer ranging from 2 to 6
- y is an integer ranging from 4 to 30.
- R 4 is in particular a hydrogen.
- A is an integer ranging from 20 to 105
- B is an integer ranging from 2 to 10
- y is an integer ranging from 10 to 20.
- silicone compounds of formula (II) that may also be mentioned include the compounds of formula (IV):
- A′ and y are integers ranging from 10 to 20.
- Dimethicone copolyols that may be used include those sold under the names DC 5329, DC 7439-146, DC 2-5695 and Q4-3667 by the company Dow Corning; KF-6013, KF-6015, KF-6016 and KF-6017 by the company Shin-Etsu.
- the compounds DC 5329, DC 7439-146 and DC2-5695 are compounds of formula (III) in which, respectively, A is 22, B is 2 and y is 12; A is 103, B is 10 and y is 12; A is 27, B is 3 and y is 12.
- Nonionic surfactants that may also be mentioned include fatty acid esters of polyols, for instance sorbitol or glyceryl mono-, di-, tri- or sesqui-oleates or stearates, glyceryl or polyethylene glycol laurates; fatty acid esters of polyethylene glycol (polyethylene glycol monostearate or monolaurate); polyoxyethylenated fatty acid esters (stearate or oleate) of sorbitol; polyoxyethylenated alkyl (lauryl, cetyl, stearyl or octyl)ethers.
- fatty acid esters of polyols for instance sorbitol or glyceryl mono-, di-, tri- or sesqui-oleates or stearates, glyceryl or polyethylene glycol laurates
- fatty acid esters of polyethylene glycol polyethylene glycol monostearate or monolaurate
- Anionic surfactants that may be mentioned include carboxylates (sodium 2-(2-hydroxyalkyloxy)acetate)), amino acid derivatives (N-acylglutamates, N-acylglycinates or acylsarcosinates), alkyl sulfates, alkyl ether sulfates and oxyethylenated derivatives thereof, sulfonates, isethionates and N-acylisethionates, taurates and N-acyl N-methyltaurates, sulfosuccinates, alkylsulfoacetates, phosphates and alkyl phosphates, polypeptides, anionic derivatives of alkyl polyglycoside (acyl-D-galactoside uronate), and fatty acid soaps, and mixtures thereof.
- carboxylates sodium 2-(2-hydroxyalkyloxy)acetate
- amino acid derivatives N-acylglutamates, N-acylgly
- Amphoteric and zwitterionic surfactants that may be used include betaines, N-alkylamidobetaines and derivatives thereof, glycine derivatives, sultaines, alkyl polyaminocarboxylates and alkylamphoacetates, and mixtures thereof.
- the surfactant may be present in the composition according to the invention in a content ranging from 0.1% to 10% by weight, preferably ranging from 0.5% to 8% by weight and preferentially ranging from 0.5% to 7% by weight, relative to the total weight of the composition.
- composition according to the invention may comprise at least one dyestuff.
- the dyestuff may be chosen from pulverulent dyestuffs (especially pigments and nacres) and water-soluble dyestuffs.
- pigments should be understood as meaning white or coloured, mineral or organic particles of any form, which are insoluble in the physiological medium, and which are intended to colour the composition.
- nacres should be understood as meaning iridescent particles of any form, produced especially by certain molluscs in their shell, or else synthesized.
- the pigments may be white or coloured, and mineral and/or organic.
- mineral pigments that may be mentioned are titanium dioxide, optionally surface-treated, zirconium oxide or cerium oxide, and also zinc oxide, iron oxide (black, yellow or red) or chromium oxide, manganese violet, ultramarine blue, chromium hydrate and ferric blue, and metal powders, for instance aluminium powder or copper powder.
- organic pigments that may be mentioned are carbon black, pigments of D & C type, and lakes based on cochineal carmine or on barium, strontium, calcium or aluminium.
- pigments with an effect such as particles comprising a natural or synthetic, organic or mineral substrate, for example glass, acrylic resins, polyester, polyurethane, polyethylene terephthalate, ceramics or aluminas, the said substrate being uncoated or coated with metallic substances, for instance aluminium, gold, silver, platinum, copper or bronze, or with metal oxides, for instance titanium dioxide, iron oxide or chromium oxide, and mixtures thereof.
- a natural or synthetic, organic or mineral substrate for example glass, acrylic resins, polyester, polyurethane, polyethylene terephthalate, ceramics or aluminas
- metallic substances for instance aluminium, gold, silver, platinum, copper or bronze
- metal oxides for instance titanium dioxide, iron oxide or chromium oxide, and mixtures thereof.
- the nacreous pigments may be chosen from white nacreous pigments such as mica coated with titanium or with bismuth oxychloride, coloured nacreous pigments such as titanium mica coated with iron oxides, titanium mica coated especially with ferric blue or with chromium oxide, titanium mica coated with an organic pigment of the abovementioned type, and also nacreous pigments based on bismuth oxychloride.
- Interference pigments especially liquid-crystal or multilayer interference pigments, may also be used.
- alkyl mentioned in the compounds cited above especially denotes an alkyl group containing from 1 to 30 carbon atoms and preferably containing from 5 to 16 carbon atoms.
- Hydrophobic-treated pigments are described especially in patent application EP-A-1 086 683.
- the water-soluble dyes are, for example, beetroot juice or methylene blue.
- the synthetic or natural liposoluble dyes are, for example, DC Red 17, DC Red 21, DC Red 27, DC Green 6, DC Yellow 11, DC Violet 2, DC Orange 5, Sudan red, carotenes ( ⁇ -carotene, lycopene), xanthophylls (capsanthin, capsorubin, lutein), palm oil, Sudan brown, quinoline yellow, annatto and curcumin.
- the dyestuffs in particular the pigments treated with a hydrophobic agent, may be present in the composition in a content ranging from 0.1% to 50% by weight, preferably ranging from 0.5% to 30% by weight and preferentially ranging from 1% to 20% by weight, relative to the total weight of the composition.
- composition according to the invention may comprise at least one filler.
- the term “filler” denotes solid particles of any form, which are in an insoluble form and dispersed in the medium of the composition, even at temperatures that may be up to the melting point of all the fatty substances of the composition.
- the fillers used according to the invention are colourless or white, namely non-pigmentary, i.e. they are not used to give a particular colour or shade to the composition according to the invention, even though their use may inherently lead to such a result.
- These fillers serve especially to modify the rheology or texture of the composition.
- organic pigmentary materials for instance carbon black, pigments of D&C type, and lakes based on cochineal carmine or on barium, strontium, calcium or aluminium
- inorganic pigmentary materials for instance titanium dioxide, zirconium oxide or cerium oxide, and also iron oxides (black, yellow or red), chromium oxide, manganese violet, ultramarine blue, chromium hydrate and ferric blue, which are, themselves, used to give a shade and coloration to the compositions incorporating them.
- fillers which thus covers non-pigmentary fillers, which may be organic or inorganic.
- non-pigmentary fillers used in the compositions according to the present invention may be of lamellar, globular or spherical form, of fibre type, or of any intermediate form between these defined forms.
- the size of the particles i.e. their granulometry, is chosen so as to ensure the good dispersion of the fillers in the composition according to the invention.
- the granulometry of the particles may be distributed within the range from 5 ⁇ m to 10 nm and in particular from 10 ⁇ m to 10 nm.
- the fillers according to the invention may or may not be surface-coated, in particular surface-treated with silicones, amino acids, fluoro derivatives or any other substance that promotes the dispersion and compatibility of the filler in the composition.
- non-pigmentary mineral fillers that may be used in the compositions according to the invention, mention may be made of talc, mica, silica, perlite, which is especially commercially available from the company World Minerals Europe under the trade name Perlite P1430, Perlite P2550 or Perlite P204, kaolin, precipitated calcium carbonate, magnesium carbonate, magnesium hydrogen carbonate, hydroxyapatite, boron nitride, hollow silica microspheres (Silica Beads® from Maprecos), and glass or ceramic microcapsules, and mixtures thereof.
- the cosmetic composition according to the invention comprises at least one non-pigmentary mineral filler chosen from the group comprising kaolin, talc, silica, perlite and clay, and mixtures thereof.
- organic fillers that may be mentioned are polyamide powder (Orgasol® Nylon® from Atochem), poly- ⁇ -alanine powder and polyethylene powder, lauroyllysine, starch, tetrafluoroethylene polymer powders (Teflon®), hollow polymer microspheres such as those of polyvinylidene chloride/acrylonitrile, for instance Expancel® (Nobel Industrie) or of acrylic acid copolymer (such as Polytrap (Dow Corning)), acrylate copolymers, PMMA, 12-hydroxystearic acid oligomer stearate and silicone resin microbeads (for example Tospearls® from Toshiba), magnesium carbonate, magnesium hydrogen carbonate, and metal soaps derived from organic carboxylic acids containing from 8 to 22 carbon atoms and preferably from 12 to 18 carbon atoms, for example zinc stearate, magnesium stearate, lithium stearate, zinc laurate or magnesium myristate, and mixtures thereof.
- the organic fillers are different from the pigments.
- They may also be particles comprising a copolymer, the said copolymer comprising trimethylol hexyl lactone.
- it may be a hexamethylene diisocyanate/trimethylol hexyl lactone copolymer.
- Such particles are especially commercially available, for example under the name Plastic Powder D-400® or Plastic Powder D-800® from the company Toshiki.
- a composition of the invention may comprise at least one filler chosen from talc, silica, starch, clay, kaolin and perlite, and mixtures thereof.
- One or more dispersants may be used, where appropriate, to protect the dispersed fillers or particles against aggregation or flocculation. They may be added independently of the solid fillers or particles or in the form of a colloidal dispersion of particles.
- the concentration of dispersants is chosen so as to obtain satisfactory dispersion of the solid particles (without flocculation).
- This dispersant may be a surfactant, an oligomer, a polymer or a mixture of several thereof, bearing one or more functionalities with strong affinity for the surface of the particles to be dispersed.
- poly(12-hydroxystearic acid) esters are used, such as poly(12-hydroxystearic acid) stearate with a molecular weight of about 750 g/mol, such as the product sold under the name Solsperse 21 000® by the company Avecia, esters of poly(12-hydroxystearic acid) with polyols such as glycerol or diglycerol, such as polyglyceryl-2 dipolyhydroxystearate (CTFA name) sold under the reference Dehymuls PGPH® by the company Henkel (or diglyceryl poly(12-hydroxystearate)), or alternatively poly(12-hydroxystearic acid), such as the product sold under the reference Arlacel P100 by the company Uniqema, and mixtures thereof.
- dispersants that may be used in the composition of the invention, mention may be made of quaternary ammonium derivatives of polycondensate fatty acids, for instance Solsperse 17 000® sold by the company Avecia, and mixtures of polydimethylsiloxane/oxypropylene such as those sold by the company Dow Corning under the references DC2-5185 and DC2-5225 C.
- a composition of the invention should be cosmetically or dermatologically acceptable, i.e. it should contain a non-toxic physiologically acceptable medium that can be applied to human lips.
- cosmetically acceptable refers to a composition of pleasant appearance, odour and feel.
- composition according to the invention may also contain ingredients commonly used in cosmetics, such as vitamins, thickeners, trace elements, softeners, sequestrants, fragrances, acidifying agents, basifying agents, preserving agents, sunscreens, surfactants, antioxidants, hair-loss counteractants, antidandruff agents and propellants, or mixtures thereof.
- ingredients commonly used in cosmetics such as vitamins, thickeners, trace elements, softeners, sequestrants, fragrances, acidifying agents, basifying agents, preserving agents, sunscreens, surfactants, antioxidants, hair-loss counteractants, antidandruff agents and propellants, or mixtures thereof.
- the invention also relates to a cosmetic assembly comprising:
- composition placed inside the said compartment, the composition being in accordance with the invention.
- the container may be in any adequate form. It may especially be in the form of a bottle, a tube, a jar, a case, a box, a sachet or a carton.
- the closing member may be in the form of a removable stopper, a lid, a cap, a tear-off strip or a capsule, especially of the type comprising a body attached to the container and a cover cap articulated on the body. It may also be in the form of a member for selectively closing the container, especially a pump, a valve or a flap valve.
- the container may be combined with an applicator, especially in the form of a brush comprising an arrangement of bristles maintained by a twisted wire.
- a twisted brush is described especially in U.S. Pat. No. 4,887,622.
- It may also be in the form of a comb comprising a plurality of application members, obtained especially by moulding. Such combs are described, for example, in patent FR 2 796 529.
- the applicator may be in the form of a fine brush, as described, for example, in patent FR 2 722 380.
- the applicator may be in the form of a block of foam or of elastomer, a felt or a spatula.
- the applicator may be free (tuft or sponge) or securely fastened to a rod borne by the closing member, as described, for example, in U.S. Pat. No. 5,492,426.
- the applicator may be securely fastened to the container, as described, for example, in patent FR 2 761 959.
- the product may be contained directly in the container, or indirectly.
- the product may be arranged on an impregnated support, especially in the form of a wipe or a pad, and arranged (individually or in plurality) in a box or in a sachet.
- an impregnated support especially in the form of a wipe or a pad, and arranged (individually or in plurality) in a box or in a sachet.
- Such a support incorporating the product is described, for example, in patent application WO 01/03538.
- the closing member may be coupled to the container by screwing.
- the coupling between the closing member and the container is done other than by screwing, especially via a bayonet mechanism, by click-fastening, gripping, welding, bonding or by magnetic attraction.
- click-fastening in particular means any system involving the crossing of a bead or cord of material by elastic deformation of a portion, especially of the closing member, followed by return to the elastically unconstrained position of the said portion after the crossing of the bead or cord.
- the container may be at least partially made of thermoplastic material.
- thermoplastic materials that may be mentioned include polypropylene or polyethylene.
- the container is made of non-thermoplastic material, especially glass or metal (or alloy).
- the container may have rigid walls or deformable walls, especially in the form of a tube or a tubular bottle.
- the container may comprise means for distributing or facilitating the distribution of the composition.
- the container may have deformable walls so as to allow the composition to exit in response to a positive pressure inside the container, this positive pressure being caused by elastic (or non-elastic) squeezing of the walls of the container.
- the product may be driven out by a piston mechanism.
- the container may comprise a mechanism, especially a rack mechanism, a threaded-rod mechanism or a helical groove mechanism, and may be capable of moving a stick in the direction of the said aperture.
- a mechanism is described, for example, in patent FR 2 806 273 or in patent FR 2 775 566.
- Such a mechanism for a liquid product is described in patent FR 2 727 609.
- the container may be formed from a carton with a base delimiting at least one housing containing the composition, and a lid, especially articulated on the base, and capable of at least partially covering the said base.
- a carton is described, for example, in patent application WO 03/018423 or in patent FR 2 791 042.
- the container may be equipped with a drainer arranged in the region of the aperture of the container.
- a drainer makes it possible to wipe the applicator and possibly the rod to which it may be securely fastened.
- Such a drainer is described, for example, in patent FR 2 792 618.
- the composition may be at atmospheric pressure inside the container (at room temperature) or pressurized, especially by means of a propellent gas (aerosol).
- a propellent gas especially by means of a propellent gas (aerosol).
- the container is equipped with a valve (of the type used for aerosols).
- the transfer index of the deposit obtained with the composition according to the invention is determined according to the measuring protocol described below.
- a support (40 mm ⁇ 70 mm rectangle) consisting of an acrylic coating (hypoallergenic acrylic adhesive on polyethylene film sold under the name Blenderme ref. FH5000-55113 by the company 3M Santé) bonded onto a layer of polyethylene foam that is adhesive on the side opposite the one to which the adhesive plaster is fixed (foam layer sold under the name RE 40 ⁇ 70EP3 from the company Joint Technique Lyonnais Ind.) is prepared.
- the colour L* 0 a* 0 b* 0 of the support, on the acrylic coating side, is measured using a Minolta CR300 colorimeter.
- the support thus prepared is preheated on a hotplate maintained at a temperature of 40° C. so that the surface of the support is maintained at a temperature of 33° C. ⁇ 1° C.
- the composition While leaving the support on the hotplate, the composition is applied to the entire non-adhesive surface of the support (i.e. to the surface of the acrylic coating), spreading it out with a brush to obtain a deposit of the composition of about 15 ⁇ m, and this deposit is then left to dry for 10 minutes.
- the support is then bonded via its adhesive face (adhesive face of the foam layer) to an anvil 20 mm in diameter and equipped with a screw pitch.
- a sample of the support/deposit assembly is then cut out using a sample punch 18 mm in diameter.
- the anvil is then screwed onto a press (Statif Manuel Imada SV-2 from the company Someco) equipped with a tensile testing machine (Imada DPS-20 from the company Someco).
- a strip 33 mm wide and 29.7 cm long is drawn on a sheet of white photocopier paper with a basis weight of 80 g/m 2 , a first line is marked 2 cm from the edge of the sheet, and a second line is then marked 5 cm from the edge of the sheet, the first and second lines thus delimiting a box on the strip; next, a first mark and a second mark located in the strip at reference points 8 cm and 16 cm, respectively, from the second mark, are applied. 20 ⁇ l of water are placed on the first mark and 10 ⁇ l of refined sunflower oil (sold by the company Lesieur) are placed on the second mark.
- the white paper is placed on the base of the press and the sample placed on the box of the strip of paper is then pressed at a pressure of about 300 g/cm 2 exerted for 30 seconds.
- the press is then opened and the sample is again placed just after the second mark (i.e. next to the box), a pressure of about 300 g/cm 2 is again exerted, and the paper is displaced, in a rectilinear manner as soon as the contact is made, at a speed of 1 cm/s over the entire length of the strip such that the sample passes through the water and oil deposits.
- the transfer index of the composition is equal to the ratio:
- the measurement is performed on 6 supports in succession and the transfer index corresponds to the mean of the six measurements obtained with the six supports.
- the present invention also relates to a cosmetic product for making up and/or caring for keratin materials, comprising at least two compositions that can be applied successively to keratin materials, especially to the lips.
- the present invention also relates to a process for making up the face and the body using these two compositions. They are preferably applied successively to the keratin materials: the first composition and then the second composition.
- compositions are conventionally known as a topcoat and a basecoat.
- the invention relates to a product (also known as a kit) for making up and/or caring for keratin materials, especially the lips, comprising a first composition and a second composition conditioned in separate containers,
- the first composition containing, in a physiologically acceptable medium:
- a siloxane resin comprising the following units:
- R 1 , R 2 and R 3 independently representing an alkyl group containing from 1 to 8 carbon atoms, an aryl group, a carbinol group or an amino group,
- a being between 0.05 and 0.5
- d being between 0.05 and 0.6
- the second composition which is different from the first, comprising at least one fatty substance.
- the fatty substance of the second composition is preferably chosen from waxes and non-volatile oils.
- the second composition comprises at least one wax and at least one non-volatile oil.
- the wax is a sunflower wax.
- the non-volatile oil is an oil such as caprylic/capric acid triglycerides.
- the presence of a second composition applied over the first composition onto the keratin materials can especially improve the gloss and/or comfort properties.
- the invention is illustrated in greater detail by the examples described below, which are given as non-limiting illustrations.
- the percentages are weight percentages.
- the MQ resin was manufactured according to the techniques described by Daudt in U.S. Pat. No. 2,676,182.
- Propyl resin a propyl silsesquioxane resin at 74.8% by weight in toluene.
- the propyl silsesquioxane resin was obtained by hydrolysis of propyltrichlorosilane.
- An MQ resin, a T propyl resin, xylene and 1M KOH in water in the proportions presented in Table 1 are introduced into a 3-necked flask equipped with a stirrer, a temperature probe and Dean-Stark apparatus mounted with a condenser.
- Xylene is pre-introduced into the Dean-Stark apparatus so as to ensure maintenance of a level of solids of 50% in the reactor.
- the mixture in the reactor is refluxed (between 100 and 140° C.) for at least 3 hours. Any water formed in the reaction mixture is continuously removed and trapped in the form of an azeotrope in the Dean-Stark apparatus. After refluxing for 3 hours, the water is removed from the apparatus and heating is continued for a further 30 minutes.
- the mixture is maintained at 90° C. for 1 hour 30 minutes.
- the mixture is maintained at 90° C. for 3 hours, and is then cooled.
- a polymer comprising a first poly(isobornyl acrylate/isobornyl methacrylate) rigid block with a Tg of 110° C., a second poly(isobutyl acrylate/acrylic acid) flexible block with a Tg of ⁇ 9° C. and an intermediate block, which is an isobornyl acrylate/isobornyl methacrylate/isobutyl acrylate/acrylic acid random polymer, is obtained.
- compositions 3 and 4 below were prepared:
- Composition Composition 4 3 comparative Weight Weight percentages percentages Compounds % % 2-Octyldodecanol 9.43 8.23 Refined plant perhydrosqualene 5.05 4.21 (Phytosqualan from Sophim) Brilliant yellow FCF aluminium lake on 2.58 2.58 alumina (42/58) (CI: 15985:1 + 77002) Brilliant blue FCF aluminium lake on 0.16 0.16 alumina (12/88) (CI: 42090:2 + 77002) Calcium salt of Lithol B Red 0.59 0.59 Rutile titanium oxide treated with 2.74 2.74 alumina/silica/trimethylolpropane (CI: 77891) Black iron oxide (CI: 77499) 0.32 0.32 Mixture of isopropyl, isobutyl and n- 0.65 0.65 butyl p-hydroxybenzoates (40/30/30) (Liquapar Oil from ISP) Polyphenyltrimethylsiloxydiphenylsiloxane 25.03 25.03 (
- Composition 3 according to the invention contains 15% polymer (weight of solids) prepared according to Example 2 and 3.5% (weight of solids) of siloxane resin prepared according to Example 1-C), i.e. in total 18.5% of film-forming polymer.
- Comparative composition 4 contains 18.5% of polymer (weight of solids) prepared according to Example 2.
- Comparative composition 4 is very viscous and tacky and is very thick on application. It is uncomfortable on the lips since the film formed is very thick and tacky.
- Composition 3 according to the invention is much more pleasant (slippery) on application and it forms a much finer and more comfortable deposit.
- Composition 5 according to the Comparative invention (Weight composition 6 Compounds percentages %) (Weight percentages %) Butyl acrylate copolymer containing 31.25 37.5 dendritic silicone side chains: [tris(trimethylsiloxy)siloxyethyldimethyl- siloxy]silylpropyl methacrylate in isododecane: 40/60 (Dow Corning FA 4002 ID Silicone Acrylate from Dow Corning) MQ-T propyl resin (30/70) in isododecane as 4 — prepared in Example 1-C above, from Dow Corning Isododecane from Ineos 1.3 — Refined plant perhydrosqualene 12.02 12.02 (Phytosqualan from Sophim) Octyldodecanol 14.2 13.62 Polybutene (Indopol H 100 from Ineos) 10.65 10.65 Isopropyl paraben (and) isobutyl paraben 0.65 0.
- compositions 5 and 6 are evaluated according to the transfer index measurement protocol described above. The results obtained are as follows:
- compositions 5 and 6 have substantially the same solids content of film-forming polymer (siloxane resin vinyl polymer containing carbosiloxane dendrimer-based units).
- composition 5 according to the invention containing a vinyl polymer comprising at least one carbosiloxane dendrimer-based unit and an MQ-T propyl siloxane resin, a transfer index value of 31.81 ⁇ 7.87 is measured.
Abstract
The present invention relates to a process for making up and/or caring for keratin materials, in which a composition is applied to the keratin materials, and especially to the lips, this composition containing, in a physiologically acceptable medium:
-
- a) a siloxane resin comprising the following units:
- (i) (R1 3SiO1/2)a
- (ii) (R2 2SiO2/2)b
- (iii) (R3SiO3/2)c and
- (iv) (SiO4/2)d
with - R1, R2 and R3 independently representing an alkyl group containing from 1 to 8 carbon atoms, an aryl group, a carbinol group or an amino group,
- a being between 0.05 and 0.5,
- b being between 0 and 0.3,
- c being greater than 0,
- d being between 0.05 and 0.6,
- a+b+c+d=1,
- on condition that more than 40 mol % of the groups R3 of the siloxane resin are propyl groups, and
- b) at least one liquid fatty phase, and
- c) at least one film-forming polymer chosen from the group comprising:
- a film-forming block ethylenic polymer, comprising at least a first block and at least a second block, is characterized in that the first block is obtained from at least one acrylate monomer of formula CH2═CH—COOR2 in which R2 represents a C4 to C12 cycloalkyl group and at least one methacrylate monomer of formula CH2═C(CH3)—COOR2 in which R′2 represents a C4 to C12 cycloalkyl group, and characterized in that the second block is obtained from an acrylic acid monomer and from at least one monomer with a glass transition temperature of less than or equal to 20° C.,
- a vinyl polymer comprising at least one carbosiloxane dendrimer-based unit,
- a dispersion of acrylic or vinyl radical homopolymer or copolymer particles dispersed in the said liquid fatty phase.
Description
- The invention relates to a cosmetic composition for keratin materials, especially the skin, the hair and the nails. The invention relates in particular to makeup compositions for keratin materials.
- One of the objects of the patent application is to produce makeup compositions for keratin materials (skin, mucous membranes, fibre, eyelashes and integuments) that allow the application of a total transfer-resistant film with good staying power.
- In the field of lipsticks and makeup in general, formulators are in search of compositions that have good staying-power properties, to satisfy consumers' expectations. These compositions should also be transfer-resistant, while at the same time offering good comfort properties.
- Formulators are thus in search of starting materials and/or systems for obtaining compositions whose application is characterized by improved staying power and a good level of comfort. The term “comfort” means the comfort on application, i.e. a composition that is easy to apply in terms of glidance and of amount applied, without, however, the applied film being too thick and/or tacky. The term “comfort” also means the comfort after application, so that the user does not experience any tautness or drying out, in particular.
- It is known to those skilled in the art to use polymers to obtain these staying-power properties in the course of the day.
- These polymers are of very diverse chemical nature and are conveyed either in a fatty phase or in an aqueous phase.
- Examples that may be mentioned include silicone resins, especially of MQ type, polyacrylates, latices, etc.
- Although these polymers do indeed afford staying-power properties, in particular transfer resistance, they are usually accompanied by discomfort either during the application of the product (difficult spreading, tackiness, etc.) or during the day (tautness, mask effect, etc.).
- It is thus necessary to search for a technical solution for obtaining these staying-power properties while at the same time maintaining comfortable use.
- These objects, and others, are achieved by means of a composition containing, in a physiologically acceptable medium, a) a siloxane resin comprising the following units:
- (i) (R1 3SiO1/2)a
- (ii) (R2 2SiO2/2)b
- (iii)(R3SiO3/2)c and
- (iv) (SiO4/2)d
- with
- R1, R2 and R3 independently representing an alkyl group containing from 1 to 8 carbon atoms, an aryl group, a carbinol group or an amino group,
- a being between 0.05 and 0.5,
- b being between 0 and 0.3,
- c being greater than 0,
- d being between 0.05 and 0.6,
- a+b+c+d=1,
- on condition that more than 40 mol % of the groups R2 of the siloxane resin are propyl groups, b) at least one liquid fatty phase and c) at least one film-forming polymer chosen from the group comprising:
-
- a film-forming block ethylenic polymer, which preferably comprises at least a first block and at least a second block with different glass transition temperatures (Tg), the said first and second blocks being linked together via an intermediate block comprising at least one constituent monomer of the first block and at least one constituent monomer of the second block,
- a vinyl polymer comprising at least one carbosiloxane dendrimer-based unit,
- a dispersion of acrylic or vinyl radical homopolymer or copolymer particles dispersed in the said liquid fatty phase.
- Preferably, the block ethylenic film-forming polymer, comprising at least a first block and at least a second block, is characterized in that the first block is obtained from at least one acrylate monomer of formula CH2═CH—COOR2 in which R2 represents a C4 to C12 cycloalkyl group and at least one methacrylate monomer of formula CH2═C(CH3)—COOR2 in which R′2 represents a C4 to C12 cycloalkyl group, and characterized in that the second block is obtained from an acrylic acid monomer and from at least one monomer with a glass transition temperature of less than or equal to 20° C. Such polymers and the process for preparing them are described, for example, in document EP 1 882 709.
- Preferably, the siloxane resin comprises the following units:
- (i) (R1 3SiO1/2)a
- (iii) (R3SiO3/2)c and
- (iv) (SiO4/2)d
- with
- R1 and R3 independently representing an alkyl group containing from 1 to 8 carbon atoms, R1 preferably being a methyl group and R3 preferably being a propyl group,
- a being between 0.05 and 0.5 and preferably between 0.15 and 0.4,
- c being greater than zero, preferably between 0.15 and 0.4,
- d being between 0.05 and 0.6, preferably between 0.2 and 0.6 or alternatively between 0.2 and 0.55,
- a+b+c+d=1,
- on condition that more than 40 mol % of the groups R3 of the siloxane resin are propyl groups.
- The siloxane resins that may be used according to the invention may be obtained via a process comprising the reaction of:
-
- A) an MQ resin comprising at least 80 mol % of units (R1 3SiO1/2)a and (SiO4/2)d
- R1 representing an alkyl group containing from 1 to 8 carbon atoms, an aryl group, a carbinol group or an amino group,
- a and d being greater than zero,
- the ratio a/d being between 0.5 and 1.5;
- and
- B) a propyl resin T comprising at least 80 mol % of units (R3SiO3/2)c,
- R3 representing an alkyl group containing from 1 to 8 carbon atoms, an aryl group, a carbinol group or an amino group,
- c being greater than zero,
- on condition that at least 40 mol % of the groups R3 are propyl groups,
in which the mass ratio A/B is between 95/5 and 15/85 and preferably the mass ratio A/B is 30/70.
- A) an MQ resin comprising at least 80 mol % of units (R1 3SiO1/2)a and (SiO4/2)d
- Advantageously, the mass ratio A/B is between 95/5 and 15/85. Preferably, the ratio A/B is less than or equal to 70/30. These preferred ratios have proven to afford comfortable deposits due to the absence of percolation of the rigid particles of MQ resin in the deposit.
- The compositions according to the invention may be in various forms, especially in the form of a powder, an anhydrous dispersion, a water/oil, water/wax, oil/water, multiple or wax/water emulsion, or a gel.
- The compositions according to the invention are found to have very good staying-power and transfer-resistance properties while at the same time maintaining a comfortable deposit, especially when it is applied to the lips.
- The resins that may be used according to the invention are especially those described in patent application WO 2005/075 542, the content of which is incorporated herein by reference.
- According to a first embodiment, the composition according to the invention is liquid.
- According to a second embodiment, the composition according to the invention is solid.
- The term “solid” characterizes the state of the composition at room temperature (25° C.) and at atmospheric pressure (760 mmHg).
- Preferably, the composition according to the invention has, when it is solid, a hardness of between 30 and 300 g, or even from 50 to 200 g.
- Protocol for Measuring the Shear:
- The measurement is performed according to the following protocol:
- A sample of the composition under consideration is hot-cast into a stick mould 12.7 mm in diameter. The mould is then cooled in a freezer for about one hour. The stick of lipstick is then stored at 20° C.
- The hardness of the samples is measured after an interval of 24 hours.
- The hardness of the samples of compositions of the invention, expressed in grams, is measured using a DFGS2 tensile testing machine sold by the company Indelco-Chatillon.
- The hardness corresponds to the maximum shear force exerted by a rigid tungsten wire 250 μm in diameter, advancing at a rate of 100 mm/minute.
- The technique described above is usually referred to as the “cheese wire” method.
- Preferably, the composition according to the invention comprises less than 3% and better still less than 1% by weight of water relative to the total weight of the composition. More preferably, the composition is totally anhydrous. The term “anhydrous” especially means that water is preferably not deliberately added to the composition, but may be present in trace amount in the various compounds used in the composition.
- According to another aspect, the present invention relates to a makeup and/or care process in which the composition as defined previously is applied to keratin materials, and especially to the lips.
- Film-Forming Polymer:
- In the present invention, the term “film-forming polymer” means a polymer that is capable, by itself or in the presence of an auxiliary film-forming agent, of forming a macroscopically continuous film that adheres to keratin materials, and preferably a cohesive film, better still a film whose cohesion and mechanical properties are such that the said film can be isolated and manipulated individually, for example when the said film is prepared by pouring onto a non-stick surface such as a Teflon-coated or silicone-coated surface.
- Block Ethylenic Polymer
- According to a first embodiment of the invention, the film-forming polymer present in the composition according to the invention is a film-forming block ethylenic polymer (which is preferably essentially linear), which preferably comprises at least a first block and at least a second block with different glass transition temperatures (Tg), the said first and second blocks being linked together via an intermediate block comprising at least one constituent monomer of the first block and at least one constituent monomer of the second block.
- The term “at least one block” means one or more blocks.
- The term “block polymer” means a polymer comprising at least two different blocks and preferably at least three different blocks.
- Advantageously, the first and second blocks of the block polymer are advantageously mutually incompatible.
- Such polymers are described, for example, in documents EP 1 411 069 or WO 04/028 488. Patent application EP 1 411 069 describes the possibility of preparing block polymers from acrylate monomers or methacrylate monomers.
- Preferably, according to this embodiment, the film-forming polymer present in the composition according to the invention is a block polymer, comprising at least a first block and at least a second block, characterized in that the first block is obtained from at least one acrylate monomer of formula CH2═CH—COOR2 in which R2 represents a C4 to C12 cycloalkyl group, and from at least one methacrylate monomer of formula CH2═C(CH3)—COOR2 in which R′2 represents a C4 to C12 cycloalkyl group, and characterized in that the second block is obtained from an acrylic acid monomer and from at least one monomer with a glass transition temperature of less than or equal to 20° C. Such polymers and the process for preparing them are described, for example, in document EP 1 882 709.
- The first block and the second block of the polymer of the invention may be advantageously mutually incompatible.
- The term “mutually incompatible blocks” means that the blend formed from a polymer corresponding to the first block and from a polymer corresponding to the second block is not miscible in the polymerization solvent that is the majority amount by weight of the block polymer, at room temperature (25° C.) and atmospheric pressure (105 Pa), for a polymer blend content of greater than or equal to 5% by weight, relative to the total weight of the blend and of the said polymerization solvent, it being understood that:
-
- i) the said polymers are present in the blend in a content such that the respective weight ratio ranges from 10/90 to 90/10, and that
- ii) each of the polymers corresponding to the first and second blocks has an average (weight-average or number-average) molecular mass equal to that of the block polymer ±15%.
- In the case of a mixture of polymerization solvents, and should two or more solvents be present in identical mass proportions, the said polymer blend is immiscible in at least one of them.
- Needless to say, in the case of a polymerization performed in a single solvent, this solvent is the majority solvent.
- The said first and second blocks may be advantageously linked together via an intermediate block comprising at least one constituent monomer of the first block and at least one constituent monomer of the second block.
- The intermediate block is a block comprising at least one constituent monomer of the first block and at least one constituent monomer of the second block of the polymer, which enables these blocks to be “compatibilized”.
- Advantageously, the intermediate block comprising at least one constituent monomer of the first block and at least one constituent monomer of the second block of the polymer is a random polymer.
- Preferably, the intermediate block is derived essentially from constituent monomers of the first block and of the second block.
- The term “essentially” means at least 85%, preferably at least 90%, better still 95% and even better still 100%.
- Advantageously, the intermediate block has a glass transition temperature Tg that is between the glass transition temperatures of the first and second blocks.
- The block polymer according to the invention is advantageously a film-forming block ethylenic polymer.
- The term “ethylenic polymer” means a polymer obtained by polymerization of ethylenically unsaturated monomers.
- The term “film-forming polymer” means a polymer that is capable of forming, by itself or in the presence of a film-forming auxiliary agent, a continuous film that adheres to a support, especially to keratin materials.
- Preferentially, the polymer used in the composition according to the invention comprises no silicon atoms in its backbone. The term “backbone” means the main chain of the polymer, as opposed to the pendent side chains.
- Preferably, the polymer according to the invention is not water-soluble, i.e. the polymer is not soluble in water or in a mixture of water and of linear or branched lower monoalcohols containing from 2 to 5 carbon atoms, for instance ethanol, isopropanol or n-propanol, without pH modification, at an active material content of at least 1% by weight, at room temperature (25° C.).
- Preferably, the polymer according to the invention is not an elastomer.
- The term “non-elastomeric polymer” means a polymer which, when it is subjected to a constraint intended to pull it (for example by 30% relative to its initial length), does not return to a length substantially identical to its initial length when the constraint ceases.
- More specifically, the term “non-elastomeric polymer” denotes a polymer with an instantaneous recovery Ri<50% and a delayed recovery R2h<70% after having been subjected to a 30% elongation. Preferably, Ri is <30% and R2h<50%.
- More specifically, the non-elastomeric nature of the polymer is determined according to the following protocol:
- A polymer film is prepared by pouring a solution of the polymer in a Teflon-coated mould, followed by drying for 7 days in an environment conditioned at 23±5° C. and 50±10% relative humidity.
- A film about 100 μm thick is thus obtained, from which are cut rectangular specimens (for example using a punch) 15 mm wide and 80 mm long.
- This sample is subjected to a tensile stress using a machine sold under the reference Zwick, under the same temperature and humidity conditions as for the drying.
- The specimens are pulled at a speed of 50 mm/min and the distance between the jaws is 50 mm, which corresponds to the initial length (l0) of the specimen.
- The instantaneous recovery Ri is determined in the following manner:
-
- the specimen is pulled by 30% (εmax), i.e. about 0.3 times its initial length (10)
- the constraint is released by applying a return speed equal to the tensile speed, i.e. 50 mm/min, and the residual elongation of the specimen is measured as a percentage, after returning to zero constraint (εi).
- The percentage instantaneous recovery (Ri) is given by the following formula:
-
R i=(εmax−εi)/(εmax)×100 - To determine the delayed recovery, the percentage residual elongation of the specimen (ε2h) is measured after 2 hours (2 hours after returning to zero stress load).
- The percentage delayed recovery (R2h) is given by the following formula:
-
R 2h=(εmax−ε2h)/(εmax)×100 - Purely as a guide, a polymer according to one embodiment of the invention has an instantaneous recovery Ri of 10% and a delayed recovery R2h of 30%.
- The polydispersity index of the polymer of the invention is advantageously greater than 2.
- The polydispersity index I of the polymer is equal to the ratio of the weight-average mass Mw to the number-average mass Mn.
- The weight-average molar mass (Mw) and number-average molar mass (Mn) are determined by gel permeation liquid chromatography (THF solvent, calibration curve established with linear polystyrene standards, refractometric detector).
- The weight-average mass (Mw) of the polymer according to the invention is preferably less than or equal to 300 000; it ranges, for example, from 35 000 to 200 000 and better still from 45 000 to 150 000 g/mol.
- The number-average mass (Mn) of the polymer according to the invention is preferably less than or equal to 70 000; it ranges, for example, from 10 000 to 60 000 and better still from 12 000 to 50 000 g/mol.
- Preferably, the polydispersity index of the polymer according to the invention is advantageously greater than 2, for example ranging from 2 to 9, preferably greater than or equal to 2.5, for example ranging from 2.5 to 8, and better still greater than or equal to 2.8, especially from 2.8 to 6.
- The block polymer of the invention comprises at least a first block and at least a second block.
- The first block is advantageously obtained from at least one acrylate monomer of formula CH2═CH—COOR2 and from at least one methacrylate monomer of formula CH2═C(CH3)—COOR2 in which R2 represents a C4 to C12 cycloalkyl group. The monomers and the proportions thereof are preferably chosen such that the glass transition temperature of the first block is greater than 20° C.
- The second block is advantageously obtained from an acrylic acid monomer and from at least one monomer with a glass transition temperature of less than or equal to 20° C.
- The monomers and the proportions thereof are preferably chosen such that the glass transition temperature of the second block is less than or equal to 20° C.
- The glass transition temperatures indicated for the first and second blocks may be theoretical Tg values determined from the theoretical Tg values of the constituent monomers of each of the blocks, which may be found in a reference manual such as the Polymer Handbook, 3rd Edition, 1989, John Wiley, according to the following relationship, known as Fox's law:
-
-
ω i being the mass fraction of the monomer i in the block under consideration and Tgi being the glass transition temperature of the homopolymer of the monomer i. - Unless otherwise indicated, the Tg values indicated for the first and second blocks in the present patent application are theoretical Tg values.
- The difference between the glass transition temperatures of the first and second blocks is generally greater than 10° C., preferably greater than 20° C. and better still greater than 30° C.
- In the present invention, the expression: “between . . . and . . . ” is intended to denote a range of values for which the limits mentioned are excluded, and “from . . . to . . . ” and “ranging from . . . to . . . ” are intended to denote a range of values for which the limits are included.
- First Block
- The first block preferably has a Tg of greater than 20° C., for example a Tg ranging from 20 to 170° C. and preferably greater than or equal to 50° C., for example ranging from 50° C. to 160° C., especially ranging from 90° C. to 130° C.
- According to one embodiment, the first block is obtained from at least one acrylate monomer of formula CH2═CH—COOR2 in which R2 represents a C4 to C12 cycloalkyl group, and from at least one methacrylate monomer of formula CH2═C(CH3)—COOR′2 in which R′2 represents a C4 to C12 cycloalkyl group.
- The first block may be obtained exclusively from the said acrylate monomer and from the said methacrylate monomer.
- The acrylate monomer and the methacrylate monomer are preferably in mass proportions of between 30/70 and 70/30, preferably between 40/50 and 50/40 and especially of the order of 50/50.
- The proportion of the first block advantageously ranges from 20% to 90%, better still from 30% to 80% and even better still from 60% to 80% by weight of the polymer.
- According to one embodiment, the first block is obtained by polymerization of isobornyl methacrylate and isobornyl acrylate.
- The first block may also comprise:
-
- (meth)acrylic acid, preferably acrylic acid,
- tert-butyl acrylate,
- the methacrylates of formula CH2═C(CH3)—COOR1, in which R1 represents a linear or branched unsubstituted alkyl group containing from 1 to 4 carbon atoms, such as a methyl, ethyl, propyl or isobutyl group,
- the (meth)acrylamides of formula:
- in which R7 and R8, which may be identical or different, each represent a hydrogen atom or a linear or branched C1 to C12 alkyl group, such as an n-butyl, t-butyl, isopropyl, isohexyl, isooctyl or isononyl group; or R7 represents H and R8 represents a 1,1-dimethyl-3-oxobutyl group,
and R′ denotes H or methyl. Examples of monomers that may be mentioned include N-butylacrylamide, N-t-butylacrylamide, N-isopropylacrylamide, N,N-dimethylacrylamide and N,N-dibutylacrylamide, -
- and mixtures thereof.
- Second Block
- The second block advantageously has a glass transition temperature Tg of less than or equal to 20° C., for example a Tg ranging from −100 to 20° C., preferably less than or equal to 15° C., especially ranging from −80° C. to 15° C. and better still less than or equal to 10° C., for example ranging from −100° C. to 10° C., especially ranging from −30° C. to 10° C.
- The second block is obtained from an acrylic acid monomer and from another monomer with a Tg of less than or equal to 20° C.
- The monomer with a Tg of less than or equal to 20° C. is preferably chosen from the following monomers:
-
- the acrylates of formula CH2═CH—COOR3,
R3 representing a linear or branched, unsubstituted C1-C12 alkyl group, with the exception of a tert-butyl group, in which is (are) optionally intercalated one or more heteroatoms chosen from O, N and S; - the methacrylates of formula CH2═C(CH3)—COOR4,
R4 representing a linear or branched, unsubstituted C6-C12 alkyl group, in which is (are) optionally intercalated one or more heteroatoms chosen from O, N and S; - vinyl esters of formula R5—CO—O—CH═CH2,
in which R5 represents a linear or branched C4-C12 alkyl group; - (C4-C12 alkyl) vinyl ethers;
- N—(C4-C12 alkyl)acrylamides, such as N-octylacrylamide;
- and mixtures thereof.
- the acrylates of formula CH2═CH—COOR3,
- The preferred monomers with a Tg of less than or equal to 20° C. are isobutyl acrylate and 2-ethylhexyl acrylate, or mixtures thereof in all proportions.
- Each of the first and second blocks may contain in minor proportion at least one constituent monomer of the other block.
- Thus, the first block may contain at least one constituent monomer of the second block, and vice versa.
- Each of the first and/or second blocks may comprise, in addition to the monomers indicated above, one or more other monomers known as additional monomers, which are different from the main monomers mentioned above.
- The nature and amount of this or these additional monomer(s) are chosen such that the block in which they are present has the desired glass transition temperature.
- This additional monomer is chosen, for example, from:
-
- ethylenically unsaturated monomers comprising at least one tertiary amine function, for instance 2-vinylpyridine, 4-vinylpyridine, dimethylaminoethyl methacrylate, diethylaminoethyl methacrylate and dimethylaminopropylmethacrylamide, and salts thereof,
- methacrylates of formula CH2═C(CH3)—COOR6,
in which R6 represents a linear or branched alkyl group containing from 1 to 4 carbon atoms, such as a methyl, ethyl, propyl or isobutyl group, the said alkyl group being substituted with one or more substituents chosen from hydroxyl groups (for instance 2-hydroxypropyl methacrylate and 2-hydroxyethyl methacrylate) and halogen atoms (Cl, Br, I or F), such as trifluoroethyl methacrylate, - methacrylates of formula CH2═C(CH3)—COOR9,
- R9 representing a linear or branched C6 to C12 alkyl group in which one or more heteroatoms chosen from O, N and S is (are) optionally intercalated, the said alkyl group being substituted with one or more substituents chosen from hydroxyl groups and halogen atoms (Cl, Br, I or F);
-
- acrylates of formula CH2═CHCOOR10,
- R10 representing a linear or branched C1 to C12 alkyl group substituted with one or more substituents chosen from hydroxyl groups and halogen atoms (Cl, Br, I or F), such as 2-hydroxypropyl acrylate and 2-hydroxyethyl acrylate, or R10 represents a C1 to C12 alkyl-O-POE (polyoxyethylene) with repetition of the oxyethylene unit 5 to 30 times, for example methoxy-POE, or R10 represents a polyoxyethylenated group comprising from 5 to 30 ethylene oxide units.
- The additional monomer may represent 0.5% to 30% by weight relative to the weight of the polymer. According to one embodiment, the polymer of the invention does not contain any additional monomer.
- Preferably, the polymer of the invention comprises at least one of the isobornyl acrylate and isobornyl methacrylate monomers in the first block and isobutyl acrylate and acrylic acid monomers in the second block.
- Preferably, the polymer comprises at least one of the isobornyl acrylate and isobornyl methacrylate monomers in equivalent weight proportion in the first block and isobutyl acrylate and acrylic acid monomers in the second block.
- Preferably, the polymer comprises at least isobornyl acrylate and isobornyl methacrylate monomers in equivalent weight proportion in the first block, and isobutyl acrylate and acrylic acid monomers in the second block, the first block representing 70% of the weight of the polymer.
- Preferably, the polymer comprises at least isobornyl acrylate and isobornyl methacrylate monomers in equivalent weight proportion in the first block, and isobutyl acrylate and acrylic acid monomers in the second block, the block with a Tg of greater than 20° C. representing 70% of the weight of the polymer, and acrylic acid representing 5% of the weight of the polymer.
- Vinyl Polymer Grafted with a Carbosiloxane Dendrimer
- According to a second embodiment of the invention, the film-forming polymer present in the composition according to the invention is a vinyl polymer comprising at least one carbosiloxane dendrimer-based unit.
- The vinyl polymer may especially have a backbone and at least one side chain, which comprises a carbosiloxane dendrimer structure. The term “carbosiloxane dendrimer structure” in the context of the present invention represents a structure with branched groups of high molecular masses with high regularity in the radial direction starting from the simple backbone. Such carbosiloxane dendrimer structures are described in the form of a highly branched siloxane-silylalkylene copolymer in the laid-open Japanese patent application Kokai 9-171 154.
- The vinyl polymer contains carbosiloxane dendrimer-based units that may be represented by the following general formula:
- in which R1 represents an aryl group or an alkyl group containing from 1 to 10 carbon atoms, and X′ represents a silylalkyl group which, when i=1, is represented by the formula:
- in which R1 is the same as defined above, R2 represents an alkylene group containing from 2 to 10 carbon atoms, R3 represents an alkyl group containing from 1 to 10 carbon atoms, Xi+1 represents a hydrogen atom, an alkyl group containing from 1 to 10 carbon atoms, an aryl group or the silylalkyl group defined above with i=i+1; i is an integer from 1 to 10 which represents the generation of the said silylalkyl group, and ai is an integer from 0 to 3; Y represents an organic group that may be polymerized using radicals chosen from the group consisting of an organic group that contains a methacrylic group or an acrylic group and that is represented by the formulae:
- in which R4 represents a hydrogen atom or an alkyl group, R5 represents an alkylene group containing from 1 to 10 carbon atoms, such as a methylene group, an ethylene group, a propylene group or a butylene group, the methylene group and the propylene group being preferred; and
- an organic group that contains a styryl group and that is represented by the formula:
- in which R6 represents a hydrogen atom or an alkyl group, R7 represents an alkyl group containing from 1 to 10 carbon atoms, such as a methyl group, an ethyl group, a propyl group or a butyl group, the methyl group being preferred, R8 represents an alkylene group containing from 1 to 10 carbon atoms, such as a methylene group, an ethylene group, a propylene group or a butylene group, the ethylene group being preferred, b is an integer from 0 to 4, and c is 0 or 1 such that if c is 0, —(R8)c— represents a bond,
- R1 represents an aryl group or an alkyl group containing from 1 to 10 carbon atoms, in which the alkyl group is preferably represented by a methyl group, an ethyl group, a propyl group, a butyl group, a pentyl group, an isopropyl group, an isobutyl group, a cyclopentyl group or a cyclohexyl group, and in which the aryl group is preferably represented by a phenyl group and a naphthyl group, in which the methyl and phenyl groups are more particularly preferred, and the methyl group is preferred among all.
- The vinyl polymer that contains a carbosiloxane dendrimer structure may be the product of polymerization of
- (A) from 0 to 99.9 parts by weight of a monomer of vinyl type; and
- (B) from 100 to 0.1 parts by weight of a carbosiloxane dendrimer containing an organic group that may be polymerized using radicals, represented by the general formula:
- in which Y represents an organic group that may be polymerized using radicals, R1 represents an aryl group or an alkyl group containing from 1 to 10 carbon atoms, and X1 represents a silylalkyl group which, when i=1, is represented by the formula:
- in which R1 is the same as defined above, R2 represents an alkylene group containing from 2 to 10 carbon atoms, R3 represents an alkyl group containing from 1 to 10 carbon atoms, Xi+1 represents a hydrogen atom, an alkyl group containing from 1 to 10 carbon atoms, an aryl group, or the silylalkyl group defined above with i=i+1; i is an integer from 1 to 10 that represents the generation of the said silylalkyl group, and ai is an integer from 0 to 3; in which the said organic group that may be polymerized with radicals contained in the component (B) is chosen from the group consisting of an organic group that contains a methacrylic group or an acrylic group and that is represented by the formulae:
- in which R4 represents a hydrogen atom or an alkyl group, R5 represents an alkylene group containing from 1 to 10 carbon atoms; and
- an organic group that contains a styryl group and that is represented by the formula:
- in which R6 represents a hydrogen atom or an alkyl group, R7 represents an alkyl group containing from 1 to carbon atoms, R8 represents an alkylene group containing from 1 to 10 carbon atoms, b is an integer from 0 to 4, and c is 0 or 1. When c is 0, —(R8)c-represents a bond.
- The monomer of vinyl type that is the component (A) in the vinyl polymer is a monomer of vinyl type that contains a radical-polymerizable vinyl group. There is no particular limitation as regards the type of such a monomer. The following are examples of this type of vinyl monomer: methyl methacrylate, ethyl methacrylate, n-propyl methacrylate, isopropyl methacrylate or a methacrylate of a lower alkyl analogue; glycidyl methacrylate; n-butyl methacrylate, isobutyl methacrylate, tert-butyl methacrylate, n-hexyl methacrylate, methacrylic acid, cyclohexyl methacrylate, 2-ethylhexyl methacrylate, octyl methacrylate, lauryl methacrylate, stearyl methacrylate or a higher-analogue methacrylate; vinyl acetate, vinyl propionate or a vinyl ester of a lower fatty acid analogue; vinyl caproate, vinyl 2-ethylhexoate, vinyl laurate, vinyl stearate or an ester of a higher fatty acid analogue; styrene, vinyltoluene, benzyl methacrylate, phenoxyethyl methacrylate, vinyl-pyrrolidone or similar vinylaromatic monomers; methacrylamide, N-methylolmethacrylamide, N-methoxy-methylmethacrylamide, isobutoxymethoxymethacrylamide, N,N-dimethylmethacrylamide or similar monomers of vinyl type containing amide groups; hydroxyethyl methacrylate, hydroxypropyl methacrylate or similar monomers of vinyl type containing hydroxyl groups; methacrylic acid, itaconic acid, crotonic acid, fumaric acid, maleic acid or similar monomers of vinyl type containing a carboxylic acid group; tetrahydrofurfuryl methacrylate, butoxyethyl methacrylate, ethoxydiethylene glycol methacrylate, polyethylene glycol methacrylate, polypropylene glycol monomethacrylate, hydroxybutyl vinyl ether, cetyl vinyl ether, 2-ethylhexyl vinyl ether or a similar monomer of vinyl type with ether bonds; methacryloxypropyl-trimethoxysilane, polydimethylsiloxane containing a methacrylic group on one of its molecular ends, polydimethylsiloxane containing a styryl group on one of its molecular ends, or a similar silicone compound containing unsaturated groups; butadiene; vinyl chloride; vinylidene chloride; methacrylonitrile; dibutyl fumarate; anhydrous maleic acid; anhydrous succinic acid; methacryl glycidyl ether; an organic salt of an amine, an ammonium salt, and an alkali metal salt of methacrylic acid, of itaconic acid, of crotonic acid, of maleic acid or of fumaric acid; a radical-polymerizable unsaturated monomer containing a sulfonic acid group such as a styrenesulfonic acid group; a quaternary ammonium salt derived from methacrylic acid, such as 2-hydroxy-3-methacryloxypropyltrimethylammonium chloride; and a methacrylic acid ester of an alcohol containing a tertiary amine group, such as a methacrylic acid ester of diethylamine.
- Multifunctional monomers of vinyl type may also be used. The following represent examples of such compounds: trimethylolpropane trimethacrylate, pentaerythrityl trimethacrylate, ethylene glycol dimethacrylate, tetraethylene glycol dimethacrylate, polyethylene glycol dimethacrylate, 1,4-butanediol dimethacrylate, 1,6-hexanediol dimethacrylate, neopentyl glycol dimethacrylate, trimethylolpropane-trioxyethyl methacrylate, tris(2-hydroxyethyl)-isocyanurate dimethacrylate, tris(2-hydroxyethyl)-isocyanurate trimethacrylate, polydimethylsiloxane capped with styryl groups containing divinylbenzene groups on both ends, or similar silicone compounds containing unsaturated groups.
- The carbosiloxane dendrimer, which is the component (B), is represented by the following formula:
- The following represent the preferred examples of radical-polymerizable organic group Y: an acryloxymethyl group, a 3-acryloxypropyl group, a methacryloxymethyl group, a 3-methacryloxypropyl group, a 4-vinylphenyl group, a 3-vinylphenyl group, a 4-(2-propenyl)phenyl group, a 3-(2-propenyl)phenyl group, a 2-(4-vinylphenyl)ethyl group, a 2-(3-vinyl-phenyl)ethyl group, a vinyl group, an allyl group, a methallyl group and a 5-hexenyl group.
- R1 represents an alkyl group or an aryl group containing from 1 to 10 carbon atoms, in which the alkyl group may be a methyl group, an ethyl group, a propyl group, a butyl group, a pentyl group, an isopropyl group, an isobutyl group, a cyclopentyl group or a cyclohexyl group; and the aryl group may be a phenyl group or a naphthyl group. The methyl and phenyl groups are particularly preferred, the methyl group being preferred among all. X1 represents a silylalkyl group that is represented by the following formula, when i is equal to 1:
- in which R2 represents an alkylene group containing from 2 to 10 carbon atoms, such as an ethylene group, a propylene group, a butylene group, a hexylene group or a similar linear alkylene group; a methylmethylene group, a methylethylene group, a 1-methylpentylene group, a 1,4-dimethylbutylene group or a similar branched alkylene group. The ethylene, methylethylene, hexylene, 1-methylpentylene and 1,4-dimethylbutylene groups are preferred among all. R3 represents an alkyl group containing from 1 to 10 carbon atoms, such as methyl, ethyl, propyl, butyl and isopropyl groups. R1 is the same as defined above. Xi+1 represents a hydrogen atom, an alkyl group containing from 1 to 10 carbon atoms, an aryl group or the silylalkyl group with i=i+1. ai is an integer from 0 to 3, and i is an integer from 1 to 10 that indicates the generation number, which represents the number of repetitions of the silylalkyl group.
- For example, when the generation number is equal to 1, the carbosiloxane dendrimer may be represented by the first general formula shown below, in which Y, R1, R2 and R3 are the same as defined above, R12 represents a hydrogen atom or is identical to R1; a1 is identical to ai. Preferably, the mean total number of groups OR3 in a molecule is within the range from 0 to 7. When the generation number is equal to 2, the carbosiloxane dendrimer may be represented by the second general formula shown below, in which Y, R1, R2, R3 and R12 are the same as defined above; a1 and a2 represent the ai of the indicated generation. Preferably, the mean total number of groups OR3 in a molecule is within the range from 0 to 25. When the generation number is equal to 3, the carbosiloxane dendrimer is represented by the third general formula shown below, in which Y, R2, R3 and R12 are the same as defined above; a1, a2 and a3 represent the ai of the indicated generation. Preferably, the total mean number of groups OR3 in a molecule is within the range from 0 to 79.
- A carbosiloxane dendrimer that contains a radical-polymerizable organic group may be represented by the following mean structural formulae:
- The carbosiloxane dendrimer may be manufactured according to the process for manufacturing a branched silalkylene siloxane described in Japanese patent application Hei 9-171 154. For example, it may be produced by subjecting an organosilicon compound containing a hydrogen atom linked to a silicon atom, represented by the following general formula:
- and an organosilicon compound containing an alkenyl group, to a hydrosilylation reaction. In the above formula, the organosilicon compound may be represented by 3-methacryloxypropyltris(dimethyl-siloxy)silane, 3-acryloxypropyltris(dimethylsiloxy)-silane and 4-vinylphenyltris(dimethylsiloxy)silane. The organosilicon compound that contains an alkenyl group may be represented by vinyltris(trimethylsiloxy)silane, vinyltris(dimethylphenylsiloxy)silane, and 5-hexenyl-tris(trimethylsiloxy)silane. The hydrosilylation reaction is performed in the presence of a chloroplatinic acid, a complex of vinylsiloxane and of platinum, or a similar transition metal catalyst.
- In the vinyl polymer that contains a dendrimer structure, the polymerization ratio between the components (A) and (B), in terms of the weight ratio between (A) and (B), may be within the range from 0/100 to 99.9/0.1 and preferably within the range from 1/99 to 99/1. A ratio between the components (A) and (B) of 0/100 means that the compound becomes a homopolymer of component (B).
- The vinyl polymer contains a carbosiloxane dendrimer structure and this polymer may be obtained by copolymerization of the components (A) and (B), or by polymerization of the component (B) alone. The polymerization may be a free-radical polymerization or an ionic polymerization, but free-radical polymerization is preferred. The polymerization may be performed by bringing about a reaction between the components (A) and (B) in a solution for a period of from 3 to 20 hours in the presence of a radical initiator at a temperature of from 50° C. to 150° C. A suitable solvent for this purpose is hexane, octane, decane, cyclohexane or a similar aliphatic hydrocarbon; benzene, toluene, xylene or a similar aromatic hydrocarbon; diethyl ether, dibutyl ether, tetrahydrofuran, dioxane or similar ethers; acetone, methyl ethyl ketone, methyl isobutyl ketone, diisobutyl ketone or similar ketones; methyl acetate, ethyl acetate, butyl acetate, isobutyl acetate or similar esters; methanol, ethanol, isopropanol, butanol or similar alcohols; octamethylcyclotetrasiloxane, decamethylcyclopentasiloxane, hexamethyldisiloxane, octamethyltrisiloxane or a similar organosiloxane oligomer. A radical initiator may be any compound known in the art for standard free-radical polymerization reactions. The specific examples of such radical initiators are 2,2′-azobis(isobutyronitrile), 2,2′-azobis(2-methylbutyronitrile), 2,2′-azobis(2,4-dimethylvaleronitrile) or similar compounds of azobis type; benzoyl peroxide, lauroyl peroxide, tert-butyl peroxybenzoate, tert-butyl peroxy-2-ethylhexanoate or a similar organic peroxide. These radical initiators may be used alone or in a combination of two or more. The radical initiators may be used in an amount of from 0.1 to 5 parts by weight per 100 parts by weight of the components (A) and (B). A chain-transfer agent may be added. The chain-transfer agent may be 2-mercaptoethanol, butyl mercaptan, n-dodecyl mercaptan, 3-mercaptopropyltrimethoxysilane, a polydimethyl-siloxane containing a mercaptopropyl group or a similar compound of mercapto type; methylene chloride, chloroform, carbon tetrachloride, butyl bromide, 3-chloropropyltrimethoxysilane or a similar halogenated compound. In the manufacture of the polymer of vinyl type, after the polymerization, the residual unreacted vinyl monomer may be removed under conditions of heating under vacuum.
- To facilitate the preparation of the mixture of the starting material of cosmetic products, the number-average molecular mass of the vinyl polymer containing a carbosiloxane dendrimer may be chosen within the range between 3000 and 2 000 000 and preferably between 5000 and 800 000. It may be a liquid, a gum, a paste, a solid, a powder or any other form. The preferred forms are solutions consisting of the dilution of a dispersion or of a powder in solvents.
- The vinyl polymer may be a dispersion of a polymer of vinyl type having a carbosiloxane dendrimer structure in its side molecular chain, in a liquid such as a silicone oil, an organic oil, an alcohol or water.
- The vinyl polymer having a carbosiloxane dendrimer structure in its side molecular chain, in this embodiment, is the same as that described above. The liquid may be a silicone oil, an organic oil, an alcohol or water. The silicone oil may be a dimethylpolysiloxane with the two molecular ends capped with trimethylsiloxy groups, a copolymer of methylphenylsiloxane and of dimethylsiloxane having the two molecular ends capped with trimethylsiloxy groups, a copolymer of methyl-3,3,3-trifluoropropylsiloxane and of dimethylsiloxane having the two molecular ends capped with trimethylsiloxy groups, or similar unreactive linear silicone oils, and also hexamethyl-cyclotrisiloxane, octamethylcyclotetrasiloxane, decamethylcyclopentasiloxane, dodecamethylcyclo-hexasiloxane or a similar cyclic compound. In addition to the unreactive silicone oils, modified polysiloxanes containing functional groups such as silanol groups, amino groups and polyether groups on the ends or within the side molecular chains may be used.
- The organic oils may be liquid paraffin, isoparaffin, hexyl laurate, isopropyl myristate, myristyl myristate, cetyl myristate, 2-octyldodecyl myristate; isopropyl palmitate, 2-ethylhexyl palmitate, butyl stearate, decyl oleate, 2-octyldodecyl oleate, myristyl lactate, cetyl lactate, lanolin acetate, stearyl alcohol, cetostearyl alcohol, oleyl alcohol, avocado oil, almond oil, olive oil, cocoa oil, jojoba oil, gum oil, sunflower oil, soybean oil, camellia oil, squalane, castor oil, mink oil, cottonseed oil, coconut oil, egg yolk oil, beef tallow, lard, polypropylene glycol monooleate, neopentyl glycol 2-ethylhexanoate or a similar glycol ester oil; triglyceryl isostearate, the triglyceride of a fatty acid of coconut oil, or a similar oil of a polyhydric alcohol ester; polyoxyethylene lauryl ether, polyoxypropylene cetyl ether or a similar polyoxyalkylene ether.
- The alcohol may be any type that is suitable for use in combination with a cosmetic product starting material. For example, it may be methanol, ethanol, butanol, isopropanol or similar lower alcohols. A solution or a dispersion of the alcohol should have a viscosity within the range from 10 to 109 mPa at 25° C. To improve the sensory use properties in a cosmetic product, the viscosity should be within the range from 100 to 5×108 mPa·s.
- The solutions and dispersions may be readily prepared by mixing the vinyl polymer having a carbosiloxane dendrimer structure with a silicone oil, an organic oil, an alcohol or water. The liquids may be present in the step of polymerization of the polymer of vinyl type having a carbosiloxane dendrimer structure. In this case, the unreacted residual vinyl monomer should be completely removed by heat treatment of the solution or dispersion under atmospheric pressure or reduced pressure. In the case of a dispersion, the dispersity of the polymer of vinyl type may be improved by adding a surfactant. Such an agent may be hexyl-benzenesulfonic acid, octylbenzenesulfonic acid, decylbenzenesulfonic acid, dodecylbenzenesulfonic acid, cetylbenzenesulfonic acid, myristylbenzenesulfonic acid or anionic surfactants of the sodium salts of these acids; octyltrimethylammonium hydroxide, dodecyltrimethylammonium hydroxide, hexadecyltrimethyl-ammonium hydroxide, octyldimethylbenzylammonium hydroxide, decyldimethylbenzylammonium hydroxide, dioctadecyldimethylammonium hydroxide, beef tallow-trimethylammonium hydroxide, coconut oil-trimethylammonium hydroxide, or a similar cationic surfactant; a polyoxyalkylene alkyl ether, a polyoxyalkylenealkylphenol, a polyoxyalkylene alkyl ester, the sorbitol ester of polyoxyalkylene, polyethylene glycol, polypropylene glycol, an ethylene oxide additive of diethylene glycol trimethylnonanol, and nonionic surfactants of polyester type, and also mixtures. In addition, the solvents and dispersions may be combined with iron oxide suitable for use with cosmetic products, or a similar pigment, and also zinc oxide, titanium oxide, silicon oxide, mica, talc or similar mineral oxides in powder form. In the dispersion, a mean particle diameter of the polymer of vinyl type may be within a range of between 0.001 and 100 microns and preferably between 0.01 and 50 microns. The reason for this is that, outside the recommended range, a cosmetic product mixed with the emulsion will not have a nice enough feel on the skin or to the touch, or sufficient spreading properties or a pleasant feel.
- The vinyl polymer contained in the dispersion or the solution may have a concentration in the range between 0.1% and 95% by weight and preferably between 5% and 85% by weight. However, to facilitate the handling and the preparation of the mixture, the range should preferably be between 10% and 75% by weight.
- The vinyl polymer may be one of the polymers described in the examples of patent application EP 0 963 751 or, for example, the product TIB-4-200 sold by Dow Corning.
- According to one embodiment, the vinyl polymer also comprises at least one organofluorine group.
- Structures in which the polymerized vinyl units constitute the backbone and carbosiloxane dendritic structures and also organofluorine groups are attached to side chains are particularly preferred.
- The organofluorine groups may be obtained by replacing with fluorine atoms all or some of the hydrogen atoms of methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tert-butyl, pentyl, neopentyl, hexyl, cyclohexyl, heptyl, octyl, nonyl, decyl, undecyl, dodecyl, tridecyl, tetradecyl, hexadecyl and octadecyl groups and other alkyl groups of 1 to 20 carbon atoms, and also alkyloxyalkylene groups of 6 to 22 carbon atoms.
- The groups represented by the formula: —(CH2)x—(CF2)y—R13 are suggested as examples of fluoroalkyl groups obtained by substituting fluorine atoms for hydrogen atoms of alkyl groups. In the formula, the index “x” is 0, 1, 2 or 3 and “y” is an integer from 1 to 20. R13 is an atom or a group chosen from a hydrogen atom, a fluorine atom, —CH(CF3)2— and CF(CF3)2. Such fluorine-substituted alkyl groups are exemplified by linear or branched polyfluoroalkyl or perfluoroalkyl groups represented by the formulae presented below.
- —CF3, —C2F5, -nC3F7, —CF (CF3)2, -nC4F9, CF2CF(CF3)2,-nC5F11, -nC6F13, -nC8F17, —CH2CF3, —CH(CF3)2, CH2CH(CF3)2—CH2(CF2)2F, —CH2(CF2)3F, —CH2(CF2)4F, —CH2(CF2)6F, —CH2(CF2)8F, —CH2CH2CF3, —CH2CH2(CF2)2F, —CH2CH2(CF2)3F, —CH2CH2(CF2)4F, —CH2CH2(CF2)6F, —CH2CH2(CF2)8F, —CH2CH2(CF2)10F, —CH2CH2(CF2)12F, —CH2CH2(CF2)14F, —CH2CH2(CF2)16F, —CH2CH2CH2CF3, —CH2CH2CH2(CF2)2F, —CH2CH2CH2(CF2)2H, —CH2(CF2)4H, and —CH2CH2(CF2)3H.
- The groups represented by —CH2CH2— (CF2)m—CFR14—[OCF2CF (CF3)]n—OC3F7 are suggested as fluoroalkyloxyfluoroalkylene groups obtained by substituting fluorine atoms for hydrogen atoms of alkyloxyalkylene groups. In the formula, the index “m” is 0 or 1, “n” is 0, 1, 2, 3, 4 or 5, and R14 is a fluorine atom CF3. Such fluoroalkyloxyfluoroalkylene groups are exemplified by the perfluoroalkyloxy-fluoroalkylene groups represented by the formulae presented below:
-
—CH2CH2CF(CF3)—[OCF2CF(CF3)]n—OC3F7, —CH2CH2CF2CF2—[OCF2CF(CF3)]n—OC3F7. - The number-average molecular weight of the vinyl polymer used in the present invention may be between 3000 and 2 000 000 and more preferably between 5000 and 800 000.
- This type of fluorinated vinyl polymer may be obtained by addition
-
- of a vinyl monomer (B) not containing any organofluorine groups in the molecule
- to a vinyl monomer containing organofluorine groups in the molecule (A), and
- a carbosiloxane dendrimer (C) containing radical-polymerizable organic groups represented by the general formula (III):
- in which Y is a radical-polymerizable organic group and R1 and Xi are as above, and by subjecting them to a copolymerization.
- The vinyl monomers (A) containing organofluorine groups in the molecule are preferably monomers represented by the general formula: —(CH2)═CR15COORf. In the formula, R15 is a hydrogen atom or a methyl group and Rf is an organofluorine group exemplified by the fluoroalkyl and fluoroalkyloxyfluoroalkylene groups described above. The compounds represented by the formulae presented below are suggested as specific examples of the component (A). In the formulae presented below “z” is an integer from 1 to 4.
- CH2═CCH3COO—CF3. CH2═CCH3COO—C2F5. CH2═CCH3COO-nC3F7 CH2 CCH3COO—CF(CF3)2. CH2═CCH3COO-nC4F9. CH2═CCH3COO—CF2CF (CF3)2. CH2═CCH3COO-nC5F11. CH2═CCH3COO-nC6F13. CH2═CCH3COO-nC8F17. CH2═CCH3COO—CH2CF3. CH2═CCH3COO—CH(CF3)2. CH2═CCH3COO—CH2CH(CF3)2. CH2═CCH3COO—CH2(CF2)2F. CH2═CCH3COO—CH2(CF2)3F. CH2═CCH3COO—CH2(CF2)4F. CH2═CCH3COO—CH2(CF2)6F. CH2═CCH3COO—CH2(CF2)8F. CH2═CCH3COO—CH2CH2CF3. CH2═CCH3COO—CH2CH2(CF2)2F. CH2═CCH3COO—CH2CH2(CF2)3F. CH2═CCH3COO—CH2CH2(CF2)4F. CH2═CCH3COO—CH2CH2(CF2)6F. CH2═CCH3COO—CH2CH2(CF2)8F. CH2═CCH3COO—CH2CH2(CF2)10F. CH2═CCH3COO—CH2CH2(CF2)12F. CH2═CCH3COO—CH2CH2(CF2)14F. CH2═CCH3COO—CH2CH2(CF2)16F. CH2═CCH3COO—CH2CH2CH2CF3. CH2═CCH3COO—CH2CH2CH2(CF2)2F. CH2 CCH3COO—CH2CH2CH2(CF2)2H. CH2═CCH3COO—CH2(CF2)4H. CH2═CCH3COO—CH2CH2(CF2)3H. CH2═CCH3COO—CH2CH2CF (CF3)—[OCF2CF(CF3)]z—OC3F7. CH2═CCH3COO—CH2CH2CF2CF2[OCF2CF (CF3)]z—OC3F7. CH2═CHCOO—CF3. CH2═CHCOO—C2F5. CH2═CHCOO-nC3F7. CH2═CHCOO—CF (CF3)2. CH2═CHCOO-nC4F9. CH2═CHCOO—CF2CF (CF3)2. CH2═CHCOO-nC5F11. CH2═CHCOO-nC6F13. CH2═CHCOO-nC8F17. CH2═CHCOO—CH2CF3. CH2═CHCOO—CH(CF3)2. CH2═CHCOO—CH2CH(CF3)2. CH2═CHCOO—CH2(CF2)2F. CH2═CHCOO—CH2(CF2)3F. CH2═CHCOO—CH2(CF2)4F. CH2═CHCOO—CH2(CF2)6F. CH2═CHCOO—CH2(CF2)8F. CH2═CHCOO—CH2CH2CF3. CH2═CHCOO—CH2CH2(CF2)2F. CH2═CHCOO—CH2CH2(CF2)3F. CH2═CHCOO—CH2CH2(CF2)4F. CH2═CHCOO—CH2CH2(CF2)6F. CH2═CHCOO—CH2CH2(CF2). CH2═CHCOO—CH2CH2(CF2)10F. CH2═CHCOO—CH2CH2(CF2)12F. CH2═CHCOO—CH2CH2(CF2)14F. CH2═CHCOO—CH2CH2(CF2)16F. CH2═CHCOO—CH2CH2CH2CF3. CH2═CHCOO—CH2CH2CH2(CF2)2F. CH2═CHCOO—CH2CH2CH2(CF)2H. CH2═CHCOO—CH2(CF2)4H. CH2═CHCOO—CH2CH2(CF2)3H. CH2═CHCOO—CH2CH2CF(CF3)—[OCF2CF(CF3)]z—OC3F7. CH2═CHCOO—CH2CH2CF2CF2—[OCF2CF (CF3)]z—OC3F7.
- Among these, the vinyl polymers represented by the formulae presented below are preferable:
- CH2═CHCOO—CH2CH2(CF2)6F. CH2═CHCOO—CH2CH2(CF2) CH2═CCH3COO—CH2CH2(CF2)6F. CH2═CCH3COO—CH2CH2(CF2)8F. CH2═CHCOO—CH2CF3. CH2═CCH3COO—CH2CF3
- The vinyl polymers represented by the formulae presented below are particularly preferable.
- CH2═CHCOO—CH2CF3. CH2═CCH3COO—CH2CF3.
- The vinyl monomers (B) not containing any organofluorine groups in the molecule may be any monomer containing radical-polymerizable vinyl groups illustrated, for example, by methyl acrylate, methyl methacrylate, ethyl acrylate, ethyl methacrylate, n-propyl acrylate, n-propyl methacrylate, isopropyl acrylate, isopropyl methacrylate, and other lower alkyl acrylates or methacrylates; glycidyl acrylate, glycidyl methacrylate; n-butyl acrylate, n-butyl methacrylate, isobutyl acrylate, isobutyl methacrylate, tert-butyl acrylate, tert-butyl methacrylate, n-hexyl acrylate, n-hexyl methacrylate, n-hexyl acrylate, n-hexyl methacrylate, cyclohexyl acrylate, cyclohexyl methacrylate, 2-ethylhexyl acrylate, 2-ethylhexyl methacrylate, octyl acrylate, octyl methacrylate, lauryl acrylate, lauryl methacrylate, stearyl acrylate, stearyl methacrylate, and other higher acrylates and methacrylates; vinyl acetate, vinyl propionate and other lower fatty acid vinyl esters; vinyl butyrate, vinyl caproate, vinyl 2-ethylhexanoate, vinyl laurate, vinyl stearate, and other higher fatty acid esters; styrene, vinyltoluene, benzyl acrylate, benzyl methacrylate, phenoxyethyl acrylate, phenoxyethyl methacrylate, vinylpyrrolidone, and other vinylaromatic monomers; dimethylaminoethyl acrylate, dimethylaminoethyl methacrylate, diethylaminoethyl acrylate, diethylaminoethyl methacrylate, and other aminovinyl monomers, acrylamide, methacrylamide, N-methylolacrylamide, N-methylolmethacrylamide, N-methoxymethylacrylamide, N-methoxymethyl-methacrylamide, isobutoxymethoxyacrylamide, isobutoxymethoxymethacrylamide, N,N-dimethylacrylamide, N,N-dimethylmethacrylamide, and other vinylamide monomers; hydroxyethyl acrylate, hydroxyethyl methacrylate, acrylic acid hydroxypropyl alcohol, methacrylic acid hydroxypropyl alcohol, and other hydroxyvinyl monomers; acrylic acid, methacrylic acid, itaconic acid, crotonic acid, fumaric acid, maleic acid, and other vinylcarboxylic acid monomers; tetra-hydrofurfuryl acrylate, tetrahydrofurfuryl methacrylate, butoxyethyl acrylate, butoxyethyl methacrylate, ethoxydiethylene glycol acrylate, ethoxy-diethylene glycol methacrylate, polyethylene glycol acrylate, polyethylene glycol methacrylate, polypropylene glycol monoacrylate, polypropylene glycol monomethacrylate, hydroxybutyl vinyl ether, cetyl vinyl ether, 2-ethylhexyl vinyl ether, and other vinyl monomers containing an ether bond; acryloxypropyl-trimethoxysilane, methacryloxypropyltrimethoxysilane, polydimethylsiloxanes containing acryl or methacryl groups at one of the ends, polydimethylsiloxanes containing alkenylaryl groups at one of the ends and other silicone compounds containing unsaturated groups; butadiene; vinyl chloride; vinylidene chloride, acrylonitrile, methacrylonitrile; dibutyl fumarate; maleic anhydride; dodecylsuccinic anhydride; acryl glycidyl ether, methacryl glycidyl ether, 3,4-epoxy-cyclohexylmethyl acrylate, 3,4-epoxycyclohexylmethyl methacrylate, alkali metal salts, ammonium salts and organic amine salts of acrylic acid, of methacrylic acid, of itaconic acid, of crotonic acid, of fumaric acid, of maleic acid and of other radical-polymerizable unsaturated carboxylic acids, radical-polymerizable unsaturated monomers containing sulfonic acid groups, such as styrene sulfonic acid and also the alkali metal salts thereof, the ammonium salts thereof and the organic amine salts thereof; the quaternary ammonium salts derived from acrylic acid or methacrylic acid, such as 2-hydroxy-3-methacryloxypropyltrimethylammonium chloride, methacrylic acid esters of a tertiary amine alcohol, such as the diethylamine ester of methacrylic acid and quaternary ammonium salts thereof.
- In addition, it is also possible to use as vinyl monomers (B) the polyfunctional vinyl monomers illustrated, for example, by trimethylolpropane triacrylate, trimethylolpropane trimethacrylate, pentaerythrityl triacrylate, pentaerythrityl trimethacrylate, ethylene glycol diacrylate, ethylene glycol dimethacrylate, tetraethylene glycol diacrylate, tetraethylene glycol dimethacrylate, polyethylene glycol diacrylate, polyethylene glycol dimethacrylate, 1,4-butanediol diacrylate, 1,4-butanediol dimethacrylate, 1,6-hexanediol diacrylate, 1,6-hexane-diol dimethacrylate, neopentyl glycol diacrylate, neopentyl glycol dimethacrylate, trimethylolpropane-trioxyethyl acrylate, trimethylolpropanetrioxyethyl methacrylate, tris(2-hydroxyethyl)isocyanurate diacrylate, tris(2-hydroxyethyl)isocyanurate dimethacrylate, tris(2-hydroxyethyl)isocyanurate triacrylate, tris(2-hydroxyethyl)isocyanurate trimethacrylate, polydimethylsiloxane in which the two ends of the molecular chain are blocked with alkenylaryl groups, and other silicone compounds containing unsaturated groups.
- As regards the ratio mentioned above in which the component (A) and the component (B) are copolymerized, the weight ratio of compound (A) to compound (B) should be within the range from 0.1:99.9 to 100:0 and preferably within the range 1:99 to 100:0.
- The carbosiloxane dendrimer (C) is represented by the general formula (III) indicated above. In formula (III), Y is a radical-polymerizable organic group, the type of which is not subject to any special limitations provided that it is an organic group capable of undergoing a radical addition reaction. Organic groups containing acryl and methacryl, organic groups containing alkenylaryl, or alkenyl groups of 2 to 10 carbon atoms represented by the general formulae presented below are suggested as specific examples.
- In the formulae, R4 and R6 are hydrogen atoms or methyl groups, R5 and R8 are alkylene groups of 1 to 10 carbon atoms, and R7 is an alkyl group of 1 to 10 carbon atoms. The index “b” is an integer from 0 to 4 and “c” is 0 or 1. Acryloxymethyl, 3-acryloxypropyl, methacryloxymethyl, 3-methacryloxypropyl, 4-vinylphenyl, 3-vinylphenyl, 4-(2-propenyl)phenyl, 3-(2-propenyl)phenyl, 2-(4-vinylphenyl)ethyl, 2-(3-vinylphenyl)enyl, vinyl, allyl, methallyl, and 5-hexenyl are suggested as examples of such radical-polymerizable organic groups. The index “i” in formula (II), which is an integer from 1 to 10, is the number of generations of the said silylalkyl group, in other words the number of times that the silylalkyl group is repeated. Thus, the carbosiloxane dendrimer of this component with a generation number of 1 is represented by the general formula:
- (in which Y, R1, R2 and R3 are as above and R12 is a hydrogen atom or such as R1 described above. The index “a1” is an integer from 0 to 3, the total mean of “a1” per molecule being from 0 to 7). The carbosiloxane dendrimers of this component with a generation number of 2 are represented by the general formula:
- (in which Y, R1, R2, R3 and R12 are as above and the indices “a1” and “a2” are integers from 0 to 3, the total mean of “a1” and of “a2” per molecule being from 0 to 25).
- The carbosiloxane dendrimers of this component with a generation number of 3 are represented by the general formula:
- (in which Y, R1, R2, R3 and R12 are as above and the indices “a1” and “a2” and “a3” are integers from 0 to 3, the total mean of “a1”, “a2” and “a3” per molecule being from 0 to 79).
- The component (C) is illustrated by carbosiloxane dendrimers represented by formulae of mean composition represented below.
- The carbosiloxane dendrimers of the component (C) may be prepared using the process for preparing siloxane/silylalkylene branched copolymers described in document EP 1 055 674. For example, they may be prepared by subjecting organic alkenyl silicones and silicone compounds comprising hydrogen atoms linked to silicon, represented by the general formula:
- (in which R1 and Y are as above) to a hydrosilylation reaction. For example, 3-methacryloxypropyltris(dimethylsiloxy)silane, 3-acryloxypropyltris(dimethylsiloxy)silane and 4-vinylphenyltris(dimethylsiloxy)silane are used as silicon compounds represented by the above formula. Vinyltris(trimethylsiloxy)silane, vinyltris(dimethyl-phenylsiloxy)silane and 5-hexenyltris(trimethylsiloxy)-silane are used as organosilicon alkenyl compounds. In addition, it is preferable to perform the hydrosilylation reaction in the presence of a transition metal catalyst such as chloroplatinic acid and the platinum/vinylsiloxane complex.
- The copolymerization ratio of the component (C), in terms of its weight ratio relative to the total weight of compounds (A) and (B) should be within the range from 0.1:99.9 to 99.9:0.1, preferably within the range from 1:99 to 99:1 and even more preferably within the range from 5:95 to 95:5.
- Amino groups may be introduced into the side chains of the vinyl polymer using, included in the component (B), vinyl monomers containing amino groups, such as dimethylaminoethyl acrylate, dimethylaminoethyl methacrylate, diethylaminoethyl acrylate and diethyl-aminoethyl methacrylate, followed by performing a modification with potassium acetate monochloride, ammonium acetate monochloride, the aminomethylpropanol salt of monochloroacetic acid, the triethanolamine salt of monobromoacetic acid, sodium monochloropropionate, and other alkali metal salts of halogenated fatty acids; otherwise, carboxylic acid groups may be introduced into the side chains of the vinyl polymer using, included in the component (B), vinyl monomers containing carboxylic acids, such as acrylic acid, methacrylic acid, itaconic acid, crotonic acid, fumaric acid and maleic acid, and the like, followed by neutralizing the product with triethylamine, diethylamine, triethanolamine and other amines.
- The fluorinated vinyl polymer may be one of the polymers described in the examples of patent application WO 03/045337 or, for example, the product TIB-4-100 sold by Dow Corning.
- The vinyl polymer may be present in a content ranging from 0.1% to 70% by weight, relative to the total weight of the composition, preferably ranging from 0.5% to 50% by weight, preferentially ranging from 1% to 40% by weight and more preferably ranging from 5% to 15% by weight.
- The vinyl polymer may be present in the composition in a proportion of at least 3% by weight in the composition, preferably between 5% and 25% by weight, more preferably between 5% and 15% by weight and especially about 10% by weight.
- Polymer in Dispersion
- According to a third embodiment of the invention, the film-forming polymer present in the composition according to the invention is a dispersion of homopolymer particles or of acrylic or vinyl radical copolymers dispersed in the liquid fatty phase of the composition.
- According to the invention, the polymer in the form of particles dispersed in the volatile liquid fatty phase is a solid that is insoluble in the liquid fatty phase of the composition even at its softening point, unlike a wax even of polymeric origin, which is itself soluble in the liquid organic phase (or fatty phase) at its melting point.
- The composition according to the invention advantageously comprises at least one stable dispersion of generally spherical polymer particles of one or more polymers, in a volatile liquid fatty phase. These dispersions may especially be in the form of polymer nanoparticles in stable dispersion in the said liquid organic phase. The nanoparticles preferably have a mean size of between 5 and 800 nm and better still between and 500 nm. However, it is possible to obtain polymer particles ranging up to 1 μm in size.
- Preferably, the polymer particles in dispersion are insoluble in water-soluble alcohols, for instance ethanol.
- The polymers in dispersion that may be used in the composition of the invention preferably have a molecular weight of about from 2000 to 10 000 000 g/mol and a Tg of from −100° C. to 300° C., better still from −50° C. to 100° C. and preferably from −10° C. to 50° C.
- It is possible to use film-forming polymers preferably having a low Tg, of less than or equal to skin temperature and especially less than or equal to 40° C.
- Preferably, the polymer used is film-forming, i.e. it is capable of forming an isolable film, by itself or in combination with a plasticizer. It is, however, possible to use a non-film-forming polymer.
- The term “non-film-forming polymer” means a polymer that is incapable of forming an isolable film by itself. This polymer can, in combination with a non-volatile compound of the oil type, form a continuous, uniform deposit on the skin and/or the lips.
- Among the film-forming polymers that may be mentioned are acrylic or vinyl free-radical homopolymers or copolymers, preferably with a Tg of less than or equal to 40° C. and especially ranging from −10° C. to 30° C., used alone or as a mixture.
- Among the non-film-forming polymers that may be mentioned are optionally crosslinked vinyl or acrylic free-radical homopolymers or copolymers preferably with a Tg of greater than 40° C. and especially ranging from 45° C. to 150° C., used alone or as a mixture.
- The term “free-radical polymer” means a polymer obtained by polymerization of unsaturated and especially ethylenic monomers, each monomer being capable of homopolymerizing (unlike polycondensates). The free-radical polymers may especially be vinyl polymers or copolymers, especially acrylic polymers.
- The acrylic polymers may result from the polymerization of ethylenically unsaturated monomers containing at least one acid group and/or esters of these acid monomers and/or amides of these acids.
- Monomers bearing an acid group that may be used include α,β-ethylenic unsaturated carboxylic acids such as acrylic acid, (meth)acrylic acid, crotonic acid, maleic acid or itaconic acid. (Meth)acrylic acid and crotonic acid are preferably used, and more preferably (meth)acrylic acid.
- The acid monomer esters are advantageously chosen from (meth)acrylic acid esters (also known as (meth)acrylates), for instance alkyl (meth)acrylates, in particular of a C1-C20 and preferably C1-C8 alkyl, aryl (meth)acrylates, in particular of a C6-C10 aryl, and hydroxyalkyl (meth)acrylates, in particular of a C2-C6 hydroxyalkyl. Alkyl (meth)acrylates that may be mentioned include methyl, ethyl, butyl, isobutyl, 2-ethylhexyl and lauryl (meth)acrylate. Hydroxyalkyl (meth)acrylates that may be mentioned include hydroxyethyl (meth)acrylate and 2-hydroxypropyl (meth)acrylate. Aryl (meth)acrylates that may be mentioned include benzyl or phenyl acrylate.
- The (meth)acrylic acid esters that are particularly preferred are the alkyl (meth)acrylates.
- Free-radical polymers that are preferably used include copolymers of (meth)acrylic acid and of alkyl (meth)acrylate, especially of a C1-C4 alkyl. Methyl acrylates optionally copolymerized with acrylic acid may more preferentially be used.
- Amides of the acid monomers that may be mentioned include (meth)acrylamides, especially N-alkyl(meth)-acrylamides, in particular of a C2-C12 alkyl, such as N-ethylacrylamide, N-t-butylacrylamide and N-octylacrylamide; N-di(C1-C4)alkyl(meth)acrylamides.
- The acrylic polymers may also result from the polymerization of ethylenically unsaturated monomers containing at least one amine group, in free form or in partially or totally neutralized form, or alternatively in partially or totally quaternized form. Such monomers may be, for example, dimethylaminoethyl (meth)acrylate, dimethylaminoethyl(meth)acrylamide, vinylamine, vinylpyridine or diallyldimethylammonium chloride.
- The vinyl polymers may also result from the homopolymerization or copolymerization of at least one monomer chosen from vinyl esters and styrene monomers. In particular, these monomers may be polymerized with acid monomers and/or esters thereof and/or amides thereof, such as those mentioned previously. Examples of vinyl esters that may be mentioned include vinyl acetate, vinyl propionate, vinyl neodecanoate, vinyl pivalate, vinyl benzoate and vinyl t-butylbenzoate. Styrene monomers that may be mentioned include styrene and α-methylstyrene.
- The list of monomers given is not limiting, and it is possible to use any monomer known to those skilled in the art included in the categories of acrylic and vinyl monomers (including monomers modified with a silicone chain).
- As other vinyl monomers that may be used, mention may also be made of:
-
- N-vinylpyrrolidone, vinylcaprolactam, vinyl-N—(C1-C6)alkylpyrroles, vinyloxazoles, vinylthiazoles, vinylpyrimidines and vinylimidazoles,
- olefins such as ethylene, propylene, butylene, isoprene or butadiene.
- The vinyl polymer may be crosslinked with one or more difunctional monomers especially comprising at least two ethylenic unsaturations, such as ethylene glycol di(meth)acrylate or diallyl phthalate.
- The polymer(s) in dispersion in the organic liquid phase may represent, as solids, from 5% to 40%, preferably from 5% to 35% and better still from 8% to 30% of the weight of the composition.
- It is preferably chosen to use a dispersion of film-forming polymer particles, the particles being dispersed in a volatile oil.
- According to one embodiment, the composition contains a stabilizer that is solid at room temperature. The polymer particles are preferably surface-stabilized by means of a stabilizer that may be a block polymer, a grafted polymer and/or a random polymer, alone or as a mixture. The stabilization may take place by any known means, and in particular by direct addition of the block polymer, grafted polymer and/or random polymer during the polymerization.
- The stabilizer is preferably also present in the mixture before polymerization. However, it is also possible to add it continuously, especially when the monomers are also added continuously.
- 2-30% by weight and preferably 5-20% by weight of stabilizer may be used relative to the initial monomer mixture.
- Among the grafted polymers that may be mentioned are silicone polymers grafted with a hydrocarbon-based chain; hydrocarbon-based polymers grafted with a silicone chain.
- Thus, grafted-block or block copolymers comprising at least one block of polyorganosiloxane type and at least one block of a free-radical polymer, for instance grafted copolymers of acrylic/silicone type, may thus be used, which may be used especially when the non-aqueous medium is silicone-based.
- It is also possible to use grafted-block or block copolymers comprising at least one block of polyorganosiloxane type and at least one block of a polyether. The polyorganosiloxane block may especially be a polydimethylsiloxane or a poly(C2-C18)alkylmethyl-siloxane; the polyether block may be a poly(C2-C10-alkylene, in particular polyoxyethylene and/or polyoxy-propylene. In particular, dimethicone copolyols or (C2-C18)alkyldimethicone copolyols such as those sold under the name Dow Corning 3225C by the company Dow Corning, and lauryl methicones such as those sold under the name Dow Corning Q2-5200 by the company Dow Corning, may be used.
- Grafted-block or block copolymers that may also be mentioned include those comprising at least one block resulting from the polymerization of at least one ethylenic monomer containing one or more optionally conjugated ethylenic bonds, for instance ethylene or dienes such as butadiene and isoprene, and of at least one block of a vinyl polymer and better still a styrene polymer. When the ethylenic monomer comprises several optionally conjugated ethylenic bonds, the residual ethylenic unsaturations after the polymerization are generally hydrogenated. Thus, in a known manner, the polymerization of isoprene leads, after hydrogenation, to the formation of an ethylene-propylene block, and the polymerization of butadiene leads, after hydrogenation, to the formation of an ethylene-butylene block. Among these polymers that may be mentioned are block copolymers, especially of “diblock” or “triblock” type such as polystyrene/polyisoprene (SI), polystyrene/polybutadiene (SB) such as those sold under the name Luvitol HSB by BASF, of the type such as polystyrene/copoly(ethylene-propylene) (SEP) such as those sold under the name Kraton by Shell Chemical Co. or of the type such as polystyrene/copoly(ethylene-butylene) (SEB). Kraton G1650 (SEBS), Kraton G1651 (SEBS), Kraton G1652 (SEBS), Kraton G1657X (SEBS), Kraton G1701X (SEP), Kraton G1702X (SEP), Kraton G1726X (SEW, Kraton D-1101 (SBS), Kraton D-1102 (SBS) and Kraton D-1107 (SIS) may be used in particular. The polymers are generally known as hydrogenated or non-hydrogenated diene copolymers.
- Gelled Permethyl 99A-750, 99A-753-59 and 99A-753-(mixture of triblock and of star polymer), Versagel 5960 from Penreco (triblock+star polymer); OS129880, OS129881 and OS84383 from Lubrizol (styrene/(meth)acrylate copolymer) may also be used.
- As grafted-block or block copolymers comprising at least one block resulting from the polymerization of at least one ethylenic monomer containing one or more ethylenic bonds and of at least one block of an acrylic polymer, mention may be made of poly(methyl (meth)acrylate)/polyisobutylene diblock or triblock copolymers or grafted copolymers containing a poly(methyl(meth)acrylate) skeleton and polyisobutylene grafts.
- As grafted-block or block copolymers comprising at least one block resulting from the polymerization of at least one ethylenic monomer containing one or more ethylenic bonds and of at least one block of a polyether such as a C2-C18 polyalkylene (especially polyethylene and/or polyoxypropylene), mention may be made of polyoxyethylene/polybutadiene or polyoxyethylene/polyisobutylene diblock or triblock copolymers.
- Copolymers based on alkyl acrylates or (meth)acrylates derived from C1-C4 alcohols and on alkyl acrylates or (meth)acrylates derived from C8-C30 alcohols may thus be used. Mention may be made in particular of stearyl (meth)acrylate/methyl (meth)acrylate copolymer.
- When the liquid synthesis solvent comprises at least one silicone oil, the stabilizer is preferably chosen from the group consisting of grafted-block or block copolymers comprising at least one block of polyorganosiloxane type and at least one block of a free-radical polymer or of a polyether or of a polyester, for instance polyoxypropylene and/or polyoxyethylene blocks.
- When the liquid organic phase does not comprise any silicone oil, the stabilizer is preferably chosen from the group formed by:
-
- (a) grafted-block or block copolymers comprising at least one block of polyorganosiloxane type and at least one block of a free-radical polymer or of a polyether or a polyester,
- (b) copolymers of alkyl acrylates or (meth)acrylates derived from C1-C4 alcohols and of alkyl acrylates or (meth)acrylates derived from C8-C30 alcohols,
- (c) grafted-block or block copolymers comprising at least one block resulting from the polymerization of at least one ethylenic monomer containing conjugated ethylenic bonds,
- and at least one block of a vinyl or acrylic polymer or of a polyether or of a polyester, or mixtures thereof.
- Diblock polymers are preferably used as stabilizer.
- When the polymer has a glass transition temperature that is too high for the intended application, a plasticizer may be combined therewith. The plasticizer may be chosen from the plasticizers usually used in the field of application and especially from compounds liable to be solvents for the polymer. Coalescers may also be used in order to aid the polymer to form a continuous and homogeneous deposit.
- The coalescers or plasticizers that may be used in the invention are especially those mentioned in document FR-A-2 782 917.
- The composition may contain a polymer plasticizer, so as to lower the Tg of the polymer film and to improve the adhesion of the polymer film to its support, in particular to keratin materials. The plasticizer especially lowers the glass transition temperature of the polymer by at least 2, 3 or 4° C. and preferably from 5° C. to 20° C. In one preferred embodiment, the plasticizer especially lowers the glass transition temperature of the polymer by at least 2, 3 or 4° C. and preferably from 5° C. to 20° C., when the plasticizer represents not more than 10% by weight of the polymer.
- According to one embodiment, the compound may be chosen from esters of at least one carboxylic acid comprising 1 to 7 carbon atoms and of a polyol comprising at least four hydroxyl groups.
- The polyol according to the invention may be a saccharide or a saccharide-based polyol, such as erythritol, xylitol or sorbitol. The polymer may be a monosaccharide or a polysaccharide comprising 1 to 10 saccharides, preferably from 1 to 4 and more preferably or 2 saccharides. The polyol may be chosen from erythritol, xylitol, sorbitol, glucose and sucrose.
- The polyol according to the invention is preferably a disaccharide. Among the disaccharides that may be mentioned are sucrose (α-D-glucopyranosyl-(1-2)-β-D-fructofuranose), lactose (β-D-galactopyranosyl-(1-4)-β-D-glucopyranose) and maltose (α-D-glucopyranosyl-(1-4)-β-D-glucopyranose).
- The plasticizer may be formed from a polyol substituted with at least two different monocarboxylic acids, or with at least three different monocarboxylic acids. The acid is preferably a monocarboxylic acid chosen in particular from acids comprising 1 to 7 carbon atoms and preferably 1 to 5 carbon atoms, for example acetic acid, n-propanoic acid, isopropanoic acid, n-butanoic acid, isobutanoic acid, tert-butanoic acid, n-pentanoic acid and benzoic acid.
- According to one preferred embodiment, the ester is sucrose diacetate hexakis(2-methylpropanoate).
- Synthesis Solvent for the Polymer Particles
- The polymer dispersion may be manufactured as described in document EP-A-749 747.
- A mixture comprising the initial monomers and also a free-radical initiator is prepared. This mixture is dissolved in a solvent referred to hereinbelow in the present description as the “synthesis solvent”. When the fatty phase is a non-volatile oil, the polymerization may be performed in an apolar organic solvent (synthesis solvent), followed by adding the non-volatile oil (which should be miscible with the said synthesis solvent) and selectively distilling off the synthesis solvent.
- A synthesis solvent which is such that the initial monomers and the free-radical initiator are soluble therein, and the polymer particles obtained are insoluble therein, so that they precipitate during their formation, is chosen. In particular, the synthesis solvent may be chosen from alkanes such as heptane, isododecane and cyclohexane.
- When the fatty phase chosen is a volatile oil, the polymerization may be performed directly in the said oil, which thus also acts as synthesis solvent. The monomers should also be soluble therein, as should the free-radical initiator, and the polymer obtained should be insoluble therein.
- The monomers are preferably present in the synthesis solvent, before polymerization, in a proportion of 5-201 by weight of the reaction mixture. The total amount of monomers may be present in the solvent before the start of the reaction, or part of the monomers may be added gradually as the polymerization reaction proceeds.
- The free-radical initiator may especially be azobisisobutyronitrile or tert-butylperoxy-2-ethyl hexanoate.
- The volatile phase of the composition may be formed from or comprise the synthesis solvent for the dispersed polymer particles.
- Additional Polymer:
- The compositions according to the invention may contain, besides the film-forming polymers described previously, an additional film-forming or non-film-forming polymer.
- The composition may comprise an aqueous phase and the additional polymer may be present in this aqueous phase. In this case, the polymer will preferably be a polymer in dispersion or an amphiphilic or associative polymer.
- The term “polymer in dispersion” means the water-insoluble polymers present in the form of particles of variable size. The polymer may or may not be crosslinked. The size of the polymer particles is typically between 25 and 500 nanometres and preferably between 50 and 200 nanometres. The following polymers in aqueous dispersion may be used: Ultrasol 2075 from Ganz Chemical, Daitosol 5000 AD from Daito Kasei, Avalure UR 450 from Noveon, DynamX from National Starch, Syntran 5760 from Interpolymer, Acusol OP 301 and from Rohm & Haas, and Neocryl A 1090 from Avecia.
- The acrylic dispersions sold under the names Neocryl XK-90®, Neocryl A-1070®, Neocryl A-1090®, Neocryl BT-62®, Neocryl A-1079® and Neocryl A-523® by the company Avecia-Neoresins, Dow Latex 432® by the company Dow Chemical, Daitosol 5000 AD® or Daitosol 5000 SJ® by the company Daito Kasey Kogyo; Syntran 5760® by the company Interpolymer, Soltex OPT by the company Rohm & Haas, aqueous dispersions of acrylic or styrene/acrylic polymers sold under the brand name Joncryl® by the company Johnson Polymer, or the aqueous dispersions of polyurethane sold under the names Neorez R-981® and Neorez R-974® by the company Avecia-Neoresins, Avalure UR-405®, Avalure UR-410®, Avalure UR-425®, Avalure UR-450®, Sancure 875®, Sancure 861®, Sancure 878® and Sancure 2060® by the company Goodrich, Impranil 85® by the company Bayer and Aquamere H-1511® by the company Hydromer; the sulfopolyesters sold under the brand name Eastman AQ® by the company Eastman Chemical Products, and vinyl dispersions, for instance Mexomer PAM® from the company Chimex, and mixtures thereof, are other examples of aqueous dispersions of water-dispersible film-forming polymer particles.
- The term “amphiphilic or associative polymers” means polymers comprising one or more hydrophilic parts that make them partially water-soluble and one or more hydrophobic parts via which the polymers associate or interact. The following associative polymers may be used: Nuvis FX 1100 from Elementis, Aculyn 22, Aculyn 44 and Aculyn 46 from Rohm & Haas, Viscophobe DB 1000 from Amerchol. Diblock copolymers formed from a hydrophilic block (polyacrylate or polyethylene glycol) and from a hydrophobic block (polystyrene or polysiloxane) may also be used.
- Polymers that are soluble in an aqueous phase containing monodisperse particles may be avoided, since they may cause aggregation of the monodisperse particles. The film-forming polymer may thus be insoluble in such an aqueous phase.
- The composition may comprise an oily phase and the film-forming polymer may be present in this oily phase. The polymer may then be in dispersion or in solution. Microgels (for example KSG) may be used, as may polymers of the type PS-PA or styrene-based copolymers (Kraton, Regalite).
- As examples of lipodispersible non-aqueous film-forming polymer dispersions in the form of non-aqueous dispersions of polymer particles in one or more silicone and/or hydrocarbon-based oils, which may be surface-stabilized with at least one stabilizer, especially a block, grafted or random polymer, mention may be made of acrylic dispersions in isododecane, for instance Mexomer PAP from the company Chimex, and dispersions of particles of a grafted ethylenic polymer, preferably an acrylic polymer, in a liquid fatty phase, the ethylenic polymer advantageously being dispersed in the absence of additional stabilizer at the surface of the particles as described especially in document WO 04/055 081.
- Among the additional film-forming polymers that may be used in the composition of the present invention, mention may be made of synthetic polymers, of free-radical type or of polycondensate type, and polymers of natural origin, and mixtures thereof.
- The expression “free-radical film-forming polymer” means a polymer obtained by polymerization of unsaturated and especially ethylenically unsaturated monomers, each monomer being capable of homopolymerizing (unlike polycondensates).
- The film-forming polymers of free-radical type may be, in particular, vinyl polymers or copolymers, in particular acrylic polymers.
- The vinyl film-forming polymers may result from the polymerization of ethylenically unsaturated monomers containing at least one acidic group and/or esters of these acidic monomers and/or amides of these acidic monomers.
- Monomers bearing an acidic group which may be used are α,β-ethylenic unsaturated carboxylic acids such as acrylic acid, methacrylic acid, crotonic acid, maleic acid or itaconic acid. (Meth)acrylic acid and crotonic acid are preferably used, and more preferably (meth)acrylic acid.
- The esters of acidic monomers are advantageously chosen from (meth)acrylic acid esters (also known as (meth)acrylates), especially (meth)acrylates of an alkyl, in particular of a C1-C30 and preferably C1-C20 alkyl, (meth)acrylates of an aryl, in particular of a C6-C10 aryl, and (meth)acrylates of a hydroxyalkyl, in particular of a C2-C6 hydroxyalkyl.
- Among the alkyl (meth)acrylates that may be mentioned are methyl methacrylate, ethyl methacrylate, butyl methacrylate, isobutyl methacrylate, 2-ethylhexyl methacrylate, lauryl methacrylate and cyclohexyl methacrylate.
- Among the hydroxyalkyl (meth)acrylates that may be mentioned are hydroxyethyl acrylate, 2-hydroxypropyl acrylate, hydroxyethyl methacrylate and 2-hydroxypropyl methacrylate.
- Among the aryl (meth)acrylates that may be mentioned are benzyl acrylate and phenyl acrylate.
- The (meth)acrylic acid esters that are particularly preferred are the alkyl (meth)acrylates.
- According to the present invention, the alkyl group of the esters may be either fluorinated or perfluorinated, i.e. some or all of the hydrogen atoms of the alkyl group are substituted with fluorine atoms.
- Examples of amides of the acid monomers that may be mentioned are (meth)acrylamides, and especially N-alkyl(meth)acrylamides, in particular of a C2-C12 alkyl. Among the N-alkyl(meth)acrylamides that may be mentioned are N-ethylacrylamide, N-t-butylacrylamide, N-t-octylacrylamide and N-undecylacrylamide.
- The vinyl film-forming polymers may also result from the homopolymerization or copolymerization of monomers chosen from vinyl esters and styrene monomers. In particular, these monomers may be polymerized with acid monomers and/or esters thereof and/or amides thereof, such as those mentioned above.
- Examples of vinyl esters that may be mentioned are vinyl acetate, vinyl neodecanoate, vinyl pivalate, vinyl benzoate and vinyl t-butylbenzoate.
- Styrene monomers that may be mentioned are styrene and α-methylstyrene.
- Among the film-forming polycondensates that may be mentioned are polyurethanes, polyesters, polyester-amides, polyamides, epoxyester resins and polyureas.
- The polyurethanes may be chosen from anionic, cationic, nonionic and amphoteric polyurethanes, polyurethane-acrylics, polyurethane-polyvinyl-pyrrolidones, polyester-polyurethanes, polyether-polyurethanes, polyureas and polyurea-polyurethanes, and mixtures thereof.
- The polyesters may be obtained, in a known manner, by polycondensation of dicarboxylic acids with polyols, in particular diols.
- The dicarboxylic acid may be aliphatic, alicyclic or aromatic. Examples of such acids that may be mentioned are: oxalic acid, malonic acid, dimethylmalonic acid, succinic acid, glutaric acid, adipic acid, pimelic acid, 2,2-dimethylglutaric acid, azelaic acid, suberic acid, sebacic acid, fumaric acid, maleic acid, itaconic acid, phthalic acid, dodecanedioic acid, 1,3-cyclohexanedicarboxylic acid, 1,4-cyclohexanedicarboxylic acid, isophthalic acid, terephthalic acid, 2,5-norbornanedicarboxylic acid, diglycolic acid, thiodipropionic acid, 2,5-naphthalene-dicarboxylic acid or 2,6-naphthalenedicarboxylic acid. These dicarboxylic acid monomers may be used alone or as a combination of at least two dicarboxylic acid monomers. Among these monomers, the ones preferentially chosen are phthalic acid, isophthalic acid and terephthalic acid.
- The diol may be chosen from aliphatic, alicyclic and aromatic diols. The diol used is preferably chosen from: ethylene glycol, diethylene glycol, triethylene glycol, 1,3-propanediol, cyclohexanedimethanol and 4-butanediol. Other polyols that may be used are glycerol, pentaerythritol, sorbitol and trimethylol-propane.
- The polyesteramides may be obtained in a manner analogous to that of the polyesters, by polycondensation of diacids with diamines or amino alcohols. Diamines that may be used are ethylenediamine, hexamethylenediamine and meta- or para-phenylenediamine. An amino alcohol that may be used is monoethanolamine.
- The polyester may also comprise at least one monomer bearing at least one group —SO3M, with M representing a hydrogen atom, an ammonium ion NH4 + or a metal ion such as, for example, an Na+, Li+, K+, Mg2+, Ca2+, Cu2+, Fe2+ or Fe3+ ion. A difunctional aromatic monomer comprising such a group —SO3M may be used in particular.
- The aromatic nucleus of the difunctional aromatic monomer also bearing a group —SO3M as described above may be chosen, for example, from benzene, naphthalene, anthracene, biphenyl, oxybiphenyl, sulfonylbiphenyl and methylenebiphenyl nuclei. As examples of difunctional aromatic monomers also bearing a group —SO3M, mention may be made of: sulfoisophthalic acid, sulfoterephthalic acid, sulfophthalic acid, 4-sulfonaphthalene-2,7-dicarboxylic acid.
- According to one example of a composition according to the invention, the film-forming polymer may be a polymer dissolved in a liquid fatty phase comprising organic solvents or oils (the film-forming polymer is thus said to be a liposoluble polymer). The liquid fatty phase preferably comprises a volatile oil, optionally mixed with a non-volatile oil.
- Examples of liposoluble polymers that may be mentioned are copolymers of vinyl ester (the vinyl group being directly linked to the oxygen atom of the ester group and the vinyl ester containing a saturated, linear or branched hydrocarbon-based radical of 1 to 19 carbon atoms, linked to the carbonyl of the ester group) and of at least one other monomer which may be a vinyl ester (other than the vinyl ester already present), an α-olefin (containing from 8 to 28 carbon atoms), an alkyl vinyl ether (in which the alkyl group comprises from 2 to 18 carbon atoms) or an allylic or methallylic ester (containing a saturated, linear or branched hydrocarbon-based radical of 1 to 19 carbon atoms, linked to the carbonyl of the ester group).
- These copolymers may be crosslinked with the aid of crosslinking agents, which may be either of the vinyl type or of the allylic or methallylic type, such as tetraallyloxyethane, divinylbenzene, divinyl octane-dioate, divinyl dodecanedioate and divinyl octadecane-dioate.
- Examples of these copolymers that may be mentioned are the following copolymers: vinyl acetate/allyl stearate, vinyl acetate/vinyl laurate, vinyl acetate/vinyl stearate, vinyl acetate/octadecene, vinyl acetate/octadecyl vinyl ether, vinyl propionate/allyl laurate, vinyl propionate/vinyl laurate, vinyl stearate/1-octadecene, vinyl acetate/1-dodecene, vinyl stearate/ethyl vinyl ether, vinyl propionate/cetyl vinyl ether, vinyl stearate/allyl acetate, vinyl 2,2-dimethyloctanoate/vinyl laurate, allyl 2,2-dimethylpentanoate/vinyl laurate, vinyl dimethylpropionate/vinyl stearate, allyl dimethylpropionate/vinyl stearate, vinyl propionate/vinyl stearate, crosslinked with 0.2% divinylbenzene, vinyl dimethylpropionate/vinyl laurate, crosslinked with 0.2% divinylbenzene, vinyl acetate/octadecyl vinyl ether, crosslinked with 0.2% tetraallyloxyethane, vinyl acetate/allyl stearate, crosslinked with 0.2% divinyl-benzene, vinyl acetate/1-octadecene, crosslinked with 0.2% divinylbenzene, and allyl propionate/allyl stearate, crosslinked with 0.26 divinylbenzene.
- Examples of liposoluble film-forming polymers that may be mentioned include copolymers of a vinyl ester and of at least one other monomer that may be a vinyl ester, especially vinyl neodecanoate, vinyl benzoate and vinyl t-butylbenzoate, an α-olefin, an alkyl vinyl ether or an allylic or methallylic ester.
- Examples of liposoluble film-forming polymers that may also be mentioned are liposoluble copolymers, and in particular those resulting from the copolymerization of vinyl esters containing from 9 to 22 carbon atoms or of alkyl acrylates or methacrylates, and alkyl radicals containing from 10 to 20 carbon atoms.
- Such liposoluble copolymers may be chosen from copolymers of polyvinyl stearate, polyvinyl stearate crosslinked with the aid of divinylbenzene, of diallyl ether or of diallyl phthalate, polystearyl (meth)acrylate, polyvinyl laurate and polylauryl (meth)acrylate, it being possible for these poly(meth)acrylates to be crosslinked with the aid of ethylene glycol dimethacrylate or tetraethylene glycol dimethacrylate.
- The liposoluble copolymers defined above are known and are described in particular in patent application FR-A-2 232 303; they may have a weight-average molecular weight ranging from 2000 to 500 000 and preferably from 4000 to 200 000.
- As liposoluble film-forming polymers that may be used in the invention, mention may also be made of polyalkylenes and in particular copolymers of C2-C20 alkenes, such as polybutene, alkylcelluloses with a linear or branched, saturated or unsaturated C1-C8 alkyl radical, for instance ethylcellulose and propylcellulose, copolymers of vinylpyrrolidone (VP) and in particular copolymers of vinylpyrrolidone and of C2 to C40 and better still C3 to C20 alkene. As examples of VP copolymers which may be used in the invention, mention may be made of the copolymers of VP/vinyl acetate, VP/ethyl methacrylate, butylated polyvinyl-pyrrolidone (PVP), VP/ethyl methacrylate/methacrylic acid, VP/eicosene, VP/hexadecene, VP/triacontene, VP/styrene or VP/acrylic acid/lauryl methacrylate.
- Mention may also be made of silicone resins, which are generally soluble or swellable in silicone oils, which are crosslinked polyorganosiloxane polymers. The nomenclature of silicone resins is known under the name “MDTQ”, the resin being described as a function of the various siloxane monomer units it comprises, each of the letters “MDTQ” characterizing a type of unit.
- Examples of commercially available polymethyl-silsesquioxane resins that may be mentioned include those sold by the company Wacker under the reference Resin MK, such as Belsil PMS MK, or by the company Shin-Etsu under the reference KR-220L.
- Examples of commercially available polypropyl-silsesquioxane resins that may be mentioned include those sold under the reference DC670 by the company Dow Corning.
- Siloxysilicate resins that may be mentioned include trimethyl siloxysilicate (TMS) resins such as those sold under the reference SR 1000 by the company General Electric or under the reference TMS 803 by the company Wacker. Mention may also be made of the trimethyl siloxysilicate resins sold in a solvent such as cyclomethicone, sold under the name KF-7312J by the company Shin-Etsu, and DC 749 and DC 593 by the company Dow Corning.
- In the case of skin makeup or care compositions, the combination of a resin according to the invention with a trimethyl siloxysilicate resin or a polypropylsilsesquioxane resin makes it possible to improve the durability of the transfer resistance.
- Mention may also be made of silicone resin copolymers such as those mentioned above with polydimethylsiloxanes, for instance the pressure-sensitive adhesive copolymers sold by the company Dow Corning under the reference Bio-PSA and described in document U.S. Pat. No. 5,162,410, or the silicone copolymers derived from the reaction of a silicone resin, such as those described above, and of a diorganosiloxane, as described in document WO 2004/073 626.
- The film-forming polymer may be chosen from block or random polymers and/or copolymers especially comprising polyurethanes, polyacrylics, silicones, fluoro polymers, butyl rubbers, ethylene copolymers, natural gums and polyvinyl alcohols, and mixtures thereof. The monomers of the block or random copolymers comprising at least one combination of monomers whose resulting polymer has a glass transition temperature of less than room temperature (25° C.) may be chosen especially from butadiene, ethylene, propylene, acrylic, methacrylic, isoprene, isobutene and a silicone, and mixtures thereof.
- The film-forming polymer may also be present in the first and/or second composition in the form of particles dispersed in an aqueous phase or in a non-aqueous solvent phase, which is generally known as a latex or pseudolatex. The techniques for preparing these dispersions are well known to those skilled in the art.
- The composition according to the invention may comprise a plasticizer that promotes the formation of a film with the film-forming polymer. Such a plasticizer may be chosen from any compound known to those skilled in the art as being capable of satisfying the desired function.
- As other examples of film-forming systems that may be used in the compositions according to the invention, mention may be made of systems in which the film is formed in situ at the time of application of the composition or of a mixture of compositions containing two silicone compounds that react together when they are placed in contact. Such systems are described especially in patent application WO 2007/071 706, the content of which is incorporated herein by reference. Systems of this type are also described in patent applications US 2007/142 575 and US 2007/142 599, the content of which is also incorporated herein by reference.
- Other Polymers:
- The compositions according to the invention may contain an elastomer, especially a polyglycerolated silicone elastomer. By way of example, use is made of an elastomeric crosslinked organopolysiloxane that may be obtained by a crosslinking addition reaction of a diorganopolysiloxane containing at least one hydrogen bonded to silicon and of polyglycerolated compounds containing ethylenically unsaturated groups, especially in the presence of a platinum catalyst.
- Polyglycerolated silicone elastomers that may be used include those sold under the names KSG-710, KSG-810, KSG-820, KSG-830 and KSG-840 by the company Shin-Etsu.
- The compositions according to the invention may also comprise an additional emulsifying silicone elastomer.
- By way of example, use may be made of polyoxyalkylenated elastomers as described especially in U.S. Pat. No. 5,236,986, U.S. Pat. No. 5,412,004, U.S. Pat. No. 5,837,793 and U.S. Pat. No. 5,811,487, the content of which is incorporated by reference.
- Polyoxyalkylenated silicone elastomers that may be used include those sold under the names KSG-21, KSG-20, KSG-30, KSG-31, KSG-32, KSG-33, KSG-210, KSG-310, KSG-320, KSG-330, KSG-340 and X-226146 by the company Shin-Etsu, and DC9010 and DC9011 by the company Dow Corning.
- When they are in combination with the resins according to the invention, these particular elastomers may make it possible to improve the transfer-resistance and comfort (suppleness) properties of the compositions comprising them.
- The compositions according to the invention may also comprise a non-emulsifying elastomer.
- Non-emulsifying elastomers are especially described in patent applications JP-A-61-194 009, EP-A-242 219, EP-A-285 886 and EP-A-765 656, the content of which is incorporated by reference.
- Spherical non-emulsifying elastomers that may be used include those sold under the names DC9040, DC9041, DC9509, DC9505 and DC9506 by the company Dow Corning.
- The spherical non-emulsifying silicone elastomer may also be in the form of an elastomeric crosslinked organopolysiloxane powder coated with silicone resin, especially with silsesquioxane resin, as described, for example, in U.S. Pat. No. 5,538,793, the content of which is incorporated by reference. Such elastomers are sold under the names KSP-100, KSP-101, KSP-102, KSP-103, KSP-104 and KSP-105 by the company Shin-Etsu.
- Other elastomeric crosslinked organopolysiloxanes in the form of spherical powders may be powders of a hybrid silicone functionalized with fluoroalkyl groups, sold especially under the name KSP-200 by the company Shin-Etsu; powders of a hybrid silicone functionalized with phenyl groups, sold especially under the name KSP-300 by the company Shin-Etsu.
- Silicone elastomers bearing a group MQ, such as those sold by the company Wacker under the names Belsil RG100, Belsil RPG33 and, preferentially, RG80, may also be used in the compositions according to the invention. These particular elastomers, when they are in combination with the resins according to the invention, may make it possible to improve the transfer-resistance properties of the compositions comprising them.
- The Oils:
- The composition according to the invention comprises at least one liquid fatty phase comprising at least one oil.
- The oil may be chosen from hydrocarbon-based oils, silicone oils and fluoro oils.
- The oil may be chosen from volatile oils and non-volatile oils, and mixtures thereof.
- The term “hydrocarbon-based oil” means an oil formed essentially from, or even consisting of, carbon and hydrogen atoms, and possibly oxygen and nitrogen atoms, and containing no silicon or fluorine atoms; it may contain ester, ether, amine or amide groups.
- The term “silicone oil” means an oil containing at least one silicon atom, and especially containing Si—O groups.
- The term “fluoro oil” means an oil containing at least one fluorine atom.
- The composition according to the invention may comprise at least one volatile oil.
- The term “volatile oil” means an oil (or non-aqueous medium) capable of evaporating on contact with the skin in less than one hour, at room temperature and atmospheric pressure. The volatile oil is a volatile cosmetic oil, which is liquid at room temperature, especially having a non-zero vapour pressure, at room temperature and atmospheric pressure, in particular having a vapour pressure ranging from 0.13 Pa to 40 000 Pa (10−3 to 300 mmHg), preferably ranging from 1.3 Pa to 13 000 Pa (0.01 to 100 mmHg) and preferentially ranging from 1.3 Pa to 1300 Pa (0.01 to 10 mmHg).
- In addition, the volatile oil generally has a boiling point, measured at atmospheric pressure, ranging from 150° C. to 260° C. and preferably ranging from 170° C. to 250° C.
- The composition according to the invention may comprise a volatile hydrocarbon-based oil chosen especially from hydrocarbon-based oils with a flash point ranging from 40° C. to 102° C., preferably ranging from 40° C. to 55° C. and preferentially ranging from 40° C. to 50° C.
- Volatile hydrocarbon-based oils that may be mentioned include volatile hydrocarbon-based oils containing from 8 to 16 carbon atoms and mixtures thereof, and especially branched C8-C16 alkanes, for instance C8-C16 isoalkanes (also known as isoparaffins), isododecane, isodecane, isohexadecane and, for example, the oils sold under the trade name Isopar or Permethyl, branched C8-C16 esters, for instance isohexyl neopentanoate, and mixtures thereof. Preferably, the volatile hydrocarbon-based oil is chosen from volatile hydrocarbon-based oils containing from 8 to 16 carbon atoms, and mixtures thereof, in particular from isododecane, isodecane and isohexadecane, and is especially isododecane.
- For skin makeup products, especially foundations and lipsticks, linear hydrocarbon-based volatile oils containing from 8 to 16 carbon atoms will advantageously be used.
- Volatile silicone oils that may be mentioned include linear or cyclic silicones containing from 2 to 7 silicon atoms, these silicones optionally comprising alkyl or alkoxy groups containing from 1 to 10 carbon atoms. As volatile silicone oils that may be used in the invention, mention may be made especially of octamethylcyclotetrasiloxane, decamethylcyclopentasiloxane, dodecamethylcyclohexasiloxane, heptamethyl-hexyltrisiloxane, heptamethyloctyltrisiloxane, octamethyltrisiloxane and decamethyltetrasiloxane, and mixtures thereof.
- The volatile oil may be present in the composition according to the invention in a content ranging from 0.1% to 90% by weight, preferably ranging from 1% to 70% by weight and preferentially ranging from 5% to 50% by weight, relative to the total weight of the composition.
- The composition according to the invention may comprise at least one non-volatile oil.
- Non-volatile hydrocarbon-based oils that may be used include liquid paraffin (or petroleum jelly), squalane, hydrogenated polyisobutylene (Parleam oil), perhydrosqualene, mink oil, turtle oil, soybean oil, sweet almond oil, beauty-leaf oil, palm oil, grapeseed oil, sesame seed oil, corn oil, arara oil, rapeseed oil, sunflower oil, cottonseed oil, apricot oil, castor oil, avocado oil, jojoba oil, olive oil or cereal germ oil; linoleic acid, oleic acid, lauric acid or stearic acid esters; fatty esters, especially of C12-C36, such as isopropyl myristate, isopropyl palmitate, butyl stearate, hexyl laurate, diisopropyl adipate, isononyl isononanoate, 2-ethylhexyl palmitate, 2-hexyldecyl laurate, 2-octyldecyl palmitate, 2-octyldodecyl myristate or lactate, bis(2-ethylhexyl) succinate, diisostearyl malate, and glyceryl or diglyceryl triisostearate; behenic acid, oleic acid, linoleic acid, linolenic acid or isostearic acid; higher fatty alcohols, especially of C16-C22, such as cetanol, oleyl alcohol, linoleyl alcohol, linolenyl alcohol, isostearyl alcohol or octyldodecanol; and mixtures thereof.
- The non-volatile oil may be present in a content ranging from 0.1% to 70% by weight, preferably ranging from 0.5% to 60% by weight and preferentially ranging from 1% to 50% by weight relative to the total weight of the non-volatile liquid fatty phase.
- For skin makeup products, especially foundations and lipsticks, volatile or non-volatile linear silicone oils will advantageously be used. The combination of a resin according to the invention and of a linear silicone oil may especially improve the transfer resistance.
- For skin makeup products, especially lipsticks, phenyl silicone oils will advantageously be used. The combination of a resin according to the invention and of a phenyl silicone oil may especially improve the gloss and comfort and reduce the tacky sensation.
- Structuring Agents:
- The composition according to the invention may comprise a structuring agent.
- The term “structuring agent” means a compound capable of increasing the viscosity of the composition. The structuring agent makes it possible especially to obtain a composition that can have a texture ranging from fluid to solid textures.
- The structuring agent may be present in the composition in a content ranging from 0.05% to 40% by weight, preferably ranging from 0.1% to 30% by weight and preferentially ranging from 0.1% to 25% by weight, relative to the total weight of the composition.
- The structuring agent may be chosen especially from thickeners (oily-medium thickeners; aqueous-medium thickeners), organogelling agents, waxes, pasty compounds and gums.
- The aqueous-medium thickener may be chosen from:
-
- hydrophilic clays,
- hydrophilic fumed silica,
- water-soluble cellulose-based thickeners,
- guar gum, xanthan gum, carob gum, scleroglucan gum, gellan gum, rhamsan gum, karaya gum or carrageenan gum,
- alginates, maltodextrins, starch and its derivatives, and hyaluronic acid and its salts,
- the polyglyceryl (meth)acrylate polymers sold under the names “Hispagel” or “Lubragel” by the companies Hispano Quimica or Guardian,
- polyvinylpyrrolidone,
- polyvinyl alcohol,
- crosslinked acrylamide polymers and copolymers, such as those sold under the names PAS 5161 or Bozepol C by the company Hoechst, Sepigel 305 by the company SEPPIC by the company Allied Colloid, or alternatively
- the crosslinked methacryloyloxyethyltrimethyl-ammonium chloride homopolymers sold under the name “Salcare SC95” by the company Allied Colloid,
- associative polymers and especially associative polyurethanes.
- Such thickeners are described especially in patent application EP-A-1 400 234, the content of which is incorporated by reference.
- The oily-medium thickener may be chosen from:
-
- carboxylate silicones,
- saccharide silicones,
- organophilic clays;
- hydrophobic fumed silicas;
- alkyl guar gums (with a C1-C6 alkyl group), such as those described in EP-A-708 114;
- hydrophobic celluloses,
- oil-gelling polymers, for instance triblock polymers or star polymers resulting from the polymerization or copolymerization of at least one monomer containing an ethylenic group, for instance the polymers sold under the name Kraton;
- polymers with a weight-average molecular mass of less than 100 000, comprising a) a polymer skeleton containing hydrocarbon-based repeating units containing at least one heteroatom, and optionally b) at least one pendent fatty chain and/or at least one terminal fatty chain, which are optionally functionalized, containing from 6 to 120 carbon atoms and being linked to these hydrocarbon-based units, as described in patent applications WO-A-02/056847 and WO-A-02/47619, the content of which is incorporated by reference; in particular, polyamide resins (especially comprising alkyl groups containing from 12 to 22 carbon atoms) such as those described in U.S. Pat. No. 5,783,657, the content of which is incorporated by reference;
- the silicone-based polyamide resins as described in patent application EP-A-1 266 647 and in the French patent application filed under the number 0 216 039, the content of which is incorporated by reference.
- Such thickeners are especially described in patent application EP-A-1 400 234, the content of which is incorporated by reference.
- The organogelling agents may be chosen from those described in patent application WO-A-03/105 788, the content of which is incorporated by reference.
- In particular, it may be advantageous to combine the resins according to the invention with particular organogelling agents, and especially:
- the bis-urea derivatives of general formula (I):
- in which:
- A is a group of formula:
- with R′ being a linear or branched C1 to C4 alkyl radical and the *s symbolizing the points of attachment of the group A to each of the two nitrogen atoms of the rest of the compound of general formula (I), and
-
- R is a saturated or unsaturated, non-cyclic, mono-branched C6 to C16 alkyl radical whose hydrocarbon-based chain is optionally interrupted with 1 to 3 heteroatoms chosen from O, S and N, or
- a salt or isomer thereof, described especially in patent application FR-A-2 892 303,
- the silicone bis-urea derivatives of general formula (I), or a salt and/or isomer thereof:
- in which:
-
- A is a group of formula (II):
- with R1 being a linear or branched C1-C4 alkyl radical, and the *s symbolizing the points of attachment of the group A to each of the two nitrogen atoms of the rest of the compound of general formula (I), and
-
- R and R′, which may be identical or different, are chosen from:
- i) the radicals of formula (III):
- in which:
-
- L is a single bond or a divalent carbon-based radical, especially a linear, branched and/or cyclic, saturated or unsaturated hydrocarbon-based radical (alkylene), containing 1 to 18 carbon atoms, and possibly comprising 1 to 4 heteroatoms chosen from N, O and S;
- Ra is:
- a) a carbon-based radical, especially a linear, branched and/or cyclic, saturated or unsaturated hydrocarbon-based radical (alkyl), containing 1 to 18 carbon atoms, and possibly comprising 1 to 8 heteroatoms chosen from N, O, Si and S; or
- b) a silicone radical of formula:
- with n being between 0 and 100, especially between 1 and 80, or even 2 to 20;
- and R2 to R6 being, independently of each other, carbon-based radicals, especially linear or branched hydrocarbon-based radicals (alkyl) containing 1 to 12 and especially 1 to 6 carbon atoms, and possibly comprising 1 to 4 heteroatoms, especially O;
-
- Rb and Rc are, independently of each other, chosen from:
- a) carbon-based radicals, especially linear, branched and/or cyclic, saturated or unsaturated hydrocarbon-based radicals (alkyl), containing 1 to 18 carbon atoms, and possibly comprising 1 to 4 heteroatoms chosen from N, O, Si and S;
- b) the radicals of formula:
- with n being between 0 and 100, especially between 1 and 80, or even 2 to 20;
- and R′2 to R′6 being, independently of each other, carbon-based radicals, especially linear or branched hydrocarbon-based radicals (alkyl), containing 1 to 12 and especially 1 to 6 carbon atoms, and possibly comprising 1 to 4 heteroatoms, especially O; and
-
- ii) linear, branched and/or cyclic, saturated or unsaturated C1-C30 alkyl radicals, optionally comprising 1 to 3 heteroatoms chosen from O, S, F and N;
- it being understood that at least one of the radicals R and/or R′ is of formula (III), such as those described in patent application FR-A-2 900 819,
- the bis-urea derivatives described in patent application FR-A-2 899 4476.
- Wax(es)
- The composition may comprise at least one solid fatty substance chosen from waxes, as structuring agent.
- The wax under consideration in the context of the present invention is generally a lipophilic compound that is solid at room temperature (25° C.), with a solid/liquid reversible change of state, having a melting point of greater than or equal to 30° C., which may be up to 200° C. and in particular up to 120° C.
- In particular, the waxes that are suitable for the invention may have a melting point of greater than or equal to 45° C. and in particular greater than or equal to 55° C.
- For the purposes of the invention, the melting point corresponds to the temperature of the most endothermic peak observed by thermal analysis (DSC) as described in standard ISO 11357-3; 1999. The melting point of the wax may be measured using a differential scanning calorimeter (DSC), for example the calorimeter sold under the name MDSC 2920 by the company TA Instruments.
- The measuring protocol is as follows:
- A sample of 5 mg of wax placed in a crucible is subjected to a first temperature rise ranging from −20° C. to 100° C., at a heating rate of 10° C./minute, it is then cooled from 100° C. to −20° C. at a cooling rate of 10° C./minute and is finally subjected to a second temperature increase ranging from −20° C. to 100° C. at a heating rate of 5° C./minute. During the second temperature increase, the variation of the difference in power absorbed by the empty crucible and by the crucible containing the sample of wax is measured as a function of the temperature. The melting point of the compound is the temperature value corresponding to the top of the peak of the curve representing the variation in the difference in absorbed power as a function of the temperature.
- The waxes that may be used in the compositions according to the invention are chosen from waxes that are solid at room temperature of animal, plant, mineral or synthetic origin, and mixtures thereof.
- As illustrations of waxes that are suitable for the invention, mention may be made especially of hydrocarbon-based waxes, for instance beeswax, lanolin wax, Chinese insect waxes, rice bran wax, carnauba wax, candelilla wax, ouricury wax, esparto grass wax, berry wax, shellac wax, Japan wax and sumach wax; montan wax, orange wax and lemon wax, microcrystalline waxes, paraffins and ozokerite; polyethylene waxes, the waxes obtained by Fischer-Tropsch synthesis and waxy copolymers, and also esters thereof.
- Mention may also be made of waxes obtained by catalytic hydrogenation of animal or plant oils containing linear or branched C8-C32 fatty chains. Among these waxes that may especially be mentioned are isomerized jojoba oil such as the trans-isomerized partially hydrogenated jojoba oil manufactured or sold by the company Desert Whale under the commercial reference Iso-Jojoba-50®, hydrogenated sunflower oil, hydrogenated castor oil, hydrogenated coconut oil, hydrogenated lanolin oil and bis(1,1,1-trimethylol-propane) tetrastearate sold under the name Hest 2T-4S® by the company Heterene.
- Mention may also be made of silicone waxes (C30-45 alkyl dimethicone) and fluoro waxes.
- The waxes obtained by hydrogenation of castor oil esterified with cetyl alcohol, sold under the names Phytowax ricin 16L64® and 22L73® by the company Sophim, may also be used. Such waxes are described in patent application FR-A-2 792 190.
- A wax that may be used is a C20-C40 alkyl (hydroxystearyloxy)stearate (the alkyl group containing from 20 to 40 carbon atoms), alone or as a mixture.
- Such a wax is especially sold under the names Kester Wax K 82 P®, Hydroxypolyester K 82 P®, Kester Wax K 80 P® and Kester Wax K 82H® by the company Koster Keunen.
- As microwaxes that may be used in the compositions according to the invention, mention may be made especially of carnauba microwaxes, such as the product sold under the name MicroCare 350® by the company Micro Powders, synthetic microwaxes, such as the product sold under the name MicroEase 114S® by the company Micro Powders, microwaxes consisting of a mixture of carnauba wax and polyethylene wax, such as the products sold under the names Micro Care 300® and 310® by the company Micro Powders, microwaxes consisting of a mixture of carnauba wax and of synthetic wax, such as the product sold under the name Micro Care 325® by the company Micro Powders, polyethylene microwaxes, such as the products sold under the names Micropoly 200®, 220®, 220L® and 250S® by the company Micro Powders, and polytetrafluoroethylene microwaxes, such as the products sold under the names Microslip 519® and 519 L® by the company Micro Powders.
- The composition according to the invention may have a wax content ranging from 0.1% to 50% by weight and better still from 1% to 30% by weight relative to the total weight of the composition.
- Pasty Compounds
- The composition according to the invention may comprise at least one pasty compound as structuring agent. Pasty fatty substances are considered as solid fatty substances for the purposes of the present invention.
- For the purposes of the present invention, the term “pasty” means a lipophilic fatty compound that undergoes a reversible solid/liquid change of state and that comprises in the solid state an anisotropic crystal organization, and comprises, at a temperature of 23° C., a liquid fraction and a solid fraction.
- In other words, the starting melting point of the pasty compound is less than 23° C. The liquid fraction of the pasty compound measured at 23° C. may represent 9% to 97% by weight of the compound. This liquid fraction at 23° C. preferably represents between 15% and 85% and more preferably between 40% and 85% by weight.
- The liquid fraction by weight of the pasty compound at 23° C. is equal to the ratio of the heat of fusion consumed at 23° C. to the heat of fusion of the pasty compound.
- The heat of fusion of the pasty compound is the heat consumed by the compound to change from the solid state to the liquid state. The pasty compound is said to be in the solid state when all of its mass is in solid form. The pasty compound is said to be in the liquid state when all of its mass is in liquid form.
- The heat of fusion of the pasty compound is equal to the area under the curve of the thermogram obtained using a differential scanning calorimeter (DSC), such as the calorimeter sold under the name MDSC 2920 by the company TA Instrument, with a temperature rise of 5 or 10° C. per minute, according to standard ISO 11357-3:1999. The heat of fusion of the pasty compound is the amount of energy required to make the compound change from the solid state to the liquid state. It is expressed in J/g.
- The heat of fusion consumed at 23° C. is the amount of energy absorbed by the sample to change from the solid state to the state that it has at 23° C., constituted of a liquid fraction and a solid fraction.
- The liquid fraction of the pasty compound, measured at 32° C., preferably represents from 30% to 100% by weight of the compound, preferably from 50% to 100% and more preferably from 60% to 100% by weight of the compound. When the liquid fraction of the pasty compound measured at 32° C. is equal to 100%, the temperature of the end of the melting range of the pasty compound is less than or equal to 32° C.
- The liquid fraction of the pasty compound measured at 32° C. is equal to the ratio of the heat of fusion consumed at 32° C. to the heat of fusion of the pasty compound. The heat of fusion consumed at 32° C. is calculated in the same manner as the heat of fusion consumed at 23° C.
- The pasty compound is preferably chosen from synthetic compounds and compounds of plant origin. A pasty compound may be obtained by synthesis from starting materials of plant origin.
- The pasty compound may advantageously be chosen from:
-
- i) lanolin and derivatives thereof,
- ii) polymer or non-polymer silicone compounds,
- iii) polymer or non-polymer fluoro compounds,
- iv) vinyl polymers, especially:
- v) olefin homopolymers
- vi) olefin copolymers
- vii) hydrogenated diene homopolymers and copolymers
- viii) linear or branched oligomers, which are homopolymers or copolymers of alkyl (meth)acrylates preferably containing a C8-C30 alkyl group
- ix) oligomers, which are homopolymers and copolymers of vinyl esters containing C8-C30 alkyl groups
- x) oligomers, which are homopolymers and copolymers of vinyl ethers containing C8-C30 alkyl groups,
- xi) liposoluble polyethers resulting from the polyetherification between one or more C2-C100 and preferably C2-C50 diols,
- xii) esters,
- xiii) and mixtures thereof.
- Among the esters that are especially preferred are:
-
- xiv) esters of a glycerol oligomer, especially diglycerol esters, in particular condensates of adipic acid and of glycerol, for which some of the hydroxyl groups of the glycerols have reacted with a mixture of fatty acids such as stearic acid, capric acid, stearic acid and isostearic acid, and 12-hydroxystearic acid, especially such as the product sold under the brand name Softisan 649 by the company Sasol,
- xv) arachidyl propionate sold under the brand name Waxenol 801 by Alzo,
- xvi) phytosterol esters,
- xvii) fatty acid triglycerides and derivatives thereof,
- xviii) pentaerythritol esters,
- xix) non-crosslinked polyesters resulting from polycondensation between a linear or branched C4-C50 dicarboxylic acid or polycarboxylic acid and a C2-C50 diol or polyol,
- xx) aliphatic esters of an ester resulting from the esterification of an aliphatic hydroxycarboxylic acid with an aliphatic carboxylic acid,
- xxi) polyesters resulting from the esterification, with a polycarboxylic acid, of an ester of an aliphatic hydroxycarboxylic acid, the said ester comprising at least two hydroxyl groups, such as the products Risocast DA-H® and Risocast DA-L®,
- xxii) esters of a diol dimer and of a diacid dimer, where appropriate esterified on their free alcohol or acid function(s) with acid or alcohol radicals, such as Plandool-G,
- xxiii) and mixtures thereof.
- Among the pasty compounds of plant origin, a mixture of soybean sterols and of oxyethylenated (5 EO) oxypropylenated (5 PO) pentaerythritol, sold under the reference Lanolide by the company Vevy, will preferably be chosen.
- Preferably, the composition comprises a total content of pasty fatty substances ranging from 0.5% to 50% by weight, preferably from 1% to 40% by weight and better still from 5% to 30% by weight relative to the weight of the composition.
- The gums are generally polydimethylsiloxanes (PDMS) of high molecular weight or cellulose gums or polysaccharides.
- Surfactants
- The composition according to the invention may comprise at least one surfactant.
- The surfactant may be lipophilic and/or hydrophilic, used alone or in combination.
- The surfactant may be chosen from nonionic, anionic, cationic and amphoteric surfactants.
- The nonionic surfactant may be chosen from:
-
- a C8-C22 alkyl dimethicone copolyol, i.e. an oxypropylenated and/or oxyethylenated polymethyl (C8-C22)alkyl dimethyl methyl siloxane.
- The C8-C22 alkyl dimethicone copolyol is advantageously a compound of formula (I) below:
- in which:
-
- PE represents (—C2H4O)x— (C3H6O)y—R, R being chosen from a hydrogen atom and an alkyl radical of 1 to 4 carbon atoms, x ranging from 0 to 100 and y ranging from 0 to 80, x and y not simultaneously being 0
- m ranging from 1 to 40
- n ranging from 10 to 200
- o ranging from 1 to 100
- p ranging from 7 to 21
- q ranging from 0 to 4
- and preferably:
- R═H
- m=1 to 10
- n=10 to 100
- o=1 to 30
- p=15
- q=3.
- A C8-C22 alkyl dimethicone copolyol that may be mentioned is cetyl dimethicone copolyol, for instance the product sold under the name Abil EM-90 by the company Goldschmidt.
-
- a dimethicone copolyol, i.e. an oxypropylenated and/or oxyethylenated polydimethyl methyl siloxane. It contains no alkyl groups with a chain length of more than 8 carbon atoms, especially C8-C22.
- Dimethicone copolyols that may be used include those corresponding to formula (II) below:
- in which:
- R1, R2 and R3, independently of each other, represent a C1-C6 alkyl radical or a radical —(CH2)X—(OCH2CH2)y—(OCH2CH2CH2)z—OR4, at least one radical R1, R2 or R3 not being an alkyl radical; R4 being a hydrogen, a C1-C2 alkyl radical or a C2-C4 acyl radical;
- A is an integer ranging from 0 to 200;
- B is an integer ranging from 0 to 50; on condition that A and B are not simultaneously equal to zero;
- x is an integer ranging from 1 to 6;
- y is an integer ranging from 1 to 30;
- z is an integer ranging from 0 to 5.
- According to one preferred embodiment of the invention, in the compound of formula (II), R1═R3=methyl radical, x is an integer ranging from 2 to 6 and y is an integer ranging from 4 to 30. R4 is in particular a hydrogen.
- Examples of compounds of formula (II) that may be mentioned include the compounds of formula (III):
- in which A is an integer ranging from 20 to 105, B is an integer ranging from 2 to 10 and y is an integer ranging from 10 to 20.
- Examples of silicone compounds of formula (II) that may also be mentioned include the compounds of formula (IV):
-
HO—(CH2CH2O)y—(CH2)3-[(CH3)2SiO]A′-[(CH3)2Si]-(CH2)3—(OCH2CH2)y—OH (Iv) - in which A′ and y are integers ranging from 10 to 20.
- Dimethicone copolyols that may be used include those sold under the names DC 5329, DC 7439-146, DC 2-5695 and Q4-3667 by the company Dow Corning; KF-6013, KF-6015, KF-6016 and KF-6017 by the company Shin-Etsu.
- The compounds DC 5329, DC 7439-146 and DC2-5695 are compounds of formula (III) in which, respectively, A is 22, B is 2 and y is 12; A is 103, B is 10 and y is 12; A is 27, B is 3 and y is 12.
- Nonionic surfactants that may also be mentioned include fatty acid esters of polyols, for instance sorbitol or glyceryl mono-, di-, tri- or sesqui-oleates or stearates, glyceryl or polyethylene glycol laurates; fatty acid esters of polyethylene glycol (polyethylene glycol monostearate or monolaurate); polyoxyethylenated fatty acid esters (stearate or oleate) of sorbitol; polyoxyethylenated alkyl (lauryl, cetyl, stearyl or octyl)ethers.
- Anionic surfactants that may be mentioned include carboxylates (sodium 2-(2-hydroxyalkyloxy)acetate)), amino acid derivatives (N-acylglutamates, N-acylglycinates or acylsarcosinates), alkyl sulfates, alkyl ether sulfates and oxyethylenated derivatives thereof, sulfonates, isethionates and N-acylisethionates, taurates and N-acyl N-methyltaurates, sulfosuccinates, alkylsulfoacetates, phosphates and alkyl phosphates, polypeptides, anionic derivatives of alkyl polyglycoside (acyl-D-galactoside uronate), and fatty acid soaps, and mixtures thereof.
- Amphoteric and zwitterionic surfactants that may be used include betaines, N-alkylamidobetaines and derivatives thereof, glycine derivatives, sultaines, alkyl polyaminocarboxylates and alkylamphoacetates, and mixtures thereof.
- Such surfactants are described especially in patent application WO-A-02/056 854, the content of which is incorporated by reference.
- The surfactant may be present in the composition according to the invention in a content ranging from 0.1% to 10% by weight, preferably ranging from 0.5% to 8% by weight and preferentially ranging from 0.5% to 7% by weight, relative to the total weight of the composition.
- Dyestuffs:
- The composition according to the invention may comprise at least one dyestuff.
- The dyestuff may be chosen from pulverulent dyestuffs (especially pigments and nacres) and water-soluble dyestuffs.
- The term “pigments” should be understood as meaning white or coloured, mineral or organic particles of any form, which are insoluble in the physiological medium, and which are intended to colour the composition.
- The term “nacres” should be understood as meaning iridescent particles of any form, produced especially by certain molluscs in their shell, or else synthesized.
- The pigments may be white or coloured, and mineral and/or organic. Among the mineral pigments that may be mentioned are titanium dioxide, optionally surface-treated, zirconium oxide or cerium oxide, and also zinc oxide, iron oxide (black, yellow or red) or chromium oxide, manganese violet, ultramarine blue, chromium hydrate and ferric blue, and metal powders, for instance aluminium powder or copper powder.
- Among the organic pigments that may be mentioned are carbon black, pigments of D & C type, and lakes based on cochineal carmine or on barium, strontium, calcium or aluminium.
- Mention may also be made of pigments with an effect, such as particles comprising a natural or synthetic, organic or mineral substrate, for example glass, acrylic resins, polyester, polyurethane, polyethylene terephthalate, ceramics or aluminas, the said substrate being uncoated or coated with metallic substances, for instance aluminium, gold, silver, platinum, copper or bronze, or with metal oxides, for instance titanium dioxide, iron oxide or chromium oxide, and mixtures thereof.
- The nacreous pigments may be chosen from white nacreous pigments such as mica coated with titanium or with bismuth oxychloride, coloured nacreous pigments such as titanium mica coated with iron oxides, titanium mica coated especially with ferric blue or with chromium oxide, titanium mica coated with an organic pigment of the abovementioned type, and also nacreous pigments based on bismuth oxychloride. Interference pigments, especially liquid-crystal or multilayer interference pigments, may also be used.
- The term “alkyl” mentioned in the compounds cited above especially denotes an alkyl group containing from 1 to 30 carbon atoms and preferably containing from 5 to 16 carbon atoms.
- Hydrophobic-treated pigments are described especially in patent application EP-A-1 086 683.
- The water-soluble dyes are, for example, beetroot juice or methylene blue.
- The synthetic or natural liposoluble dyes are, for example, DC Red 17, DC Red 21, DC Red 27, DC Green 6, DC Yellow 11, DC Violet 2, DC Orange 5, Sudan red, carotenes (β-carotene, lycopene), xanthophylls (capsanthin, capsorubin, lutein), palm oil, Sudan brown, quinoline yellow, annatto and curcumin.
- The dyestuffs, in particular the pigments treated with a hydrophobic agent, may be present in the composition in a content ranging from 0.1% to 50% by weight, preferably ranging from 0.5% to 30% by weight and preferentially ranging from 1% to 20% by weight, relative to the total weight of the composition.
- Fillers:
- The composition according to the invention may comprise at least one filler.
- For the purposes of the present invention, the term “filler” denotes solid particles of any form, which are in an insoluble form and dispersed in the medium of the composition, even at temperatures that may be up to the melting point of all the fatty substances of the composition.
- Generally, the fillers used according to the invention are colourless or white, namely non-pigmentary, i.e. they are not used to give a particular colour or shade to the composition according to the invention, even though their use may inherently lead to such a result. These fillers serve especially to modify the rheology or texture of the composition.
- In this respect, they are different from nacres, organic pigmentary materials, for instance carbon black, pigments of D&C type, and lakes based on cochineal carmine or on barium, strontium, calcium or aluminium, and inorganic pigmentary materials, for instance titanium dioxide, zirconium oxide or cerium oxide, and also iron oxides (black, yellow or red), chromium oxide, manganese violet, ultramarine blue, chromium hydrate and ferric blue, which are, themselves, used to give a shade and coloration to the compositions incorporating them.
- For the purposes of the invention, such compounds are not covered by the definition of fillers, which thus covers non-pigmentary fillers, which may be organic or inorganic.
- The non-pigmentary fillers used in the compositions according to the present invention may be of lamellar, globular or spherical form, of fibre type, or of any intermediate form between these defined forms.
- The size of the particles, i.e. their granulometry, is chosen so as to ensure the good dispersion of the fillers in the composition according to the invention. The granulometry of the particles may be distributed within the range from 5 μm to 10 nm and in particular from 10 μm to 10 nm.
- The fillers according to the invention may or may not be surface-coated, in particular surface-treated with silicones, amino acids, fluoro derivatives or any other substance that promotes the dispersion and compatibility of the filler in the composition.
- Mineral Fillers
- For the purposes of the present invention, the terms “mineral” and “inorganic” are used interchangeably.
- Among the non-pigmentary mineral fillers that may be used in the compositions according to the invention, mention may be made of talc, mica, silica, perlite, which is especially commercially available from the company World Minerals Europe under the trade name Perlite P1430, Perlite P2550 or Perlite P204, kaolin, precipitated calcium carbonate, magnesium carbonate, magnesium hydrogen carbonate, hydroxyapatite, boron nitride, hollow silica microspheres (Silica Beads® from Maprecos), and glass or ceramic microcapsules, and mixtures thereof.
- According to one embodiment, the cosmetic composition according to the invention comprises at least one non-pigmentary mineral filler chosen from the group comprising kaolin, talc, silica, perlite and clay, and mixtures thereof.
- Organic Fillers
- Among the organic fillers that may be mentioned are polyamide powder (Orgasol® Nylon® from Atochem), poly-β-alanine powder and polyethylene powder, lauroyllysine, starch, tetrafluoroethylene polymer powders (Teflon®), hollow polymer microspheres such as those of polyvinylidene chloride/acrylonitrile, for instance Expancel® (Nobel Industrie) or of acrylic acid copolymer (such as Polytrap (Dow Corning)), acrylate copolymers, PMMA, 12-hydroxystearic acid oligomer stearate and silicone resin microbeads (for example Tospearls® from Toshiba), magnesium carbonate, magnesium hydrogen carbonate, and metal soaps derived from organic carboxylic acids containing from 8 to 22 carbon atoms and preferably from 12 to 18 carbon atoms, for example zinc stearate, magnesium stearate, lithium stearate, zinc laurate or magnesium myristate, and mixtures thereof.
- For the purposes of the present invention, the organic fillers are different from the pigments.
- They may also be particles comprising a copolymer, the said copolymer comprising trimethylol hexyl lactone. In particular, it may be a hexamethylene diisocyanate/trimethylol hexyl lactone copolymer. Such particles are especially commercially available, for example under the name Plastic Powder D-400® or Plastic Powder D-800® from the company Toshiki.
- According to one embodiment, a composition of the invention may comprise at least one filler chosen from talc, silica, starch, clay, kaolin and perlite, and mixtures thereof.
- One or more dispersants may be used, where appropriate, to protect the dispersed fillers or particles against aggregation or flocculation. They may be added independently of the solid fillers or particles or in the form of a colloidal dispersion of particles.
- The concentration of dispersants is chosen so as to obtain satisfactory dispersion of the solid particles (without flocculation).
- This dispersant may be a surfactant, an oligomer, a polymer or a mixture of several thereof, bearing one or more functionalities with strong affinity for the surface of the particles to be dispersed. In particular, poly(12-hydroxystearic acid) esters are used, such as poly(12-hydroxystearic acid) stearate with a molecular weight of about 750 g/mol, such as the product sold under the name Solsperse 21 000® by the company Avecia, esters of poly(12-hydroxystearic acid) with polyols such as glycerol or diglycerol, such as polyglyceryl-2 dipolyhydroxystearate (CTFA name) sold under the reference Dehymuls PGPH® by the company Henkel (or diglyceryl poly(12-hydroxystearate)), or alternatively poly(12-hydroxystearic acid), such as the product sold under the reference Arlacel P100 by the company Uniqema, and mixtures thereof.
- As other dispersants that may be used in the composition of the invention, mention may be made of quaternary ammonium derivatives of polycondensate fatty acids, for instance Solsperse 17 000® sold by the company Avecia, and mixtures of polydimethylsiloxane/oxypropylene such as those sold by the company Dow Corning under the references DC2-5185 and DC2-5225 C.
- A composition of the invention should be cosmetically or dermatologically acceptable, i.e. it should contain a non-toxic physiologically acceptable medium that can be applied to human lips. For the purposes of the invention, the term “cosmetically acceptable” refers to a composition of pleasant appearance, odour and feel.
- The composition according to the invention may also contain ingredients commonly used in cosmetics, such as vitamins, thickeners, trace elements, softeners, sequestrants, fragrances, acidifying agents, basifying agents, preserving agents, sunscreens, surfactants, antioxidants, hair-loss counteractants, antidandruff agents and propellants, or mixtures thereof.
- Needless to say, a person skilled in the art will take care to select this or these optional additional compounds, and/or the amount thereof, such that the advantageous properties of the corresponding composition according to the invention are not, or are not substantially, adversely impaired by the envisaged addition.
- According to another aspect, the invention also relates to a cosmetic assembly comprising:
- i) a container delimiting at least one compartment, the said container being closed by a closing member; and
- ii) a composition placed inside the said compartment, the composition being in accordance with the invention.
- The container may be in any adequate form. It may especially be in the form of a bottle, a tube, a jar, a case, a box, a sachet or a carton.
- The closing member may be in the form of a removable stopper, a lid, a cap, a tear-off strip or a capsule, especially of the type comprising a body attached to the container and a cover cap articulated on the body. It may also be in the form of a member for selectively closing the container, especially a pump, a valve or a flap valve.
- The container may be combined with an applicator, especially in the form of a brush comprising an arrangement of bristles maintained by a twisted wire. Such a twisted brush is described especially in U.S. Pat. No. 4,887,622. It may also be in the form of a comb comprising a plurality of application members, obtained especially by moulding. Such combs are described, for example, in patent FR 2 796 529. The applicator may be in the form of a fine brush, as described, for example, in patent FR 2 722 380. The applicator may be in the form of a block of foam or of elastomer, a felt or a spatula. The applicator may be free (tuft or sponge) or securely fastened to a rod borne by the closing member, as described, for example, in U.S. Pat. No. 5,492,426. The applicator may be securely fastened to the container, as described, for example, in patent FR 2 761 959.
- The product may be contained directly in the container, or indirectly. By way of example, the product may be arranged on an impregnated support, especially in the form of a wipe or a pad, and arranged (individually or in plurality) in a box or in a sachet. Such a support incorporating the product is described, for example, in patent application WO 01/03538.
- The closing member may be coupled to the container by screwing. Alternatively, the coupling between the closing member and the container is done other than by screwing, especially via a bayonet mechanism, by click-fastening, gripping, welding, bonding or by magnetic attraction. The term “click-fastening” in particular means any system involving the crossing of a bead or cord of material by elastic deformation of a portion, especially of the closing member, followed by return to the elastically unconstrained position of the said portion after the crossing of the bead or cord.
- The container may be at least partially made of thermoplastic material. Examples of thermoplastic materials that may be mentioned include polypropylene or polyethylene.
- Alternatively, the container is made of non-thermoplastic material, especially glass or metal (or alloy).
- The container may have rigid walls or deformable walls, especially in the form of a tube or a tubular bottle.
- The container may comprise means for distributing or facilitating the distribution of the composition. By way of example, the container may have deformable walls so as to allow the composition to exit in response to a positive pressure inside the container, this positive pressure being caused by elastic (or non-elastic) squeezing of the walls of the container. Alternatively, especially when the product is in the form of a stick, the product may be driven out by a piston mechanism. Still in the case of a stick, especially of makeup product (lipstick, foundation, etc.), the container may comprise a mechanism, especially a rack mechanism, a threaded-rod mechanism or a helical groove mechanism, and may be capable of moving a stick in the direction of the said aperture. Such a mechanism is described, for example, in patent FR 2 806 273 or in patent FR 2 775 566. Such a mechanism for a liquid product is described in patent FR 2 727 609.
- The container may be formed from a carton with a base delimiting at least one housing containing the composition, and a lid, especially articulated on the base, and capable of at least partially covering the said base. Such a carton is described, for example, in patent application WO 03/018423 or in patent FR 2 791 042.
- The container may be equipped with a drainer arranged in the region of the aperture of the container. Such a drainer makes it possible to wipe the applicator and possibly the rod to which it may be securely fastened. Such a drainer is described, for example, in patent FR 2 792 618.
- The composition may be at atmospheric pressure inside the container (at room temperature) or pressurized, especially by means of a propellent gas (aerosol). In the latter case, the container is equipped with a valve (of the type used for aerosols).
- Protocol for Measuring the Transfer:
- The transfer index of the deposit obtained with the composition according to the invention is determined according to the measuring protocol described below.
- A support (40 mm×70 mm rectangle) consisting of an acrylic coating (hypoallergenic acrylic adhesive on polyethylene film sold under the name Blenderme ref. FH5000-55113 by the company 3M Santé) bonded onto a layer of polyethylene foam that is adhesive on the side opposite the one to which the adhesive plaster is fixed (foam layer sold under the name RE 40×70EP3 from the company Joint Technique Lyonnais Ind.) is prepared.
- The colour L*0a*0b*0 of the support, on the acrylic coating side, is measured using a Minolta CR300 colorimeter.
- The support thus prepared is preheated on a hotplate maintained at a temperature of 40° C. so that the surface of the support is maintained at a temperature of 33° C.±1° C.
- While leaving the support on the hotplate, the composition is applied to the entire non-adhesive surface of the support (i.e. to the surface of the acrylic coating), spreading it out with a brush to obtain a deposit of the composition of about 15 μm, and this deposit is then left to dry for 10 minutes.
- After drying, the colour L*a*b* of the film thus obtained is measured.
- The colour difference ΔE1 between the colour of the film relative to the colour of the naked support is then determined via the following relationship:
-
ΔE1=√{square root over ((L*−L 0*)2+(a*−a 0*)2+(b*−b 0*)2)}{square root over ((L*−L 0*)2+(a*−a 0*)2+(b*−b 0*)2)}{square root over ((L*−L 0*)2+(a*−a 0*)2+(b*−b 0*)2)} - The support is then bonded via its adhesive face (adhesive face of the foam layer) to an anvil 20 mm in diameter and equipped with a screw pitch. A sample of the support/deposit assembly is then cut out using a sample punch 18 mm in diameter. The anvil is then screwed onto a press (Statif Manuel Imada SV-2 from the company Someco) equipped with a tensile testing machine (Imada DPS-20 from the company Someco).
- A strip 33 mm wide and 29.7 cm long is drawn on a sheet of white photocopier paper with a basis weight of 80 g/m2, a first line is marked 2 cm from the edge of the sheet, and a second line is then marked 5 cm from the edge of the sheet, the first and second lines thus delimiting a box on the strip; next, a first mark and a second mark located in the strip at reference points 8 cm and 16 cm, respectively, from the second mark, are applied. 20 μl of water are placed on the first mark and 10 μl of refined sunflower oil (sold by the company Lesieur) are placed on the second mark.
- The white paper is placed on the base of the press and the sample placed on the box of the strip of paper is then pressed at a pressure of about 300 g/cm2 exerted for 30 seconds. The press is then opened and the sample is again placed just after the second mark (i.e. next to the box), a pressure of about 300 g/cm2 is again exerted, and the paper is displaced, in a rectilinear manner as soon as the contact is made, at a speed of 1 cm/s over the entire length of the strip such that the sample passes through the water and oil deposits.
- After removing the sample, some of the deposit has transferred onto the paper. The colour L*', a*', b*' of the deposit remaining on the sample is then measured.
- The colour difference ΔE2 between the colour of the deposit remaining on the sample relative to the colour of the naked support is then determined via the following relationship:
-
ΔE2=√{square root over ((L*′−L 0*)2+(a*′−a 0*)2+(b*′−b 0*)2)}{square root over ((L*′−L 0*)2+(a*′−a 0*)2+(b*′−b 0*)2)}{square root over ((L*′−L 0*)2+(a*′−a 0*)2+(b*′−b 0*)2)} - The transfer index of the composition, expressed as a percentage, is equal to the ratio:
-
100×ΔE2/ΔE1 - The measurement is performed on 6 supports in succession and the transfer index corresponds to the mean of the six measurements obtained with the six supports.
- The present invention also relates to a cosmetic product for making up and/or caring for keratin materials, comprising at least two compositions that can be applied successively to keratin materials, especially to the lips.
- The present invention also relates to a process for making up the face and the body using these two compositions. They are preferably applied successively to the keratin materials: the first composition and then the second composition.
- These two compositions are conventionally known as a topcoat and a basecoat.
- Thus, according to this embodiment, the invention relates to a product (also known as a kit) for making up and/or caring for keratin materials, especially the lips, comprising a first composition and a second composition conditioned in separate containers,
- the first composition containing, in a physiologically acceptable medium:
- a) a siloxane resin comprising the following units:
- (i) (R1 3SiO1/2)a
- (ii) (R2 2SiO2/2)b
- (iii) (R3SiO3/2)c and
- (iv) (SiO4/2)d
- with
- R1, R2 and R3 independently representing an alkyl group containing from 1 to 8 carbon atoms, an aryl group, a carbinol group or an amino group,
- a being between 0.05 and 0.5,
- b being between 0 and 0.3,
- c being greater than 0,
- d being between 0.05 and 0.6,
- a+b+c+d=1,
- on condition that more than 40 mol % of the groups R3 of the siloxane resin are propyl groups, and
- b) at least one liquid fatty phase, and
- c) at least one film-forming polymer chosen from the group comprising:
-
- a film-forming block ethylenic polymer, comprising at least a first block and at least a second block, is characterized in that the first block is obtained from at least one acrylate monomer of formula CH2═CH—COOR2 in which R2 represents a C4 to C12 cycloalkyl group and at least one methacrylate monomer of formula CH2═C(CH3)—COOR2 in which R′2 represents a C4 to C12 cycloalkyl group, and characterized in that the second block is obtained from an acrylic acid monomer and from at least one monomer with a glass transition temperature of less than or equal to 20° C.,
- a vinyl polymer comprising at least one carbosiloxane dendrimer-based unit,
- a dispersion of acrylic or vinyl radical homopolymer or copolymer particles dispersed in the said liquid fatty phase,
- and the second composition, which is different from the first, comprising at least one fatty substance.
- The fatty substance of the second composition is preferably chosen from waxes and non-volatile oils.
- According to one preferred embodiment, the second composition comprises at least one wax and at least one non-volatile oil.
- Advantageously, the wax is a sunflower wax.
- Preferably, the non-volatile oil is an oil such as caprylic/capric acid triglycerides.
- The presence of a second composition applied over the first composition onto the keratin materials can especially improve the gloss and/or comfort properties.
- The content of all the patents or patent applications cited previously is incorporated by reference into the present patent application.
- In the patent application, unless specifically mentioned otherwise, the contents are expressed on a weight basis relative to the total weight of the composition.
- The invention is illustrated in greater detail by the examples described below, which are given as non-limiting illustrations. The percentages are weight percentages.
- The following resins are used:
- MQ resin=an MQ resin of formula M0.43Q0.57 and of Mn=3230 dissolved in xylene to a proportion of 70.8% by weight of solids. The MQ resin was manufactured according to the techniques described by Daudt in U.S. Pat. No. 2,676,182.
- T Propyl resin=a propyl silsesquioxane resin at 74.8% by weight in toluene. The propyl silsesquioxane resin was obtained by hydrolysis of propyltrichlorosilane.
- Preparation of the MQTPr Resins
- An MQ resin, a T propyl resin, xylene and 1M KOH in water in the proportions presented in Table 1 are introduced into a 3-necked flask equipped with a stirrer, a temperature probe and Dean-Stark apparatus mounted with a condenser. Xylene is pre-introduced into the Dean-Stark apparatus so as to ensure maintenance of a level of solids of 50% in the reactor. The mixture in the reactor is refluxed (between 100 and 140° C.) for at least 3 hours. Any water formed in the reaction mixture is continuously removed and trapped in the form of an azeotrope in the Dean-Stark apparatus. After refluxing for 3 hours, the water is removed from the apparatus and heating is continued for a further 30 minutes. After cooling the mixture, an excess of acetic acid is added to neutralize the KOH in the mixture. The mixture is then filtered to remove the salts formed, by passing it through a filter under pressure. Solvent exchange is performed by heating the mixture in a rotary evaporator under vacuum. After removing the majority of the xylene, decamethylcyclopentasiloxane (or isododecane) is added while continuing to remove any residual aromatic solvent. The structures of the resulting siloxane resins are characterized by 29Si NMR and GPC, and the results are summarized in Table 2 below.
-
TABLE 1 Weight Weight Weight Weight Mass ratio % of % of T Weight % of % of of MQ/TPr MQ propyl % of 1M acetic Example # resins added resin resin xylene KOH acid 1-a (85:15) 59.4 10.5 29.1 0.9 0.2 1-b (50:50) 34.9 34.8 29.1 0.9 0.2 1-c (30:70) 20.9 48.8 29.2 0.9 0.2 1-d (95:5) 67.1 3.5 28.3 0.9 0.2 1-e (100:0) 69.3 0 28.8 0.9 0.2 -
TABLE 2 Resin structure Weight according to NMR % of Example # characterization OH Mn Mw Mw/Mn MQ resin M0.43Q0.57 3230 1516 4.7 T Propyl TPr 1.0 7.0 3470 11 400 3.3 resin 1-a M0.374Q0.529:TPr 0.097 1.4 5880 271 000 46.1 1-b M0.248Q0.341:TPr 0.412 2.1 6640 3 860 000 581.3 1-c M0.162Q0.217:TPr 0.621 1.5 7600 25 300 000 3329 1-d M0.419Q0.5485:TPr 0.03 1.5 1-e MQ 1.7 5200 28 900 5.6 - 300 g of isododecane are introduced into a 1-litre reactor and the temperature is then raised so as to pass from room temperature (25° C.) to 90° C. over 1 hour.
- 105 g of isobornyl methacrylate (manufactured by Arkema), 105 g of isobornyl acrylate (manufactured by Arkema) and 1.8 g of 2,5-bis(2-ethylhexanoylperoxy)-2,5-dimethylhexane (Trigonox® 141 from Akzo Nobel) are then added, at 90° C. and over 1 hour.
- The mixture is maintained at 90° C. for 1 hour 30 minutes.
- 75 g of isobutyl acrylate (manufactured by Fluka), g of acrylic acid and 1.2 g of 2,5-bis(2-ethylhexanoylperoxy)-2,5-dimethylhexane are then introduced into the preceding mixture, still at 90° C. and over 30 minutes.
- The mixture is maintained at 90° C. for 3 hours, and is then cooled.
- A solution containing 50% polymer active material in isododecane is obtained.
- A polymer comprising a first poly(isobornyl acrylate/isobornyl methacrylate) rigid block with a Tg of 110° C., a second poly(isobutyl acrylate/acrylic acid) flexible block with a Tg of −9° C. and an intermediate block, which is an isobornyl acrylate/isobornyl methacrylate/isobutyl acrylate/acrylic acid random polymer, is obtained.
- Compositions 3 and 4 below were prepared:
-
Composition Composition 4 3 comparative Weight Weight percentages percentages Compounds % % 2-Octyldodecanol 9.43 8.23 Refined plant perhydrosqualene 5.05 4.21 (Phytosqualan from Sophim) Brilliant yellow FCF aluminium lake on 2.58 2.58 alumina (42/58) (CI: 15985:1 + 77002) Brilliant blue FCF aluminium lake on 0.16 0.16 alumina (12/88) (CI: 42090:2 + 77002) Calcium salt of Lithol B Red 0.59 0.59 Rutile titanium oxide treated with 2.74 2.74 alumina/silica/trimethylolpropane (CI: 77891) Black iron oxide (CI: 77499) 0.32 0.32 Mixture of isopropyl, isobutyl and n- 0.65 0.65 butyl p-hydroxybenzoates (40/30/30) (Liquapar Oil from ISP) Polyphenyltrimethylsiloxydiphenylsiloxane 25.03 25.03 (viscosity: 1000 cSt-MW: 3000 - g/mol) (Belsil PDM 1000 from Wacker) Mica-titanium dioxide-brown iron oxide 2 2 (77/21/4) (size: 16-128 microns) Fragrance 0.3 0.3 Polybutylene (monoolefins/isoparaffins) 10.65 10.65 (MW: 920) (Indopol H 100 from Ineos) Poly(isobornyl methacrylate-co-isobornyl 30 37.04 acrylate-co-isobutyl acrylate-co-acrylic acid) as prepared in Example 2 above (50% of polymer in 50% of isododecane) MQ-T propyl resin (30:70) in isododecane 5.1 — as prepared in Example 1 above, from Dow Corning Hydrophilic fumed silica (Aerosil 200 0.5 0.5 from Evonik Degussa) Hydrophobic fumed silica, surface-treated 5 5 with dimethylsilane (Aerosil 972 from Evonik Degussa) Total: 100 100 - Procedure:
-
- a. The fillers and pigments optionally present are ground in part of the oily phase.
- b. The rest of the liposoluble ingredients are then mixed together at a temperature of about 100° C. The ground mixture is then added to the oily phase.
- c. The mixture is stirred with a Rayneri blender for 45 minutes, and the siloxane resin is added at room temperature.
- d. The formulation is poured into isododecane-leaktight heating bags.
- Composition 3 according to the invention contains 15% polymer (weight of solids) prepared according to Example 2 and 3.5% (weight of solids) of siloxane resin prepared according to Example 1-C), i.e. in total 18.5% of film-forming polymer.
- Comparative composition 4 contains 18.5% of polymer (weight of solids) prepared according to Example 2.
- Comparative composition 4 is very viscous and tacky and is very thick on application. It is uncomfortable on the lips since the film formed is very thick and tacky.
- Composition 3 according to the invention is much more pleasant (slippery) on application and it forms a much finer and more comfortable deposit.
- The following lipstick formulation was prepared (same procedure as that described previously).
-
Composition 5 according to the Comparative invention (Weight composition 6 Compounds percentages %) (Weight percentages %) Butyl acrylate copolymer containing 31.25 37.5 dendritic silicone side chains: [tris(trimethylsiloxy)siloxyethyldimethyl- siloxy]silylpropyl methacrylate in isododecane: 40/60 (Dow Corning FA 4002 ID Silicone Acrylate from Dow Corning) MQ-T propyl resin (30/70) in isododecane as 4 — prepared in Example 1-C above, from Dow Corning Isododecane from Ineos 1.3 — Refined plant perhydrosqualene 12.02 12.02 (Phytosqualan from Sophim) Octyldodecanol 14.2 13.62 Polybutene (Indopol H 100 from Ineos) 10.65 10.65 Isopropyl paraben (and) isobutyl paraben 0.65 0.65 (and) butyl paraben (Liquapar Oil from ISP) Red 7 (Unipure Red LC 3079 OR from LCW 0.23 0.23 (Sensient)) Iron oxides (Sunpuro Black Iron Oxide C33- 0.05 0.05 7001 from Sun) Mica (and) titanium dioxide (and) iron 1 1 oxides (Cloisonne Sparkle Gold 222 J from Engelhard) Calcium aluminium borosilicate (and) silver 2.5 2.5 (Metashine ME 2040 PS from NError! Hyperlink reference not valid.) Trimethylsiloxyphenyl Dimethicone (Belsil 16.65 16.3 PDM 1000 from Wacker) Silica Dimethyl Silylate Aerosil R 972 from 5 5 EError! Hyperlink reference not valid. Silica (Aerosil 200 from EError! Hyperlink 0.5 0.5 reference not valid.) Total 100 100 - The staying power of compositions 5 and 6 is evaluated according to the transfer index measurement protocol described above. The results obtained are as follows:
- The two compositions 5 and 6 have substantially the same solids content of film-forming polymer (siloxane resin vinyl polymer containing carbosiloxane dendrimer-based units).
- For composition 5 according to the invention containing a vinyl polymer comprising at least one carbosiloxane dendrimer-based unit and an MQ-T propyl siloxane resin, a transfer index value of 31.81±7.87 is measured.
- For comparative composition 6 containing no siloxane resin, a transfer index value of 18.32±0.56 is measured.
Claims (15)
1. A process for making up and/or caring for keratin materials, comprising:
applying to the keratin materials a composition, comprising in a physiologically acceptable medium:
a) a siloxane resin comprising the following units:
(i) (R1 3SiO1/2)a
(ii) (R2 2SiO2/2)b
(iii) (R3SiO3/2)c and
(iv) (SiO4/2)d
wherein
R1, R2 and R3 are each independently an alkyl group containing from 1 to 8 carbon atoms, an aryl group, a carbinol group or an amino group,
a is between 0.05 and 0.5,
b is between 0 and 0.3,
c is greater than 0,
d is between 0.05 and 0.6,
a+b+c+d=1,
with the proviso that more than 40 mol % of the groups R3 of the siloxane resin are propyl groups, and
b) at least one liquid fatty phase, and
c) at least one film-forming polymer chosen from the group comprising:
a film-forming block ethylenic polymer, comprising at least a first block and at least a second block,
wherein
the first block is obtained from at least one acrylate monomer of formula CH2═CH—COOR2
R2 represents a C4 to C12 cycloalkyl group and at least one methacrylate monomer of formula CH2═C(CH3)—COOR′2
wherein R′2 represents a C4 to C12 cycloalkyl group, and
the second block is obtained from an acrylic acid monomer and from at least one monomer with a glass transition temperature of less than or equal to 20° C.,
a vinyl polymer comprising at least one carbosiloxane dendrimer-based unit,
a dispersion of acrylic or vinyl radical homopolymer or copolymer particles dispersed in the said liquid fatty phase.
2. The process for making up and/or caring for keratin materials according to claim 1 , wherein the said siloxane resin comprises:
(i) (R1 3SiO1/2)a
(iii) (R3SiO3/2)c and
(iv) (SiO4/2)d
wherein
R1 and R3 each independently is an alkyl group containing from 1 to 8 carbon atoms,
a is between 0.05 and 0.5 ,
c is greater than zero,
d is between 0.05 and 0.6,
a+b+c+d=1,
with the proviso that more than 40 mol % of the groups R3 of the siloxane resin are propyl groups.
3. The process for making up and/or caring for keratin materials according to claim 1 wherein the said siloxane resin is obtained via a process comprising reaction between:
A) an MQ resin comprising at least 80 mol % of units (R1 3SiO1/2)a and (SiO4/2)d
wherein
R1 is a methyl group,
a and d are greater than zero,
a ratio a/d is between 0.5 and 1.5;
and
B) a T propyl resin comprising at least 80 mol % of units (R3SiO3/2)c,
wherein
R3 is a propyl group,
c is greater than zero,
in which a mass ratio A/B is between 95/5 and 15/85 .
4. The process for making up and/or caring for keratin materials according to claim 1 , wherein the film-forming block ethylenic polymer comprises an intermediate block comprising at least one constituent monomer of the first block and at least one constituent monomer of the second block, and a polydispersity index of the film-forming block ethylenic polymer is greater than 2.
5. The process for making up and/or caring for keratin materials according to claim 1 , wherein, the second block of the film-forming block ethylenic polymer is obtained from polymerization of acrylic acid and isobutyl acrylate, and the first block is obtained from polymerization of isobornyl acrylate and isobornyl methacrylate.
6. The process for making up and/or caring for keratin materials according to claim 1 , wherein the vinyl polymer comprises at least one carbosiloxane dendrimer of formula (I):
wherein
Z is a divalent organic group,
p is 0 or 1,
R1 is an aryl or alkyl group of 1 to 10 carbon atoms and
Xi is a silylalkyl group of formula (II):
wherein
R1 is an aryl or alkyl group of 1 to 10 carbon atoms,
R2 is an alkylene group of 1 to 10 carbon atoms,
R3 is an alkyl group of 1 to 10 carbon atoms, and
Xi+1 is a group selected from the group consisting of hydrogen atoms, aryl groups and alkyl groups containing up to 10 carbon atoms, and silylalkyl groups the index i is an integer from 1 to 10 indicating the generation of the silylalkyl group starting in each carbosiloxane dendritic structure with a value of 1 for the group Xi in formula (I) and
the index ai is an integer from 0 to 3.
7. The process for making up and/or caring for keratin materials according to claim 1 , wherein the dispersion of homopolymer or copolymer particles comprises polymer particles in dispersion selected from acrylic polymers or copolymers which are insoluble in water-soluble alcohols.
8. The process for making up and/or caring for keratin materials according to claim 1 , wherein a content of the film-forming polymer is from 0.1% to 60% by weight of film-forming polymer solids.
9. The process for making up and/or caring for keratin materials according to claim 1 , wherein a total solids content of the siloxane resin is from 1% to 80% by weight relative to the total weight of the composition.
10. The process for making up and/or caring for keratin materials claim 1 , wherein the composition comprises less than 3% by weight of water relative to the total weight of the composition.
11. The process for making up and/or caring for keratin materials according to claim 1 , wherein the composition further comprises at least one structuring agent selected from the group consisting of thickeners, organogelling agents, waxes, pasty fatty substances and gums.
12. The process for making up and/or caring for keratin materials according to claim 1 , wherein the composition further comprises at least one other oil, which is non-volatile.
13. The process for making up and/or caring for keratin materials according to claim 1 , wherein the composition further comprises at least one dyestuff and/or at least one filler.
14. A composition for making up and/or caring for keratin materials, comprising, in a physiologically acceptable medium:
a) a siloxane resin comprising the following units:
(i) (R1 3SiO1/2)a
(ii) (R2 2SiO2/2)b
(iii) (R3SiO3/2)c and
(iv) (SiO4/2)d
(i) (R1 3SiO1/2)a
(ii) (R2 2SiO2/2)b
(iii) (R3SiO3/2)c and
(iv) (SiO4/2)d
wherein
R1, R2 and R3 are each independently an alkyl group containing from 1 to 8 carbon atoms, an aryl group, a carbinol group or an amino group,
a is between 0.05 and 0.5,
b is between 0 and 0.3,
c is greater than 0,
d is between 0.05 and 0.6,
a+b+c+d=1,
with the proviso that more than 40 mol % of the groups R3 of the siloxane resin are propyl groups, and
b) at least one liquid fatty phase, and
c) at least one film-forming polymer chosen from the group comprising:
a film-forming block ethylenic polymer, comprising at least a first block and at least a second block,
wherein
the first block is obtained from at least one acrylate monomer of formula CH2═CH—COOR2
R2 represents a C4 to C12 cycloalkyl group and at least one methacrylate monomer of formula CH2═C(CH3)—COOR′2 in which
wherein R′2 represents a C4 to C12 cycloalkyl group, and
the second block is obtained from an acrylic acid monomer and from at least one monomer with a glass transition temperature of less than or equal to 20° C.,
a vinyl polymer comprising at least one carbosiloxane dendrimer-based unit,
a dispersion of acrylic or vinyl radical homopolymer or copolymer particles dispersed in the said liquid fatty phase.
15. A product for making up and/or caring for keratin materials, comprising a first composition and a second composition conditioned in separate containers, wherein
the first composition comprises, in a physiologically acceptable medium:
a) a siloxane resin comprising the following units:
(i) (R1 3SiO1/2)a
(ii) (R2 2SiO2/2)b
(iii) (R3SiO3/2)c and
(iv) (SiO4/2)d
wherein
R1, R2 and R3 are each independently an alkyl group containing from 1 to 8 carbon atoms, an aryl group, a carbinol group or an amino group,
a is between 0.05 and 0.5,
b is between 0 and 0.3,
c is greater than 0,
d is between 0.05 and 0.6,
a+b+c+d=1,
with the proviso that more than 40 mol % of the groups R3 of the siloxane resin are propyl groups, and
b) at least one liquid fatty phase, and
c) at least one film-forming polymer selected from the group consisting of:
a film-forming block ethylenic polymer, comprising at least a first block and at least a second block,
wherein
the first block is obtained from at least one acrylate monomer of formula CH2═CH—COOR2
R2 represents a C4 to C12 cycloalkyl group and at least one methacrylate monomer of formula CH2═C(CH3)—COOR′2
wherein R′2 represents a C4 to C12 cycloalkyl group, and
the second block is obtained from an acrylic acid monomer and from at least one monomer with a glass transition temperature of less than or equal to 20° C.;
a vinyl polymer comprising at least one carbosiloxane dendrimer-based unit; and
a dispersion of acrylic or vinyl radical homopolymer or copolymer particles dispersed in the said liquid fatty phase;
and the second composition, which is different from the first, comprises at least one fatty substance.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/746,282 US20100310489A1 (en) | 2007-12-05 | 2008-12-05 | Cosmetic makeup and/or care process using a siloxane resin and a film-forming polymer |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US99235707P | 2007-12-05 | 2007-12-05 | |
| US12/746,282 US20100310489A1 (en) | 2007-12-05 | 2008-12-05 | Cosmetic makeup and/or care process using a siloxane resin and a film-forming polymer |
| PCT/FR2008/052228 WO2009080960A2 (en) | 2007-12-05 | 2008-12-05 | Cosmetic make-up and/or care method using a siloxane resin and a film-forming polymer |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20100310489A1 true US20100310489A1 (en) | 2010-12-09 |
Family
ID=40566065
Family Applications (8)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/746,428 Abandoned US20100260700A1 (en) | 2007-12-05 | 2008-12-05 | Cosmetic method using a composition comprising a siloxane resin and a mineral filler |
| US12/746,454 Expired - Fee Related US9023335B2 (en) | 2007-12-05 | 2008-12-05 | Cosmetic method using a composition comprising a siloxane resin and a volatile hydrocarbon-based solvent |
| US12/746,324 Abandoned US20100310490A1 (en) | 2007-12-05 | 2008-12-05 | Cosmetic makeup and/or care process using a siloxane resin and a polar wax |
| US12/746,413 Abandoned US20110002869A1 (en) | 2007-12-05 | 2008-12-05 | Cosmetic makeup and/or care process using a siloxane resin and a non-volatile oil |
| US12/746,613 Abandoned US20100316587A1 (en) | 2007-12-05 | 2008-12-05 | Cosmetic makeup and/or care method using a siloxane resin and a filler |
| US12/746,282 Abandoned US20100310489A1 (en) | 2007-12-05 | 2008-12-05 | Cosmetic makeup and/or care process using a siloxane resin and a film-forming polymer |
| US12/746,285 Expired - Fee Related US8367083B2 (en) | 2007-12-05 | 2008-12-05 | Cosmetic makeup and/or care method using a siloxane resin and a phenyl silicone oil |
| US14/665,002 Abandoned US20150250705A1 (en) | 2007-12-05 | 2015-03-23 | Cosmetic method using a composition comprising a siloxane resin and a volatile hydrocarbon-based solvent |
Family Applications Before (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/746,428 Abandoned US20100260700A1 (en) | 2007-12-05 | 2008-12-05 | Cosmetic method using a composition comprising a siloxane resin and a mineral filler |
| US12/746,454 Expired - Fee Related US9023335B2 (en) | 2007-12-05 | 2008-12-05 | Cosmetic method using a composition comprising a siloxane resin and a volatile hydrocarbon-based solvent |
| US12/746,324 Abandoned US20100310490A1 (en) | 2007-12-05 | 2008-12-05 | Cosmetic makeup and/or care process using a siloxane resin and a polar wax |
| US12/746,413 Abandoned US20110002869A1 (en) | 2007-12-05 | 2008-12-05 | Cosmetic makeup and/or care process using a siloxane resin and a non-volatile oil |
| US12/746,613 Abandoned US20100316587A1 (en) | 2007-12-05 | 2008-12-05 | Cosmetic makeup and/or care method using a siloxane resin and a filler |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/746,285 Expired - Fee Related US8367083B2 (en) | 2007-12-05 | 2008-12-05 | Cosmetic makeup and/or care method using a siloxane resin and a phenyl silicone oil |
| US14/665,002 Abandoned US20150250705A1 (en) | 2007-12-05 | 2015-03-23 | Cosmetic method using a composition comprising a siloxane resin and a volatile hydrocarbon-based solvent |
Country Status (5)
| Country | Link |
|---|---|
| US (8) | US20100260700A1 (en) |
| EP (7) | EP2231109B1 (en) |
| CN (7) | CN101951881B (en) |
| ES (2) | ES2403429T3 (en) |
| WO (18) | WO2009080955A2 (en) |
Cited By (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100330012A1 (en) * | 2009-06-29 | 2010-12-30 | L'oreal S.A. | Hydrating cream foundation in emulsion form |
| US20110020263A1 (en) * | 2009-06-01 | 2011-01-27 | L'oreal | Composition containing a block polymer and a nonvolatile ester oil |
| US20110020257A1 (en) * | 2009-06-29 | 2011-01-27 | L'oreal S.A. | Composition comprising a polyol, a sugar silicone surfactant and a oil-soluble polar modified polymer |
| US20110020261A1 (en) * | 2009-06-29 | 2011-01-27 | L'ORéAL S.A. | Long wear, waterproof mascara composition with water washability |
| US20110038819A1 (en) * | 2009-06-29 | 2011-02-17 | L'ORéAL S.A. | Composition comprising a sugar silicone surfactant and a oil-soluble polar modified polymer |
| US8647611B2 (en) | 2009-06-29 | 2014-02-11 | L'oréal | Composition containing a polyol and a reaction product |
| US8663667B2 (en) | 2009-06-29 | 2014-03-04 | L'oreal | Refreshing cream foundation in gel form |
| US8747868B2 (en) | 2010-12-30 | 2014-06-10 | L'oreal | Reaction product of a polar modified polymer and an alkoxysilane and a composition containing the reaction product |
| US8871229B2 (en) | 2012-02-28 | 2014-10-28 | L'oreal | Cosmetic composition for long-wearing properties |
| US20140348769A1 (en) * | 2011-12-30 | 2014-11-27 | L'oreal | Compositions containing silicon resin, oil and gelling agent |
| US8932566B2 (en) | 2009-06-29 | 2015-01-13 | L'oreal | Composition comprising a polyol and a oil-soluble polar modified polymer |
| US8992903B2 (en) | 2002-09-26 | 2015-03-31 | L'oreal | Composition comprising at least one block polymer and at least one gelling agent |
| US9023387B2 (en) | 2008-12-09 | 2015-05-05 | L'oreal | Transfer-resistant emulsion containing a surfactant |
| US9040593B2 (en) | 2008-12-16 | 2015-05-26 | L'oreal | Water-insoluble reaction product of a polyamine and an oil-soluble high carbon polar modified polymer |
| US9192561B2 (en) | 2010-05-14 | 2015-11-24 | L'oreal | Compositions containing hyperbranched polyol and acrylic film former |
| US9226888B2 (en) | 2011-12-30 | 2016-01-05 | L'oreal | Compositions containing an acrylic film former, a tackifier and an ester |
| US20160038402A1 (en) * | 2013-03-25 | 2016-02-11 | L'oreal | Lipstick composition in emulsion form comprising a particular film-forming polymer and treatment process employing same |
| US9308396B2 (en) | 2008-12-16 | 2016-04-12 | L'oreal | Transfer-resistant and long wear foundation in emulsion form containing oil absorbing powders |
| US9345657B2 (en) | 2011-12-30 | 2016-05-24 | L'oreal | Compositions containing a silicon resin and a tackifying agent |
| US20170020792A1 (en) * | 2013-12-23 | 2017-01-26 | L'oreal | Solid composition with a vinyl polymer bearing a carbosiloxane dendrimer unit and volatile hydrocarbon-based oils, and treatment process |
| US9732191B2 (en) | 2014-01-08 | 2017-08-15 | Dow Corning Corporation | Method for capping MQ-type silicone resins |
| US20180214368A1 (en) * | 2017-01-31 | 2018-08-02 | L'oréal | Long-wear compositions containing silicone acrylate copolymer and surface-treated pigment |
| US20180214367A1 (en) * | 2017-01-31 | 2018-08-02 | L'oréal | Long-wear compositions containing silicone acrylate copolymer, silicone elastomer resin and surface-treated pigment |
| US10653600B2 (en) | 2011-06-23 | 2020-05-19 | L'oreal | Cosmetic composition comprising a supramolecular compound capable of establishing hydrogen bonds, and two particular distinct silicone oils |
| US20200305576A1 (en) * | 2016-03-31 | 2020-10-01 | L'oreal | Device for packaging and applying an emulsion comprising a film-forming agent and non-volatile oils and composition |
| EP3983340B1 (en) * | 2019-06-12 | 2023-08-02 | Nouryon Chemicals International B.V. | Method for isolating carboxylic acid from an aqueous side stream |
Families Citing this family (90)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8604151B2 (en) | 2004-02-02 | 2013-12-10 | Dow Corning Corporation | Bodied siloxane resin including M, Q, and T-propyl units and capped with additional M units |
| CN101677923B (en) * | 2007-06-19 | 2015-07-15 | 考格尼斯知识产权管理有限责任公司 | Hydrocarbon mixtures and use thereof |
| DE102008017031A1 (en) * | 2007-06-19 | 2008-12-24 | Cognis Ip Management Gmbh | Hydrocarbon mixtures and their use |
| US8895039B2 (en) * | 2007-06-19 | 2014-11-25 | Cognis Ip Management Gmbh | Cosmetic preparations containing hydrocarbons |
| ES2628970T3 (en) * | 2007-06-19 | 2017-08-04 | Cognis Ip Management Gmbh | Hydrocarbon mixtures and their use |
| GB0712767D0 (en) * | 2007-07-02 | 2007-08-08 | Dow Corning | Foamable compositions |
| WO2009080955A2 (en) * | 2007-12-05 | 2009-07-02 | L'oreal | Cosmetic make-up and/or care method using a siloxane resin and a polar wax |
| EP2116221A1 (en) * | 2008-05-07 | 2009-11-11 | Cognis IP Management GmbH | Cosmetic preparation containing hydrocarbons |
| FR2931670B1 (en) * | 2008-06-02 | 2015-05-22 | Oreal | POLYESTER COMPOSITIONS IN A FATTY PHASE AND USES THEREOF. |
| FR2934129B1 (en) * | 2008-07-24 | 2014-05-02 | Oreal | COSMETIC TREATMENT PROCESS. |
| US8298517B2 (en) | 2008-07-24 | 2012-10-30 | Dow Corning Corporation | Personal care compositions having improved compatibility and providing improved sun protection |
| KR101783487B1 (en) * | 2009-05-01 | 2017-10-23 | 나노시스, 인크. | Functionalized matrixes for dispersion of nanostructures |
| WO2010146147A2 (en) * | 2009-06-18 | 2010-12-23 | L'oreal | Composition for treating keratin fibres comprising a block copolymer, a siloxane resin and a volatile solvent |
| FR2946877B1 (en) * | 2009-06-18 | 2011-07-29 | Oreal | COMPOSITION FOR TREATING KERATIN FIBERS COMPRISING A SEQUENCE POLYMER, A SILOXANE RESIN AND A SURFACTANT |
| FR2953399B1 (en) * | 2009-12-09 | 2012-01-13 | Oreal | SOLID COSMETIC COMPOSITION COMPRISING A POLYESTER AND A SELECTIVE ETHYLENIC COPLYMER |
| FR2954157B1 (en) * | 2009-12-21 | 2012-01-13 | Oreal | METHOD OF MAKE-UP OF SKIN AND / OR LIP |
| FR2957253B1 (en) * | 2010-03-10 | 2012-03-16 | Oreal | PREPARATION OF PRE-MIXTURES OF FILMOGENIC POLYMERS IN VOLATILE LINEAR ALKANES AND USE IN COSMETICS |
| JP5827790B2 (en) | 2010-04-28 | 2015-12-02 | 東レ・ダウコーニング株式会社 | Cosmetic and skin external preparation containing higher alcohol-modified silicone |
| MX357168B (en) * | 2010-05-17 | 2018-06-28 | Mary Kay Inc | Topical skin formulation. |
| DK3222266T3 (en) | 2010-08-27 | 2018-06-06 | Sienna Biopharmaceuticals Inc | COMPOSITIONS AND METHODS OF TARGETED THERMO REGULATION |
| US9572880B2 (en) | 2010-08-27 | 2017-02-21 | Sienna Biopharmaceuticals, Inc. | Ultrasound delivery of nanoparticles |
| FR2968973B1 (en) * | 2010-12-16 | 2012-12-28 | Oreal | AQUEOUS COSMETIC COMPOSITION COMPRISING ALKYLCELLULOSE AND SILICONE RESIN |
| DE202011110589U1 (en) | 2010-09-20 | 2015-02-05 | L'oréal | Alkylcellulose-containing aqueous cosmetic composition |
| PL2913044T3 (en) | 2010-09-20 | 2018-09-28 | L'oréal | Aqueous cosmetic composition comprising alkylcellulose |
| DE102010041887A1 (en) | 2010-10-01 | 2012-04-05 | Beiersdorf Ag | Care products for protecting colored hair with silicone resins |
| FR2966156B1 (en) * | 2010-10-18 | 2014-01-03 | Oreal | PROCESS FOR PREPARING A SILICONE RESIN AND USE IN COSMETICS OF THE RESIN |
| FR2968985B1 (en) * | 2010-12-17 | 2014-06-06 | Oreal | MAKE-UP AND / OR CARE PRODUCT IN HEART-SKIN FORM |
| FR2968950B1 (en) | 2010-12-20 | 2013-06-07 | Oreal | COSMETIC COMPOSITION COMPRISING A CUCURBIC ACID COMPOUND |
| FR2968951B1 (en) | 2010-12-20 | 2012-12-28 | Oreal | COSMETIC COMPOSITION COMPRISING A CUCURBIC ACID COMPOUND |
| CN102085168B (en) * | 2011-01-26 | 2012-05-09 | 珠海市时代经典化妆品有限公司 | Anti-aging cosmetic |
| KR101257628B1 (en) | 2011-03-24 | 2013-04-29 | (주)아모레퍼시픽 | Cosmetics comprising cosmetic composition impregnated in urethane foam |
| FR2973691B1 (en) | 2011-04-05 | 2013-03-29 | Oreal | COSMETIC COMPOSITION COMPRISING A CUCURBIC ACID COMPOUND AND A SEMICRYSTALLINE ACRYLIC POLYMER |
| CN103442679A (en) | 2011-04-05 | 2013-12-11 | 莱雅公司 | Cosmetic composition including a cucurbic acid compound and a mixture of sulfonic and acrylic polymers |
| FR2973694B1 (en) | 2011-04-08 | 2013-03-29 | Oreal | COSMETIC COMPOSITION COMPRISING A CUCURBIC ACID COMPOUND AND A MIXTURE OF POLYMERS |
| US9221848B2 (en) | 2011-05-03 | 2015-12-29 | Dow Corning Corporation | Method of forming an MT-propyl siloxane resin |
| FR2976487B1 (en) * | 2011-06-15 | 2013-06-28 | Oreal | COSMETIC COMPOSITION FOR COATING KERATINIC FIBERS OF THE EMULSION TYPE AND METHOD FOR COATING KERATIN FIBERS |
| FR2980108B1 (en) * | 2011-09-21 | 2013-08-30 | Oreal | SOLID COSMETIC COMPOSITION IN THE FORM OF COMPACT POWDER |
| US20160023433A1 (en) * | 2011-12-21 | 2016-01-28 | Adc Acquisition Company | Thermoplastic composite prepreg for automated fiber placement |
| FR2985176B1 (en) | 2012-01-02 | 2015-05-29 | Oreal | AQUEOUS LIQUID COSMETIC COMPOSITION COMPRISING ALKYLCELLULOSE, NON-VOLATILE OILS AND AT LEAST ONE SURFACTANT |
| FR2986426B1 (en) * | 2012-02-06 | 2014-01-24 | Oreal | COSMETIC COMPOSITION COMPRISING SILICA AEROGEL PARTICLES AND A SEMI-CRYSTALLINE POLYMER |
| WO2013123324A1 (en) * | 2012-02-17 | 2013-08-22 | Revlon | Gelling agent for use in cosmetic compositions |
| KR20130116182A (en) | 2012-04-12 | 2013-10-23 | (주)아모레퍼시픽 | Foamed material of improvement in use |
| EP2837374B1 (en) * | 2012-04-13 | 2017-12-13 | Amorepacific Corporation | Cosmetic composition carrier comprising foams |
| FR2989893B1 (en) | 2012-04-26 | 2014-05-16 | Oreal | COSMETIC COMPOSITION COMPRISING A PHOSPHORYLATED OLIGOSACCHARIDE AND A SULFONIC COPOLYMER |
| ES2629903T3 (en) | 2012-10-11 | 2017-08-16 | Nanocomposix, Inc. | Compositions and method of silver nanoplates |
| CN104853718B (en) * | 2012-12-24 | 2018-09-11 | 荷兰联合利华有限公司 | Cosmetic composition |
| FR3004647B1 (en) * | 2013-04-19 | 2015-07-03 | Oreal | COMPOSITION CONTAINING EMULSION, THE OILY PHASE COMPRISING A COMPOUND HAVING A SILICONE ELASTOMER AND A SURFACTANT, A SILICONE ELASTOMER POWDER AND A POLY ALKYL (METH) ACRYLATE |
| FR3008313B1 (en) * | 2013-07-11 | 2020-07-24 | Thorel Jean Noel | TOPICAL COMPOSITION PROTECTIVE COSMETIC AGAINST XENOBIOTICS |
| FR3009957B1 (en) | 2013-08-30 | 2018-06-29 | Oreal | COSMETIC COMPOSITION COMPRISING A MIXTURE OF SULFONIC AND ACRYLIC POLYMERS |
| CN103816059A (en) * | 2013-12-04 | 2014-05-28 | 任保林 | Low-toxic nail polish formula |
| FR3015872B1 (en) | 2013-12-27 | 2017-03-24 | Oreal | MAKE-UP DEVICE COMPRISING A PLURALITY OF COSMETIC INKS |
| FR3015870B1 (en) | 2013-12-27 | 2016-02-05 | Oreal | DEVICE FOR MAKE-UP BY TRANSFERRING KERATINIC MATERIALS. |
| FR3015890B1 (en) | 2013-12-27 | 2016-02-05 | Oreal | DEVICE FOR MAKE-UP BY TRANSFERRING KERATINIC MATERIALS |
| FR3015889B1 (en) | 2013-12-27 | 2016-02-05 | Oreal | DEVICE FOR MAKE-UP BY TRANSFERRING KERATINIC MATERIALS |
| FR3015887B1 (en) | 2013-12-27 | 2017-03-24 | Oreal | DEVICE AND METHOD FOR MAKE-UP BY TRANSFERRING KERATINIC MATERIALS |
| FR3015888B1 (en) | 2013-12-27 | 2017-03-31 | Oreal | DEVICE FOR MAKE-UP BY TRANSFERRING KERATINIC MATERIALS |
| DE102014210575A1 (en) | 2014-06-04 | 2015-12-17 | Beiersdorf Ag | Cosmetic shampoos containing silicone resins dispersed in silicone oil |
| US9789055B2 (en) * | 2014-06-18 | 2017-10-17 | L'oreal | Solid lipstick composition having improved hardness |
| FR3025100B1 (en) | 2014-08-28 | 2016-12-09 | Oreal | GEL-TYPE COSMETIC COMPOSITION IMPROVED |
| CN104606093A (en) * | 2014-12-31 | 2015-05-13 | 上海创馨化妆品有限公司 | Emulsified lipstick |
| JP7046472B2 (en) * | 2015-03-27 | 2022-04-04 | ロレアル | Water-in-oil emulsion composition |
| FR3041530B1 (en) * | 2015-09-25 | 2020-01-17 | L'oreal | ANHYDROUS COMPOSITION COMPRISING A NON-VOLATILE OIL, A VOLATILE HYDROCARBON OIL, A PARTICULAR LIPOPHILIC FILM-FORMING POLYMER, A MONO-ALCOHOL AND A PARTICULATE MATERIAL |
| WO2017083601A1 (en) | 2015-11-12 | 2017-05-18 | The Procter & Gamble Company | Hair conditioning composition providing improved hair manageability |
| US11351105B2 (en) | 2015-12-21 | 2022-06-07 | L'oreal | Composition comprising alkylcellulose, incompatible hydrocarbon and silicone oils and method employing it |
| US11931379B2 (en) * | 2015-12-22 | 2024-03-19 | Johnson & Johnson Consumer Inc. | Stable foaming composition and method of use |
| WO2017107066A1 (en) * | 2015-12-22 | 2017-06-29 | L'oreal | Composition for brightening or whitening keratin materials |
| WO2017173267A1 (en) * | 2016-03-31 | 2017-10-05 | L'oreal | Cosmetic compositions capable of forming a multilayer structure after application to a keratinous material |
| CN109310610B (en) * | 2016-06-10 | 2021-11-02 | 联合利华知识产权控股有限公司 | Personal care compositions |
| US20180116947A1 (en) | 2016-10-31 | 2018-05-03 | L'oréal | Water in oil emulsion providing skin mattity and true color |
| US20180214369A1 (en) | 2017-01-31 | 2018-08-02 | L'oréal | Long-wear compositions containing silicone resin and silicone elastomer resin |
| CN111133074B (en) | 2017-07-28 | 2023-01-03 | 艾利丹尼森公司 | Pressure sensitive adhesives and articles having hyperbranched silsesquioxane cores and methods of making the same |
| US10881601B2 (en) | 2017-09-29 | 2021-01-05 | L'oreal | Cosmetic compositions capable of forming a multilayer structure after application to a keratinous material |
| US10952954B2 (en) | 2017-09-29 | 2021-03-23 | L'oreal | Cosmetic compositions capable of forming a multilayer structure after application to a keratinous material |
| CN111247210B (en) * | 2017-11-20 | 2021-12-17 | 瓦克化学股份公司 | Silicone elastomer gels containing natural oils |
| FR3075013B1 (en) * | 2017-12-14 | 2021-09-17 | Lvmh Rech | ARTICLE FOR DECORATING THE SKIN, LIPS OR NAIL OF AN INDIVIDUAL, FIXER FOR SUCH AN ARTICLE AND PROCESS FOR DECORATING THE SKIN, LIPS OR NAIL |
| JP7097976B2 (en) * | 2017-12-28 | 2022-07-08 | ロレアル | Compositions for caring for and / or making up keratin substances |
| US10786439B2 (en) | 2018-06-29 | 2020-09-29 | L'oréal | Satin lip compositions |
| KR20220103991A (en) * | 2019-11-21 | 2022-07-25 | 다우 글로벌 테크놀로지스 엘엘씨 | Personal Care Compositions Comprising Multi-Level Polymers |
| FR3104958A1 (en) * | 2019-12-19 | 2021-06-25 | L V M H Recherche | Ultra-long-lasting foundation |
| WO2021148986A1 (en) * | 2020-01-22 | 2021-07-29 | Shiseido Company, Ltd. | Cosmetic composition |
| CN114539526B (en) * | 2020-11-24 | 2023-11-07 | 陈才虎 | Preparation method of liquid propyl silicone resin blend for personal care |
| CN112778911B (en) * | 2021-01-04 | 2022-08-23 | 上海晖研材料科技有限公司 | Application of surface-modified cerium oxide particles as polishing solution abrasive particles |
| CN112778970B (en) * | 2021-01-04 | 2022-05-10 | 上海晖研材料科技有限公司 | Method for preparing surface-modified cerium oxide particles and polishing solution containing same |
| WO2022148990A1 (en) * | 2021-01-07 | 2022-07-14 | L'oreal | Anhydrous eyelash compositions containing silicone resins and plasticizer |
| FR3131211A1 (en) * | 2021-12-23 | 2023-06-30 | L'oreal | Cosmetic composition comprising a polyhydroxyalkanoate copolymer with an (un)saturated hydrocarbon chain, and a clay |
| FR3131205A1 (en) * | 2021-12-23 | 2023-06-30 | L'oreal | Cosmetic composition comprising a polyhydroxyalkanoate copolymer with an (un)saturated hydrocarbon chain, and a silicone polymer |
| FR3131202A1 (en) * | 2021-12-23 | 2023-06-30 | L'oreal | Cosmetic composition comprising a polyhydroxyalkanoate copolymer with an (un)saturated hydrocarbon chain, and a natural resin |
| WO2024003588A1 (en) | 2022-06-30 | 2024-01-04 | L'oreal | Water-in-oil emulsions suitable as eyeliners |
| WO2024015451A1 (en) | 2022-07-14 | 2024-01-18 | L'oreal | Vegan semi-solid cosmetic composition |
| CN116059135B (en) * | 2023-04-06 | 2023-07-07 | 山东安得医疗用品股份有限公司 | Water-blocking and bacteria-blocking skin protective agent and preparation method thereof |
Citations (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5851517A (en) * | 1995-06-21 | 1998-12-22 | L'oreal | Composition including a dispersion of polymer particles in a non-aqueous medium |
| US6280748B1 (en) * | 1998-06-12 | 2001-08-28 | Dow Corning Toray Silicone, Ltd. | Cosmetic raw material cosmetic product and method for manufacturing cosmetic products |
| US20050008597A1 (en) * | 2001-11-28 | 2005-01-13 | Haruhiko Furukawa | Cosmetic raw material cosmetic product and method for manufacturing a cosmetic product |
| US20060093568A1 (en) * | 2002-09-26 | 2006-05-04 | Xavier Blin | Composition comprising a block polymer and a film-forming agent |
| US20060292096A1 (en) * | 2005-06-28 | 2006-12-28 | L'oreal | Cosmetic compositions having enhanced wear properties |
| US20070134181A1 (en) * | 2005-12-08 | 2007-06-14 | L'oreal | Cosmetic composition comprising an ester of dimerdilinoleic acid and of polyol(s) and a silicone surfactant |
| US20080025934A1 (en) * | 2006-07-27 | 2008-01-31 | L'oreal | Cosmetic composition combining a copolymer, a non-volatile oil and a glossy oil |
| US20080031837A1 (en) * | 2006-07-27 | 2008-02-07 | Celine Farcet | Block polymers and their process of preparation |
| US7482419B2 (en) * | 2004-04-12 | 2009-01-27 | Dow Corning Corporation | Silsesquioxane resin wax |
| US20090130037A1 (en) * | 2004-10-05 | 2009-05-21 | L'oreal | Method of Applying Makeup to a Surface and a Kit for Implementing such a Method |
| US20110020263A1 (en) * | 2009-06-01 | 2011-01-27 | L'oreal | Composition containing a block polymer and a nonvolatile ester oil |
| US20110280817A1 (en) * | 2010-05-14 | 2011-11-17 | L'ORéAL S.A. | Compositions containing hyperbranched polyol and acrylic film former |
| US8119110B2 (en) * | 2003-09-26 | 2012-02-21 | L'oreal S.A. | Cosmetic composition comprising a block polymer and a non-volatile silicone oil |
| US8124710B2 (en) * | 2004-02-02 | 2012-02-28 | Dow Corning Corporation | MQ-T propyl siloxane resins |
| US20120171139A1 (en) * | 2010-12-30 | 2012-07-05 | L'oreal S.A. | Comfortable, long-wearing, transfer-resistant colored cosmetic compositions |
| US20120171137A1 (en) * | 2010-12-30 | 2012-07-05 | L'oreal S.A. | Comfortable, long-wearing, transfer-resistant colored cosmetic compositions having a non-tacky feel |
Family Cites Families (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ATE225643T1 (en) * | 1995-11-07 | 2002-10-15 | Procter & Gamble | METHOD FOR IMPROVING THE PERFORMANCE OF LONG USE COSMETIC PRODUCTS |
| US6071503A (en) * | 1995-11-07 | 2000-06-06 | The Procter & Gamble Company | Transfer resistant cosmetic compositions |
| US5874069A (en) * | 1997-01-24 | 1999-02-23 | Colgate-Palmolive Company | Cosmetic composition containing silicon-modified amides as thickening agents and method of forming same |
| US6387405B1 (en) * | 1998-02-27 | 2002-05-14 | E-L Management Corp. | Velvety hydrocarbon based cosmetic compositions |
| JP4066497B2 (en) * | 1998-03-23 | 2008-03-26 | ダイキン工業株式会社 | Cosmetics comprising a fluorinated copolymer |
| US6103250A (en) * | 1999-07-06 | 2000-08-15 | Revlon Consumer Products Corporation | Anhydrous cosmetic compositions containing emulsifying siloxane elastomer |
| FR2796276B1 (en) * | 1999-07-15 | 2003-05-16 | Oreal | SOLID COMPOSITION COMPRISING AN OIL AND A PARTICULAR GELLING COMPOUND, COSMETIC PROCESSING METHOD AND USE OF THE SAME |
| JP2002097366A (en) * | 2000-06-19 | 2002-04-02 | L'oreal Sa | Cosmetic composition |
| FR2813185B1 (en) * | 2000-08-30 | 2003-02-14 | Oreal | NON-TRANSFER COSMETIC COMPOSITION COMPRISING A NON-VOLATILE SILICONE COMPOUND, A NON-VOLATILE HYDROCARBON OIL AND AN INERT PARTICULATE PHASE |
| US8080257B2 (en) * | 2000-12-12 | 2011-12-20 | L'oreal S.A. | Cosmetic compositions containing at least one hetero polymer and at least one film-forming silicone resin and methods of using |
| WO2002056847A1 (en) * | 2001-01-17 | 2002-07-25 | L'oreal | Cosmetic composition comprising a polymer and a fluorinated oil |
| FR2823100B1 (en) * | 2001-04-10 | 2003-05-30 | Oreal | TWO-LAYER MAKEUP PROCESS AND MAKEUP KIT CONTAINING FIRST AND SECOND COMPOSITIONS |
| US6881416B2 (en) * | 2002-04-08 | 2005-04-19 | Wacker Chemical Corporation | Alkyl group-substituted organopolysiloxane gels |
| US20030232030A1 (en) * | 2002-06-12 | 2003-12-18 | L'oreal | Compositions containing at least one oil structured with at least one silicone-polyamide polymer, and at least one gelling agent and methods of using the same |
| US20040247549A1 (en) * | 2002-06-12 | 2004-12-09 | L'oreal S.A. | Cosmetic emulsions containing at least one hetero polymer and at least one sunscreen and methods of using the same |
| AU2003294029A1 (en) * | 2002-11-06 | 2004-06-07 | L'oreal | Composition containing a semi-crystalline polymer and a pasty fatty substance |
| JP2006509811A (en) * | 2002-12-12 | 2006-03-23 | ロレアル | Non-transfer cosmetic composition comprising a dispersion of grafted ethylenic polymer |
| FR2848422B1 (en) * | 2002-12-17 | 2009-07-03 | Oreal | TRANSPARENT OR TRANSLUCENT COSMETIC COMPOSITION OF CARE AND / OR MAKE-UP, STRUCTURED BY SILICONE POLYMERS |
| JP4064843B2 (en) * | 2003-02-05 | 2008-03-19 | 株式会社日本ナチュラルプロダクツ | Oily solid cosmetic |
| US20040151680A1 (en) * | 2003-02-05 | 2004-08-05 | Patil Anjali Abhimanyu | Cosmetic compositions containing phenyl silicones |
| US20040161395A1 (en) * | 2003-02-14 | 2004-08-19 | Patil Anjali Abhimanyu | Cosmetic compositions containing composite siloxane polymers |
| FR2855043B1 (en) * | 2003-05-22 | 2006-08-11 | Oreal | MAKE-UP COMPOSITION FOR THE SKIN, AND MORE PARTICULARLY A FLUID FOUNDATION-TYPE COMPOSITION, WITH OPTIMIZED APPLICATION QUALITIES |
| JP2004292373A (en) * | 2003-03-27 | 2004-10-21 | Kose Corp | Oily cosmetic |
| US20050026795A1 (en) * | 2003-07-17 | 2005-02-03 | L'oreal | Polyester-containing composition, uses thereof |
| FR2857590B1 (en) * | 2003-07-17 | 2006-01-27 | Oreal | COSMETIC COMPOSITION OF CARE OR MAKE-UP OF KERATINIC MATERIAL COMPRISING A POLYESTER AND USES |
| TW200530337A (en) * | 2003-12-26 | 2005-09-16 | Dow Corning Toray Silicone | Organopolysiloxane gelling agent and gelled compositions |
| CN100480329C (en) | 2004-02-02 | 2009-04-22 | 陶氏康宁公司 | MQ and T-propyl siloxane resins compositions |
| KR20070004852A (en) * | 2004-03-16 | 2007-01-09 | 다우 코닝 코포레이션 | Alkyl-phenyl silsesquioxane resins compositions |
| FR2873582B1 (en) * | 2004-07-28 | 2006-11-24 | Oreal | COSMETIC COMPOSITION COMPRISING AT LEAST ONE APOLAR WAX AND AT LEAST ONE VOLATILE ALKYLTRISILOXANE OIL |
| US20060045893A1 (en) * | 2004-08-27 | 2006-03-02 | Yu Warren Hwa-Lin | Long-wearing cosmetic compositions |
| WO2006080924A1 (en) * | 2005-01-27 | 2006-08-03 | C.B. Fleet Company Incorporated | Feminine anti-itch gel |
| US7494513B2 (en) * | 2005-04-29 | 2009-02-24 | L'oreal | Direct emulsion for bleaching hair |
| EP1798213A1 (en) * | 2005-12-14 | 2007-06-20 | Cognis IP Management GmbH | Process for the production of hydrocarbons |
| FR2894817B1 (en) * | 2005-12-20 | 2008-05-09 | Oreal | A METHOD OF MAKE-UP OR CARE OF KERATINIC MATERIALS COMPRISING THE APPLICATION OF COMPOUNDS A AND B OF WHICH AT LEAST ONE IS SILICONE |
| FR2899100B1 (en) * | 2006-04-04 | 2008-08-08 | Oreal | COSMETIC OR PHARMACEUTICAL COMPOSITION COMPRISING A POLYCONDENSATE, COSMETIC PROCESSING METHOD EMPLOYING THE SAME, PLYCONDENSATE AND PROCESS FOR PREPARING THE SAME |
| US8557230B2 (en) * | 2006-05-03 | 2013-10-15 | L'oreal | Cosmetic compositions containing block copolymers, tackifiers and shine enhancing agents |
| FR2900819B1 (en) * | 2006-05-09 | 2010-10-15 | Oreal | BIS-UREE TYPE COMPOUND, COMPOSITION COMPRISING SAME, USE AND METHOD OF COSMETIC TREATMENT |
| FR2902653B1 (en) * | 2006-06-22 | 2008-09-12 | Oreal | COSMETIC OR PHARMACEUTICAL COMPOSITION COMPRISING A POLYCONDENSATE, COSMETIC PROCESSING METHOD EMPLOYING SAID COMPOSITION, POLYCONDENSATE AND PREPARATION METHOD |
| CN101808611A (en) * | 2007-09-26 | 2010-08-18 | 陶氏康宁公司 | silicone organic elastomer gels from organopolysiloxane resins |
| WO2009080955A2 (en) | 2007-12-05 | 2009-07-02 | L'oreal | Cosmetic make-up and/or care method using a siloxane resin and a polar wax |
| FR2926022B1 (en) * | 2008-01-08 | 2013-02-15 | Oreal | COSMETIC COMPOSITION, IN PARTICULAR OF MAKE-UP OF KERATINIC MATERIALS, WITH IMPROVED COSMETIC PROPERTIES. |
-
2008
- 2008-12-05 WO PCT/FR2008/052222 patent/WO2009080955A2/en active Application Filing
- 2008-12-05 WO PCT/FR2008/052235 patent/WO2009080965A2/en active Application Filing
- 2008-12-05 WO PCT/FR2008/052229 patent/WO2009080961A2/en active Application Filing
- 2008-12-05 CN CN2008801251809A patent/CN101951881B/en not_active Expired - Fee Related
- 2008-12-05 WO PCT/FR2008/052218 patent/WO2009080951A2/en active Application Filing
- 2008-12-05 US US12/746,428 patent/US20100260700A1/en not_active Abandoned
- 2008-12-05 WO PCT/FR2008/052225 patent/WO2009080957A2/en active Application Filing
- 2008-12-05 EP EP08864950A patent/EP2231109B1/en not_active Not-in-force
- 2008-12-05 WO PCT/FR2008/052220 patent/WO2009080953A2/en active Application Filing
- 2008-12-05 US US12/746,454 patent/US9023335B2/en not_active Expired - Fee Related
- 2008-12-05 WO PCT/FR2008/052228 patent/WO2009080960A2/en active Application Filing
- 2008-12-05 WO PCT/FR2008/052234 patent/WO2009080964A2/en active Application Filing
- 2008-12-05 CN CN2008801262894A patent/CN101980694A/en active Pending
- 2008-12-05 WO PCT/FR2008/052231 patent/WO2009080962A2/en active Application Filing
- 2008-12-05 EP EP08865291A patent/EP2229220A2/en not_active Withdrawn
- 2008-12-05 WO PCT/EP2008/066887 patent/WO2009071662A2/en active Application Filing
- 2008-12-05 EP EP08865611A patent/EP2229144A2/en not_active Withdrawn
- 2008-12-05 WO PCT/FR2008/052224 patent/WO2009080956A2/en active Application Filing
- 2008-12-05 US US12/746,324 patent/US20100310490A1/en not_active Abandoned
- 2008-12-05 US US12/746,413 patent/US20110002869A1/en not_active Abandoned
- 2008-12-05 CN CN2008801262841A patent/CN102026682A/en active Pending
- 2008-12-05 CN CN2008801262875A patent/CN101980693A/en active Pending
- 2008-12-05 ES ES08864950T patent/ES2403429T3/en active Active
- 2008-12-05 CN CN2008801262860A patent/CN101980749A/en active Pending
- 2008-12-05 EP EP08865749A patent/EP2231283B1/en not_active Not-in-force
- 2008-12-05 WO PCT/FR2008/052237 patent/WO2009080967A2/en active Application Filing
- 2008-12-05 US US12/746,613 patent/US20100316587A1/en not_active Abandoned
- 2008-12-05 WO PCT/FR2008/052221 patent/WO2009080954A2/en active Application Filing
- 2008-12-05 US US12/746,282 patent/US20100310489A1/en not_active Abandoned
- 2008-12-05 CN CN2008801263030A patent/CN101939056A/en active Pending
- 2008-12-05 CN CN200880125179.6A patent/CN101951992B/en not_active Expired - Fee Related
- 2008-12-05 EP EP08863917A patent/EP2229218A2/en not_active Withdrawn
- 2008-12-05 WO PCT/FR2008/052236 patent/WO2009080966A2/en active Application Filing
- 2008-12-05 ES ES08865749T patent/ES2392125T3/en active Active
- 2008-12-05 WO PCT/FR2008/052226 patent/WO2009080958A2/en active Application Filing
- 2008-12-05 EP EP08864985A patent/EP2229143A2/en not_active Withdrawn
- 2008-12-05 WO PCT/FR2008/052219 patent/WO2009080952A2/en active Application Filing
- 2008-12-05 EP EP08863987A patent/EP2229219A2/en not_active Withdrawn
- 2008-12-05 WO PCT/FR2008/052223 patent/WO2009077709A2/en active Application Filing
- 2008-12-05 US US12/746,285 patent/US8367083B2/en not_active Expired - Fee Related
- 2008-12-05 WO PCT/FR2008/052233 patent/WO2009080963A2/en active Application Filing
-
2015
- 2015-03-23 US US14/665,002 patent/US20150250705A1/en not_active Abandoned
Patent Citations (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5851517A (en) * | 1995-06-21 | 1998-12-22 | L'oreal | Composition including a dispersion of polymer particles in a non-aqueous medium |
| US6280748B1 (en) * | 1998-06-12 | 2001-08-28 | Dow Corning Toray Silicone, Ltd. | Cosmetic raw material cosmetic product and method for manufacturing cosmetic products |
| US20050008597A1 (en) * | 2001-11-28 | 2005-01-13 | Haruhiko Furukawa | Cosmetic raw material cosmetic product and method for manufacturing a cosmetic product |
| US7875265B2 (en) * | 2002-09-26 | 2011-01-25 | L'oreal | Cosmetic composition comprising a sequenced polymer and a plasticizer |
| US20060093568A1 (en) * | 2002-09-26 | 2006-05-04 | Xavier Blin | Composition comprising a block polymer and a film-forming agent |
| US20060147402A1 (en) * | 2002-09-26 | 2006-07-06 | Xavier Blin | Composition comprising a sequenced polymer and a gelling agent |
| US20060115444A1 (en) * | 2002-09-26 | 2006-06-01 | Xavier Blin | Glossy liquid composition comprising a sequenced polymer |
| US8119110B2 (en) * | 2003-09-26 | 2012-02-21 | L'oreal S.A. | Cosmetic composition comprising a block polymer and a non-volatile silicone oil |
| US8124710B2 (en) * | 2004-02-02 | 2012-02-28 | Dow Corning Corporation | MQ-T propyl siloxane resins |
| US7482419B2 (en) * | 2004-04-12 | 2009-01-27 | Dow Corning Corporation | Silsesquioxane resin wax |
| US20090130037A1 (en) * | 2004-10-05 | 2009-05-21 | L'oreal | Method of Applying Makeup to a Surface and a Kit for Implementing such a Method |
| US20060292096A1 (en) * | 2005-06-28 | 2006-12-28 | L'oreal | Cosmetic compositions having enhanced wear properties |
| US20070134181A1 (en) * | 2005-12-08 | 2007-06-14 | L'oreal | Cosmetic composition comprising an ester of dimerdilinoleic acid and of polyol(s) and a silicone surfactant |
| US20080031837A1 (en) * | 2006-07-27 | 2008-02-07 | Celine Farcet | Block polymers and their process of preparation |
| US20080025934A1 (en) * | 2006-07-27 | 2008-01-31 | L'oreal | Cosmetic composition combining a copolymer, a non-volatile oil and a glossy oil |
| US20110020263A1 (en) * | 2009-06-01 | 2011-01-27 | L'oreal | Composition containing a block polymer and a nonvolatile ester oil |
| US20110280817A1 (en) * | 2010-05-14 | 2011-11-17 | L'ORéAL S.A. | Compositions containing hyperbranched polyol and acrylic film former |
| US20120171139A1 (en) * | 2010-12-30 | 2012-07-05 | L'oreal S.A. | Comfortable, long-wearing, transfer-resistant colored cosmetic compositions |
| US20120171137A1 (en) * | 2010-12-30 | 2012-07-05 | L'oreal S.A. | Comfortable, long-wearing, transfer-resistant colored cosmetic compositions having a non-tacky feel |
Cited By (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8992903B2 (en) | 2002-09-26 | 2015-03-31 | L'oreal | Composition comprising at least one block polymer and at least one gelling agent |
| US9017704B2 (en) | 2002-09-26 | 2015-04-28 | L'oreal | Composition comprising a block polymer and a film-forming agent |
| US9023387B2 (en) | 2008-12-09 | 2015-05-05 | L'oreal | Transfer-resistant emulsion containing a surfactant |
| US9308396B2 (en) | 2008-12-16 | 2016-04-12 | L'oreal | Transfer-resistant and long wear foundation in emulsion form containing oil absorbing powders |
| US9040593B2 (en) | 2008-12-16 | 2015-05-26 | L'oreal | Water-insoluble reaction product of a polyamine and an oil-soluble high carbon polar modified polymer |
| US20110020263A1 (en) * | 2009-06-01 | 2011-01-27 | L'oreal | Composition containing a block polymer and a nonvolatile ester oil |
| US9895561B2 (en) | 2009-06-01 | 2018-02-20 | L'oreal | Composition containing a block polymer and a nonvolatile ester oil |
| US8663609B2 (en) | 2009-06-29 | 2014-03-04 | L'oreal | Composition comprising a polyol, a sugar silicone surfactant and a oil-soluble polar modified polymer |
| US20110020257A1 (en) * | 2009-06-29 | 2011-01-27 | L'oreal S.A. | Composition comprising a polyol, a sugar silicone surfactant and a oil-soluble polar modified polymer |
| US20100330012A1 (en) * | 2009-06-29 | 2010-12-30 | L'oreal S.A. | Hydrating cream foundation in emulsion form |
| US8663667B2 (en) | 2009-06-29 | 2014-03-04 | L'oreal | Refreshing cream foundation in gel form |
| US8828366B2 (en) | 2009-06-29 | 2014-09-09 | L'oreal | Hydrating cream foundation in emulsion form |
| US20110020261A1 (en) * | 2009-06-29 | 2011-01-27 | L'ORéAL S.A. | Long wear, waterproof mascara composition with water washability |
| US20110038819A1 (en) * | 2009-06-29 | 2011-02-17 | L'ORéAL S.A. | Composition comprising a sugar silicone surfactant and a oil-soluble polar modified polymer |
| US8597626B2 (en) | 2009-06-29 | 2013-12-03 | L'oreal | Long wear, waterproof mascara composition with water washability |
| US8932566B2 (en) | 2009-06-29 | 2015-01-13 | L'oreal | Composition comprising a polyol and a oil-soluble polar modified polymer |
| US8652451B2 (en) | 2009-06-29 | 2014-02-18 | L'oreal | Composition comprising a sugar silicone surfactant and a oil-soluble polar modified polymer |
| US8647611B2 (en) | 2009-06-29 | 2014-02-11 | L'oréal | Composition containing a polyol and a reaction product |
| US9192561B2 (en) | 2010-05-14 | 2015-11-24 | L'oreal | Compositions containing hyperbranched polyol and acrylic film former |
| US8747868B2 (en) | 2010-12-30 | 2014-06-10 | L'oreal | Reaction product of a polar modified polymer and an alkoxysilane and a composition containing the reaction product |
| US8846062B2 (en) | 2010-12-30 | 2014-09-30 | L'oreal | Reaction product of a polar modified polymer and an alkoxysilane and a composition containing the reaction product |
| US10653600B2 (en) | 2011-06-23 | 2020-05-19 | L'oreal | Cosmetic composition comprising a supramolecular compound capable of establishing hydrogen bonds, and two particular distinct silicone oils |
| US9226888B2 (en) | 2011-12-30 | 2016-01-05 | L'oreal | Compositions containing an acrylic film former, a tackifier and an ester |
| US9345657B2 (en) | 2011-12-30 | 2016-05-24 | L'oreal | Compositions containing a silicon resin and a tackifying agent |
| US9351919B2 (en) * | 2011-12-30 | 2016-05-31 | L'oreal | Compositions containing silicon resin, oil and gelling agent |
| US20140348769A1 (en) * | 2011-12-30 | 2014-11-27 | L'oreal | Compositions containing silicon resin, oil and gelling agent |
| US8871229B2 (en) | 2012-02-28 | 2014-10-28 | L'oreal | Cosmetic composition for long-wearing properties |
| US20160038402A1 (en) * | 2013-03-25 | 2016-02-11 | L'oreal | Lipstick composition in emulsion form comprising a particular film-forming polymer and treatment process employing same |
| US20170087080A1 (en) * | 2013-03-25 | 2017-03-30 | L'oreal | Lipstick composition in emulsion form comprising a particular film-forming polymer and treatment process employing same |
| US20170020792A1 (en) * | 2013-12-23 | 2017-01-26 | L'oreal | Solid composition with a vinyl polymer bearing a carbosiloxane dendrimer unit and volatile hydrocarbon-based oils, and treatment process |
| US9732191B2 (en) | 2014-01-08 | 2017-08-15 | Dow Corning Corporation | Method for capping MQ-type silicone resins |
| US20200305576A1 (en) * | 2016-03-31 | 2020-10-01 | L'oreal | Device for packaging and applying an emulsion comprising a film-forming agent and non-volatile oils and composition |
| US20180214368A1 (en) * | 2017-01-31 | 2018-08-02 | L'oréal | Long-wear compositions containing silicone acrylate copolymer and surface-treated pigment |
| US20180214367A1 (en) * | 2017-01-31 | 2018-08-02 | L'oréal | Long-wear compositions containing silicone acrylate copolymer, silicone elastomer resin and surface-treated pigment |
| US11058627B2 (en) * | 2017-01-31 | 2021-07-13 | L'oreal | Long-wear compositions containing silicone acrylate copolymer and surface-treated pigment |
| US11058626B2 (en) * | 2017-01-31 | 2021-07-13 | L'oreal | Long-wear compositions containing silicone acrylate copolymer, silicone elastomer resin and surface-treated pigment |
| EP3983340B1 (en) * | 2019-06-12 | 2023-08-02 | Nouryon Chemicals International B.V. | Method for isolating carboxylic acid from an aqueous side stream |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20100310489A1 (en) | Cosmetic makeup and/or care process using a siloxane resin and a film-forming polymer | |
| JP3981132B2 (en) | Composition comprising an arrayed polymer and a gelling agent | |
| US7378103B2 (en) | Cosmetic composition comprising a polyglycerolated silicone elastomer | |
| EP0388582B2 (en) | Cosmetic composition | |
| US8747828B2 (en) | Cosmetic method using a composition containing siloxane resins and specific non-ionic surfactant | |
| US20050287103A1 (en) | Cosmetic composition comprising at least one ester and at least one film-forming polymer | |
| US20060165626A1 (en) | Cosmetic composition comprising at least one ester of alkoxylated alcohol and at least one film-forming polymer | |
| US20060013791A1 (en) | Cosmetic composition comprising a defined silicone polymer and a film former | |
| EP2512427B1 (en) | Cosmetic composition comprising a supramolecular compound capable of establishing hydrogen bonds, and a particular additional ingredient | |
| JP2005350466A (en) | Cosmetic composition containing ester and film-forming agent | |
| WO2006058793A1 (en) | Cosmetic composition comprising a vinyl polymer containing at least one carbosiloxane dendrimer-based unit | |
| WO2006058795A1 (en) | Two-coat cosmetic product, uses thereof and makeup kit containing this product | |
| WO2005058274A1 (en) | Composition comprising a dispersion of particles of a grafted ethylenic polymer and a film-forming agent | |
| JP4317185B2 (en) | Cosmetic composition comprising an ester of an alkoxylated alcohol and a film-forming polymer |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: L'OREAL, FRANCE Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:BARBA, CLAUDIA;REEL/FRAME:024943/0216 Effective date: 20100705 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |