US6391336B1 - Inorganic-polymer complexes for the controlled release of compounds including medicinals - Google Patents
Inorganic-polymer complexes for the controlled release of compounds including medicinals Download PDFInfo
- Publication number
- US6391336B1 US6391336B1 US08/935,300 US93530097A US6391336B1 US 6391336 B1 US6391336 B1 US 6391336B1 US 93530097 A US93530097 A US 93530097A US 6391336 B1 US6391336 B1 US 6391336B1
- Authority
- US
- United States
- Prior art keywords
- composition
- active agent
- medicinal
- matrix
- agent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- XLYOFNOQVPJJNP-UHFFFAOYSA-N O Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 0 *n1cc(C(=O)O)c(=O)c2cc(F)c(N3CCC([H])CC3)cc21.*n1cc(C(=O)OC)c(=O)c2cc(F)c(N3CCC([H])CC3)cc21.II Chemical compound *n1cc(C(=O)O)c(=O)c2cc(F)c(N3CCC([H])CC3)cc21.*n1cc(C(=O)OC)c(=O)c2cc(F)c(N3CCC([H])CC3)cc21.II 0.000 description 1
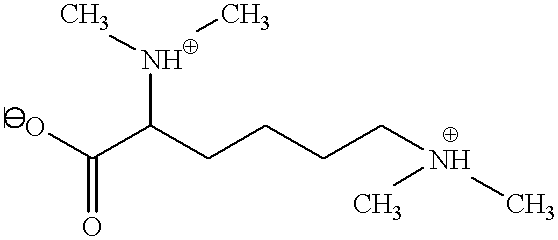
- LMHSVMFRKQENAQ-UHFFFAOYSA-N CC(C)C(CCCCN(C)C)C(=O)O Chemical compound CC(C)C(CCCCN(C)C)C(=O)O LMHSVMFRKQENAQ-UHFFFAOYSA-N 0.000 description 1
- WQULIGQUKIXWNP-UBDWQDLISA-N COc1ccc2cc(C(C)C(=O)OC(=O)C(CCCCC(C)C)C(C)C)ccc2c1.II.[H]N(C(=O)c1cccc2ccccc12)[C@]1([H])C(=O)N2C(C(=O)OC(=O)C(CCCCC(C)C)C(C)C)C(C)(C)SC21[H] Chemical compound COc1ccc2cc(C(C)C(=O)OC(=O)C(CCCCC(C)C)C(C)C)ccc2c1.II.[H]N(C(=O)c1cccc2ccccc12)[C@]1([H])C(=O)N2C(C(=O)OC(=O)C(CCCCC(C)C)C(C)C)C(C)(C)SC21[H] WQULIGQUKIXWNP-UBDWQDLISA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0014—Skin, i.e. galenical aspects of topical compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/02—Inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/58—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. poly[meth]acrylate, polyacrylamide, polystyrene, polyvinylpyrrolidone, polyvinylalcohol or polystyrene sulfonic acid resin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1652—Polysaccharides, e.g. alginate, cellulose derivatives; Cyclodextrin
Definitions
- This invention relates generally to the production and use of inorganic-polymer complexes for the controlled release of compounds including medicinals.
- Systemic antibiotic treatment is often unsatisfactory in cases of osteomyelitis as well as infections in devitalized tissue, avascular scar tissue, and other areas with insufficient blood supply.
- Increasing blood levels of antibiotics can result in toxicity.
- aminoglycosides can produce ototoxicity and nephrotoxicity.
- Another problem with long-term systemic treatment with antibiotics is the selection of drug-resistant mutants. In poorly vascularized areas, the infectious organism may encounter concentrations below the minimum lethal concentration which provides the opportunity for selection of a resistant form. Also, in large-animal veterinary practice, the cost of the antibiotic for systemic use can be an issue.
- Antibiotic formulations of polymethylmethacrylate have been employed as antiseptic bone cement and as beads either free or attached to a wire which is used for percutaneous removal [H. W. Bucholz, et al, Chiburg , 43, 446 (1970)].
- PMMA is not bioerodible.
- POP plaster of Paris
- Polymethylmethacrylate and POP have been compared with regard to release profiles. Release rates from POP tend to be very fast.
- Both polymethylmethacrylate and POP can be used to produce dimensionally stable beads and other structures.
- the acrylate cements or beads are formed by mixing pre-formed polymethylmethacrylate polymer, methylmethacrylate monomer, and a free-radical initiator. An exothermic reaction ensues which results in matrix temperatures as high as 100° C. Many antibiotics such as polymyxin and tetracycline are inactivated by these conditions [G. J. Popham, et al, Orth. Rev ., 20, 331 (1991)].
- polymethylmethacrylate is biocompatible but not resorbable. Therefore, beads used to treat local infection must be retrieved by surgery which is accompanied by the risk of reinfection.
- POP beads or pellets are resorbable but show inferior drug release profiles [G. W. Bowyer, et al, J. Trauma , 36, 331 (1994)].
- compositions containing hyaluronic acid have been used for topical administration of pharmacological substances [F. Della Valle, et al, U.S. Pat. No. 5,166,331 and U.S. Pat. No. 4,736,024].
- the subject invention relates to a delivery system comprising:
- the system comprises a complexing agent and a medicinal. Included within the invention are methods of producing sustained release of a medicinal in a mammal by administering the system with a medicinal to a mammal.
- a still further embodiment of the invention is a method of diagnosing disease in a mammal by administering a radiopaque matrix to the mammal.
- the subject invention relates to a resorbable matrix with favorable release kinetics.
- Inorganic compounds such as CaSO 4 .1/2 H 2 O can be combined with biopolymers in the presence of a bioactive agent including medicinals to produce a matrix.
- matrix polymer refers to a polymer (often a biopolymer) which serves to control the erosion rate, setting time, and influences the release profile by raising the viscosity of the medium in the pores and channels of the delivery system.
- complexing agent refers to an agent (often a biopolymer), which is used to form a salt or conjugate with the active agent which in effect raises the molecular weight of the active agent and lowers its rate of efflux.
- the complexing agent is typically a small molecule capable of aggregation which has affinity for the active agent.
- Pharmacologically acceptable hydrophobic medicinal complexing agents include proteins such as albumin, lipids or cyclodextrins which can be used to complex neutral medicinal molecules or charged molecules which contain an apolar moiety. Liposomes containing a medicinal can be entrapped within the calcium sulfate matrix.
- the consistency and viscosity of the slurry is dependent on the amount and nature of the matrix biopolymer.
- the slurry can be injected with subsequent formation of a solid in vivo.
- a medicinal can exist in the inorganic-biopolymer complex either free or complexed to the medicinal complexing agent.
- the free compound is released relatively fast.
- the complexed medicinal is released relatively slowly often contingent on the bioerosion of the inorganic-biopolymer complex.
- Antibiotics and local anesthetics are used to illustrate this principle:
- the resorbable inorganic-biopolymer complex can contain free antibiotic (e.g., as the sodium salt) or in the form of a biopolymer complex with a polycation such as polylysine or polymyxin B.
- Lidocaine is conveniently employed as the hydrochloride, the free base, or complexed as the salt of chondroitin sulfate or polyglutamate.
- the delivery system of the subject invention for use with medicinals must meet the following requirements:
- Safety non-toxic, non-immunogenic, non-pyrogenic, non-allergenic.
- the matrix should be sterilizable and precursors should have an acceptable shelf-life.
- Cast forms should be dimensionally stable.
- An inorganic compound for example, CaSO 4 .1/2H 2 O
- Matrix polymer for example, hyaluronic acid or dextran
- Complexing agent for example, chondroitin sulfate, polylysine, or cyclodextrin.
- Calcium sulfate.1/2H 2 O (hemihydrate) is the preferred inorganic component.
- the hemihydrate takes up water and crystallizes as the higher hydrate.
- Unadulterated calcium sulfate matrix exhibits poor drug release profiles. With matrix polymers and complexing agent-active agent complexes the release profiles are improved.
- Other inorganics may be employee such as calcium silicates, aluminates, hydroxides and/or phosphates (see pages 72, 95, 327 in Reference Book of Inorganic Chemistry (1951) Latimer, W. H., and Hildebrand, J. M., Macmillan, New York, hereby incorporated by reference in its entirety).
- the inorganic compound goes from slurry to solid in a reasonable time period, i.e., 10 minutes-two hours.
- the matrix biopolymer influences the setting time and the release profile.
- polymers In order to slow the efflux of active agent, e.g., medicinal, from the dosage form, polymers, often biopolymers, are included in the matrix to raise the viscosity.
- Hyaluronic acid e.g., 1-5%
- proteins e.g., collagen (gelatin)
- fibrinogen which form viscous solutions
- dextran e.g., 1-50%)
- Viscosity can be changed as a function of time.
- Hydrolytic enzymes such as a protease, can be included to lower the viscosity as a function of time to speed the efflux and compensate for the decrease in the medicinal gradient. This feature provides for a desirable release profile.
- biopolymers polymers of biological origin
- polymers which are known to be safe are employed.
- Polymers useful for this purpose include, but are not limited to, the following:
- glycosaminoglycans such as chondroitin sulfate
- the polymers should be assimilable for use in veterinary or human medicine.
- advantageous polymers include polylysine, polyornithine, and polymyxins.
- neutral complexing agents are employed. Examples include cyclodextrins and proteins which bind the medicinals. Small molecules which aggregate and bind the medicinals are alternatives.
- Apolar molecules which form multi-molecular aggregates can be employed. This type is exemplified by liposomes.
- a series of active medicinals which possess varying degrees of apolar character can be advantageously employed with the apolar complexing agent. Such a series is exemplified by hydrocortisone hemisuccinate-sodium, hydrocortisone, hydrocortisone acetate, and hydrocortisone octanoate.
- the medicinal complexing agent serves to delay the release of the medicinal.
- the medicinal complexing agents can be in the form of a cationic polymer such as polylysine or polyoptithine, an anionic polymer such as chondroitin sulfate and a neutral compound such as cyclodextrin or a lipid or mixture of lipids. Also, chondroitin sulfate can be used with a tetramethyl-lysine linker
- Cationic medicinals may be analogously bound to progressively larger carboxylate (sulfate) containing compounds.
- An enzymatic digest of chondroitin sulfate constitutes a random series of sizes and is conveniently prepared.
- a complexing agent and a medicinal only (without an inorganic); see e.g., Table 1 compositions E, H, J, K, L and O.
- a matrix polymer and a medicinal only for example, hyaluronic acid and a medicinal such as an antibiotic or anesthetic.
- Complexing agents for non-medicinals are discussed in section V “Non-medical Applications.”
- the basis for formation of the inorganic-biopolymer complex matrix can be expressed in the following reaction:
- the drug free and complexed to a medicinal complexing agent, is conveniently mixed with calcium sulfate as a finely ground solid.
- the matrix biopolymer is included to influence the setting time and the drug release profile.
- the setting time can be adjusted so that the user can administer the inorganic-biopolymer complex matrix in the form of a liquid using a syringe with a 23 gauge needle or larger.
- the matrix will solidify soon thereafter. It is convenient to transfer the slurry to the barrel of a syringe using a spatula or second syringe.
- the plunger is inserted and the inorganic-biopolymer complex matrix is injected after expulsion of air.
- Subcutaneous injections are routinely done with a syringe fitted with a 25-gauge needle.
- Dispensing into molds can be accomplished using a syringe fitted with a blunt needle or in some cases a pipette.
- the liquid injection can be s.c., i.m., or i.p.
- the administration is done by parenteral injection.
- Administration of the solid matrix can be by surgical implant, oral, i.p., i.a. or p.a. Specific sites can be targeted for administration of the medicinal such as an anesthetic or anti-inflammatory.
- the drug is conveniently employed as a solid which can be finely ground and mixed with the calcium sulfate.
- the matrix polymer is routinely used as a solution. In a representative formulation the following proportions and ingredients are used:
- the calcium sulfate amount is set at 1 g, the amount of drug used is in the range of 1-200 mg and the matrix biopolymer in the range of 0.4-3 ml.
- the concentration of the matrix biopolymer ranges from 0.1-50%.
- Dextran (clinical grade) is a convenient accelerator at low concentrations.
- the solutions are less viscous than HA solutions and dextran is inexpensive.
- the inorganic-biopolymer complex can be formed as spheres, granules, cylinders, tablets and beads (including microbeads) for injection or for use in capsules.
- the latter can be formed by dispersing the slurry into a rapidly stirring water-immiscible medium.
- the size of the beads can be determined by the amount and nature of the surfactant and the stirring rate.
- the inorganic-biopolymer complex matrix can be molded and or carved into specific shapes to conform to, voids in bone structures. Just prior to formation of the intractable solid, the material is plastic and can be conveniently shaped to fit openings of irregular geometry.
- An idealized release profile has three phases.
- the burst phase is not necessary for many drugs but would be advantageous for anesthetics and antimicrobics.
- the maintenance, or zero-order phase is a desirable result of the delayed release of the complexed drug.
- the drop-off referred to as the closing phase, occurs as the bioerosion process comes to a conclusion. Sub-batches of beads of varying size, drug load, and release profile can be blended to provide the desired release profile.
- the use of the medicinal complexing agent will change the effective molecular weight of the medicinal.
- the matrix density and composition will influence the internal viscosity of the delivery system.
- the shape of the delivery device will dictate the surface area.
- the surface area of a sphere is given by
- V 4 3 ⁇ ⁇ ⁇ ⁇ r 3 ( 4 )
- Another means to control the release profile involves drug precursors. As the precursor is converted to the native compound, its avidity (affinity) for the medicinal complexing agent decreases which in turn raises its diffusivity, thus creating a biphasic release profile. As opposed to release of a molecule that is covalently linked to a polymer, this embodiment is dependent on a change in polarity.
- Compound I is positively charged at physiological pH. It is strongly bound to chondroitin sulfate. As it hydrolyzes to form Compound II, the net charge becomes zero and as a consequence the release is accelerated.
- a biphasic release profile is the result when free II is included in the dosage form.
- the release profile can be controlled by the nature of the hydrolyzable group attached to the carboxyl group.
- the hydrolyzable group can be an ester, an anhydride or other labile functionalities.
- the delivery systems described herein are well suited for sustained release of: an analgesic, an anesthetic, an antialcohol preparation, an anti-microbic, an antiseptic (e.gs. silver ion, and silver sulfadiazine), an anticoagulant, an antineoplastic, an antidepressant, an anti-diabetic agent, an antihypertensive drug, an anti-inflammatory agent, an antinauseant, an anorexic, an antiulcer drug, a cardiovascular drug, a contraceptive, an antihistamine, a diuretic, a hormone/antihormone, an immunosuppressive, a narcotic detoxification agent, a uricosuric agent, and a wound healing promoter.
- an analgesic e.gs. silver ion, and silver sulfadiazine
- an anticoagulant e.gs. silver ion, and silver sulfadiazine
- an anticoagulant e.gs
- a logical alternative to systemic treatment is to employ delivery systems for local release of antibiotics.
- levels much greater than the minimum lethal concentration can be achieved in the therapeutic compartment while blood levels remain low.
- Inorganic biopolymer complexes can be implanted as beads after surgical debridement or the matrix can be injected as a liquid with subsequent solidification.
- the inorganic-biopolymer complexes containing antibiotics are especially useful in filling cavities in bone produced by osteomyelitis. Placement of antibiotic-inorganic-biopolymer complexes in the vicinity of infected bone or other tissue results in eradication of the micro-organism and permits aseptic healing accompanied by resorption of the inorganic-biopolymer complex. When treating bone lesions, bone morphogenic proteins can also be included to promote growth of new bone.
- Inorganic biopolymer complexes are effective for treatment of other local infections, such as joint sepsis, surgical infections, wound infections, uterine infections, oral-dental-periodontal infections, vaginitis, and localized abscesses.
- Likely infectious agents include Aeromonas, Capnocytophaga, Citrobacter, Clostridium, Edwardsiella, Eichenella, Enterobacter, Enteroccus, E.
- Coli Fusobacterium, Hafnia, Kingella, Klebsiella, Moraxella, Morganella, Mycobacterium, Pasturella, Peptostreptococcus, Plesimonas, Proteus, Pseudomonas, Staphylococcus, Streptococcus, and Vibrio.
- Anti-microbics of special interest include cefazolin, piperacillin, nafcillin, cephalexin, imipenem, amikacin, gentamicin, norfloxacin, enrofloxacin ciprofloxacin, vancomycin, nystatin, and amphotericin B.
- the antibiotic inorganic-biopolymer complexes can be used prophylactically.
- antibiotic beads can be distributed to provide antibiotic coverage at critical points. Placing antibiotic beads under the incision is often advantageous.
- Inorganic biopolymer complexes for local delivery of anti-inflammatory drugs hold great promise for treatment of osteoarthritis, degenerative joint disease, and other such afflictions.
- Neutral and charged forms are advantageously employed together.
- free hydrocortisone and hydrocortisone succinate complexed to polymyxin is a useful combination.
- the anti-inflammatory inorganic-biopolymer complexes are placed adjacent to diseased joints, tendon sheaths, etc. Use can accompany arthroscopic procedures both as an injectable and as pre-formed implants.
- NSAIDs are also of interest including naproxen, and disalicylate.
- NSAIDS e.g., analgesics such as aspirin, and other medicinals can be formulated in tablet or capsule form for oral administration.
- Inorganic-biopolymer complexes for pain control are primarily based on free and complexed cationic anesthetics such as lidocaine, buvicaine, bupivacaine, chloroprocaine, procaine, etidocaine, prilocaine, dezocine, hydromorphone, etc.
- An advantageous medicinal complexing agent is chondroitin sulfate. Tablets or beads are especially useful following arthroscopic procedures. Implants are placed next to the joint capsule laterally and medially. Pain relief is provided for 3-5 days which obviates or greatly reduces systemic use of narcotics.
- analgesia and tranquilization can be provided by the use of a complex of chondroitin sulfate and two bio-active compounds—fentanyl and droperidol.
- the simultaneous use of free and bound forms of the active agents provides rapid onset of the desired effects followed by sustained release from the polymeric salt.
- Antineoplastics such as ifosfamide, cytoxan, carboplatin, cis-platin, leuprolide, doxorubicin, carmustine, bleomycin, and fluorouracil can be formulated in inorganic-biopolymer complexes for regional chemotherapy. In situations in which locally disseminated tumor is discovered and surgical removal is deemed inadvisable, administration of inorganic-biopolymer complex via injection is advantageous. Charged agents can be employed as salts with medicinal complexing agents as well as free. Neutral molecules can be formulated with cyclodextrins and emulsifiers. Also, following resection, antineoplastic inorganic-biopolymer complexes can placed in the void left by the tumor as a preventative of recurrence.
- Radiopaque inorganic-biopolymer complexes can be produced by inclusion of BaSO 4 , iodipamide, or other imaging agents in the complex. These formulations can be readily visualized radlographically during and after surgical procedures.
- Pre-formed beads and tablets can be used as prophylactic anti-infectives and as pain control agents. These inorganic-biopolymer complexes are especially useful at the conclusion of orthopedic procedures such as joint arthroscopy and arthroplasty.
- the term “medicinal” includes proteins as well as small molecules.
- the term “protein” includes naturally occurring proteins, recombinant proteins, protein derivatives, chemically synthesized proteins, and synthetic peptides.
- Medicinal proteins useful in the subject invention include colony stimulating factors (CSF) including G-CSF, GM-CSF, and M-CSF; erythropoietin; interleukins, IL-2,IL-4,IL-6,etc; interferons; growth factors (GF) including epidermal-GF, nerve-GF; tumor necrosis factor (TNF); hormones/bioactive peptides; ACTH; angiotensin, atrial natriuretic peptides, bradykynin, dynorphins/endorphins/ ⁇ -lipotropin fragments, enkephalin; gastrointestinal peptides including gastrin and glucacon; growth hormone and growth hormone releasing factors; luteinizing hormone and releasing hormone: melaniocyte stimulating hormone
- G-CSF Medicinal Clinical Indication G-CSF Adjunct to myelosuppressive chemo- therapy Erythropoietin Anemia, kidney disease “Replacement” enzymes Heritable genetic deficiencies of enzymes Hormones endocrine gland failure, treatment of hormone sensitive cancers, contra- ception, growth promotion Cytokines such as colony Immunoadjuvants stimulating factors, e.g., GM-CSF, interferons, e.gs., IFN-alpha, IFN-beta, interleukins, e.gs., IL-1, IL-2 and IL-6 and TNF Vaccine antigens Immunization-preventative and therapeutic BMP-2 Bone replacement Wound healing promoters burns, trauma rh-Lysozyme antimicrobic Growth Factors growth promotion
- Cytokines such as colony Immunoadjuvants stimulating factors, e.g., GM-CSF, interferons, e.g
- the matrix polymer can be selected from the following: polyethyleneglycol, polyvinylpyrrolidone, polyvinylalcohol, starch, xanthan, cellulose and cellulose derivatives (e.g., carboxymethylcellulose).
- non-ionic complexing agents include polyoxyethylene esters and ethers, and surfactants of either biological or non-biological origin.
- ionic complexing agents include polyacrylic acid, alginic acid, dextran sulfate, polyvinylpyridine, polyvinylamine, polyethyleneimine as well as synthetic lipid compounds.
- CaSO 4 .1/2H 2 O is sterilized by heating at 120° C. for 4 hours and then divided into 1 g aliquots which are stored in individual plastic containers in a desiccator.
- Calcium sulfate(1 mg), 50 mg norfloxacin, and 110 mg iodipamide, all finely ground, are mixed thoroughly.
- To this mixture is added 0.6 ml of cold hyaluronic acid solution (2%).
- the slurry is mixed to an even consistency and is loaded into the barrel of a 3 ml syringe with a spatula. The plunger is replaced and the air expelled.
- the needle is attached to the syringe and the inorganic-biopolymer complex is ready for administration or casting in a mold.
- Chondroitin sulfate solution (sodium salt, 5%) is converted to the acid form by passage over a column of DOWEX-50 (sulfonated polystrene). Assuming a residue molecular weight of 500, a stoichiometric amount of amikacin free base is added at 0-4° C. The H is adjusted to 7 and the product is frozen. Alternatively, the product is freeze-dried and stored in a desiccator. Using chondroitin sulfate as the medicinal complexing agent, other complexes can be made by this procedure. Lidocaine, morphine, gentamicin, clindamycin, and doxorubicin are examples.
- Example 1 Calcium sulfate (1 g) is mixed with 50 mg of finely ground cis-platin (cis-diaminedichloroplatinum). To this mixture 0.6 ml of hyaluronic acid solution (2%) is added and the slurry is transferred to a 3 ml syringe as described in Example 1. Using a 20-gauge blunt end needle, the inorganic-biopolymer complex is injected into a teflon mold with spherical holes which are 3.2 mm in diameter. After 48 hours at room temperature, the mold is split and the beads are removed with a dental explorer under sterile conditions. Beads are placed in slits made surgically around a tumor or around the site of tumor removal in an effort to prevent recurrence.
- Polymyxin sulfate solution (10%) is cooled to 0-4° C.
- a stoichiometric amount of barium hydroxide solution is added to produce the free base of polymyxin and insoluble barium sulfate.
- cefazolin dissolved in 50% THF, are added. After trituration, the suspension is filtered to remove the barium sulfate. The residue is washed to recover all of the conjugate. The solvent of the combined filtrate and washing is evaporated and the polymyxin-cefazolin salt is used as the solid.
- Calcium sulfate (1 g) is mixed with 100 mg of polymyxin-cefazolin salt and 50 mg of cefazolin-sodium.
- hyaluronic acid 2%
- the slurry is administered directly or placed in a bead or tablet mold.
- Polylysine, polyomnithine, or polyarginine may be used in place of polymnyxin.
- Penicillin G is employed simultaneously as the salt of potassium, procaine, benzathine, and polymyxin. To 1 g of calcium sulfate is added 100 mg of penicillin G-potassium plus 100 mg procaine-penicillin and 50 mg each of polymyxin-penicillin and polylysine-penicillin. After thorough mixing, 0.6 ml of 20% dextran is added and the slurry handled as described above.
- apolar medicinal complexing agent such as Polysorb 80 is employed with the following forms of hydrocortisone:
- Dinoseb is conjugated with polyethyleneimine (PEI) using water as a solvent.
- PEI polyethyleneimine
- This mixture 600 mg is combined with 1 g of calcium sulfate and the slurry used to produce beads with a water-immiscible medium such as sesame oil.
- Naphthalene acetic acid can be used in place of dinoseb to produce a long-lasting root growth stimulator.
- a matrix including norfloxacin (formulation A of Table 1) was used to treat the infection. After thorough debridement of the cavity, the void was filled with freshly prepared matrix. No surgical intervention was necessary after the treatment. The infection was eradicated and no sign of lameness appeared after 1 month.
- Chondroitin sulfate (1 g) is dissolved in 4 ml distilled water at 04° C. TCA (1 ml ml, 32%) at 0C is added with stirring. The solution is poured into 20 ml of cold ethanol; the precipitate is collected on a filter, washed and dried. One equivalent of solid amikacin (free base) is added. The solution is adjusted to pH 7.4. It can be used as is or supplemented with amikacin sulfate.
Abstract
Description
| TABLE 1 | ||||
| Complexing | ||||
| Formulation | CaSO4 ½H2O | Matrix polymer | agent | Medicinal |
| A | 1 g | HA - 0.6 ml (2%) | 50 mg NF | |
| R/100 mg Ia | ||||
| B | 1 g | Dextran - 0.6 ml (20%) | lecithin - | 50 mg NF |
| R/100 mg Ia | 100 mg | |||
| C | 1 g | HA, 0.6 ml (2%) | polyglutamic | 100 mg lidocaine |
| acid | ||||
| D | 1 g | HA, 0.6 ml (2%) | chon S | 100 mg amikacin |
| E | — | HA, 0.6 ml (2%) | chon S | Amikacin 100 mg |
| F | 1 g | Dextran - 6 ml (20%) | polylysine | Cef 100 mg |
| HA, 0.6 ml (2%) | ||||
| G | 1 g | HA, 0.6 ml (2%) | 500 mg HC | |
| (10% a.i.) | ||||
| H | — | HA, 0.6 ml (2%) | 500 mg HC | |
| (10% a.i.) | ||||
| I | 1 g | HA, 0.6 ml (2%) | 50 mg cis-platin | |
| J | — | HA, 0.6 ml (2%) | chon S | Lidocaine 100 mg |
| K | — | HA, 0.6 ml (2%) | chon S | Morphine 100 mg |
| L | — | HA, 0.6 ml (2%) | chon S | Hydromorphone |
| 100 mg | ||||
| M | 1 g | HA, 0.6 ml (2%) | 50 mg Imip | |
| N | 1 g | HA, 0.6 ml (2%) | 5 mg BMP-2 | |
| O | — | HA, 0.6 ml (2%) | polylysine | 100 mg Imip |
| P | 1 g | — | 0.6 ml chon S | lidocaine 24 mg |
| Q | .5 g | HA, 1 ml (2%) | HA | |
| R* | 1 g | Dextran 200 mg (solid) | — | Lidocaine 100 mg |
| (solid) | ||||
| S | 1 g | Gelatin (10%) 0.6 ml | — | Lidocaine 100 mg |
| (solid) | ||||
| R = radiopaque | ||||
| Ia = iodipamide | ||||
| HA = hyaluronic acid, sodium salt | ||||
| NF = norfloxacin | ||||
| Imip = imipenem | ||||
| Cef = cefazolin | ||||
| HC = hydrocortisone | ||||
| CD = 2-hydroxypropyl-(3-cyclodextrin) | ||||
| Chon S = chondroitin sulfate | ||||
| LD = lidocaine | ||||
| *Slurry is made with 0.6 ml of water. | ||||
| Ingredient | Amount | ||
| Calcium sulfate | 1 | g | ||
| Drug | 50 | mg | ||
| matrix biopolymer at 2% | 0.6 | ml | ||
| TABLE 2 |
| Change of setting time by matrix biopolymers |
| A. Hyaluronic acid (HA) | ||
| Calcium sulfate | HA (%) | Setting time (min) |
| 1 g | 0.6 ml (0) | 75 |
| 1 g | 0.6 ml (.2) | 60 |
| 1 g | 0.6 ml (2) | 20 |
| B. Dextran | ||
| Calcium sulfate | Dextran (%) | Setting time (min) |
| 1 g | 0.6 ml (0) | 75 |
| 1 g | 0.6 ml (10) | 15 |
| 1 g | 0.6 ml (20) | 25 |
| 1 g | 0.6 ml (50) | 80 |
| Medicinal | Clinical Indication |
| G-CSF | Adjunct to myelosuppressive chemo- |
| therapy | |
| Erythropoietin | Anemia, kidney disease |
| “Replacement” enzymes | Heritable genetic deficiencies of |
| enzymes | |
| Hormones | endocrine gland failure, treatment of |
| hormone sensitive cancers, contra- | |
| ception, growth promotion | |
| Cytokines such as colony | Immunoadjuvants |
| stimulating factors, e.g., | |
| GM-CSF, interferons, e.gs., | |
| IFN-alpha, IFN-beta, interleukins, | |
| e.gs., IL-1, IL-2 and IL-6 and TNF | |
| Vaccine antigens | Immunization-preventative and |
| therapeutic | |
| BMP-2 | Bone replacement |
| Wound healing promoters | burns, trauma |
| rh-Lysozyme | antimicrobic |
| Growth Factors | growth promotion |
| TABLE 3 |
| Release of Lidocaine for Matrices with (B) and |
| without (A) the Matrix Biopolymer. |
| Matrix A | Matrix B (11% Dextran) |
| Day | % Release | Day | % Release | ||
| 1 | 85 | 1 | 24 | ||
| 2 | 10 | 2 | 26 | ||
| 3 | 1 | 3 | 22 | ||
| 4 | 1 | 4 | 15 | ||
| 5 | 1 | 5 | 6 | ||
Claims (41)
Priority Applications (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US08/935,300 US6391336B1 (en) | 1997-09-22 | 1997-09-22 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| CA002303884A CA2303884C (en) | 1997-09-22 | 1998-09-22 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| AU94925/98A AU758803B2 (en) | 1997-09-22 | 1998-09-22 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| US09/509,016 US6630486B1 (en) | 1997-09-22 | 1998-09-22 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| PCT/US1998/019528 WO1999015150A1 (en) | 1997-09-22 | 1998-09-22 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| JP2000512521A JP5259030B2 (en) | 1997-09-22 | 1998-09-22 | Inorganic-polymer complex for controlled release of drug-containing compounds |
| EP98948335A EP1017364A4 (en) | 1997-09-22 | 1998-09-22 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| US10/365,419 US6869976B2 (en) | 1997-09-22 | 2003-02-13 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| US10/838,303 US20040208934A1 (en) | 1997-09-22 | 2004-05-05 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US08/935,300 US6391336B1 (en) | 1997-09-22 | 1997-09-22 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
Related Child Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US1998/019528 Continuation-In-Part WO1999015150A1 (en) | 1997-09-22 | 1998-09-22 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| US09/509,016 Continuation-In-Part US6630486B1 (en) | 1997-09-22 | 1998-09-22 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| US09509016 Continuation-In-Part | 1998-09-26 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US6391336B1 true US6391336B1 (en) | 2002-05-21 |
Family
ID=25466890
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US08/935,300 Expired - Lifetime US6391336B1 (en) | 1997-09-22 | 1997-09-22 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| US09/509,016 Expired - Fee Related US6630486B1 (en) | 1997-09-22 | 1998-09-22 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| US10/365,419 Expired - Lifetime US6869976B2 (en) | 1997-09-22 | 2003-02-13 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| US10/838,303 Abandoned US20040208934A1 (en) | 1997-09-22 | 2004-05-05 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/509,016 Expired - Fee Related US6630486B1 (en) | 1997-09-22 | 1998-09-22 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| US10/365,419 Expired - Lifetime US6869976B2 (en) | 1997-09-22 | 2003-02-13 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| US10/838,303 Abandoned US20040208934A1 (en) | 1997-09-22 | 2004-05-05 | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
Country Status (6)
| Country | Link |
|---|---|
| US (4) | US6391336B1 (en) |
| EP (1) | EP1017364A4 (en) |
| JP (1) | JP5259030B2 (en) |
| AU (1) | AU758803B2 (en) |
| CA (1) | CA2303884C (en) |
| WO (1) | WO1999015150A1 (en) |
Cited By (39)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030091635A1 (en) * | 2001-09-26 | 2003-05-15 | Baichwal Anand R. | Opioid formulations having reduced potential for abuse |
| US20030129234A1 (en) * | 2001-07-06 | 2003-07-10 | Penwest Pharmaceuticals Company | Methods of making sustained release formulations of oxymorphone |
| US20030194438A1 (en) * | 2002-04-11 | 2003-10-16 | Albert Prescott | Extended release analgesic for pain control |
| US20040180072A1 (en) * | 2003-03-12 | 2004-09-16 | Howmedica Osteonics Corp. | Prosthesis with sustained release analgesic |
| US6800245B1 (en) * | 2000-11-28 | 2004-10-05 | Vita Special Purpose Corporation | Sterile polymerizable systems and kits and methods of their manufacture and use |
| US20040208934A1 (en) * | 1997-09-22 | 2004-10-21 | Royer Biomedical, Inc. | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| US20060034926A1 (en) * | 2003-03-20 | 2006-02-16 | Kristine Fraatz | Controlled release system |
| US20060153893A1 (en) * | 2002-04-08 | 2006-07-13 | Denki Kagaku Kogyo Kabushiki Kaisha | Therapeutic composition for bone infectious disease |
| US20060189572A1 (en) * | 2001-01-10 | 2006-08-24 | Showa Yakuhin Kako Co., Ltd. | Composition for local anesthesia |
| US20060204533A1 (en) * | 2005-03-14 | 2006-09-14 | Biotegra, Inc. | Drug Delivery Compositions and Related Methods |
| US20060228415A1 (en) * | 2003-08-08 | 2006-10-12 | Biovail Laboratories International S.R.L. | Modified release tablet of bupropion hydrochloride |
| US20060269604A1 (en) * | 1993-11-23 | 2006-11-30 | Purdue Pharma L.P. | Method of treating pain by administering 24 hour oral opioid formulations exhibiting rapid rate of initial rise of plasma drug level |
| US20060293287A1 (en) * | 2005-06-28 | 2006-12-28 | Jadhav Prakash M | Storage stable formulation and a process for its preparation |
| US20070098794A1 (en) * | 2001-07-06 | 2007-05-03 | Haui-Hung Kao | Oxymorphone controlled release formulations |
| US20070098793A1 (en) * | 2001-07-06 | 2007-05-03 | Haui-Hung Kao | Oxymorphone controlled release formulations |
| US20070128118A1 (en) * | 2005-12-05 | 2007-06-07 | Nitto Denko Corporation | Polyglutamate-amino acid conjugates and methods |
| US20070212414A1 (en) * | 2006-03-08 | 2007-09-13 | Penwest Pharmaceuticals Co. | Ethanol-resistant sustained release formulations |
| US20080063681A1 (en) * | 2006-09-11 | 2008-03-13 | Ebi, L.P. | Therapeutic bone replacement material |
| US20080181852A1 (en) * | 2007-01-29 | 2008-07-31 | Nitto Denko Corporation | Multi-functional Drug Carriers |
| US20080188819A1 (en) * | 2006-07-07 | 2008-08-07 | Kloke Tim M | Beaded Wound Spacer Device |
| US20080233165A1 (en) * | 2000-08-07 | 2008-09-25 | Biolok International, Inc. | Time release calcium sulfate and growth factor matrix for bone augmentation |
| US20080253969A1 (en) * | 2007-04-10 | 2008-10-16 | Nitto Denko Corporation | Multi-functional polyglutamate drug carriers |
| US20080279778A1 (en) * | 2007-05-09 | 2008-11-13 | Nitto Denko Corporation | Polyglutamate conjugates and polyglutamate-amino acid conjugates having a plurality of drugs |
| US20080279777A1 (en) * | 2007-05-09 | 2008-11-13 | Nitto Denko Corporation | Compositions that include a hydrophobic compound and a polyamino acid conjugate |
| US20080279782A1 (en) * | 2007-05-09 | 2008-11-13 | Nitto Denko Corporation | Polymers conjugated with platinum drugs |
| US20090028922A1 (en) * | 2007-07-24 | 2009-01-29 | Haggard Warren O | Local Delivery Method and Composition |
| US20090081610A1 (en) * | 2007-09-14 | 2009-03-26 | Discus Dental, Llc | Dental prophylaxis devices |
| US20090118215A1 (en) * | 2006-03-14 | 2009-05-07 | Lidds Ab | Bioresorbable Controlled-Release Composition |
| US20090124650A1 (en) * | 2007-06-21 | 2009-05-14 | Endo Pharmaceuticals, Inc. | Method of Treating Pain Utilizing Controlled Release Oxymorphone Pharmaceutical Compositions and Instructions on Effects of Alcohol |
| US20090202609A1 (en) * | 2008-01-06 | 2009-08-13 | Keough Steven J | Medical device with coating composition |
| US20090226393A1 (en) * | 2008-03-06 | 2009-09-10 | Nitto Denko Corporation | Polymer paclitaxel conjugates and methods for treating cancer |
| US20100029486A1 (en) * | 2008-07-31 | 2010-02-04 | Michael Dean Willis | Extended release tablet and method for making and using same |
| US20110177149A1 (en) * | 2008-08-13 | 2011-07-21 | Messina James J | Broad spectrum animal repellent and method |
| US8124118B2 (en) | 2003-10-22 | 2012-02-28 | Lidds Ab | Composition comprising biodegradable hydrating ceramics for controlled drug delivery |
| US9155671B2 (en) | 2012-10-16 | 2015-10-13 | Surmodics, Inc. | Wound packing device and methods |
| US9271486B2 (en) | 2011-11-10 | 2016-03-01 | James J. Messina | Combination animal repellents |
| US10201457B2 (en) | 2014-08-01 | 2019-02-12 | Surmodics, Inc. | Wound packing device with nanotextured surface |
| WO2019135420A1 (en) * | 2018-01-03 | 2019-07-11 | 김배용 | Composite using porous material and polymer, and use thereof |
| WO2019170912A1 (en) | 2018-03-09 | 2019-09-12 | Lidds Ab | Bioresorbable controlled-release compositions with sting modulating molecules |
Families Citing this family (100)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4234803B2 (en) * | 1997-10-27 | 2009-03-04 | 久光製薬株式会社 | Pharmaceutical composition with controlled drug release rate |
| US7371408B1 (en) | 1999-06-07 | 2008-05-13 | Wright Medical Technology, Inc. | Bone graft substitute composition |
| AUPQ259399A0 (en) | 1999-09-01 | 1999-09-23 | Lustre Investments Pte Ltd | Therapeutic agents |
| US7582311B1 (en) * | 1999-10-15 | 2009-09-01 | Genentech, Inc. | Injection vehicle for polymer-based formulations |
| WO2002030469A2 (en) * | 2000-10-12 | 2002-04-18 | Orchid Chemicals And Pharmaceuticals Limited | Beta-lactam antibiotic-polysaccharide complex |
| WO2002032396A2 (en) * | 2000-10-16 | 2002-04-25 | Massachusetts Institute Of Technology | Lipid-protein-sugar particles for delivery of nucleic acids |
| US6497901B1 (en) * | 2000-11-02 | 2002-12-24 | Royer Biomedical, Inc. | Resorbable matrices for delivery of bioactive compounds |
| DE10064219B9 (en) | 2000-12-22 | 2009-02-12 | Nasalis Pain Relief International Gmbh | Novel pharmaceutical composition containing fentanyl and / or its derivatives |
| WO2003011214A2 (en) * | 2001-07-31 | 2003-02-13 | Royer Biomedical, Inc. | Novel methods and formulations for administration of active agents |
| US7371409B2 (en) | 2001-09-06 | 2008-05-13 | Wright Medical Technology, Inc. | Bone graft substitute composition |
| US6946137B2 (en) * | 2001-10-19 | 2005-09-20 | Idexx Laboratories, Inc. | Methods for the controlled delivery of pharmacologically active compounds |
| EA200401111A1 (en) | 2002-02-25 | 2005-02-24 | Диффьюжн Фармасьютикалз Ллс | TRANS-CAROTINOID BIPOLAR SALTS AND THEIR APPLICATION |
| US7759506B2 (en) | 2002-02-25 | 2010-07-20 | Diffusion Pharmaceuticals Llc | Bipolar trans carotenoid salts and their uses |
| ATE490745T1 (en) | 2002-03-29 | 2010-12-15 | Wright Medical Tech Inc | BONE TRANSPLANT REPLACEMENT COMPOSITION |
| US7291179B2 (en) | 2002-06-24 | 2007-11-06 | Wright Medical Technology, Inc. | Bone graft substitute composition |
| JP4569080B2 (en) * | 2002-07-17 | 2010-10-27 | 大正製薬株式会社 | Nasal composition |
| NZ538628A (en) * | 2002-08-12 | 2008-06-30 | Dynavax Tech Corp | Immunomodulatory compositions, methods of making, and methods of use thereof |
| US7338433B2 (en) | 2002-08-13 | 2008-03-04 | Allergan, Inc. | Remotely adjustable gastric banding method |
| JP4425791B2 (en) * | 2002-08-16 | 2010-03-03 | 電気化学工業株式会社 | Injection for treatment of isolated arthropathy |
| DE60331457D1 (en) | 2002-08-28 | 2010-04-08 | Allergan Inc | TEMPTING MAGNETIC BANDING DEVICE |
| DE10255106A1 (en) * | 2002-11-24 | 2004-06-09 | Novosom Ag | Liposomal glucocorticoids |
| FR2861734B1 (en) | 2003-04-10 | 2006-04-14 | Corneal Ind | CROSSLINKING OF LOW AND HIGH MOLECULAR MASS POLYSACCHARIDES; PREPARATION OF INJECTABLE SINGLE PHASE HYDROGELS; POLYSACCHARIDES AND HYDROGELS OBTAINED |
| JP2007527279A (en) | 2004-01-23 | 2007-09-27 | アラーガン、インコーポレイテッド | One-piece adjustable gastric band that can be fixed removably |
| ES2333024T3 (en) | 2004-03-08 | 2010-02-16 | Allergan Medical S.A. | CLOSURE SYSTEM FOR TUBULAR ORGANS. |
| US7250550B2 (en) | 2004-10-22 | 2007-07-31 | Wright Medical Technology, Inc. | Synthetic bone substitute material |
| EP1853544A4 (en) | 2005-02-24 | 2010-06-16 | Diffusion Pharmaceuticals Llc | Trans carotenoids, their synthesis, formulation and uses |
| AU2006228675B2 (en) * | 2005-03-31 | 2012-05-17 | Lidds Ab | Method for treating prostate diseases based on local delivery of active substances |
| US8251888B2 (en) | 2005-04-13 | 2012-08-28 | Mitchell Steven Roslin | Artificial gastric valve |
| US8025903B2 (en) | 2005-09-09 | 2011-09-27 | Wright Medical Technology, Inc. | Composite bone graft substitute cement and articles produced therefrom |
| CN103349793B (en) | 2005-09-09 | 2016-02-10 | 阿格诺沃斯健康关爱公司 | Composite bone graft substitute cement and the goods obtained by it |
| US20070154448A1 (en) | 2005-11-22 | 2007-07-05 | Ted Reid | Methods and compositions using Substance P to promote wound healing |
| US8043206B2 (en) | 2006-01-04 | 2011-10-25 | Allergan, Inc. | Self-regulating gastric band with pressure data processing |
| US20100203136A1 (en) * | 2006-05-04 | 2010-08-12 | Royer Biomedical, Inc. | METHOD FOR DELIVERING A HUMAN CHORIONIC GONADOTROPIN (Hcg) VACCINE FOR LONG-ACTING ANTIBODY PROTECTION |
| EP2155235B1 (en) | 2007-04-09 | 2016-04-06 | Wake Forest University Health Sciences | Oxygen-generating compositions for enhancing cell and tissue survival in vivo |
| US8293804B2 (en) | 2007-04-13 | 2012-10-23 | Diffusion Pharmaceuticals Llc | Use of bipolar trans carotenoids as a pretreatment and in the treatment of peripheral vascular disease |
| WO2008128189A1 (en) | 2007-04-13 | 2008-10-23 | The Penn State Research Foundation | Anti-cancer compositions and methods |
| US8071119B2 (en) * | 2007-05-14 | 2011-12-06 | Sustained Nano Systems Llc | Controlled release implantable dispensing device and method |
| US8114668B2 (en) * | 2007-05-14 | 2012-02-14 | Cardiac Pacemakers, Inc. | Composition for cold storage of stem cells |
| US20090148498A1 (en) * | 2007-05-14 | 2009-06-11 | Sustained Nano Systems Llc | Controlled release implantable dispensing device and method |
| IN266731B (en) * | 2007-05-14 | 2015-05-28 | Sustained Nano Systems Llc | |
| US20080293637A1 (en) | 2007-05-23 | 2008-11-27 | Allergan, Inc. | Cross-linked collagen and uses thereof |
| US8318695B2 (en) | 2007-07-30 | 2012-11-27 | Allergan, Inc. | Tunably crosslinked polysaccharide compositions |
| US8697044B2 (en) | 2007-10-09 | 2014-04-15 | Allergan, Inc. | Crossed-linked hyaluronic acid and collagen and uses thereof |
| JP2011502125A (en) | 2007-10-31 | 2011-01-20 | ディフュージョン・ファーマシューティカルズ・エルエルシー | A new class of treatments that promote small molecule diffusion |
| JP5670196B2 (en) | 2007-11-16 | 2015-02-18 | バイセプト セラピューティクス、インク. | Compositions and methods for treating purpura |
| US8394782B2 (en) | 2007-11-30 | 2013-03-12 | Allergan, Inc. | Polysaccharide gel formulation having increased longevity |
| US8394784B2 (en) | 2007-11-30 | 2013-03-12 | Allergan, Inc. | Polysaccharide gel formulation having multi-stage bioactive agent delivery |
| CA2710515A1 (en) * | 2007-12-28 | 2009-07-09 | Khashayar Kevin Neshat | Controlled release local anesthetic for post dental surgery and method of use |
| JP5392674B2 (en) * | 2008-04-03 | 2014-01-22 | 公立大学法人大阪府立大学 | Collagen pharmaceutical composition and method for producing the same |
| US8003633B1 (en) | 2008-04-14 | 2011-08-23 | The Penn State Research Foundation | Anti-cancer compositions and methods |
| US8357795B2 (en) | 2008-08-04 | 2013-01-22 | Allergan, Inc. | Hyaluronic acid-based gels including lidocaine |
| ES2829971T3 (en) | 2008-09-02 | 2021-06-02 | Tautona Group Lp | Hyaluronic acid threads and / or derivatives thereof, methods to manufacture them and uses thereof |
| KR101258336B1 (en) | 2008-10-02 | 2013-04-25 | 밀란 인크. | Method of making a multilayer adhesive laminate |
| US20100305397A1 (en) * | 2008-10-06 | 2010-12-02 | Allergan Medical Sarl | Hydraulic-mechanical gastric band |
| US20100185049A1 (en) | 2008-10-22 | 2010-07-22 | Allergan, Inc. | Dome and screw valves for remotely adjustable gastric banding systems |
| US20100215716A1 (en) * | 2009-02-23 | 2010-08-26 | Biomet Manufacturing Corp. | Compositions and methods for coating orthopedic implants |
| US8390326B2 (en) * | 2009-05-05 | 2013-03-05 | William Marsh Rice University | Method for fabrication of a semiconductor element and structure thereof |
| CA2765697C (en) | 2009-06-22 | 2019-11-12 | Diffusion Pharmaceuticals Llc | Diffusion enhancing compounds and their use alone or with thrombolytics |
| US20110172180A1 (en) | 2010-01-13 | 2011-07-14 | Allergan Industrie. Sas | Heat stable hyaluronic acid compositions for dermatological use |
| US9114188B2 (en) | 2010-01-13 | 2015-08-25 | Allergan, Industrie, S.A.S. | Stable hydrogel compositions including additives |
| US8840541B2 (en) | 2010-02-25 | 2014-09-23 | Apollo Endosurgery, Inc. | Pressure sensing gastric banding system |
| KR101764451B1 (en) | 2010-03-12 | 2017-08-02 | 알러간 인더스트리 에스에이에스 | A Fluid Composition Comprising A Hyaluronan Polymer and Mannitol For Improving Skin Condition |
| PL3078388T3 (en) | 2010-03-22 | 2019-08-30 | Allergan, Inc. | Cross-linked hydrogels for soft tissue augmentation |
| DE102010003615A1 (en) | 2010-04-01 | 2011-10-06 | Leibniz-Institut Für Polymerforschung Dresden E.V. | Process for the preparation of a drug delivery system based on polyelectrolyte complexes |
| US9028394B2 (en) | 2010-04-29 | 2015-05-12 | Apollo Endosurgery, Inc. | Self-adjusting mechanical gastric band |
| US20110270024A1 (en) | 2010-04-29 | 2011-11-03 | Allergan, Inc. | Self-adjusting gastric band having various compliant components |
| US9044298B2 (en) | 2010-04-29 | 2015-06-02 | Apollo Endosurgery, Inc. | Self-adjusting gastric band |
| CN103124498A (en) | 2010-06-02 | 2013-05-29 | 扩散药品有限公司 | Oral formulations of bipolar trans carotenoids |
| US8517915B2 (en) | 2010-06-10 | 2013-08-27 | Allergan, Inc. | Remotely adjustable gastric banding system |
| US8883139B2 (en) | 2010-08-19 | 2014-11-11 | Allergan Inc. | Compositions and soft tissue replacement methods |
| US8889123B2 (en) | 2010-08-19 | 2014-11-18 | Allergan, Inc. | Compositions and soft tissue replacement methods |
| US9005605B2 (en) | 2010-08-19 | 2015-04-14 | Allergan, Inc. | Compositions and soft tissue replacement methods |
| US8697057B2 (en) | 2010-08-19 | 2014-04-15 | Allergan, Inc. | Compositions and soft tissue replacement methods |
| US20120059216A1 (en) | 2010-09-07 | 2012-03-08 | Allergan, Inc. | Remotely adjustable gastric banding system |
| AU2011338548B2 (en) | 2010-12-06 | 2016-09-29 | The Penn State Research Foundation | Compositions and methods relating to proliferative diseases |
| EP3679934A1 (en) | 2011-04-29 | 2020-07-15 | The Penn State Research Foundation | Small molecule trail gene induction by normal and tumor cells as an anticancer therapy |
| US9408797B2 (en) | 2011-06-03 | 2016-08-09 | Allergan, Inc. | Dermal filler compositions for fine line treatment |
| US9393263B2 (en) | 2011-06-03 | 2016-07-19 | Allergan, Inc. | Dermal filler compositions including antioxidants |
| KR102312056B1 (en) | 2011-06-03 | 2021-10-12 | 알러간 인더스트리 에스에이에스 | Dermal filler compositions including antioxidants |
| US20130096081A1 (en) | 2011-06-03 | 2013-04-18 | Allergan, Inc. | Dermal filler compositions |
| US9662422B2 (en) | 2011-09-06 | 2017-05-30 | Allergan, Inc. | Crosslinked hyaluronic acid-collagen gels for improving tissue graft viability and soft tissue augmentation |
| US20130244943A1 (en) | 2011-09-06 | 2013-09-19 | Allergan, Inc. | Hyaluronic acid-collagen matrices for dermal filling and volumizing applications |
| US8876694B2 (en) | 2011-12-07 | 2014-11-04 | Apollo Endosurgery, Inc. | Tube connector with a guiding tip |
| US8961394B2 (en) | 2011-12-20 | 2015-02-24 | Apollo Endosurgery, Inc. | Self-sealing fluid joint for use with a gastric band |
| JP2016503063A (en) | 2012-12-20 | 2016-02-01 | ザ ペン ステイト リサーチ ファンデーション | Methods and compositions for the treatment of cancer |
| US9999603B2 (en) | 2013-03-15 | 2018-06-19 | The Penn State Research Foundation | Compositions and methods including leelamine and arachidonyl trifluoromethyl ketone relating to treatment of cancer |
| US10010504B2 (en) | 2013-03-15 | 2018-07-03 | The Penn State Research Foundation | Compositions and methods including celecoxib and plumbagin relating to treatment of cancer |
| ES2761558T3 (en) | 2014-09-30 | 2020-05-20 | Allergan Ind Sas | Stable hydrogel compositions including additives |
| US9750785B2 (en) | 2015-01-30 | 2017-09-05 | Par Pharmaceutical, Inc. | Vasopressin formulations for use in treatment of hypotension |
| US9925233B2 (en) | 2015-01-30 | 2018-03-27 | Par Pharmaceutical, Inc. | Vasopressin formulations for use in treatment of hypotension |
| US9687526B2 (en) | 2015-01-30 | 2017-06-27 | Par Pharmaceutical, Inc. | Vasopressin formulations for use in treatment of hypotension |
| US9375478B1 (en) | 2015-01-30 | 2016-06-28 | Par Pharmaceutical, Inc. | Vasopressin formulations for use in treatment of hypotension |
| US9744209B2 (en) | 2015-01-30 | 2017-08-29 | Par Pharmaceutical, Inc. | Vasopressin formulations for use in treatment of hypotension |
| US9937223B2 (en) | 2015-01-30 | 2018-04-10 | Par Pharmaceutical, Inc. | Vasopressin formulations for use in treatment of hypotension |
| WO2016128783A1 (en) | 2015-02-09 | 2016-08-18 | Allergan Industrie Sas | Compositions and methods for improving skin appearance |
| WO2017143207A1 (en) | 2016-02-18 | 2017-08-24 | The Penn State Research Foundation | GENERATING GABAergic NEURONS IN BRAINS |
| KR20230014850A (en) | 2016-03-24 | 2023-01-30 | 디퓨젼 파마슈티컬즈 엘엘씨 | Use of bipolar trans carotenoids with chemotherapy and radiotherapy for treatment of cancer |
| EP4039812A1 (en) | 2017-02-28 | 2022-08-10 | The Penn State Research Foundation | Regenerating functional neurons for treatment of neural injury caused by disruption of blood flow |
| FR3071729A1 (en) * | 2017-10-04 | 2019-04-05 | Cementic | DISINFECTANT DENTAL CEMENTS COMPRISING LIPOSOMES |
| EP3958868A4 (en) | 2019-04-22 | 2023-01-18 | The Penn State Research Foundation | Methods and compositions relating to inhibition of aldehyde dehydrogenases for treatment of cancer |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4946870A (en) | 1986-06-06 | 1990-08-07 | Union Carbide Chemicals And Plastics Company Inc. | Delivery systems for pharmaceutical or therapeutic actives |
| US5385887A (en) * | 1993-09-10 | 1995-01-31 | Genetics Institute, Inc. | Formulations for delivery of osteogenic proteins |
| EP0642785A2 (en) | 1993-09-09 | 1995-03-15 | Edward Mendell Co., Inc. | Sustained release heterodisperse hydrogel systems for insoluble drugs |
| US5407686A (en) * | 1991-11-27 | 1995-04-18 | Sidmak Laboratories, Inc. | Sustained release composition for oral administration of active ingredient |
| US5697922A (en) * | 1992-11-20 | 1997-12-16 | Pfizer Inc. | Delivery device having encapsulated excipients |
| US5783214A (en) * | 1994-06-13 | 1998-07-21 | Buford Biomedical, Inc. | Bio-erodible matrix for the controlled release of medicinals |
Family Cites Families (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4020152A (en) | 1973-12-18 | 1977-04-26 | Thann & Mulhouse | Barium titanate and barium zirconate in radiological contrast products |
| DE2843963A1 (en) * | 1978-10-09 | 1980-04-24 | Merck Patent Gmbh | BODY-RESORBABLE SHAPED MATERIAL BASED ON COLLAGEN AND THEIR USE IN MEDICINE |
| US4233317A (en) * | 1978-12-18 | 1980-11-11 | Mcneilab, Inc. | Analgesic potentiation |
| IT1229075B (en) | 1985-04-05 | 1991-07-17 | Fidia Farmaceutici | Topical compsn. contg. hyaluronic acid deriv. as vehicle |
| US5166331A (en) | 1983-10-10 | 1992-11-24 | Fidia, S.P.A. | Hyaluronics acid fractions, methods for the preparation thereof, and pharmaceutical compositions containing same |
| US5085861A (en) * | 1987-03-12 | 1992-02-04 | The Beth Israel Hospital Association | Bioerodable implant composition comprising crosslinked biodegradable polyesters |
| FR2647050B1 (en) * | 1989-05-16 | 1991-11-22 | Seppic Sa | PROCESS FOR THE MANUFACTURE OF A DIRECTLY COMPRESSIBLE STARCH FOR USE IN THE MANUFACTURE OF TABLETS AND TABLETS OBTAINED |
| CA2056384C (en) * | 1989-06-05 | 1998-06-23 | Tobin N. Gerhart | Bioerodible polymers for drug delivery in bone |
| JPH04279520A (en) * | 1990-05-31 | 1992-10-05 | Eisai Co Ltd | Pharmaceutical preparation for embedding in bone |
| CA2119090A1 (en) * | 1993-03-26 | 1994-09-27 | Wayne R. Gombotz | Compositions for controlled release of biologically active tgf-.beta. |
| JPH0731673A (en) * | 1993-07-19 | 1995-02-03 | Asahi Optical Co Ltd | Bioabsorbable polymer-containing curable bone filling material |
| JPH0753388A (en) * | 1993-08-12 | 1995-02-28 | Lion Corp | Bone metabolism improver |
| US5681873A (en) * | 1993-10-14 | 1997-10-28 | Atrix Laboratories, Inc. | Biodegradable polymeric composition |
| AU3795395A (en) * | 1994-11-30 | 1996-06-06 | Ethicon Inc. | Hard tissue bone cements and substitutes |
| US5614206A (en) | 1995-03-07 | 1997-03-25 | Wright Medical Technology, Inc. | Controlled dissolution pellet containing calcium sulfate |
| US5612027A (en) * | 1995-04-18 | 1997-03-18 | Galin; Miles A. | Controlled release of miotic and mydriatic drugs in the anterior chamber |
| US5648097A (en) * | 1995-10-04 | 1997-07-15 | Biotek, Inc. | Calcium mineral-based microparticles and method for the production thereof |
| US5702715A (en) * | 1995-10-27 | 1997-12-30 | Drying Technology | Reinforced biological sealants |
| US6245351B1 (en) * | 1996-03-07 | 2001-06-12 | Takeda Chemical Industries, Ltd. | Controlled-release composition |
| US6299905B1 (en) * | 1996-04-16 | 2001-10-09 | Depuy Orthopaedics, Inc. | Bioerodable polymeric adhesives for tissue repair |
| US7041641B2 (en) * | 1997-03-20 | 2006-05-09 | Stryker Corporation | Osteogenic devices and methods of use thereof for repair of endochondral bone and osteochondral defects |
| GB9710699D0 (en) * | 1997-05-24 | 1997-07-16 | Danbiosyst Uk | Gastro-retentive controlled release system |
| US6113947A (en) * | 1997-06-13 | 2000-09-05 | Genentech, Inc. | Controlled release microencapsulated NGF formulation |
| US6391336B1 (en) * | 1997-09-22 | 2002-05-21 | Royer Biomedical, Inc. | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| US6497901B1 (en) * | 2000-11-02 | 2002-12-24 | Royer Biomedical, Inc. | Resorbable matrices for delivery of bioactive compounds |
| WO2004000276A1 (en) * | 2002-06-20 | 2003-12-31 | Royer Biomedical, Inc. | Resorbable matrices with coatings for delivery of bioactive compounds |
| AU2004249213A1 (en) * | 2003-06-20 | 2004-12-29 | Royer Biomedical, Inc. | Drug polymer complexes |
-
1997
- 1997-09-22 US US08/935,300 patent/US6391336B1/en not_active Expired - Lifetime
-
1998
- 1998-09-22 WO PCT/US1998/019528 patent/WO1999015150A1/en active IP Right Grant
- 1998-09-22 JP JP2000512521A patent/JP5259030B2/en not_active Expired - Fee Related
- 1998-09-22 CA CA002303884A patent/CA2303884C/en not_active Expired - Fee Related
- 1998-09-22 US US09/509,016 patent/US6630486B1/en not_active Expired - Fee Related
- 1998-09-22 EP EP98948335A patent/EP1017364A4/en not_active Withdrawn
- 1998-09-22 AU AU94925/98A patent/AU758803B2/en not_active Ceased
-
2003
- 2003-02-13 US US10/365,419 patent/US6869976B2/en not_active Expired - Lifetime
-
2004
- 2004-05-05 US US10/838,303 patent/US20040208934A1/en not_active Abandoned
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4946870A (en) | 1986-06-06 | 1990-08-07 | Union Carbide Chemicals And Plastics Company Inc. | Delivery systems for pharmaceutical or therapeutic actives |
| US5407686A (en) * | 1991-11-27 | 1995-04-18 | Sidmak Laboratories, Inc. | Sustained release composition for oral administration of active ingredient |
| US5697922A (en) * | 1992-11-20 | 1997-12-16 | Pfizer Inc. | Delivery device having encapsulated excipients |
| EP0642785A2 (en) | 1993-09-09 | 1995-03-15 | Edward Mendell Co., Inc. | Sustained release heterodisperse hydrogel systems for insoluble drugs |
| US5385887A (en) * | 1993-09-10 | 1995-01-31 | Genetics Institute, Inc. | Formulations for delivery of osteogenic proteins |
| US5783214A (en) * | 1994-06-13 | 1998-07-21 | Buford Biomedical, Inc. | Bio-erodible matrix for the controlled release of medicinals |
Non-Patent Citations (11)
| Title |
|---|
| Bowyer, G.W., et al, Antibiotic Release From Impregnated Pellets And Beads, The Journal of Trauma, vol. 36, No. 3, Mar. 1994, pp. 331-335. |
| Goodell, J. A., et al, Preparation and Release Characteristics of Tobramycin-Impregnated Polymethylmethacrylate Beads, American journal of hospital Pharmacy, vol. 43, Jun. 1986, pp. 1454-1461. |
| Henry, S.L., et al, Antibiotic-Impregnated Beads: A Production Technique, Contemporary Orthopaedics, vol. 19, No. 3, Sep. 1989, pp. 221-226. |
| Henry, S.L., et al, Antibiotic-Impregnated Beads-Part I: Bead Implantation Versus Systemic Therapy, Orthopaedic Review, vol. XX, No. 3, Mar. 1991, pp. 242-247. |
| Mackey, D., et al, Antibiotic Loaded Plaster of Paris Pellets: An In vitro Study of a Possible Method of Local Antibiotic Therapy in Bone Infection, Clinical Orthopedics and Related Research, No. 167, Jul. 1982, pp. 263-268. |
| Marcinko, D.E., Gentamicin-Impregnated PMMA Beads: An Introduction and Review, The Journal of Foot Surgery, vol. 24, No. 2, 1985, pp. 116-121. |
| Popham, G. Jeffrey, et al, Antibiotic-Impregnated Beads-Part II: Factors in Antibiotic Selection, Orthopaedic Review, vol. XX, No. 4, Apr. 1991, pp. 331-337. |
| Schneider, R.K., et al, Use of Antibiotic-Impregnated Polymethyl Methacrylate for Treatment of an Open Radial Fracture in a Horse, Scientific Reports, JAVMA, vol. 207, No. 11, Dec. 1, 1995, pp. 1454-1457. |
| Seligson, David, M.D., Grand Rounds-Antibiotic-Impregnated Beads in Orthopedic infectious Problems, Journal of the Kentucky Medical Association, Jan. 1984, pp. 25-29. |
| Stabile, D.E., et al, Development and Application of Antibiotic-Loaded Bone Cement Beads, Journal of the American Podiatric Medical Association, vol. 80, No. 7, Jul. 1990, pp. 354-359. |
| Torholm, Carsten, et al, Total hip Joint Arthroplasty with Gentamicin-impregnated Cement, Clinical Orthopaedics and Related Research, No. 181, Dec. 1983, pp. 99-106. |
Cited By (75)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060269604A1 (en) * | 1993-11-23 | 2006-11-30 | Purdue Pharma L.P. | Method of treating pain by administering 24 hour oral opioid formulations exhibiting rapid rate of initial rise of plasma drug level |
| US20070237833A1 (en) * | 1993-11-23 | 2007-10-11 | Purdue Pharma L.P. | Method of treating pain by administering 24 hour oral opioid formulations exhibiting rapid rate of initial rise of plasma drug level |
| US20070237832A1 (en) * | 1993-11-23 | 2007-10-11 | Purdue Pharma L.P. | Method of treating pain by administering 24 hour oral opioid formulations exhibiting rapid rate of initial rise of plasma drug level |
| US20100209514A1 (en) * | 1993-11-23 | 2010-08-19 | Sackler Richard S | Method of treating pain by administering 24 hour oral oploid formulations exhibiting rapid rate of initial rise of plasma drug level |
| US20100209351A1 (en) * | 1993-11-23 | 2010-08-19 | Sackler Richard S | Method of treating pain by administering 24 hour oral opioid formulations exhibiting rapid rate of initial rise of plasma drug level |
| US20080031963A1 (en) * | 1993-11-23 | 2008-02-07 | Purdue Pharma L.P. | Method of treating pain by administering 24 hour oral opioid formulations exhibiting rapid rate of initial rise of plasma drug level |
| US20040208934A1 (en) * | 1997-09-22 | 2004-10-21 | Royer Biomedical, Inc. | Inorganic-polymer complexes for the controlled release of compounds including medicinals |
| US20080233165A1 (en) * | 2000-08-07 | 2008-09-25 | Biolok International, Inc. | Time release calcium sulfate and growth factor matrix for bone augmentation |
| US20050008528A1 (en) * | 2000-11-28 | 2005-01-13 | Vasanth Prabhu | Sterile polymerizable systems and kits and methods of their manufacture and use |
| US20080319380A1 (en) * | 2000-11-28 | 2008-12-25 | Vita Special Purpose Corporation | Sterile Polymerizable Systems And Kits And Methods Of Their Manufacture And Use |
| US6800245B1 (en) * | 2000-11-28 | 2004-10-05 | Vita Special Purpose Corporation | Sterile polymerizable systems and kits and methods of their manufacture and use |
| US20100226820A1 (en) * | 2000-11-28 | 2010-09-09 | Vasanth Prabhu | Sterile Polymerizable Systems And Kits And Methods Of Their Manufacture And Use |
| US20060189572A1 (en) * | 2001-01-10 | 2006-08-24 | Showa Yakuhin Kako Co., Ltd. | Composition for local anesthesia |
| US8648056B2 (en) * | 2001-01-10 | 2014-02-11 | Showa Yakuhin Kako Co., Ltd. | Composition for local anesthesia |
| US8329216B2 (en) | 2001-07-06 | 2012-12-11 | Endo Pharmaceuticals Inc. | Oxymorphone controlled release formulations |
| US7276250B2 (en) | 2001-07-06 | 2007-10-02 | Penwest Pharmaceuticals Company | Sustained release formulations of oxymorphone |
| US20030129234A1 (en) * | 2001-07-06 | 2003-07-10 | Penwest Pharmaceuticals Company | Methods of making sustained release formulations of oxymorphone |
| US20070098794A1 (en) * | 2001-07-06 | 2007-05-03 | Haui-Hung Kao | Oxymorphone controlled release formulations |
| US20070098793A1 (en) * | 2001-07-06 | 2007-05-03 | Haui-Hung Kao | Oxymorphone controlled release formulations |
| US20030129230A1 (en) * | 2001-07-06 | 2003-07-10 | Penwest Pharmaceuticals Company | Sustained release formulations of oxymorphone |
| US20070134328A1 (en) * | 2001-07-06 | 2007-06-14 | Endo Pharmaceuticals, Inc. | Oxymorphone controlled release formulations |
| US8309122B2 (en) | 2001-07-06 | 2012-11-13 | Endo Pharmaceuticals Inc. | Oxymorphone controlled release formulations |
| US20070140975A1 (en) * | 2001-09-26 | 2007-06-21 | Penwest Pharmaceuticals Co. | Opioid formulations having reduced potential for abuse |
| US20030091635A1 (en) * | 2001-09-26 | 2003-05-15 | Baichwal Anand R. | Opioid formulations having reduced potential for abuse |
| US20110039764A1 (en) * | 2002-04-08 | 2011-02-17 | Hiroaki Matsuno | Therapeutic composition for bone infectious disease |
| US20060153893A1 (en) * | 2002-04-08 | 2006-07-13 | Denki Kagaku Kogyo Kabushiki Kaisha | Therapeutic composition for bone infectious disease |
| WO2005016341A1 (en) * | 2002-04-11 | 2005-02-24 | Biomedical Research Models Inc. | Extended release analgesic for pain control |
| US20030194438A1 (en) * | 2002-04-11 | 2003-10-16 | Albert Prescott | Extended release analgesic for pain control |
| US6939538B2 (en) * | 2002-04-11 | 2005-09-06 | Biomedical Research Models, Inc. | Extended release analgesic for pain control |
| US9445901B2 (en) | 2003-03-12 | 2016-09-20 | Deger C. Tunc | Prosthesis with sustained release analgesic |
| US20040180072A1 (en) * | 2003-03-12 | 2004-09-16 | Howmedica Osteonics Corp. | Prosthesis with sustained release analgesic |
| US20090246277A9 (en) * | 2003-03-20 | 2009-10-01 | Kristine Fraatz | Controlled release system |
| US20060034926A1 (en) * | 2003-03-20 | 2006-02-16 | Kristine Fraatz | Controlled release system |
| US8231903B2 (en) * | 2003-03-20 | 2012-07-31 | Bayer Animal Health Gmbh | Controlled release system |
| US20060228415A1 (en) * | 2003-08-08 | 2006-10-12 | Biovail Laboratories International S.R.L. | Modified release tablet of bupropion hydrochloride |
| US7537784B2 (en) | 2003-08-08 | 2009-05-26 | Biovail Laboratories International Srl | Modified release tablet of bupropion hydrochloride |
| US9034359B2 (en) | 2003-10-22 | 2015-05-19 | Lidds Ab | Composition comprising biodegradable hydrating ceramics for controlled drug delivery |
| US8124118B2 (en) | 2003-10-22 | 2012-02-28 | Lidds Ab | Composition comprising biodegradable hydrating ceramics for controlled drug delivery |
| US20060204533A1 (en) * | 2005-03-14 | 2006-09-14 | Biotegra, Inc. | Drug Delivery Compositions and Related Methods |
| US8486429B2 (en) | 2005-06-28 | 2013-07-16 | United Phosphorus, Ltd. | Storage stable formulation and a process for its preparation |
| US20060293287A1 (en) * | 2005-06-28 | 2006-12-28 | Jadhav Prakash M | Storage stable formulation and a process for its preparation |
| US9855338B2 (en) | 2005-12-05 | 2018-01-02 | Nitto Denko Corporation | Polyglutamate-amino acid conjugates and methods |
| US20070128118A1 (en) * | 2005-12-05 | 2007-06-07 | Nitto Denko Corporation | Polyglutamate-amino acid conjugates and methods |
| US20070212414A1 (en) * | 2006-03-08 | 2007-09-13 | Penwest Pharmaceuticals Co. | Ethanol-resistant sustained release formulations |
| US8999389B2 (en) | 2006-03-14 | 2015-04-07 | Lidds Ab | Bioresorbable controlled-release composition |
| US20090118215A1 (en) * | 2006-03-14 | 2009-05-07 | Lidds Ab | Bioresorbable Controlled-Release Composition |
| US20080188819A1 (en) * | 2006-07-07 | 2008-08-07 | Kloke Tim M | Beaded Wound Spacer Device |
| US8685421B2 (en) | 2006-07-07 | 2014-04-01 | Surmodics, Inc. | Beaded wound spacer device |
| US8697106B2 (en) | 2006-07-07 | 2014-04-15 | Surmodics, Inc. | Coating composition |
| US20080063681A1 (en) * | 2006-09-11 | 2008-03-13 | Ebi, L.P. | Therapeutic bone replacement material |
| US20080181852A1 (en) * | 2007-01-29 | 2008-07-31 | Nitto Denko Corporation | Multi-functional Drug Carriers |
| US20080253969A1 (en) * | 2007-04-10 | 2008-10-16 | Nitto Denko Corporation | Multi-functional polyglutamate drug carriers |
| US8329199B2 (en) | 2007-05-09 | 2012-12-11 | Nitto Denko Corporation | Compositions that include a hydrophobic compound and a polyamino acid conjugate |
| US20080279778A1 (en) * | 2007-05-09 | 2008-11-13 | Nitto Denko Corporation | Polyglutamate conjugates and polyglutamate-amino acid conjugates having a plurality of drugs |
| US8197828B2 (en) | 2007-05-09 | 2012-06-12 | Nitto Denko Corporation | Compositions that include a hydrophobic compound and a polyamino acid conjugate |
| US20080279782A1 (en) * | 2007-05-09 | 2008-11-13 | Nitto Denko Corporation | Polymers conjugated with platinum drugs |
| US20080279777A1 (en) * | 2007-05-09 | 2008-11-13 | Nitto Denko Corporation | Compositions that include a hydrophobic compound and a polyamino acid conjugate |
| US20090124650A1 (en) * | 2007-06-21 | 2009-05-14 | Endo Pharmaceuticals, Inc. | Method of Treating Pain Utilizing Controlled Release Oxymorphone Pharmaceutical Compositions and Instructions on Effects of Alcohol |
| US20090028922A1 (en) * | 2007-07-24 | 2009-01-29 | Haggard Warren O | Local Delivery Method and Composition |
| US7923021B2 (en) * | 2007-07-24 | 2011-04-12 | University Of Memphis Research Foundation | Local delivery method and composition |
| US20090081610A1 (en) * | 2007-09-14 | 2009-03-26 | Discus Dental, Llc | Dental prophylaxis devices |
| US20090202609A1 (en) * | 2008-01-06 | 2009-08-13 | Keough Steven J | Medical device with coating composition |
| US20090226393A1 (en) * | 2008-03-06 | 2009-09-10 | Nitto Denko Corporation | Polymer paclitaxel conjugates and methods for treating cancer |
| US8343524B2 (en) | 2008-07-31 | 2013-01-01 | Clarke Mosquito Control Products, Inc. | Extended release tablet and method for making and using same |
| US20100029486A1 (en) * | 2008-07-31 | 2010-02-04 | Michael Dean Willis | Extended release tablet and method for making and using same |
| US20110177149A1 (en) * | 2008-08-13 | 2011-07-21 | Messina James J | Broad spectrum animal repellent and method |
| US9693566B2 (en) | 2008-08-13 | 2017-07-04 | James Messina, Sr. | Broad spectrum animal repellent and method |
| US9414603B2 (en) | 2011-11-10 | 2016-08-16 | James J. Messina | Combination animal repellents |
| US9271486B2 (en) | 2011-11-10 | 2016-03-01 | James J. Messina | Combination animal repellents |
| US9572348B2 (en) | 2011-11-10 | 2017-02-21 | James J. Messina | Combination animal repellents |
| US9155671B2 (en) | 2012-10-16 | 2015-10-13 | Surmodics, Inc. | Wound packing device and methods |
| US10080688B2 (en) | 2012-10-16 | 2018-09-25 | Surmodics, Inc. | Wound packing device and method |
| US10201457B2 (en) | 2014-08-01 | 2019-02-12 | Surmodics, Inc. | Wound packing device with nanotextured surface |
| WO2019135420A1 (en) * | 2018-01-03 | 2019-07-11 | 김배용 | Composite using porous material and polymer, and use thereof |
| WO2019170912A1 (en) | 2018-03-09 | 2019-09-12 | Lidds Ab | Bioresorbable controlled-release compositions with sting modulating molecules |
Also Published As
| Publication number | Publication date |
|---|---|
| WO1999015150A1 (en) | 1999-04-01 |
| US6630486B1 (en) | 2003-10-07 |
| CA2303884A1 (en) | 1999-04-01 |
| EP1017364A4 (en) | 2006-06-28 |
| JP5259030B2 (en) | 2013-08-07 |
| JP2001517613A (en) | 2001-10-09 |
| US20040208934A1 (en) | 2004-10-21 |
| EP1017364A1 (en) | 2000-07-12 |
| US6869976B2 (en) | 2005-03-22 |
| US20030170307A1 (en) | 2003-09-11 |
| CA2303884C (en) | 2008-06-17 |
| AU9492598A (en) | 1999-04-12 |
| AU758803B2 (en) | 2003-03-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6391336B1 (en) | Inorganic-polymer complexes for the controlled release of compounds including medicinals | |
| US6497901B1 (en) | Resorbable matrices for delivery of bioactive compounds | |
| RU2271196C2 (en) | Implantable composition (variants) and method for production thereof | |
| ES2294814T3 (en) | DELAYED AND SUSTAINED RELEASE GELS. | |
| US20040109893A1 (en) | Sustained release dosage forms of anesthetics for pain management | |
| PT975333E (en) | Sustained-release alginate gels | |
| WO2005009408A2 (en) | Sustained release dosage forms of anesthetics for pain management | |
| BG64642B1 (en) | Pharmaceutical composition having continuous release of medicamentous forms capsulated in hyluronic acid microparticles | |
| JP2003517886A (en) | Liquid composition of biodegradable block copolymer for drug delivery system and method for producing the same | |
| US20010007673A1 (en) | Sustained-release delayed gels | |
| JPH03163032A (en) | Sustained release pharmaceutical for intracephalic administration | |
| US20200171166A1 (en) | Gel composition and method for producing gel composition | |
| US20050266077A1 (en) | Resorbable matrices with coatings for delivery of bioactive compounds | |
| JP2000509403A (en) | Pharmaceutical compositions for sustained release of insoluble active ingredients | |
| EP1219298A1 (en) | Process for producing protein powder | |
| WO2003011214A2 (en) | Novel methods and formulations for administration of active agents | |
| KR20000051004A (en) | Hyaluronate microparticles for sustained release of a protein drug | |
| AU2005200949B2 (en) | Sustained-Release Delayed Gels | |
| JPH01156912A (en) | Slowly releasing fine particle agent and production thereof | |
| WO1998000161A1 (en) | Fibrin-based systems for the controlled release of medicinals | |
| MXPA99010284A (en) | Sustained-release delayed gels |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: BUFORD BIOMEDICAL, INC., MARYLAND Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:ROYER, GARFIELD P.;REEL/FRAME:008985/0461 Effective date: 19980126 |
|
| AS | Assignment |
Owner name: ROYER BIOMEDICAL, INC., MARYLAND Free format text: CHANGE OF NAME;ASSIGNOR:BUFORD BIOMEDICAL, INC.;REEL/FRAME:012627/0793 Effective date: 20010323 |
|
| REMI | Maintenance fee reminder mailed | ||
| FEPP | Fee payment procedure |
Free format text: PAYOR NUMBER ASSIGNED (ORIGINAL EVENT CODE: ASPN); ENTITY STATUS OF PATENT OWNER: SMALL ENTITY |
|
| FPAY | Fee payment |
Year of fee payment: 4 |
|
| SULP | Surcharge for late payment | ||
| FEPP | Fee payment procedure |
Free format text: PETITION RELATED TO MAINTENANCE FEES FILED (ORIGINAL EVENT CODE: PMFP); ENTITY STATUS OF PATENT OWNER: SMALL ENTITY |
|
| FEPP | Fee payment procedure |
Free format text: PETITION RELATED TO MAINTENANCE FEES GRANTED (ORIGINAL EVENT CODE: PMFG); ENTITY STATUS OF PATENT OWNER: SMALL ENTITY |
|
| REMI | Maintenance fee reminder mailed | ||
| REIN | Reinstatement after maintenance fee payment confirmed | ||
| FP | Lapsed due to failure to pay maintenance fee |
Effective date: 20100521 |
|
| FPAY | Fee payment |
Year of fee payment: 8 |
|
| SULP | Surcharge for late payment | ||
| PRDP | Patent reinstated due to the acceptance of a late maintenance fee |
Effective date: 20100924 |
|
| STCF | Information on status: patent grant |
Free format text: PATENTED CASE |
|
| FPAY | Fee payment |
Year of fee payment: 12 |