USRE43327E1 - Hydrophobic polyamine analogs and methods for their use - Google Patents
Hydrophobic polyamine analogs and methods for their use Download PDFInfo
- Publication number
- USRE43327E1 USRE43327E1 US13/047,297 US200213047297A USRE43327E US RE43327 E1 USRE43327 E1 US RE43327E1 US 200213047297 A US200213047297 A US 200213047297A US RE43327 E USRE43327 E US RE43327E
- Authority
- US
- United States
- Prior art keywords
- polyamine
- aliphatic
- multiring
- unsubstituted
- substituted
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime, expires
Links
- 229920000768 polyamine Polymers 0.000 title claims abstract description 203
- 238000000034 method Methods 0.000 title abstract description 25
- 230000002209 hydrophobic effect Effects 0.000 title abstract description 22
- 239000000203 mixture Substances 0.000 claims abstract description 37
- 150000001875 compounds Chemical class 0.000 claims description 62
- -1 carbalkoxyalkyl Chemical group 0.000 claims description 45
- PFNFFQXMRSDOHW-UHFFFAOYSA-N spermine Chemical compound NCCCNCCCCNCCCN PFNFFQXMRSDOHW-UHFFFAOYSA-N 0.000 claims description 40
- 239000003112 inhibitor Substances 0.000 claims description 35
- ATHGHQPFGPMSJY-UHFFFAOYSA-N spermidine Chemical compound NCCCCNCCCN ATHGHQPFGPMSJY-UHFFFAOYSA-N 0.000 claims description 30
- 125000003118 aryl group Chemical group 0.000 claims description 28
- KIDHWZJUCRJVML-UHFFFAOYSA-N putrescine Chemical group NCCCCN KIDHWZJUCRJVML-UHFFFAOYSA-N 0.000 claims description 28
- 230000015572 biosynthetic process Effects 0.000 claims description 27
- 125000001931 aliphatic group Chemical group 0.000 claims description 25
- 229940063675 spermine Drugs 0.000 claims description 20
- 229940063673 spermidine Drugs 0.000 claims description 16
- 125000003545 alkoxy group Chemical group 0.000 claims description 15
- 239000005700 Putrescine Substances 0.000 claims description 14
- 125000004181 carboxyalkyl group Chemical group 0.000 claims description 13
- 125000000623 heterocyclic group Chemical group 0.000 claims description 13
- 125000002723 alicyclic group Chemical group 0.000 claims description 12
- 229920006395 saturated elastomer Polymers 0.000 claims description 12
- 230000000699 topical effect Effects 0.000 claims description 11
- 125000004391 aryl sulfonyl group Chemical group 0.000 claims description 10
- 125000000008 (C1-C10) alkyl group Chemical group 0.000 claims description 9
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 9
- 239000003981 vehicle Substances 0.000 claims description 8
- 229910052799 carbon Inorganic materials 0.000 claims description 6
- 239000003085 diluting agent Substances 0.000 claims description 6
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 6
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 5
- 238000007917 intracranial administration Methods 0.000 claims description 5
- 229910052739 hydrogen Inorganic materials 0.000 claims description 3
- 238000007918 intramuscular administration Methods 0.000 claims description 3
- 238000007912 intraperitoneal administration Methods 0.000 claims description 3
- 238000001990 intravenous administration Methods 0.000 claims description 3
- 238000007911 parenteral administration Methods 0.000 claims description 3
- 238000007920 subcutaneous administration Methods 0.000 claims description 3
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 claims description 2
- 229910052717 sulfur Inorganic materials 0.000 claims description 2
- 239000011593 sulfur Substances 0.000 claims description 2
- VLCYCQAOQCDTCN-UHFFFAOYSA-N eflornithine Chemical group NCCCC(N)(C(F)F)C(O)=O VLCYCQAOQCDTCN-UHFFFAOYSA-N 0.000 claims 5
- 230000032258 transport Effects 0.000 description 42
- 210000004027 cell Anatomy 0.000 description 39
- 108010078791 Carrier Proteins Proteins 0.000 description 28
- 125000003277 amino group Chemical group 0.000 description 28
- 230000005764 inhibitory process Effects 0.000 description 24
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 24
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 21
- 238000004128 high performance liquid chromatography Methods 0.000 description 21
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 21
- 238000003786 synthesis reaction Methods 0.000 description 20
- 206010028980 Neoplasm Diseases 0.000 description 19
- 238000004458 analytical method Methods 0.000 description 17
- 150000001412 amines Chemical class 0.000 description 15
- 0 [1*]C(=C)NCC(CCC([2*])=C)C(=O)CCCCCCN Chemical compound [1*]C(=C)NCC(CCC([2*])=C)C(=O)CCCCCCN 0.000 description 14
- 150000001413 amino acids Chemical class 0.000 description 14
- 230000010261 cell growth Effects 0.000 description 14
- VLTRZXGMWDSKGL-UHFFFAOYSA-N perchloric acid Chemical compound OCl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-N 0.000 description 14
- 238000006268 reductive amination reaction Methods 0.000 description 14
- 239000000523 sample Substances 0.000 description 14
- 125000001424 substituent group Chemical group 0.000 description 14
- 201000011510 cancer Diseases 0.000 description 13
- 239000002243 precursor Substances 0.000 description 13
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 12
- 125000002252 acyl group Chemical group 0.000 description 12
- 235000001014 amino acid Nutrition 0.000 description 12
- 230000000694 effects Effects 0.000 description 12
- 125000000217 alkyl group Chemical group 0.000 description 11
- 150000001408 amides Chemical class 0.000 description 11
- 239000003153 chemical reaction reagent Substances 0.000 description 11
- 230000014759 maintenance of location Effects 0.000 description 11
- 230000004048 modification Effects 0.000 description 11
- 238000012986 modification Methods 0.000 description 11
- 239000000243 solution Substances 0.000 description 11
- 239000000126 substance Substances 0.000 description 11
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 10
- 238000002360 preparation method Methods 0.000 description 10
- 239000006228 supernatant Substances 0.000 description 10
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 9
- 238000003556 assay Methods 0.000 description 9
- 239000003795 chemical substances by application Substances 0.000 description 9
- 201000010099 disease Diseases 0.000 description 9
- 108090000623 proteins and genes Proteins 0.000 description 9
- 230000004663 cell proliferation Effects 0.000 description 8
- 150000002148 esters Chemical class 0.000 description 8
- 239000007788 liquid Substances 0.000 description 8
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 7
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 7
- 238000006243 chemical reaction Methods 0.000 description 7
- 238000002347 injection Methods 0.000 description 7
- 239000007924 injection Substances 0.000 description 7
- 230000003993 interaction Effects 0.000 description 7
- 239000000047 product Substances 0.000 description 7
- 239000002904 solvent Substances 0.000 description 7
- 210000001519 tissue Anatomy 0.000 description 7
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 6
- 230000001413 cellular effect Effects 0.000 description 6
- 230000012010 growth Effects 0.000 description 6
- 150000002576 ketones Chemical class 0.000 description 6
- 239000008194 pharmaceutical composition Substances 0.000 description 6
- 235000018102 proteins Nutrition 0.000 description 6
- 102000004169 proteins and genes Human genes 0.000 description 6
- 150000003839 salts Chemical class 0.000 description 6
- 239000007787 solid Substances 0.000 description 6
- 229940124530 sulfonamide Drugs 0.000 description 6
- 150000003456 sulfonamides Chemical class 0.000 description 6
- 150000003512 tertiary amines Chemical class 0.000 description 6
- 238000012546 transfer Methods 0.000 description 6
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 5
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical compound C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 5
- 125000004429 atom Chemical group 0.000 description 5
- 125000004432 carbon atom Chemical group C* 0.000 description 5
- 230000009036 growth inhibition Effects 0.000 description 5
- 125000001183 hydrocarbyl group Chemical group 0.000 description 5
- 239000000543 intermediate Substances 0.000 description 5
- 230000000670 limiting effect Effects 0.000 description 5
- 229910052757 nitrogen Inorganic materials 0.000 description 5
- 239000002674 ointment Substances 0.000 description 5
- 125000006239 protecting group Chemical group 0.000 description 5
- 238000000746 purification Methods 0.000 description 5
- 230000000638 stimulation Effects 0.000 description 5
- 239000012808 vapor phase Substances 0.000 description 5
- KBPLFHHGFOOTCA-UHFFFAOYSA-N 1-Octanol Chemical compound CCCCCCCCO KBPLFHHGFOOTCA-UHFFFAOYSA-N 0.000 description 4
- OGNSCSPNOLGXSM-UHFFFAOYSA-N 2,4-diaminobutyric acid Chemical compound NCCC(N)C(O)=O OGNSCSPNOLGXSM-UHFFFAOYSA-N 0.000 description 4
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 4
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 4
- 239000004472 Lysine Substances 0.000 description 4
- 229940121798 Polyamine transport inhibitor Drugs 0.000 description 4
- 150000001263 acyl chlorides Chemical class 0.000 description 4
- 230000010933 acylation Effects 0.000 description 4
- 238000005917 acylation reaction Methods 0.000 description 4
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 4
- 150000001299 aldehydes Chemical class 0.000 description 4
- 230000001580 bacterial effect Effects 0.000 description 4
- 238000004364 calculation method Methods 0.000 description 4
- 150000003857 carboxamides Chemical class 0.000 description 4
- ZPUCINDJVBIVPJ-LJISPDSOSA-N cocaine Chemical compound O([C@H]1C[C@@H]2CC[C@@H](N2C)[C@H]1C(=O)OC)C(=O)C1=CC=CC=C1 ZPUCINDJVBIVPJ-LJISPDSOSA-N 0.000 description 4
- 238000010511 deprotection reaction Methods 0.000 description 4
- 239000003814 drug Substances 0.000 description 4
- 230000002538 fungal effect Effects 0.000 description 4
- 208000016354 hearing loss disease Diseases 0.000 description 4
- 235000018977 lysine Nutrition 0.000 description 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 4
- MXWHMTNPTTVWDM-NXOFHUPFSA-N mitoguazone Chemical compound NC(N)=N\N=C(/C)\C=N\N=C(N)N MXWHMTNPTTVWDM-NXOFHUPFSA-N 0.000 description 4
- 125000004433 nitrogen atom Chemical group N* 0.000 description 4
- 230000000144 pharmacologic effect Effects 0.000 description 4
- 230000003389 potentiating effect Effects 0.000 description 4
- 229940002612 prodrug Drugs 0.000 description 4
- 239000000651 prodrug Substances 0.000 description 4
- 230000009885 systemic effect Effects 0.000 description 4
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 3
- 206010011878 Deafness Diseases 0.000 description 3
- 241000588724 Escherichia coli Species 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical compound C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 description 3
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 3
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 3
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical class [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 3
- XTNRHIZWLNLONB-OAHLLOKOSA-N [H]N([H])CCCC[C@]([H])(C(=O)N([H])CCCN([H])CCCCN([H])CCCN([H])[H])N([H])[H] Chemical compound [H]N([H])CCCC[C@]([H])(C(=O)N([H])CCCN([H])CCCCN([H])CCCN([H])[H])N([H])[H] XTNRHIZWLNLONB-OAHLLOKOSA-N 0.000 description 3
- 125000002947 alkylene group Chemical group 0.000 description 3
- 239000003963 antioxidant agent Substances 0.000 description 3
- 235000006708 antioxidants Nutrition 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- 125000001584 benzyloxycarbonyl group Chemical group C(=O)(OCC1=CC=CC=C1)* 0.000 description 3
- 150000001728 carbonyl compounds Chemical class 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 238000002512 chemotherapy Methods 0.000 description 3
- 239000006071 cream Substances 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- 239000002532 enzyme inhibitor Substances 0.000 description 3
- 210000003527 eukaryotic cell Anatomy 0.000 description 3
- 210000002950 fibroblast Anatomy 0.000 description 3
- 125000005456 glyceride group Chemical group 0.000 description 3
- 230000010370 hearing loss Effects 0.000 description 3
- 231100000888 hearing loss Toxicity 0.000 description 3
- PWSKHLMYTZNYKO-UHFFFAOYSA-N heptane-1,7-diamine Chemical compound NCCCCCCCN PWSKHLMYTZNYKO-UHFFFAOYSA-N 0.000 description 3
- 230000036571 hydration Effects 0.000 description 3
- 238000006703 hydration reaction Methods 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 230000003834 intracellular effect Effects 0.000 description 3
- 210000004962 mammalian cell Anatomy 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 210000004379 membrane Anatomy 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 230000004060 metabolic process Effects 0.000 description 3
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 description 3
- 230000037361 pathway Effects 0.000 description 3
- 239000008188 pellet Substances 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 230000035755 proliferation Effects 0.000 description 3
- 230000002285 radioactive effect Effects 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 210000002966 serum Anatomy 0.000 description 3
- 239000007858 starting material Substances 0.000 description 3
- 238000006467 substitution reaction Methods 0.000 description 3
- YBBRCQOCSYXUOC-UHFFFAOYSA-N sulfuryl dichloride Chemical compound ClS(Cl)(=O)=O YBBRCQOCSYXUOC-UHFFFAOYSA-N 0.000 description 3
- 230000001629 suppression Effects 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- YWJCZGDVJQLZET-GGHFOJMISA-N (2r,3s,4r,5r)-2-[[[(z)-4-aminobut-2-enyl]-methylamino]methyl]-5-(6-aminopurin-9-yl)oxolane-3,4-diol Chemical compound O[C@@H]1[C@H](O)[C@@H](CN(C\C=C/CN)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 YWJCZGDVJQLZET-GGHFOJMISA-N 0.000 description 2
- GVJHHUAWPYXKBD-UHFFFAOYSA-N (±)-α-Tocopherol Chemical compound OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- IZHVBANLECCAGF-UHFFFAOYSA-N 2-hydroxy-3-(octadecanoyloxy)propyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)COC(=O)CCCCCCCCCCCCCCCCC IZHVBANLECCAGF-UHFFFAOYSA-N 0.000 description 2
- BWMDMTSNSXYYSP-UHFFFAOYSA-N 2-propylguanidine Chemical compound CCCNC(N)=N BWMDMTSNSXYYSP-UHFFFAOYSA-N 0.000 description 2
- PECYZEOJVXMISF-REOHCLBHSA-N 3-amino-L-alanine Chemical compound [NH3+]C[C@H](N)C([O-])=O PECYZEOJVXMISF-REOHCLBHSA-N 0.000 description 2
- WUUGFSXJNOTRMR-IOSLPCCCSA-N 5'-S-methyl-5'-thioadenosine Chemical compound O[C@@H]1[C@H](O)[C@@H](CSC)O[C@H]1N1C2=NC=NC(N)=C2N=C1 WUUGFSXJNOTRMR-IOSLPCCCSA-N 0.000 description 2
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical compound N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 2
- UJOBWOGCFQCDNV-UHFFFAOYSA-N 9H-carbazole Chemical compound C1=CC=C2C3=CC=CC=C3NC2=C1 UJOBWOGCFQCDNV-UHFFFAOYSA-N 0.000 description 2
- DLFVBJFMPXGRIB-UHFFFAOYSA-N Acetamide Chemical compound CC(N)=O DLFVBJFMPXGRIB-UHFFFAOYSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 2
- 208000000230 African Trypanosomiasis Diseases 0.000 description 2
- 208000024827 Alzheimer disease Diseases 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 2
- 108020004491 Antisense DNA Proteins 0.000 description 2
- 208000019901 Anxiety disease Diseases 0.000 description 2
- 239000004475 Arginine Substances 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Natural products OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 208000023275 Autoimmune disease Diseases 0.000 description 2
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 2
- IAODIKMMKVBCBX-VPPMUXOBSA-N CC/C=C/C/C=C/CC.CC/C=C/CC.CC/C=C\C/C=C\CC.CC/C=C\CC Chemical compound CC/C=C/C/C=C/CC.CC/C=C/CC.CC/C=C\C/C=C\CC.CC/C=C\CC IAODIKMMKVBCBX-VPPMUXOBSA-N 0.000 description 2
- ZMNPGKYVBVQYEV-UHFFFAOYSA-N CCC(CC)C(=O)CCCCCCN Chemical compound CCC(CC)C(=O)CCCCCCN ZMNPGKYVBVQYEV-UHFFFAOYSA-N 0.000 description 2
- 239000004215 Carbon black (E152) Substances 0.000 description 2
- 102000014914 Carrier Proteins Human genes 0.000 description 2
- 208000022497 Cocaine-Related disease Diseases 0.000 description 2
- 208000011231 Crohn disease Diseases 0.000 description 2
- XPDXVDYUQZHFPV-UHFFFAOYSA-N Dansyl Chloride Chemical compound C1=CC=C2C(N(C)C)=CC=CC2=C1S(Cl)(=O)=O XPDXVDYUQZHFPV-UHFFFAOYSA-N 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 102000009024 Epidermal Growth Factor Human genes 0.000 description 2
- 101800003838 Epidermal growth factor Proteins 0.000 description 2
- YLQBMQCUIZJEEH-UHFFFAOYSA-N Furan Chemical compound C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 2
- 208000010412 Glaucoma Diseases 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 201000002980 Hyperparathyroidism Diseases 0.000 description 2
- 206010020772 Hypertension Diseases 0.000 description 2
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 2
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 2
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 2
- FQPGMQABJNQLLF-VKHMYHEASA-N L-canaline Chemical compound NOCC[C@H](N)C(O)=O FQPGMQABJNQLLF-VKHMYHEASA-N 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 102100021079 Ornithine decarboxylase Human genes 0.000 description 2
- 208000001132 Osteoporosis Diseases 0.000 description 2
- 208000030852 Parasitic disease Diseases 0.000 description 2
- 208000008469 Peptic Ulcer Diseases 0.000 description 2
- 201000004681 Psoriasis Diseases 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical compound C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 description 2
- 238000004617 QSAR study Methods 0.000 description 2
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 2
- 208000006011 Stroke Diseases 0.000 description 2
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- ISRBWDUPBIXQEA-OAQYLSRUSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)C1=CC=CC=C1)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)C1=CC=CC=C1)N([H])[H] ISRBWDUPBIXQEA-OAQYLSRUSA-N 0.000 description 2
- CRCYSNDCJMMZFG-HXUWFJFHSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CCC(C)C)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CCC(C)C)N([H])[H] CRCYSNDCJMMZFG-HXUWFJFHSA-N 0.000 description 2
- USNCWPSRPOWEBG-PMERELPUSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@]([H])(CCCCN([H])C(=O)CCCCCCCCCCCCCCC)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@]([H])(CCCCN([H])C(=O)CCCCCCCCCCCCCCC)N([H])[H] USNCWPSRPOWEBG-PMERELPUSA-N 0.000 description 2
- IMBWYJPYJPAETG-LBAQZLPGSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@]([H])(CCCCN([H])C(C)CC(C)C)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@]([H])(CCCCN([H])C(C)CC(C)C)N([H])[H] IMBWYJPYJPAETG-LBAQZLPGSA-N 0.000 description 2
- 229960000583 acetic acid Drugs 0.000 description 2
- 238000010306 acid treatment Methods 0.000 description 2
- 229960000643 adenine Drugs 0.000 description 2
- 229960005305 adenosine Drugs 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 230000001093 anti-cancer Effects 0.000 description 2
- 239000002246 antineoplastic agent Substances 0.000 description 2
- 239000003816 antisense DNA Substances 0.000 description 2
- 230000036506 anxiety Effects 0.000 description 2
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 2
- 235000009697 arginine Nutrition 0.000 description 2
- 208000006673 asthma Diseases 0.000 description 2
- 206010003668 atrial tachycardia Diseases 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 102000023732 binding proteins Human genes 0.000 description 2
- 108091008324 binding proteins Proteins 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 125000001246 bromo group Chemical group Br* 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- HQABUPZFAYXKJW-UHFFFAOYSA-N butan-1-amine Chemical compound CCCCN HQABUPZFAYXKJW-UHFFFAOYSA-N 0.000 description 2
- VHRGRCVQAFMJIZ-UHFFFAOYSA-N cadaverine Chemical compound NCCCCCN VHRGRCVQAFMJIZ-UHFFFAOYSA-N 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 150000004657 carbamic acid derivatives Chemical class 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 238000009903 catalytic hydrogenation reaction Methods 0.000 description 2
- 238000004113 cell culture Methods 0.000 description 2
- 238000012512 characterization method Methods 0.000 description 2
- 239000013043 chemical agent Substances 0.000 description 2
- 125000001309 chloro group Chemical group Cl* 0.000 description 2
- 238000004587 chromatography analysis Methods 0.000 description 2
- 229960003920 cocaine Drugs 0.000 description 2
- 201000006145 cocaine dependence Diseases 0.000 description 2
- 230000002596 correlated effect Effects 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 235000019788 craving Nutrition 0.000 description 2
- 235000018417 cysteine Nutrition 0.000 description 2
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 2
- 125000001295 dansyl group Chemical group [H]C1=C([H])C(N(C([H])([H])[H])C([H])([H])[H])=C2C([H])=C([H])C([H])=C(C2=C1[H])S(*)(=O)=O 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 238000001212 derivatisation Methods 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 206010013663 drug dependence Diseases 0.000 description 2
- 238000010828 elution Methods 0.000 description 2
- 229940088598 enzyme Drugs 0.000 description 2
- 229940116977 epidermal growth factor Drugs 0.000 description 2
- 206010015037 epilepsy Diseases 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- 125000001153 fluoro group Chemical group F* 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 239000012634 fragment Substances 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 239000012362 glacial acetic acid Substances 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 230000003779 hair growth Effects 0.000 description 2
- 125000005843 halogen group Chemical group 0.000 description 2
- 208000029080 human African trypanosomiasis Diseases 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 150000002430 hydrocarbons Chemical class 0.000 description 2
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 2
- 125000001165 hydrophobic group Chemical group 0.000 description 2
- 230000000917 hyperalgesic effect Effects 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 230000008991 intestinal motility Effects 0.000 description 2
- 125000002346 iodo group Chemical group I* 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- AWJUIBRHMBBTKR-UHFFFAOYSA-N isoquinoline Chemical compound C1=NC=CC2=CC=CC=C21 AWJUIBRHMBBTKR-UHFFFAOYSA-N 0.000 description 2
- 230000004807 localization Effects 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 2
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 2
- 229960003539 mitoguazone Drugs 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 239000001788 mono and diglycerides of fatty acids Substances 0.000 description 2
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical class CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 2
- 230000004770 neurodegeneration Effects 0.000 description 2
- 208000015122 neurodegenerative disease Diseases 0.000 description 2
- 150000002825 nitriles Chemical class 0.000 description 2
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 2
- 231100000252 nontoxic Toxicity 0.000 description 2
- 230000003000 nontoxic effect Effects 0.000 description 2
- 108020004707 nucleic acids Proteins 0.000 description 2
- 150000007523 nucleic acids Chemical class 0.000 description 2
- 102000039446 nucleic acids Human genes 0.000 description 2
- VSZFWDPIWSPZON-UHFFFAOYSA-N o-(3-aminopropyl)hydroxylamine Chemical compound NCCCON VSZFWDPIWSPZON-UHFFFAOYSA-N 0.000 description 2
- 125000000636 p-nitrophenyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)[N+]([O-])=O 0.000 description 2
- 208000011906 peptic ulcer disease Diseases 0.000 description 2
- 238000005897 peptide coupling reaction Methods 0.000 description 2
- 239000012071 phase Substances 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- 229920002523 polyethylene Glycol 1000 Polymers 0.000 description 2
- 229940113116 polyethylene glycol 1000 Drugs 0.000 description 2
- 239000011148 porous material Substances 0.000 description 2
- 230000002787 reinforcement Effects 0.000 description 2
- 208000037803 restenosis Diseases 0.000 description 2
- 206010039073 rheumatoid arthritis Diseases 0.000 description 2
- 150000003335 secondary amines Chemical class 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 238000010898 silica gel chromatography Methods 0.000 description 2
- 201000002612 sleeping sickness Diseases 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 2
- 238000004809 thin layer chromatography Methods 0.000 description 2
- 229930192474 thiophene Natural products 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 238000002054 transplantation Methods 0.000 description 2
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 2
- 230000024883 vasodilation Effects 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- CYPGNVSXMAUSJY-CXUHLZMHSA-N (1e)-1-(diaminomethylidenehydrazinylidene)-2,3-dihydroindene-4-carboximidamide Chemical compound C1=CC=C(C(N)=N)C2=C1C(=N/N=C(N)N)/CC2 CYPGNVSXMAUSJY-CXUHLZMHSA-N 0.000 description 1
- UADDLKHYLGJYJV-IDTAVKCVSA-N (2R,3R,4S,5S)-2-(6-aminopurin-9-yl)-5-(butylaminosulfanylmethyl)oxolane-3,4-diol Chemical compound CCCCNSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 UADDLKHYLGJYJV-IDTAVKCVSA-N 0.000 description 1
- ZLGKOOYLCJDFHS-WOUKDFQISA-N (2r,3r,4r,5r)-5-(6-aminopurin-9-yl)-4-ethylsulfanyl-2-(hydroxymethyl)oxolan-3-ol Chemical compound CCS[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC=NC(N)=C2N=C1 ZLGKOOYLCJDFHS-WOUKDFQISA-N 0.000 description 1
- XJZIRTQHHWOZNA-ZRURSIFKSA-N (2r,3s,4r,5r)-2-(2-amino-4-hydroxybutyl)-5-(6-aminopurin-9-yl)oxolane-3,4-diol Chemical compound O[C@@H]1[C@H](O)[C@@H](CC(CCO)N)O[C@H]1N1C2=NC=NC(N)=C2N=C1 XJZIRTQHHWOZNA-ZRURSIFKSA-N 0.000 description 1
- GVSGUDGNTHCZHI-KQYNXXCUSA-N (2r,3s,4r,5r)-2-(aminomethyl)-5-(6-aminopurin-9-yl)oxolane-3,4-diol Chemical compound O[C@@H]1[C@H](O)[C@@H](CN)O[C@H]1N1C2=NC=NC(N)=C2N=C1 GVSGUDGNTHCZHI-KQYNXXCUSA-N 0.000 description 1
- MSHFOWYMHIZSJV-QYVSTXNMSA-N (2r,3s,4r,5r)-2-[(3-aminopropylamino)methyl]-5-(6-aminopurin-9-yl)oxolane-3,4-diol Chemical compound O[C@@H]1[C@H](O)[C@@H](CNCCCN)O[C@H]1N1C2=NC=NC(N)=C2N=C1 MSHFOWYMHIZSJV-QYVSTXNMSA-N 0.000 description 1
- VRAHREWXGGWUKL-IVZWLZJFSA-N (2r,3s,5r)-2-[[2-aminooxyethyl(methyl)amino]oxymethyl]-5-(6-aminopurin-9-yl)oxolan-3-ol Chemical compound C1[C@H](O)[C@@H](CON(CCON)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 VRAHREWXGGWUKL-IVZWLZJFSA-N 0.000 description 1
- PLFPTPGURSEUHE-DKWTVANSSA-N (2s)-2-aminopropanoic acid;methane Chemical compound C.C[C@H](N)C(O)=O PLFPTPGURSEUHE-DKWTVANSSA-N 0.000 description 1
- FURSDADPUPTMLG-HNQUOIGGSA-N (e)-but-1-ene-1,4-diamine Chemical compound NCC\C=C\N FURSDADPUPTMLG-HNQUOIGGSA-N 0.000 description 1
- UKAUYVFTDYCKQA-UHFFFAOYSA-N -2-Amino-4-hydroxybutanoic acid Natural products OC(=O)C(N)CCO UKAUYVFTDYCKQA-UHFFFAOYSA-N 0.000 description 1
- UYBWIEGTWASWSR-UHFFFAOYSA-N 1,3-diaminopropan-2-ol Chemical compound NCC(O)CN UYBWIEGTWASWSR-UHFFFAOYSA-N 0.000 description 1
- CBCKQZAAMUWICA-UHFFFAOYSA-N 1,4-phenylenediamine Chemical compound NC1=CC=C(N)C=C1 CBCKQZAAMUWICA-UHFFFAOYSA-N 0.000 description 1
- 238000001644 13C nuclear magnetic resonance spectroscopy Methods 0.000 description 1
- 238000005160 1H NMR spectroscopy Methods 0.000 description 1
- MPBKAOHKPAKJDA-VCHYOVAHSA-N 2-(3-aminopropyl)-1-[(e)-(2,2-difluoro-1-phenylethylidene)amino]guanidine Chemical compound NCCCN=C(N)N\N=C(\C(F)F)C1=CC=CC=C1 MPBKAOHKPAKJDA-VCHYOVAHSA-N 0.000 description 1
- DDNVYHHARMNUBE-HMMKTVFPSA-N 2-[(e)-[(1e)-1-(diaminomethylidenehydrazinylidene)butan-2-ylidene]amino]guanidine Chemical compound NC(N)=N\N=C(/CC)\C=N\N=C(N)N DDNVYHHARMNUBE-HMMKTVFPSA-N 0.000 description 1
- QCPPROTWPVZXKL-HMMKTVFPSA-N 2-[(e)-[(3e)-3-(diaminomethylidenehydrazinylidene)butan-2-ylidene]amino]guanidine Chemical compound NC(N)=N\N=C(/C)\C(\C)=N\N=C(N)N QCPPROTWPVZXKL-HMMKTVFPSA-N 0.000 description 1
- HXWLLFLQUZOBFF-FUJGBLOQSA-N 2-[(z)-[(4e)-4-(diaminomethylidenehydrazinylidene)hexan-3-ylidene]amino]guanidine Chemical compound NC(N)=N/N=C(/CC)\C(\CC)=N\N=C(N)N HXWLLFLQUZOBFF-FUJGBLOQSA-N 0.000 description 1
- OWULHUHLYMMBTL-UHFFFAOYSA-N 2-[1-(diaminomethylidenehydrazinylidene)hexan-2-ylideneamino]guanidine Chemical compound CCCCC(=NN=C(N)N)C=NN=C(N)N OWULHUHLYMMBTL-UHFFFAOYSA-N 0.000 description 1
- SRKQUQMCVHDJDD-WCEDTRLTSA-N 2-cyclohexyl-1-[(e)-[(1e)-1-[(n'-cyclohexylcarbamimidoyl)hydrazinylidene]propan-2-ylidene]amino]guanidine Chemical compound C1CCCCC1N=C(N)N\N=C(/C)\C=N\NC(N)=NC1CCCCC1 SRKQUQMCVHDJDD-WCEDTRLTSA-N 0.000 description 1
- GGQJPAQXCYUEKB-UHFFFAOYSA-N 2-methylbutane-1,4-diamine Chemical compound NCC(C)CCN GGQJPAQXCYUEKB-UHFFFAOYSA-N 0.000 description 1
- 125000005916 2-methylpentyl group Chemical group 0.000 description 1
- JVQIKJMSUIMUDI-UHFFFAOYSA-N 3-pyrroline Chemical compound C1NCC=C1 JVQIKJMSUIMUDI-UHFFFAOYSA-N 0.000 description 1
- ZXVONLUNISGICL-UHFFFAOYSA-N 4,6-dinitro-o-cresol Chemical group CC1=CC([N+]([O-])=O)=CC([N+]([O-])=O)=C1O ZXVONLUNISGICL-UHFFFAOYSA-N 0.000 description 1
- DVNIRPKXKMIDQU-WOUKDFQISA-N 5'-(dimethylsulfonio)-5'-deoxyadenosine Chemical compound O[C@@H]1[C@H](O)[C@@H](C[S+](C)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 DVNIRPKXKMIDQU-WOUKDFQISA-N 0.000 description 1
- RMAOLICYOBWFLA-VCZNENMGSA-N 5'-{[2-(aminooxy)ethyl](methyl)sulfonio}-5'-deoxyadenosine Chemical compound O[C@@H]1[C@H](O)[C@@H](C[S+](CCON)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 RMAOLICYOBWFLA-VCZNENMGSA-N 0.000 description 1
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 1
- 102000005758 Adenosylmethionine decarboxylase Human genes 0.000 description 1
- 108010070753 Adenosylmethionine decarboxylase Proteins 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 102000015790 Asparaginase Human genes 0.000 description 1
- 108010024976 Asparaginase Proteins 0.000 description 1
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical compound C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- 101000694614 Bos taurus Primary amine oxidase, liver isozyme Proteins 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 1
- 102000004031 Carboxy-Lyases Human genes 0.000 description 1
- 108090000489 Carboxy-Lyases Proteins 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- XFXPMWWXUTWYJX-UHFFFAOYSA-N Cyanide Chemical compound N#[C-] XFXPMWWXUTWYJX-UHFFFAOYSA-N 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 1
- OGNSCSPNOLGXSM-GSVOUGTGSA-N D-2,4-diaminobutyric acid Chemical compound NCC[C@@H](N)C(O)=O OGNSCSPNOLGXSM-GSVOUGTGSA-N 0.000 description 1
- ZZZCUOFIHGPKAK-UHFFFAOYSA-N D-erythro-ascorbic acid Natural products OCC1OC(=O)C(O)=C1O ZZZCUOFIHGPKAK-UHFFFAOYSA-N 0.000 description 1
- KDXKERNSBIXSRK-RXMQYKEDSA-N D-lysine Chemical compound NCCCC[C@@H](N)C(O)=O KDXKERNSBIXSRK-RXMQYKEDSA-N 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 229910004373 HOAc Inorganic materials 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- AVXURJPOCDRRFD-UHFFFAOYSA-N Hydroxylamine Chemical compound ON AVXURJPOCDRRFD-UHFFFAOYSA-N 0.000 description 1
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 102000000588 Interleukin-2 Human genes 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 1
- OGNSCSPNOLGXSM-VKHMYHEASA-N L-2,4-diaminobutyric acid Chemical compound NCC[C@H](N)C(O)=O OGNSCSPNOLGXSM-VKHMYHEASA-N 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-N L-arginine Chemical compound OC(=O)[C@@H](N)CCCN=C(N)N ODKSFYDXXFIFQN-BYPYZUCNSA-N 0.000 description 1
- 235000014852 L-arginine Nutrition 0.000 description 1
- 229930064664 L-arginine Natural products 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- 229930182816 L-glutamine Natural products 0.000 description 1
- UKAUYVFTDYCKQA-VKHMYHEASA-N L-homoserine Chemical compound OC(=O)[C@@H](N)CCO UKAUYVFTDYCKQA-VKHMYHEASA-N 0.000 description 1
- 125000001176 L-lysyl group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C([H])([H])C([H])([H])C([H])([H])C(N([H])[H])([H])[H] 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-L L-tartrate(2-) Chemical compound [O-]C(=O)[C@H](O)[C@@H](O)C([O-])=O FEWJPZIEWOKRBE-JCYAYHJZSA-L 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- WSMYVTOQOOLQHP-UHFFFAOYSA-N Malondialdehyde Chemical compound O=CCC=O WSMYVTOQOOLQHP-UHFFFAOYSA-N 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- BKAYIFDRRZZKNF-VIFPVBQESA-N N-acetylcarnosine Chemical compound CC(=O)NCCC(=O)N[C@H](C(O)=O)CC1=CN=CN1 BKAYIFDRRZZKNF-VIFPVBQESA-N 0.000 description 1
- HNCPYRUADINFLX-YCSZXMBFSA-N N-amino-N'-[[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl]methanimidamide sulfuric acid Chemical compound S(=O)(=O)(O)O.NN=CNC[C@@H]1[C@H]([C@H]([C@@H](O1)N1C=NC=2C(N)=NC=NC12)O)O HNCPYRUADINFLX-YCSZXMBFSA-N 0.000 description 1
- 229930193140 Neomycin Natural products 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- 108700005126 Ornithine decarboxylases Proteins 0.000 description 1
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 1
- ZCQWOFVYLHDMMC-UHFFFAOYSA-N Oxazole Chemical compound C1=COC=N1 ZCQWOFVYLHDMMC-UHFFFAOYSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 108010001441 Phosphopeptides Proteins 0.000 description 1
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 description 1
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 229930003268 Vitamin C Natural products 0.000 description 1
- 229930003427 Vitamin E Natural products 0.000 description 1
- DQQJIRKAXYQBEM-FSWKTMCQSA-N [H]C(NCCCC[C@]([H])(N)C(=O)NCCCNCCCCNCCCN)C12CC3CC(CC(C3)C1)C2 Chemical compound [H]C(NCCCC[C@]([H])(N)C(=O)NCCCNCCCCNCCCN)C12CC3CC(CC(C3)C1)C2 DQQJIRKAXYQBEM-FSWKTMCQSA-N 0.000 description 1
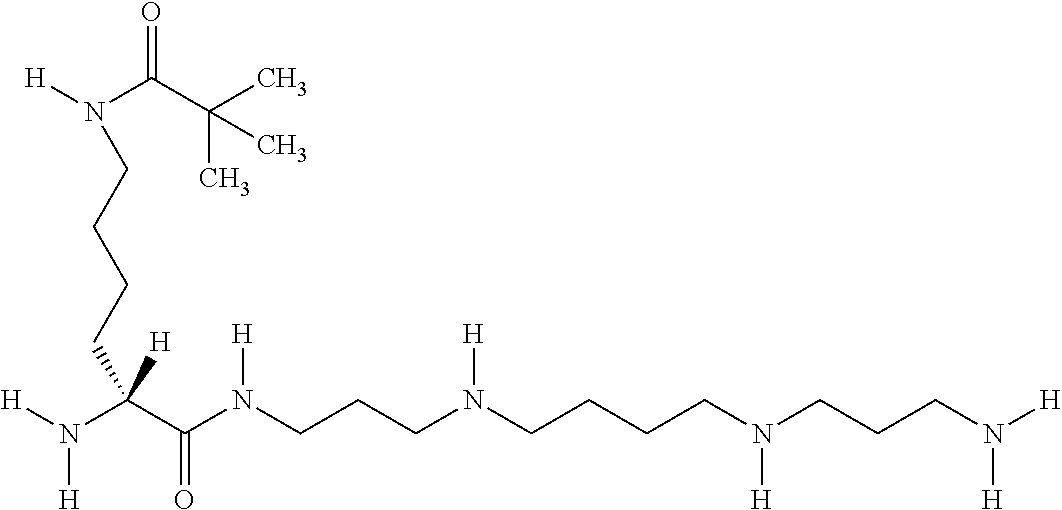
- LDPULLBCVDFNNG-IBGZPJMESA-N [H]N(CCCC[C@]([H])(N)C(=O)NCCCNCCCCNCCCN)CC(C)(C)C Chemical compound [H]N(CCCC[C@]([H])(N)C(=O)NCCCNCCCCNCCCN)CC(C)(C)C LDPULLBCVDFNNG-IBGZPJMESA-N 0.000 description 1
- SSZJNHFPCNJSKQ-HHHXNRCGSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@H](CCCCN([H])C(=O)C(C1=CC=CC=C1)C1=CC=CC=C1)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@H](CCCCN([H])C(=O)C(C1=CC=CC=C1)C1=CC=CC=C1)N([H])[H] SSZJNHFPCNJSKQ-HHHXNRCGSA-N 0.000 description 1
- MPZHJDXPCWRCQW-GOSISDBHSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)C(C)(C)C)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)C(C)(C)C)N([H])[H] MPZHJDXPCWRCQW-GOSISDBHSA-N 0.000 description 1
- MFGVTRSYKHHODA-GOSISDBHSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)C(C)C)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)C(C)C)N([H])[H] MFGVTRSYKHHODA-GOSISDBHSA-N 0.000 description 1
- RDPXHCGNIAVEIZ-ICSVULDUSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)C12CC3CC(CC(C3)C1)C2)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)C12CC3CC(CC(C3)C1)C2)N([H])[H] RDPXHCGNIAVEIZ-ICSVULDUSA-N 0.000 description 1
- ZXAJBAHPOBNRDS-HHHXNRCGSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)C1=CC=C(C2=CC=CC=C2)C=C1)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)C1=CC=C(C2=CC=CC=C2)C=C1)N([H])[H] ZXAJBAHPOBNRDS-HHHXNRCGSA-N 0.000 description 1
- WOZMLFOEMOJRQS-GOSISDBHSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)C1=CC=CO1)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)C1=CC=CO1)N([H])[H] WOZMLFOEMOJRQS-GOSISDBHSA-N 0.000 description 1
- ACQCOCCUSOOVHZ-LJQANCHMSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CC(C)(C)C)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CC(C)(C)C)N([H])[H] ACQCOCCUSOOVHZ-LJQANCHMSA-N 0.000 description 1
- DFJFTIGBTJENCQ-LYUBNGADSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CC1CC2CCC1C2)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CC1CC2CCC1C2)N([H])[H] DFJFTIGBTJENCQ-LYUBNGADSA-N 0.000 description 1
- TWGASPIGBCCTAG-HXUWFJFHSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CCCCC)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CCCCC)N([H])[H] TWGASPIGBCCTAG-HXUWFJFHSA-N 0.000 description 1
- HBEMIKVWJOGSSP-OAQYLSRUSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CCCCCC)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CCCCCC)N([H])[H] HBEMIKVWJOGSSP-OAQYLSRUSA-N 0.000 description 1
- XFWOUFHAYKZQJF-JOCHJYFZSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CCCCCCC)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CCCCCCC)N([H])[H] XFWOUFHAYKZQJF-JOCHJYFZSA-N 0.000 description 1
- TTYPRZRZVCCHQV-HSZRJFAPSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CCCCCCCC)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CCCCCCCC)N([H])[H] TTYPRZRZVCCHQV-HSZRJFAPSA-N 0.000 description 1
- USNCWPSRPOWEBG-SSEXGKCCSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CCCCCCCCCCCCCCC)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CCCCCCCCCCCCCCC)N([H])[H] USNCWPSRPOWEBG-SSEXGKCCSA-N 0.000 description 1
- OBHYCSNEGUPWJY-XDZVQPMWSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CO[C@@H]1C[C@H](C)CC[C@H]1C(C)C)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C(=O)CO[C@@H]1C[C@H](C)CC[C@H]1C(C)C)N([H])[H] OBHYCSNEGUPWJY-XDZVQPMWSA-N 0.000 description 1
- YQJVFRAWOXIQPE-YFPHNYGOSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C1C[C@H](C)CC[C@H]1C(C)C)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])C1C[C@H](C)CC[C@H]1C(C)C)N([H])[H] YQJVFRAWOXIQPE-YFPHNYGOSA-N 0.000 description 1
- LDPULLBCVDFNNG-LJQANCHMSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CC(C)(C)C)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CC(C)(C)C)N([H])[H] LDPULLBCVDFNNG-LJQANCHMSA-N 0.000 description 1
- GIPISTCBAXKQKF-LJQANCHMSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CC(C)C)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CC(C)C)N([H])[H] GIPISTCBAXKQKF-LJQANCHMSA-N 0.000 description 1
- PUZKQECCXLFRHQ-MUUNZHRXSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CC1=CC=C(C2=CC=CC=C2)C=C1)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CC1=CC=C(C2=CC=CC=C2)C=C1)N([H])[H] PUZKQECCXLFRHQ-MUUNZHRXSA-N 0.000 description 1
- IOWUZUKAROQENB-LYUBNGADSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CC1CC2C=CC1C2)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CC1CC2C=CC1C2)N([H])[H] IOWUZUKAROQENB-LYUBNGADSA-N 0.000 description 1
- NPDHPGBHHFQQNE-JOCHJYFZSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CC1CCCCC1)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CC1CCCCC1)N([H])[H] NPDHPGBHHFQQNE-JOCHJYFZSA-N 0.000 description 1
- HOPFTRVMDDRJAW-HXUWFJFHSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CCC(C)(C)C)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CCC(C)(C)C)N([H])[H] HOPFTRVMDDRJAW-HXUWFJFHSA-N 0.000 description 1
- JWXCGHXMCJNSIE-LJQANCHMSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CCCC)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CCCC)N([H])[H] JWXCGHXMCJNSIE-LJQANCHMSA-N 0.000 description 1
- RKYMYGUEVJULQT-OAQYLSRUSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CCCCCC)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CCCCCC)N([H])[H] RKYMYGUEVJULQT-OAQYLSRUSA-N 0.000 description 1
- RMKCNMCCAWMSGM-JOCHJYFZSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CCCCCCC)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CCCCCCC)N([H])[H] RMKCNMCCAWMSGM-JOCHJYFZSA-N 0.000 description 1
- SRTXRGKNBOPWCA-LOSJGSFVSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CC[C@@H](C)CCC=C(C)C)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCCN([H])CC[C@@H](C)CCC=C(C)C)N([H])[H] SRTXRGKNBOPWCA-LOSJGSFVSA-N 0.000 description 1
- AGNZAVQEJMNQKC-HXUWFJFHSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCN([H])C(=O)C1CCCCC1)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCN([H])C(=O)C1CCCCC1)N([H])[H] AGNZAVQEJMNQKC-HXUWFJFHSA-N 0.000 description 1
- GWXVBCZMHFBJSO-QGZVFWFLSA-N [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCN([H])C(=O)CCC)N([H])[H] Chemical compound [H]N([H])CCCN([H])CCCCN([H])CCCN([H])C(=O)[C@@]([H])(CCCN([H])C(=O)CCC)N([H])[H] GWXVBCZMHFBJSO-QGZVFWFLSA-N 0.000 description 1
- LZWVWIUMLWQNEX-JGCGQSQUSA-N [H][C@@](N)(CCCCNC(=O)CCCCCCCCCCCCCCC)C(=O)CCCCCCCCCNCCCN Chemical compound [H][C@@](N)(CCCCNC(=O)CCCCCCCCCCCCCCC)C(=O)CCCCCCCCCNCCCN LZWVWIUMLWQNEX-JGCGQSQUSA-N 0.000 description 1
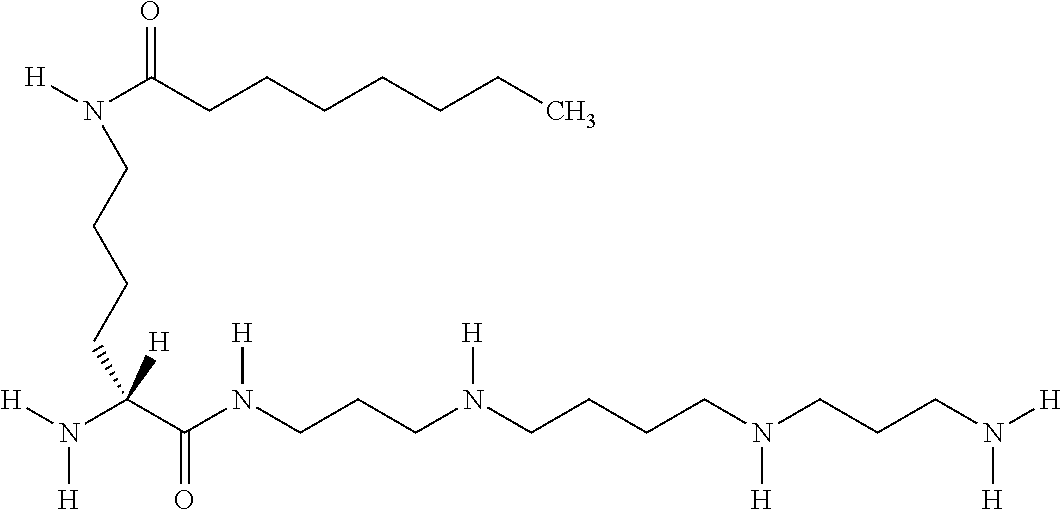
- RJNYGZBHYOCFGJ-VWLOTQADSA-N [H][C@](N)(CCCCNC(=O)CCCCCCC)C(=O)CCCCCCCCCNCCCC Chemical compound [H][C@](N)(CCCCNC(=O)CCCCCCC)C(=O)CCCCCCCCCNCCCC RJNYGZBHYOCFGJ-VWLOTQADSA-N 0.000 description 1
- TXPCTPPMAVZZJF-QFIPXVFZSA-N [H][C@](N)(CCCCNC(CCC)CCC)C(=O)NCCCNCCCCNCCCN Chemical compound [H][C@](N)(CCCCNC(CCC)CCC)C(=O)NCCCNCCCCNCCCN TXPCTPPMAVZZJF-QFIPXVFZSA-N 0.000 description 1
- BGZRFBWNWYSKRS-VWLOTQADSA-N [H][C@](N)(CCCCNCC1CCCCC1)C(=O)CCCCCCCCCNCCCC Chemical compound [H][C@](N)(CCCCNCC1CCCCC1)C(=O)CCCCCCCCCNCCCC BGZRFBWNWYSKRS-VWLOTQADSA-N 0.000 description 1
- FUNVYMXPCJVTIK-XIFFEERXSA-N [H][C@](N)(CCCCNCCCCCCCCCCCCCCCC)C(=O)CCCCCCCCCNCCCN Chemical compound [H][C@](N)(CCCCNCCCCCCCCCCCCCCCC)C(=O)CCCCCCCCCNCCCN FUNVYMXPCJVTIK-XIFFEERXSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 125000005073 adamantyl group Chemical group C12(CC3CC(CC(C1)C3)C2)* 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 229940009456 adriamycin Drugs 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 125000004390 alkyl sulfonyl group Chemical group 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 150000001370 alpha-amino acid derivatives Chemical class 0.000 description 1
- 235000008206 alpha-amino acids Nutrition 0.000 description 1
- 150000003862 amino acid derivatives Chemical class 0.000 description 1
- HAMNKKUPIHEESI-UHFFFAOYSA-N aminoguanidine Chemical compound NNC(N)=N HAMNKKUPIHEESI-UHFFFAOYSA-N 0.000 description 1
- 239000003708 ampul Substances 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000000843 anti-fungal effect Effects 0.000 description 1
- 230000002924 anti-infective effect Effects 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 230000000118 anti-neoplastic effect Effects 0.000 description 1
- 230000002141 anti-parasite Effects 0.000 description 1
- 230000001028 anti-proliverative effect Effects 0.000 description 1
- 230000000840 anti-viral effect Effects 0.000 description 1
- 229940124536 anticoccidial agent Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 229960005475 antiinfective agent Drugs 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 229940034982 antineoplastic agent Drugs 0.000 description 1
- 239000003096 antiparasitic agent Substances 0.000 description 1
- 239000003443 antiviral agent Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 229960003272 asparaginase Drugs 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-M asparaginate Chemical compound [O-]C(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-M 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 239000012752 auxiliary agent Substances 0.000 description 1
- 125000000852 azido group Chemical group *N=[N+]=[N-] 0.000 description 1
- 125000004069 aziridinyl group Chemical group 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- OGBUMNBNEWYMNJ-UHFFFAOYSA-N batilol Chemical class CCCCCCCCCCCCCCCCCCOCC(O)CO OGBUMNBNEWYMNJ-UHFFFAOYSA-N 0.000 description 1
- 229940077388 benzenesulfonate Drugs 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- RWCCWEUUXYIKHB-UHFFFAOYSA-N benzophenone Chemical group C=1C=CC=CC=1C(=O)C1=CC=CC=C1 RWCCWEUUXYIKHB-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 238000012742 biochemical analysis Methods 0.000 description 1
- 230000008687 biosynthesis inhibition Effects 0.000 description 1
- 230000006696 biosynthetic metabolic pathway Effects 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- GKFQZRDNTUZMKS-JEDNCBNOSA-N butan-1-amine;(2s)-2,6-diaminohexanoic acid Chemical compound CCCCN.NCCCC[C@H](N)C(O)=O GKFQZRDNTUZMKS-JEDNCBNOSA-N 0.000 description 1
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 1
- PASHVRUKOFIRIK-UHFFFAOYSA-L calcium sulfate dihydrate Chemical compound O.O.[Ca+2].[O-]S([O-])(=O)=O PASHVRUKOFIRIK-UHFFFAOYSA-L 0.000 description 1
- 239000012830 cancer therapeutic Substances 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 125000003739 carbamimidoyl group Chemical group C(N)(=N)* 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 239000012876 carrier material Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 238000000006 cell growth inhibition assay Methods 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 238000001516 cell proliferation assay Methods 0.000 description 1
- 238000002659 cell therapy Methods 0.000 description 1
- 230000033077 cellular process Effects 0.000 description 1
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 1
- PBAYDYUZOSNJGU-UHFFFAOYSA-N chelidonic acid Natural products OC(=O)C1=CC(=O)C=C(C(O)=O)O1 PBAYDYUZOSNJGU-UHFFFAOYSA-N 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 230000002113 chemopreventative effect Effects 0.000 description 1
- 230000000973 chemotherapeutic effect Effects 0.000 description 1
- 229940001468 citrate Drugs 0.000 description 1
- 239000003224 coccidiostatic agent Substances 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 230000000875 corresponding effect Effects 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 239000012043 crude product Substances 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 150000004985 diamines Chemical class 0.000 description 1
- 230000003292 diminished effect Effects 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 239000003596 drug target Substances 0.000 description 1
- 238000000132 electrospray ionisation Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 230000003511 endothelial effect Effects 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 238000001917 fluorescence detection Methods 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 229940014144 folate Drugs 0.000 description 1
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 1
- 235000019152 folic acid Nutrition 0.000 description 1
- 239000011724 folic acid Substances 0.000 description 1
- 238000013467 fragmentation Methods 0.000 description 1
- 238000006062 fragmentation reaction Methods 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-L fumarate(2-) Chemical compound [O-]C(=O)\C=C\C([O-])=O VZCYOOQTPOCHFL-OWOJBTEDSA-L 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 238000007306 functionalization reaction Methods 0.000 description 1
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 229940074045 glyceryl distearate Drugs 0.000 description 1
- 229940075507 glyceryl monostearate Drugs 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 210000002768 hair cell Anatomy 0.000 description 1
- 230000013632 homeostatic process Effects 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229960001680 ibuprofen Drugs 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 239000000367 immunologic factor Substances 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000012444 intercalating antibiotic Substances 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 229940079322 interferon Drugs 0.000 description 1
- PGLTVOMIXTUURA-UHFFFAOYSA-N iodoacetamide Chemical class NC(=O)CI PGLTVOMIXTUURA-UHFFFAOYSA-N 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 239000000865 liniment Substances 0.000 description 1
- 239000006194 liquid suspension Substances 0.000 description 1
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 230000000873 masking effect Effects 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 229940057917 medium chain triglycerides Drugs 0.000 description 1
- 230000008384 membrane barrier Effects 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- 210000004400 mucous membrane Anatomy 0.000 description 1
- YMUKFPITBPXIQC-UHFFFAOYSA-N n'-butan-2-ylbutane-1,4-diamine Chemical compound CCC(C)NCCCCN YMUKFPITBPXIQC-UHFFFAOYSA-N 0.000 description 1
- ONCPEIKLFULSSA-UHFFFAOYSA-N n'-ethylbutane-1,4-diamine Chemical compound CCNCCCCN ONCPEIKLFULSSA-UHFFFAOYSA-N 0.000 description 1
- VJACJUQFGYLDMG-UHFFFAOYSA-N n'-hexan-2-ylbutane-1,4-diamine Chemical compound CCCCC(C)NCCCCN VJACJUQFGYLDMG-UHFFFAOYSA-N 0.000 description 1
- GPPKGIMVZYIFNP-UHFFFAOYSA-N n'-pentan-2-ylbutane-1,4-diamine Chemical compound CCCC(C)NCCCCN GPPKGIMVZYIFNP-UHFFFAOYSA-N 0.000 description 1
- FBJWOCSWSGEYRJ-UHFFFAOYSA-N n'-propan-2-ylbutane-1,4-diamine Chemical compound CC(C)NCCCCN FBJWOCSWSGEYRJ-UHFFFAOYSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 229960004927 neomycin Drugs 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- 238000002414 normal-phase solid-phase extraction Methods 0.000 description 1
- 239000012038 nucleophile Substances 0.000 description 1
- WAQMADQIOCFJRY-UHFFFAOYSA-N o-(2-methylsulfanylethyl)hydroxylamine Chemical compound CSCCON WAQMADQIOCFJRY-UHFFFAOYSA-N 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 239000003883 ointment base Substances 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 239000006179 pH buffering agent Substances 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 239000002304 perfume Substances 0.000 description 1
- 235000019271 petrolatum Nutrition 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- USRGIUJOYOXOQJ-GBXIJSLDSA-N phosphothreonine Chemical compound OP(=O)(O)O[C@H](C)[C@H](N)C(O)=O USRGIUJOYOXOQJ-GBXIJSLDSA-N 0.000 description 1
- DCWXELXMIBXGTH-UHFFFAOYSA-N phosphotyrosine Chemical compound OC(=O)C(N)CC1=CC=C(OP(O)(O)=O)C=C1 DCWXELXMIBXGTH-UHFFFAOYSA-N 0.000 description 1
- 238000005222 photoaffinity labeling Methods 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- ZNZJJSYHZBXQSM-UHFFFAOYSA-N propane-2,2-diamine Chemical compound CC(C)(N)N ZNZJJSYHZBXQSM-UHFFFAOYSA-N 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- WGYKZJWCGVVSQN-UHFFFAOYSA-N propylamine Chemical group CCCN WGYKZJWCGVVSQN-UHFFFAOYSA-N 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 238000004366 reverse phase liquid chromatography Methods 0.000 description 1
- 238000004007 reversed phase HPLC Methods 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- SUUGLGYBZXSJAA-HRQDUAQYSA-N s-adenosyl-1,8-diamino-3-thiooctane Chemical compound O[C@@H]1[C@@H](O)[C@H](CSC(/CCCCCN)=C/CN)O[C@H]1N1C2=NC=NC(N)=C2N=C1 SUUGLGYBZXSJAA-HRQDUAQYSA-N 0.000 description 1
- 229930195734 saturated hydrocarbon Natural products 0.000 description 1
- 239000012047 saturated solution Substances 0.000 description 1
- 238000003345 scintillation counting Methods 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 238000002805 secondary assay Methods 0.000 description 1
- DUIOPKIIICUYRZ-UHFFFAOYSA-N semicarbazide Chemical compound NNC(N)=O DUIOPKIIICUYRZ-UHFFFAOYSA-N 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 229910000033 sodium borohydride Inorganic materials 0.000 description 1
- 239000012279 sodium borohydride Substances 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 235000002639 sodium chloride Nutrition 0.000 description 1
- BEOOHQFXGBMRKU-UHFFFAOYSA-N sodium cyanoborohydride Chemical compound [Na+].[B-]C#N BEOOHQFXGBMRKU-UHFFFAOYSA-N 0.000 description 1
- 239000002689 soil Substances 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 108091009298 spermidine binding proteins Proteins 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000012289 standard assay Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 238000005556 structure-activity relationship Methods 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 125000000565 sulfonamide group Chemical group 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- OBTWBSRJZRCYQV-UHFFFAOYSA-N sulfuryl difluoride Chemical class FS(F)(=O)=O OBTWBSRJZRCYQV-UHFFFAOYSA-N 0.000 description 1
- 230000001502 supplementing effect Effects 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 150000007970 thio esters Chemical class 0.000 description 1
- 230000036964 tight binding Effects 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- NOYPYLRCIDNJJB-UHFFFAOYSA-N trimetrexate Chemical compound COC1=C(OC)C(OC)=CC(NCC=2C(=C3C(N)=NC(N)=NC3=CC=2)C)=C1 NOYPYLRCIDNJJB-UHFFFAOYSA-N 0.000 description 1
- 229960001099 trimetrexate Drugs 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 230000004565 tumor cell growth Effects 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 229960005486 vaccine Drugs 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- 235000019154 vitamin C Nutrition 0.000 description 1
- 239000011718 vitamin C Substances 0.000 description 1
- 235000019165 vitamin E Nutrition 0.000 description 1
- 229940046009 vitamin E Drugs 0.000 description 1
- 239000011709 vitamin E Substances 0.000 description 1
- 235000012431 wafers Nutrition 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 238000010626 work up procedure Methods 0.000 description 1
- 238000012447 xenograft mouse model Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C311/00—Amides of sulfonic acids, i.e. compounds having singly-bound oxygen atoms of sulfo groups replaced by nitrogen atoms, not being part of nitro or nitroso groups
- C07C311/30—Sulfonamides, the carbon skeleton of the acid part being further substituted by singly-bound nitrogen atoms, not being part of nitro or nitroso groups
- C07C311/37—Sulfonamides, the carbon skeleton of the acid part being further substituted by singly-bound nitrogen atoms, not being part of nitro or nitroso groups having the sulfur atom of at least one of the sulfonamide groups bound to a carbon atom of a six-membered aromatic ring
- C07C311/38—Sulfonamides, the carbon skeleton of the acid part being further substituted by singly-bound nitrogen atoms, not being part of nitro or nitroso groups having the sulfur atom of at least one of the sulfonamide groups bound to a carbon atom of a six-membered aromatic ring having sulfur atoms of sulfonamide groups and amino groups bound to carbon atoms of six-membered rings of the same carbon skeleton
- C07C311/39—Sulfonamides, the carbon skeleton of the acid part being further substituted by singly-bound nitrogen atoms, not being part of nitro or nitroso groups having the sulfur atom of at least one of the sulfonamide groups bound to a carbon atom of a six-membered aromatic ring having sulfur atoms of sulfonamide groups and amino groups bound to carbon atoms of six-membered rings of the same carbon skeleton having the nitrogen atom of at least one of the sulfonamide groups bound to hydrogen atoms or to an acyclic carbon atom
- C07C311/42—Sulfonamides, the carbon skeleton of the acid part being further substituted by singly-bound nitrogen atoms, not being part of nitro or nitroso groups having the sulfur atom of at least one of the sulfonamide groups bound to a carbon atom of a six-membered aromatic ring having sulfur atoms of sulfonamide groups and amino groups bound to carbon atoms of six-membered rings of the same carbon skeleton having the nitrogen atom of at least one of the sulfonamide groups bound to hydrogen atoms or to an acyclic carbon atom to an acyclic carbon atom of a hydrocarbon radical substituted by carboxyl groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C237/00—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by amino groups
- C07C237/02—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by amino groups having the carbon atoms of the carboxamide groups bound to acyclic carbon atoms of the carbon skeleton
- C07C237/04—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by amino groups having the carbon atoms of the carboxamide groups bound to acyclic carbon atoms of the carbon skeleton the carbon skeleton being acyclic and saturated
- C07C237/10—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by amino groups having the carbon atoms of the carboxamide groups bound to acyclic carbon atoms of the carbon skeleton the carbon skeleton being acyclic and saturated having the nitrogen atom of at least one of the carboxamide groups bound to an acyclic carbon atom of a hydrocarbon radical substituted by nitrogen atoms not being part of nitro or nitroso groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C237/00—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by amino groups
- C07C237/02—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by amino groups having the carbon atoms of the carboxamide groups bound to acyclic carbon atoms of the carbon skeleton
- C07C237/22—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by amino groups having the carbon atoms of the carboxamide groups bound to acyclic carbon atoms of the carbon skeleton having nitrogen atoms of amino groups bound to the carbon skeleton of the acid part, further acylated
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C255/00—Carboxylic acid nitriles
- C07C255/01—Carboxylic acid nitriles having cyano groups bound to acyclic carbon atoms
- C07C255/24—Carboxylic acid nitriles having cyano groups bound to acyclic carbon atoms containing cyano groups and singly-bound nitrogen atoms, not being further bound to other hetero atoms, bound to the same saturated acyclic carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C311/00—Amides of sulfonic acids, i.e. compounds having singly-bound oxygen atoms of sulfo groups replaced by nitrogen atoms, not being part of nitro or nitroso groups
- C07C311/01—Sulfonamides having sulfur atoms of sulfonamide groups bound to acyclic carbon atoms
- C07C311/02—Sulfonamides having sulfur atoms of sulfonamide groups bound to acyclic carbon atoms of an acyclic saturated carbon skeleton
- C07C311/03—Sulfonamides having sulfur atoms of sulfonamide groups bound to acyclic carbon atoms of an acyclic saturated carbon skeleton having the nitrogen atoms of the sulfonamide groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C311/06—Sulfonamides having sulfur atoms of sulfonamide groups bound to acyclic carbon atoms of an acyclic saturated carbon skeleton having the nitrogen atoms of the sulfonamide groups bound to hydrogen atoms or to acyclic carbon atoms to acyclic carbon atoms of hydrocarbon radicals substituted by carboxyl groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C311/00—Amides of sulfonic acids, i.e. compounds having singly-bound oxygen atoms of sulfo groups replaced by nitrogen atoms, not being part of nitro or nitroso groups
- C07C311/15—Sulfonamides having sulfur atoms of sulfonamide groups bound to carbon atoms of six-membered aromatic rings
- C07C311/16—Sulfonamides having sulfur atoms of sulfonamide groups bound to carbon atoms of six-membered aromatic rings having the nitrogen atom of at least one of the sulfonamide groups bound to hydrogen atoms or to an acyclic carbon atom
- C07C311/19—Sulfonamides having sulfur atoms of sulfonamide groups bound to carbon atoms of six-membered aromatic rings having the nitrogen atom of at least one of the sulfonamide groups bound to hydrogen atoms or to an acyclic carbon atom to an acyclic carbon atom of a hydrocarbon radical substituted by carboxyl groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/02—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings
- C07D307/34—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D307/56—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D307/68—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/26—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D333/30—Hetero atoms other than halogen
- C07D333/34—Sulfur atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/04—Systems containing only non-condensed rings with a four-membered ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/12—Systems containing only non-condensed rings with a six-membered ring
- C07C2601/14—The ring being saturated
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2602/00—Systems containing two condensed rings
- C07C2602/36—Systems containing two condensed rings the rings having more than two atoms in common
- C07C2602/42—Systems containing two condensed rings the rings having more than two atoms in common the bicyclo ring system containing seven carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2603/00—Systems containing at least three condensed rings
- C07C2603/56—Ring systems containing bridged rings
- C07C2603/58—Ring systems containing bridged rings containing three rings
- C07C2603/70—Ring systems containing bridged rings containing three rings containing only six-membered rings
- C07C2603/74—Adamantanes
Definitions
- the invention in the field of chemistry and biochemistry relates to the synthesis and use of a novel class of polyamine transport inhibitor compounds. These compounds have pharmacological and/or agricultural applications as well as uses in analytical and preparative assays relating to polyamine transport. As pharmaceuticals, these compounds are used to treat disorders of undesired cell proliferation, especially in eukaryotic cells, alone or in combination with other agents such as polyamine synthesis inhibitors.
- Polyamine transport into mammalian cells is energy and temperature dependent, saturable, carrier mediated and operates against a substantial concentration gradient (Seiler, N. et al. Polyamine transport in mammalian cells. Int. J. Biochem. 1990, 22, 211-218; Khan, N. A.; Quemener, V. et al. Characterization of polyamine transport pathways, in Neuropharmacology of Polyamines (Carter, C., ed), 1994, Academic, San Diego, pp. 37-60). Ample experimental proof exists that polyamine concentration homeostasis is mediated via this transport system. Changes in the requirements for polyamines in response to growth stimulation is reflected by increases in the transport activity.
- Intracellular putrescine deprivation induces uptake of the natural polyamines and methylglyoxal bis (guanylhydrazone). Biochem. J. 1980, 192, 941-945). The cells then returned to their original rate of growth.
- a radiochemical assay is used for biochemical analysis of transport and has been used to study polyamine transport in yeast and a variety of mammalian cells (Kakinuma, Y et al., Biochem. Biophys. Res. Comm. 216:985-992, 1995; Seiler, N. et al., Int. J. Biochem. Cell Biol. 28:843-861, 1996). See, for example Huber, M. et al. Cancer Res. 55:934-943, 1995.
- the present invention is directed to novel polyamine analogs and derivatives and methods for their use as drugs, as agricultural or as environmentally useful agents.
- These novel polyamine analogs and derivatives comprise a hydrophobic moiety covalently attached to a polyamine moiety.
- These novel PA analogs can be considered to have amphipathic character (hydrophobic as well as charged portions).
- the polyamine analogs and derivatives of the invention include those that may be viewed as a polyamine acylated with a hydrophobic acyl group, where acylation is by formation of either an amide or a sulfonamide linkage. While the linkage between the hydrophobic acyl group and the polyamine moiety may occur at any amine group within the polyamine, linkages to a primary amine functionality are preferred.
- the analogs and derivatives of the invention are potent inhibitors of cellular polyamine transport. Without being bound by theory, they are inferred to bind to a cell's polyamine transporter apparatus with very high affinity. They may be used independently or in combination with the inhibition of cellular polyamine synthesis, even in the presence of exogenously supplied spermidine, to inhibit cell growth and proliferation.
- R is selected from H or from the group of a straight or branched C1-50 saturated or unsaturated aliphatic, carboxyalkyl, carbalkoxyalkyl, or alkoxy; a C1-8 alicyclic; a single or multiring aryl substituted aliphatic; an aliphatic-substituted single or multiring aromatic; a single or multiring heterocyclic; a single or multiring heterocyclic aliphatic; a C1-10 alkyl; an aryl sulfonyl; or cyano;
- X may be —CO—, —SO 2 —, or —CH 2 —, and
- polyamine may be any naturally occurring, such as putrescine, spermine or spermidine, or synthetically produced polyamine.
- R is at least about C5, at least about C10, at least about C11, at least about C12, at least about C13, at least about C14, at least about C15, at least about C16, at least about C17, at least about C18, at least about C19, at least about C20, or at least about C22.
- the linkage between X and the polyamine may be direct, wherein there are no atoms between X and the nitrogen of the amine group of the polyamine, or indirect, where there may be one or more atoms between X and the nitrogen of the amine group of the polyamine.
- the linkage between X and the polyamine may occur via any amino group within the polyamine, although a primary amino group is used in preferred embodiments of the invention.
- the intervening one or more atoms are preferably those of an amino acid or a derivative thereof.
- the intervening one or more atoms are those of lysine, aspartic acid, glutamic acid, omithine, or 2,4-diaminobutyric acid.
- Preferred compounds of this type may be represented as R—X-L-polyamine wherein R is a straight or branched C10-50 saturated or unsaturated aliphatic, carboxyalkyl, carbalkoxyalkyl, or alkoxy; a C1-8 alicyclic; a single or multiring aryl substituted or unsubstituted aliphatic; an aliphatic-substituted or unsubstituted single or multiring aromatic; a single or multiring heterocyclic; a single or multiring heterocyclic aliphatic; an aryl sulfonyl;
- X is —CO—, —SO 2 —, or —CH 2 —;
- L is a covalent bond or a naturally occurring amino acid, omithine, 2,4-diaminobutyric acid, or derivatives thereof.
- the analogs and derivatives of the invention may be optionally further substituted at one or more other positions of the polyamine. These include, but are not limited to, internal nitrogen and/or internal carbon atoms.
- preferred substituents are structures that increase polyamine transport inhibition, binding affinity or otherwise enhance the irreversibility of binding of the compound to a polyamine binding molecule, such as the polyamine transporter, an enzyme or DNA.
- additional substituents include the aziridine group and various other aliphatic, aromatic, mixed aliphatic-aromatic, or heterocyclic multi-ring structures. Reactive moieties which, like aziridine, bind covalently to a polyamine transporter or another polyamine binding molecule, are also within the scope of this invention.
- Such reactive moieties are used for affinity labeling in a diagnostic or research context, and may contribute to pharmacological activity in inhibiting polyamine transport or polyamine synthesis.
- the reactive group can be a reactive photoaffinity group such as an azido or benzophenone group.
- Chemical agents for photoaffinity labeling are well-known in the art (Flemming, S. A., Tetrahedron 1995, 51, 12479-12520).
- a preferred aspect of the invention relates to a polyamine analog or derivative that is a highly specific polyamine transport inhibitor with pharmaceutical utility as an anti-cancer chemotherapeutic.
- One class of a polyamine analog or derivative of the invention that binds to a polyamine-binding site of a molecule and/or inhibits polyamine transport, is described by the following formula II:
- a, b, and c independently range from 1 to 10; d and e independently range from 0 to 30; each X is independently either a carbon (C) or sulfur (S) atom, and R 1 and R 2 are as described below, or each of R 1 X ⁇ O ⁇ n — and R 2 X ⁇ O ⁇ n — are independently replaced by H; and * denotes a chiral carbon position.
- n is 1; if X is S, then n is 2; and if X is C, then the XO group may be CH 2 such that n is 0.
- R 1 and R 2 are independently selected from H or from the group of a straight or branched C1-50 saturated or unsaturated aliphatic, carboxyalkyl, carbalkoxyalkyl, or alkoxy; a C1-8 alicyclic; a single or multiring aryl substituted aliphatic; an aliphatic-substituted single or multiring aromatic; a single or multiring aromatic or saturated heterocyclic; a single or multiring heterocyclic aliphatic; a C1-10 alkyl; an aryl sulfonyl; or cyano.
- heterocyclic rings as used herein include, but are not limited to, pyrrole, furan, thiophene, imidazole, oxazole, thiazole, pyrazole, 3-pyrroline, pyrrolidine, pyridine, pyrimidine, purine, quinoline, isoquinoline, and carbazole.
- carboxyalkyl refers to the substituent —R′—COOH wherein R′ is alkylene; and carbalkoxyalkyl refers to —R′—COOR wherein R′ and R are alkylene and alkyl respectively.
- alkyl refers to a saturated straight- or branched-chain hydrocarbyl radical of 1-6 carbon atoms such as methyl, ethyl, n-propyl, isopropyl, n-butyl, t-butyl, n-pentyl, 2-methylpentyl, n-hexyl, and so forth.
- Alkylene is the same as alkyl except that the group is divalent.
- Aryl or alkyl sulfonyl moieties have the formula —SO 2 R, and alkoxy moieties have the formula —O—R, wherein R is alkyl, as defined above, or is aryl wherein aryl is phenyl, optionally substituted with 1-3 substituents independently selected from halo (fluoro, chloro, bromo or iodo), lower alkyl (1-6C) and lower alkoxy (1-6C).
- R 1 and R 2 are defined as above for formula II and R 3 and R 4 are independently selected from organic substituents including —CH 3 and as defined above for R 1 and R 2 in formula II above.
- This grouping of analogs is produced by reductive amination of the free amino precursor with a ketone. Some members of this group of analogs are shown in Series V (see FIG. 2 ).
- R 1 and R 2 are identical and as described for formula II. Positions R 3 and R 4 may also be identical, and all of R 1 through R 4 may also be identical. Additionally, each of positions R 1 , R 2 , R 3 and R 4 in formula III may also be independently H.
- proximal and/or the distal amino group relative to the polyamine can be di-alkylated to form tertiary amines.
- These materials can be synthesized by reductive amination with a large excess of the carbonyl component. Additionally, these materials may be produced by a conjugate addition of the amine precursor to an ⁇ , ⁇ -unsaturated carbonyl or ⁇ , ⁇ -unsaturated nitrile.
- R 1 , R 2 , R 3 and R 4 can be independently varied and are as defined as above for formula III.
- R 1 , R 2 , R 3 and R 4 may also be independently H.
- the values of a, b, c, d and e are as described above for formula III. This aspect of the invention is depicted in the following formula IV:
- Z 1 is NR 1 R 3 and Z 2 is selected from —R 1 , —CHR 1 R 2 or —CR 1 R 2 R 3 (wherein R 1 , R 2 , and R 3 are as defined above for formula III) or Z 2 is NR 2 R 4 and Z 1 is selected from —R 1 , —CHR 1 R 2 or —CR 1 R 2 R 3 (wherein R 1 , R 2 , and R 3 are as defined above for formula III)
- Values for a, b, and c independently range from 1 to 10; d and e independently range from 0 to 30.
- Compounds encompassed by formula V may be prepared by first coupling amino acid derivatives (modified to contain the non-amine containing Z group) to a polyamine followed by appropriate derivatization of the amine containing Z group. Chemistries for such reactions are known in the art and disclosed herein.
- positions R 1 , R 2 , R 3 and R 4 of all the formulas set forth above are independently selected from the following, where each of g, h, i, j, and k are independently selected from 0 to 15:
- the present invention includes the free base or acid forms, as well as salts thereof, of the polyamine analogs and derivatives described by the above formulas.
- the invention also includes the optical isomers of the above described analogs and derivatives, especially those resulting from the chiral center indicated above with a *.
- mixtures of enantiomers and/or diastereoisomers, resulting from a single preparative step, combination, or interconversion are encompassed.
- the invention also provides prodrug forms of the above described analogs and derivatives, wherein the prodrug is metabolized in vivo to produce an analog or derivative as set forth above. Indeed, some of the above described analogs or derivatives may be a prodrug for another analog or derivative.
- compositions containing the above described analogs and derivatives are provided.
- the compositions are formulated to be suitable for pharmaceutical or agricultural use by the inclusion of appropriate carriers or excipients.
- methods for the use of the above described analogs and derivatives, as well as compositions are provided. These methods include uses of the invention's polyamine compounds to inhibit polyamine transport, as well as treat human and agricultural diseases and conditions.
- human diseases and conditions include, but are not limited to, cancer, osteoporosis, asthma, autoimmune diseases, rheumatoid arthritis, systemic lupus erythematosus, Type I insulin-dependent diabetes, tissue transplantation, African sleeping sickness, psoriasis, restenosis, inhibition of unwanted hair growth as cosmetic suppression, hyperparathyroidism, inflammation, treatment of peptic ulcer, glaucoma, Alzheimer's disease, suppression of atrial tachycardias, stimulation or inhibition of intestinal motility, Crohn's disease and other inflammatory bowel diseases, high blood pressure (vasodilation), stroke, epilepsy, anxiety, neurodegenerative diseases, hyperalgesic states, protection against hearing loss (especially cancer chemotherapy induced hearing loss), and pharmac
- FIGS. 1A and 1B show Scheme 1, a pathway for the synthesis of selectively acylated lysine-spermine derivatives.
- the pathway may be readily adapted for the synthesis of other polyamine derivatives by the use of an analogous protected “NH—X—COO” starting material (wherein X is CH—(CH 2 ) d —NH—COO—CH 2 —Ph, wherein d is as described above and “Ph” is phenyl) and/or the use of any primary polyamine, including spermine.
- FIGS. 2A–2X illustrate exemplary polyamine structures encompassed by the present invention. They have been divided into Series I–VI based upon the character of the chemical moiety attached to a spermine backbone to produce exemplary analogs and derivatives of the invention. Other polyamines may also be used as the backbone.
- the structures depicted in the first, left-most column of each table represent the specific chemical starting materials utilized in the synthesis of individual polyamine structures.
- the synthetic steps used result in the end products that are carboxamides from a reaction between an acyl chloride and an amine (series I), sulfonamides from the reaction between a sulfonyl chloride and an amine (series II), carboxamides from the reaction of a DCC, HBTU or PyBOP activated carboxylic acid and an amine (series III), alkylated secondary amines from the reductive amination of the amine with an aldehyde (series IV), alkylated secondary amines with ⁇ -alkyl substituents from the reductive amination of the free amino precursor with a ketone (Series V) and di-alkylated tertiary amine products by reductive amination with a large excess of a carbonyl containing (e.g.
- Series VI aldehyde or ketone component
- Columns E and F are directed to doubly derivatized forms of the base chemical structure.
- FIGS. 3A and 3B show representative structures of polyamine analogs relating to the present invention.
- FIG. 4 shows the relationship between the length of the hydrocarbon substituent at the ⁇ -position of the L-lysine analogs and the resulting activity as polyamine transport inhibitors as defined by EC 50 (see Example IV).
- FIG. 5 representatively shows the portion of compounds for calculation of logP values.
- FIG. 6 presents calculated logP values versus HPLC retention time for dansylated derivatives of compounds shown in FIG. 2 (Series I).
- FIG. 7 presents calculated logP values versus average EC 50 values obtained for compounds with 4 cell lines (data for Series I compounds in Table 1).
- FIG. 8 presents HPLC retention time for dansylated derivatives of compounds shown in Table 2 (Series IV and V) versus average EC 50 values obtained for 4 cell lines (data in Table 1).
- FIG. 9 shows the relationship between calculated logP values and HPLC retention time for dansylated derivatives of compounds shown in Table 2 (Series IV and V).
- FIG. 10 presents calculated logP values versus average EC 50 values obtained for compounds with 4 cell lines (data for Series IV and V compounds in Table 2).
- FIG. 11 presents HPLC retention time for dansylated derivatives of compounds shown in Table 2 (Series IV and V) versus average EC 50 values obtained for 4 cell lines using data in Table 1.
- FIGS. 12A–12K show the structures of exemplary polyamine analogs and derivatives of the present invention.
- the present inventors have designed novel polyamine analogs and derivatives for the inhibition of polyamine transport and other uses. These analogs and derivatives are inferred to bind polyamine transporters with high affinity and inhibit polyamine transport, either competitively or non-competitively. Thus these compounds can alter polyamine metabolism in cells by reducing or preventing polyamine uptake.
- one or more polyamine analogs and derivatives are used in combination with polyamine synthesis inhibitors to inhibit cell growth and proliferation.
- they are useful as drugs in a number of diseases, particularly cancer and other conditions involving cellular proliferation, including, but not limited to, inflammatory diseases or conditions where components of the immune system undergo undesired proliferation.
- Non-limiting examples include asthma, autoimmune diseases, rheumatoid arthritis, systemic lupus erythematosus, Type I insulin dependent diabetes, psoriasis, restenosis, inhibition of unwanted proliferation of hair on skin, tissue transplantation, African sleeping sickness, osteoporosis, hyperparathyroidism, treatment of peptic ulcer, glaucoma, Alzheimer's disease, suppression of atrial tachycardias, stimulation or inhibition of intestinal motility, Crohn's disease and other inflammatory bowel diseases, high blood pressure (vasodilation), stroke, epilepsy, anxiety, neurodegenerative diseases, hyperalgesic states, the protection of hair cells from chemotherapy induced loss of hearing, and pharmacological manipulation of cocaine reinforcement and craving in treating cocaine addiction and overdose, and other fungal, bacterial, viral, and parasitic diseases.
- polyamine includes putrescine, spermine or spermidine, as well as longer linear polyamines, branched polyamines, and the like, which may have between 2 and about 10 nitrogens. Also included in this definition are polyamine derivatives or analogs comprising a basic polyamine chain with any of a number of functional groups bound to a C atom or a terminal or internal N atom. For modification at a primary amino group, a polyamine must, of course, contain such a group.
- Polyamine “analogs” and/or “derivatives” generally refer to any modified polyamine molecule disclosed or described herein. These molecules are generally modifications of existing polyamines, whether naturally occurring or synthetically produced, and may also be referred to as “polyamine agents”, “PA” or “agents” of the invention. Preferred PAs bind and/or inhibit cellular polyamine transport, and as such may also be referred to as “transport binding molecules” or “polyamine transport inhibitors”.
- the scope of this definition includes any modification to produce a PA from an existing polyamine or the isolation of a structurally identical PA from a naturally occurring source. Preferably, the modification is the addition of one or more chemical moieties to the polyamine.
- APA that is an “inhibitor” polyamine analog or derivative (a) binds to polyamine transporters better than a native polyamine and/or (b) by some means blocks the uptake of a polyamine into a cell or a subcellular polyamine transporter preparation.
- the invention includes PAs that efficiently inhibit polyamine transporters in different eukaryotic cell types as well as inhibit cellular growth and proliferation when used in combination with a polyamine synthesis inhibitor.
- the PAs of the invention generally have an acylated primary amine functionality and are expected to bind to a cell's polyamine transporter apparatus with a very high affinity. Measurements of K i were determined by using an assay that shows the inhibition of polyamine uptake, such as uptake of 3 H-spermidine.
- the PAs were also analyzed with a secondary assay to show inhibition of cellular polyamine uptake based on a measurement of cellular growth inhibition in combination with a potent inhibitor of polyamine biosynthesis.
- This assay was conducted in the presence of polyamines, such as spermidine, to determine a PA's ability to prevent the uptake of polyamines thereby overcoming the polyamine biosynthesis inhibition with DFMO (difluoromethylomithine). Due to the trend that polyamine mono-anides give high potency in both of these assays, it has been inferred, without limiting the invention thereto, that there is a site on the transporter protein for tight binding of the inhibitor's amide functionality.
- DFMO difluoromethylomithine
- Preferred embodiments of these PAs are the result of acylation at a polyamine molecule with two or more primary amine groups.
- the linkage between the acyl group and the primary amine group is preferably an amide linkage (indicated below as the bond between “CO” and “NH”) and results in a molecule with the following general formula. rest of acyl group-CO—NH-rest of polyamine
- polyamine in the above formula may be any polyamine with at least one primary amine group, but more preferably with two or more primary groups, for linkage to the acyl group.
- One preferred class of acyl groups for inclusion in the above formula contains two primary amines for further acylation.
- the resultant class of PAs may be described by the following formula (formula II).
- Non-limiting examples of alkyl moieties as present in these compounds include straight or branched chains of at least about 8 carbon atoms for increased hydrophobicity (or lipophilicity), such as at least about 10, at least about 12, at least about 14, at least about 16, at least about 18, at least about 20, at least about 22, at least about 24, at least about 26, at least about 28, and at least about 30.
- the chain is of at least about 19, 21, 23, 25, or 27 carbon atoms, with at least about 20 to at least about 24 or 26 as even more preferred.
- PAs encompassed by the above formula is where d is 4 and e is 0, although generally excluded from this group are PAs where R 2 X ⁇ O ⁇ n — is an H and R 1 X ⁇ O ⁇ n — is R 1 SO 2 — wherein R 1 is a thiophene moiety linked to the S atom via the 2 position, and substituted at the 5 position, of the thiophene.
- R 1 is a thiophene moiety linked to the S atom via the 2 position, and substituted at the 5 position, of the thiophene.
- the substitution at the 5 position includes an amide linkage.
- PAs wherein the amide linkage is attached to a chlorinated aromatic group, such as the compound identified as ORI 1340 in U.S. patent application Ser. No. 09/396,523, filed Sep. 15, 1999.
- the invention includes, but is not limited to, situations where the hydrophobic (lipophilic) moiety may serve as an anchor to some hydrophobic pocket on the transporter or in a region nearby. This may result in the interaction of the polyamine portion of the analog with the polyamine transporter.
- the logP coefficient is the logarithm of the ratio of distribution of a compound in a mixture of 1-octanol and H 2 O.
- Compounds with logP values greater than 1 are considered lipophilic (greater solubility in 1-octanol versus H 2 O).
- the presence of ionizable groups in the compound has a dramatic effect on this parameter. Ionization will greatly increase a compound's H 2 O solubility. For this reason, a compound's ionization potential must be taken into consideration when correlating lipophilicity with activity.
- One such computer program is ChemDraw Pro Version 5.0 from Cambridge-SoftCorporation.
- Preferred PAs of the invention with respect to Series I type compounds are those with low EC 50 values, such as those with below about 5, about 6, about 7, about 8, about 9, about 10, about 15, about 20 or about 25 minute HPLC retention times.
- Preferred PAs of the invention with respect to Series IV and V type compounds are those with low EC 50 values, such as those with below about 5, about 6, about 7, about 8, about 9, about 10, about 12, about 14, about 16, about 18, or about 20 minute HPLC retention times.
- the present invention utilized a dansylation protocol to form dansyl derivatives of the described analogs and analyzing these derivatives by fluorescence detection on C18 reverse phase HPLC. The difference between the elution of the peak due to the analog and the peak due to an internal standard (1,7-diaminoheptane) is shown for several representative analogs in Tables 1 and 2 above.
- the side chains for the twenty naturally occurring amino acids span a range of free energy values for the transfer from the vapor phase to H 2 O from 2.39 kcal/mol for hydrogen (glycine) or 1.94 kcal/mol for methane (alanine) to ⁇ 7.00 kcal/mol for n-butylamine (lysine) or ⁇ 14.6 kcal/mol for n-propylguanidine (arginine).
- hydrophilic potential scale which is correlated with the potential that a given amino acid would be present on the outside, or hydrophilic portion of a protein versus the more hydrophobic interior of a protein.
- the authors state “that the energetic cost of removing hydrophilic side chains from water is much greater than the cost of pulling hydrophobic side chains into water, and, indeed, it has been observed that hydrophobic residues occur rather often at the surfaces of proteins.”
- the present invention could use this scale to describe the lipophilicity of the substituent attached to the polyamine.
- the polyamine portion is removed before this analysis.
- any substituent with a free energy of transfer from the vapor phase to H 2 O less than that determined for n-butylamine (and thus correlated to lysine) of ⁇ 7.00 kcal/mol would be expected to be a preferred polyamine transport inhibitor in comparison to the lysine-spermine conjugate (ORI 1202).
- PAs wherein d is 4 and e is 0 includes both the L and D-stereoisomers due to the chiral carbon indicated by * in the above formula.
- Exemplary PAs such as ORI 1202 (L-Lys-spm), 1426 (D-Lys-spm), and those containing IA4 ( FIG. 2 ) demonstrated potency in both the transporter inhibition and cell growth inhibition assays described below.
- PA ORI 1202 also displayed effectiveness in several anti-cancer mouse xenograft models. See Weeks, R. S., Vanderwerf, S. M., Carlson, C. L., Burns, M. R., O'Day, C. L., Cai, C. F., Devens, B.
- Additional modification of the two primary amine groups in the acyl group in the above formula is readily accomplished by the availability of the primary amine groups for selective functionalization together with the commercial availability of orthogonally di-protected versions of H 2 N (CH 2 ) n CH(NH 2 )COOH type molecules (where n ranges from 1 to 50 for example), such as lysine and omithine.
- increases in the lipophilicity of the substituent at the above R 1 and R 2 positions may dramatically increase the affinity for the polyamine transporter.
- Increases in lipophilicity in the PAs of the invention may improve the inhibition of polyamine transport due to the presence of both hydrophilic and hydrophobic domains.
- Biological systems have a significant chemical problem when they attempt to move a very hydrophilic substance, such as polycationic polyamines, across their very hydrophobic outer membrane barriers. If the transporter moves the polyamines in their polycationic forms across this barrier, the transporter may do so via some mechanism for masking or minimizing their hydrophilicity.
- Mechanisms for this may include the formation of specific salt bridges between the polyamine and negatively charged residues on the protein or formation of a charged interior in the intermembrane pore.
- the transporter may have the task of providing a very specific polyamine shaped hydrophilic pore in the presence of the very hydrophobic environment of the membrane. For these reasons the transporter likely has hydrophobic residues for interactions with the membrane in close proximity to hydrophilic residues specific for interactions with the polyamine.
- the present invention exploits the likely characteristics of a polyamine transporter to improve transport inhibition.
- the present invention provides several series of PAs that contain both a polyamine-mimicking portion and a hydrophobic membrane-mimicking portion. These PAs have been inferred to have great affinity for the transporter, and they show substantially increased growth inhibition (in combination with a polyamine synthesis inhibitor) in comparison to PAs lacking a significantly hydrophobic domain. Possibly for very similar reasons, the present PAs are also expected to show improved bioavailability through oral administration. Increases in lipophilicity are expected to enhance absorption after oral uptake.
- FIG. 4 shows the relationship between the length of the hydrocarbon substituent at the R position and the resulting EC 50 value in the presence of a polyamine synthesis inhibitor.
- Table 3 shows the results from analysis of various exemplary PAs for their ability to inhibit cellular growth in combination with DFMO relative to control cells left untreated.
- EC 50 refers to the concentration of PA resulting in 50% of maximum cell growth inhibition in the presence of both DFMO and the PA.
- K i refers to the inhibition constant for polyamine transport based on double reciprocal Lineweaver-Burke plot analyses of four radioactive substrate concentrations (0.3–3 ⁇ M) and five inhibitor concentrations (0.01–1.0 ⁇ M) and a control.
- Compounds ORI 1202 and 1426 are included for comparison. See the Examples below.
- EC 50 values ( ⁇ M) of representative polyamine analogs determined in the presence of DFMO (1-5 mM). Also shown are the IC 50 results from analyses of various exemplary PAs. IC 50 refers to the concentration of PA that results in 50% of maximum cell growth inhibition in the presence of PA alone. Cell Line EC 50 ( ⁇ M) AVG.
- FIG. 2 A set of PAs wherein positions R 1 and R 2 of formula I are substituted by an aliphatic chain with varying degrees of unsaturation in the hydrocarbon chain are represented in FIG. 2 , Series III. These compounds include those with internal geometrically cis (zusammen or Z-form) and trans (entitch or E-form) isomers are also presented in this series.
- the invention incorporates considerations based on the charge character of the PA.
- the introduction of the R 1 X ⁇ O ⁇ n — and R 2 X ⁇ O ⁇ n — moieties reduces the number of positive charges in the analog or derivative by one.
- the vast majority of amine groups will be in their positively charged ammonium state.
- PAs based on the long-chain hydrocarbon containing carboamides ( FIG. 2 , Series I), may be prepared by incorporating the lipophilic and biologically stable sulfonamide group. These PAs are shown in FIG. 2 , Series II. Without being bound by theory, it may be that the addition of an additional carbonyl-like oxygen atom in the sulfonamide series increases the interactions at an amide-binding domain of polyamine transporters. An additional factor which may be playing a role is the increased lipophilicity in sulfonamides versus carboxamides. Additionally sulfonamides are known to be more biologically stable in comparison to carboxamides.
- the present invention also provides additional ways to increase the lipophilicity of the substituents on the PA molecule.
- Alternatives with additional alkyl groups on the acyl portion of the molecule will increase the lipophilicity of this group and thus give an analog with higher activity.
- One additional method to increase this lipophilicity is through attachment of an additional alkyl chain alpha to the amino group (substituent which is attached to the carbon atom attached to the nitrogen). These analogs are produced by reductive amination of the free amino precursor with one of the ketone reagents shown in Series V.
- An additional advantage provided by inclusion of a methyl, or other substituent, at the alpha position of the amine group is decreased rate of biological metabolism.
- An additional method to increase the lipophilicity of the analogs is through the production of a tertiary amine at the proximal or distal, or both, nitrogen atoms of the molecule.
- These molecules which are shown in Series VI, are produced via the reductive amination reaction using a free mono- or di-amine precursor and an excess of the carbonyl containing reagent shown in Series VI.
- An alternative method to produce these di-substituted tertiary amine containing molecules is the conjugate addition of the selectively protected amine precursor to an ⁇ , ⁇ -unsaturated carbonyl compound or an ⁇ , ⁇ -unsaturated nitrile compound.
- the present invention further provides methods for the synthesis of the disclosed PAs.
- an orthogonally protected diamine containing compound such as, but not limited to, certain amino acids, is coupled to a primary amine group of a polyamine followed by deprotection of one or both of the protected amine groups followed optionally by further derivatization of the amine.
- an exemplary scheme for the production of spermine based PAs according to the above formula wherein d is 4, e is 0, X is C, and either R 1 X ⁇ O ⁇ n — or R 2 X ⁇ O ⁇ n — is H is shown in FIG.
- Any appropriate protecting group(s) may be used in the practice of the invention, and the indication of Boc-(butoxycarbonyl-) and Cbz-(carbobenzoxy-) protecting groups are for illustrative purposes only.
- Other protective group strategies are known in the art (see, for example, “Protective Groups in Organic Synthesis—Third Ed. 1999, eds. T. W. Greene and P. G. M. Wuts. John Wiley and Sons, Inc. New York).
- polyamine analogs may be prepared via the coupling of distal carboxylic acid containing amino acids with suitable protecting groups on this distal carboxylic acid (e.g. methyl or benzyl ester) such as N- t Boc-Asp(OCH 3 )—OH or N- t Boc-Glu(OCH 3 )—OH with a primary amine group of a polyamine (such as, but not limited to, spermine) followed by exhaustive protection of the remaining amino groups. After purification by silica gel chromatography the distal carboxylic acid is deprotected and reacted with long chain hydrocarbon containing amines or alcohols to give amides or esters respectively.
- suitable protecting groups on this distal carboxylic acid e.g. methyl or benzyl ester
- suitable protecting groups on this distal carboxylic acid e.g. methyl or benzyl ester
- suitable protecting groups on this distal carboxylic acid e.g. methyl or benzyl ester
- n can also be greater than 2, preferably up to about 10 (including 3, 4, 5, 6, 7, 8 and 9) and R is defined as provided for R 1 and R 2 in formula II above.
- the alpha amino group of the distal carboxylic acid containing amino acid may also be derivatized as described above in Formula II. Such compounds may be described as “inverted” amide or ester derivatives of the compounds described in FIG. 2 .
- Similar hydrophobic PAs can be prepared by the use of cysteine, serine, or homo serine to link the hydrophobic and polyamine moieties indirectly.
- the hydrophobic PAs may also be linked via an ester linkage (like that possible via serine), a thioester linkage (like that possible via cysteine), a urea linkage (—N—CO—N—), a carbamate linkage (—O—CO—N— or —N—CO—O—), or an extended sulfonamide linkage (—NH—SO 2 —),
- the active ester is added to an excess of polyamine to produce a mixture of substituted and unsubstituted acyl polyamines.
- the remaining free amino groups of the polyamines can then be protected, such as via their t Boc or Cbz carbamates, and the desired orthogonally-protected products can be isolated. Full protection of the amino groups produces a more lipophilic product mixture which facilitates purification of the desired compound.
- the exemplary reaction scheme in FIG. 1 results in two synthetic intermediates, one with 4 Boc and 1 Cbz carbamates and the other with 4 Cbz and 1 Boc carbamates.
- the deprotected amino groups may then be further modified via conventional amide chemistry.
- the deprotected amino groups may be acylated or alkylated with either an acyl chloride or sulfonyl chloride to produce PAs shown in FIG. 2 as Series I and II, respectively.
- the positions may also be carboxylic acid activated with standard peptide coupling reagents such as DCC, PyPOP or HBTU (to produce Series III PAs) or aldehydes using reductive amination conditions (to produce Series IV PAs). Additional analogs are produced by reductive amination of the free amino precursor with one of the ketone reagents shown in Series V.
- Series VI analogs are produced via the reductive amination reaction using a free mono- or di-amine precursor and an excess of the carbonyl containing reagent shown in the Series VI portion of FIG. 2 .
- An alternative method to produce these di-substituted tertiary amine-containing molecules is the conjugate addition of the selectively protected amine precursor to an ⁇ , ⁇ -unsaturated carbonyl compound or an ⁇ , ⁇ -unsaturated nitrile compound.
- the above described synthetic schemes may be conducted in a parallel fashion to permit the simultaneous production of multiple PAs.
- the reaction scheme shown in FIG. 1 may be started with a mixture of L- and D-forms of Boc-Lys-(Cbz)-ONP and spermine. This results in a possible 4 different amino groups (two based on each of the L- and D-forms, and two based on each of the distal and proximal amino groups) deprotection and subsequent modification. There are also two additional possible modifications where both amino groups are simultaneously deprotected for subsequent modification. This results in a total of 6 possible routes for modification.
- compositions containing one or more PAs, as well as acceptable salts thereof, in combination with an excipient, diluent or vehicle to facilitate its use or administration to a subject Preferably, the compositions are formulated for pharmaceutical, therapeutic or agricultural uses.
- Pharmaceutically acceptable salts of the invention (which contain basic groups) are formed where appropriate with strong or moderately strong, non-toxic, organic or inorganic acids in the presence of the basic amine by methods known in the art.
- Exemplary salts include, but are not limited to, maleate, fumarate, lactate, oxalate, methanesulfonate, ethanesulfonate, benzenesulfonate, tartrate, citrate, hydrochloride, hydrobromide, sulfate, phosphate and nitrate salts.
- the PAs of the invention possess the ability to inhibit polyamine transport, a property that is exploited in the treatment of any of a number of diseases or conditions, most notably cancer.
- a composition of this invention may be active per se, or may act as a “pro-drug” that is converted in vivo to active form.
- PAs of the invention may be incorporated into convenient dosage forms, such as capsules, impregnated wafers, tablets or injectable preparations. Solid or liquid pharmaceutically acceptable carriers may also be employed. Pharmaceutical compositions designed for timed or delayed release may also be formulated.
- compositions contain anti-oxidants, surfactants and/or glycerides.
- anti-oxidants include, but not limited to, BHT, vitamin E and/or C.
- glycerides include, but are not limited to, one or more selected from acetylated or unsubstituted monoglycerides; medium chain triglycerides, such as those found in oils; and caprylocaproyl macrogol-8 glycerides.
- the compounds of the invention are administered systemically, e.g., by injection or oral administration.
- injection may be by any known route, preferably intravenous, subcutaneous, intramuscular, intracranial or intraperitoneal.
- injectables can be prepared in conventional forms, either as solutions or suspensions, solid forms suitable for solution or suspension in liquid prior to injection, or as emulsions.
- Solid carriers include starch, lactose, calcium sulfate dihydrate, terra alba, sucrose, talc, gelatin, agar, pectin, acacia, magnesium stearate and stearic acid.
- Liquid carriers include syrup, peanut oil, olive oil, saline, water, dextrose, glycerol and the like.
- the carrier or diluent may include any prolonged release material, such as glyceryl monostearate or glyceryl distearate, alone or with a wax.
- the preparation may be in the form of a syrup, elixir, emulsion, soft gelatin capsule, liquid containing capsule, sterile injectable liquid (e.g., a solution), such as an ampule, or an aqueous or nonaqueous liquid suspension.
- the pharmaceutical preparations are made following conventional techniques of pharmaceutical chemistry involving such steps as mixing, granulating and compressing, when necessary for tablet forms, or mixing, filling and dissolving the ingredients, as appropriate, to give the desired products for oral or parenteral administration.
- Other preparations for topical, transdermal, intravaginal, intranasal, intrabronchial, intracranial, intraocular, intraaural and rectal administration may also be prepared.
- the pharmaceutical compositions may also contain minor amounts of nontoxic auxiliary substances such as wetting or emulsifying agents, pH buffering agents and so forth.
- the pharmaceutical composition may be administered topically or transdermally, e.g., as an ointment, cream or gel; orally; rectally; e.g., as a suppository, parenterally, by injection or continuously by infusion; intravaginally; intranasally; intrabronchially; intracranially; intraaurally; or intraocularly.
- Intraaural formulations are particularly preferred for the treatment or alleviation of hearing loss due to chemotherapy.
- the compound may be incorporated into topically applied vehicles such as a salve or ointment.
- the carrier for the active ingredient may be either in sprayable or nonsprayable form.
- Non-sprayable forms can be semi-solid or solid forms comprising a carrier indigenous to topical application and having a dynamic viscosity preferably greater than that of water.
- Suitable formulations include, but are not limited to, solution, suspensions, emulsions, creams, ointments, powders, liniments, salves, and the like.
- auxiliary agents e.g., preservatives, stabilizers, wetting agents, buffers, or salts for influencing osmotic pressure and the like.
- Preferred vehicles for non-sprayable topical preparations include ointment bases, e.g., polyethylene glycol-1000 (PEG-1000); conventional creams; gels; as well as petroleum jelly and the like.
- Topical preparations are particularly preferred for the application of the present invention to the control of unwanted hair growth on skin.
- sprayable aerosol preparations wherein the compound, preferably in combination with a solid or liquid inert carrier material, is packaged in a squeeze bottle or in admixture with a pressurized volatile, normally gaseous propellant.
- the aerosol preparations can contain solvents, buffers, surfactants, perfumes, and/or antioxidants in addition to the compounds of the invention.
- a target area e.g., skin surface, mucous membrane, eyes, etc.
- This amount will generally range from about 0.001 mg to about 1 g per application, depending upon the area to be treated, the severity of the symptoms, and the nature of the topical vehicle employed.
- compositions of the invention may be administered alone or in combination with one or more additional compounds that are used to treat the disease or condition.
- the PAs are given in combination with anti-tumor agents, such as mitotic inhibitors, e.g., vinblastine; alkylating agents, e.g., cyclophosphamide; folate inhibitors, e.g., methotrexate, pritrexim or trimetrexate; antimetabolites, e.g., 5-fluorouracil and cytosine arabinoside; intercalating antibiotics, e.g., adriamycin and bleomycin; enzymes or enzyme inhibitors, e.g., asparaginase; topoisomerase inhibitors, e.g., etoposide; or biological response modifiers, e.g., interferon and interleukin-2.
- pharmaceutical compositions comprising any known cancer therapeutic in combination with the PAs disclosed herein are within the scope of this invention.
- the present compounds are administered in combination with one or more polyamine synthesis inhibitors such as, but not limited to, inhibitors of ornithine decarboxylase such as DFMO, aceylenic putrescine, 1-aminooxy-3-aminopropane, antizyme, 2-butylputrescine, cadaverine, L-canaline, 5′-deoxy-5′-[N-methyl-N-[3-(aminooxy)ethyl]amino]adenosine, 5′-deoxy-5′-N-methyl-N-[3-(hydrazinopropyl)amino]adenosine, diaminopropane, 1,3-diamino-2-propanol, 2-difluoromethyl putrescine, difluorophenylethyl(4-aminopropylamidinohydrazone), 2,3-dimethylputrescine, N-dimethylputrescine, 2-e
- S-adenosylmethionine decarboxylase such as SAM486A (4-aminoindanon-1-(2′amidino)hydrazone dihydrochloride monohydrate), S-adenosyl-1,8-diamino-3-thiooctane, S-(5′-adenosyl) methylthio-2-aminooxyethan, S-adenosyl-3-methylthio-1-propylamine, 5′- ⁇ [(Z)-4-amino-2-butenyl]methylamino ⁇ -5′-deoxyadenosine , 5′-amino-5′-deoxyadenosine, 5′-[(aminoiminomethyl)amino]-5′deoxyadenosine dihydrogensulphate, 1-aminooxy-3-aminopropane
- the PAs of the invention may also be used in combination with monoclonal antibodies and tumor vaccines as well as with cellular therapy in subjects undergoing treatment for human diseases such as cancer.
- the PAs may also be used for chemoprevention in subjects at risk for developing cancer wherein one or more PAs are taken alone or in combination with a polyamine synthesis inhibitor to prevent the onset or recurrence of cancer.
- compositions of the invention may also comprise one or more other medicaments such as anti-infectives including antibacterial, anti-fungal, anti-parasitic, anti-viral, and anti-coccidial agents.
- anti-infectives including antibacterial, anti-fungal, anti-parasitic, anti-viral, and anti-coccidial agents.
- Typical single dosages of the compounds of this invention are between about 1 ng and about 10 g/kg body weight.
- the dose is preferably between about 0.01 mg and about 1 g/kg body wt. and, most preferably, between about 0.1 mg and about 100 mg/kg body wt.
- dosages in the range of about 0.01-20% concentration of the compound, preferably 1-5% are suggested.
- a total daily dosage in the range of about 1-500 mg is preferred for oral administration.
- the foregoing ranges are, however, suggestive, as the number of variables in regard to an individual treatment regime is large, and considerable excursions from these recommended values are expected and may be routinely made by those skilled in the art.
- Effective amounts or doses of the compound for treating a disease or condition can be determined using recognized in vitro systems or in vivo animal models for the particular disease or condition.
- many art-recognized models are known and are representative of a broad spectrum of human tumors.
- the compounds may be tested for inhibition of tumor cell growth in culture using standard assays with any of a multitude of tumor cell lines of human or nonhuman animal origin. Many of these approaches, including animal models, are described in detail in Geran, R. I. et al., “Protocols for Screening Chemical Agents and Natural Products against Animal Tumors and Other Biological Systems (Third Edition)”, Canc. Chemother Reports, Part 3, 3:1-112.
- the present invention also provides methods of using the PAs, whether formulated in compositions or not, to inhibit cell growth and proliferation when used alone or in combination with a polyamine synthesis inhibitor. Such methods may be readily conducted by systemic or local administration of the PAs. Local delivery of a PA provides a high local concentration while reducing the likelihood of systemic effects on polyamine metabolism that may result from systemic PA administration.
- the inhibition of cellular growth and proliferation is advantageously conducted with the contemporaneous administration of one or more inhibitors of polyamine synthesis.
- Such inhibition may be applied toward a variety of cell types, including, but not limited to, bacterial cells, fungal cells, and the eukaryotic cells of higher multicellular organisms.
- one or more PAs may be used to inhibit bacterial or fungal cell growth. This embodiment may be advantageously used in both the clinic and agriculture to control bacteria or fungi.
- one or more PAs may be used in combination with an inhibitor of polyamine synthesis to inhibit the growth and/or proliferation of cancer cells, including those of solid tumors. While this latter application may be performed in any multicellular organism, most preferred are applications of the invention for use in human subjects.
- a PA may be used to identify and/or localize a polyamine transporter by virtue of physical binding between the PA and the transporter and the presence of a label linked to the PA.
- Suitable labels are well known in the art, and they permit the identification or localization of the PA either because the label itself emits a detectable signal, or by virtue of its affinity for a label-specific partner which is detectable or becomes so by binding to, or otherwise reacting with, the label.
- labels include, but are not limited to, radioactive isotopes, fluorescent tags, and proteinaceous tags.
- the methods of identification and /or localization provided by the invention may be used in whole or as part of a diagnostic or research protocol.
- the invention also provides preparative uses of the PAs.
- one or more PAs can be used to bind and isolate proteins or other cellular factors that interact with polyamines.
- An exemplar of such a method is the use of a PA to bind to a polyamine transporter and permit its isolation or purification.
- These methods can be performed in solution, where interaction between a PA and a PA binding protein or factor results in a complex that may be subsequently isolated or purified from solution, or in solid phase, where a PA is immobilized and interactions between the PA and a PA binding protein or factor results in a complex of the protein or factor with the immobilized PA.
- PAs analogs were synthesized in a parallel fashion starting from the orthogonally protected diamino containing amino acid starting materials.
- the use of the 4-nitrophenyl activated ester L-Boc-Lys-(Cbz)-ONP in FIG. 1 provides an exemplary illustration of the synthetic process.
- the active ester is added dropwise to a solution of 1.5 equivalents of polyamine in methanol to give a statistical mixture of unsubstituted, mono-substituted and di-substituted acyl polyamines.
- the remaining free amino groups in the polyamine moiety are protected either as their t Boc or Cbz carbamates. Standard workup results in a completely protected crude product mixture.
- the desired orthogonally-protected product is isolated in pure form by silica gel chromatography using standard organic solvents.
- This purification process is based on separation of polyamine molecules with the remaining amino groups being fully protected, which provides a much more lipophilic product mixture that greatly facilitates the purification process.
- the exemplary intermediates containing either 4 Boc groups or 4 Cbz groups in addition to the acyl functionality remained lipophilic enough to purify using standard solvents including a one to one mixture of ethyl acetate and hexanes containing various proportions of methanol (0 to 10%).
- the approach provides two synthetic intermediates, one with 4 Boc and 1 Cbz carbamates and the other with 4 Cbz and 1 Boc carbamates. These intermediates allow the exposure of only one amino group, either the proximal ( ⁇ -) or distal ( ⁇ -), in a selective manner. It is also possible to modify this approach such that both amino groups are exposed for further modification.
- the selective deprotection of either the proximal ( ⁇ -) or distal ( ⁇ -) amino group as shown in FIG. 1 may occur via catalytic hydrogenation or acid treatment, respectively.
- the exposed amino groups were then acylated or alkylated with either an acyl chloride or sulfonyl chloride to produce Series I and II (see FIG.
- the exposed amino groups may also be carboxylic acid activated with standard peptide coupling reagents such as DCC, PyPOP or HBTU (to produce Series III type PAs) or aldehydes under reductive amination conditions (to produce Series IV type PAs). Additional analogs are produced by reductive amination of the free amino precursor with one of the ketone reagents shown in Series V. Series VI analogs are produced via the reductive amination reaction using a free mono- or di-amine precursor and an excess of the carbonyl reagent that are shown in the Series VI chart.
- An alternative method to produce these di-substituted tertiary amine-containing molecules is the conjugate addition of the selectively protected amine precursor to an ⁇ , ⁇ -unsaturated carbonyl compound or an ⁇ , ⁇ -unsaturated nitrile compound.
- TLC thin layer chromatography
- HPLC high performance liquid chromatography
- LC-MS liquid chromatography-mass spectroscopy
- All cell lines were obtained from ATCC (Manassas, Va.) and cultured in the recommended media, serum, and CO 2 concentration. Medias were obtained from Mediatech, Inc. (Herdon, Va.) and serums from Gibco BRL (Gaithersburg, Md.). 50 U/ml penicillin, 50 ⁇ g/ml streptomycin and 2 mM L-glutamine (all from Bio Whittaker, Walkersville, Md.) were included in all cultures. DFMO was obtained from Marion Merrell Dow (Cinncinati, Ohio). When cells were cultured with polyamines or ORI compounds, 1 mM aminoguanidine (AG; Sigma) was included to inhibit serum amine oxidase activity. IC 50 refers to the concentration of PA that results in 50% of maximum cell growth inhibition in the presence of PA alone.
- Cells were plated in 96-well plates such that they would be in log growth for the duration of the assay. The day after plating, PAs were added to the cells, and growth, if any, permitted to continue for six days in the presence of 1 mM AG and 0.5 ⁇ M SPD to insure that any growth inhibition was not the result of depletion of external polyamines in the media. At the end of the six days, cell growth was measured by MTS/PMS dye assay (Cell Titer 96 Aqueous Non-Radioactive Cell Proliferation Assay; Promega, Madison, Wis.).
- EC 50 represents the concentration of PA that resulted in 50% of maximum growth inhibition achievable in the presence of both DFMO (5 mM in all cell lines except MDA) and PA (at different concentrations depending in part on the cell line used) compared to controls.
- IC 50 represents the concentration of PA that resulted in 50% maximum growth inhibition when used alone. Results are shown in Table 3 above.
- Plasma samples from blood—remove 125–150 ⁇ l sample (optimally) into a microfuge tube and mix 1:1 with 0.4M perchloric acid. Vortex and spin down sample at 13000 rpm for 10 minutes in 5° C. centrifuge. Remove 200 ⁇ l supernatant for dansylation as described in dansylation protocol. Plasma samples as small as 25 ⁇ l may be analyzed (for this and the following discussion, any sample that does not yield 200 ⁇ l supernatant for dansylation may have its volume increased to 200 ⁇ l with perchloric acid for the dansylation protocol).
- Tissues Keep samples on ice during preparation. Cut an approximately 100 mg piece from tissue sample and place into 15 ml conical tube. Add 1.2M perchloric acid in a 20:1 vol/weight ratio (i.e. 2 ml/100 mg). Homogenize tissue using a tissue grinder. Vortex sample and remove 1 ml into a microfuge tube. Spin at 13000 rpm for 10 minutes in 5° C. centrifuge. Remove 200 ⁇ l supernatant for dansylation as described in dansylation protocol.
- Alltech C-18 maxi-prep cartridges are used, one for each sample dansylated, to clean any interfering reactions from the samples. This process also places the samples in methanol for application to the HPLC system.
- Each cartridge is placed on a vacuum manifold and washed once with 3 ml MeOH followed by 3 ml H 2 O. Samples are then removed by 1 ml syringe from the glass vials and applied to the Alltech cartridges. Each cartridge is then washed with 10 ml H 2 O and dried 2 ⁇ with 30 cc syringe of air.
- loop overfill is achieved by injecting 100 ⁇ l onto a 20 ⁇ l loop. Samples are kept at 4° C. until injection by a water cooled storage rack on the 231XL auto injector.
Abstract
The disclosed invention provides new polyamine analogs and derivatives containing a hydrophobic region and a polyamine region as well as methods and compositions for their use.
Description
This application is a reissue of Ser. No. 10/296,259, filed Nov. 21, 2002, now U.S. Pat. No. 6,963,010, which is a 371 of PCT/US02/00347, filed Jan. 8, 2002; which claims benefit of Ser. No. 60/260,415, filed Jan. 8, 2001.
The invention in the field of chemistry and biochemistry relates to the synthesis and use of a novel class of polyamine transport inhibitor compounds. These compounds have pharmacological and/or agricultural applications as well as uses in analytical and preparative assays relating to polyamine transport. As pharmaceuticals, these compounds are used to treat disorders of undesired cell proliferation, especially in eukaryotic cells, alone or in combination with other agents such as polyamine synthesis inhibitors.
Decades of research on the myriad of biological activities that the polyamines, putrescine, spermidine and spermine play in cellular processes have shown the profound role they play in life (Cohen, S. S., “A Guide to the Polyamines” 1998, Oxford University Press, New York). As polycations at physiological pH, they bind tightly to and strongly modulate the biological activities of all of the anionic cellular components.
Many stimuli involved in both normal and neoplastic growth activate the polyamine biosynthetic pathway. A great number of multidisciplinary studies have shown that the intracellular concentrations of the polyamines is highly regulated at many steps in their biosynthesis, catabolism and transport. The fact that cells contain such complex apparatus for the tight control of the levels of these molecules shows that only a very narrow concentration range is tolerated.
Polyamine transport into mammalian cells is energy and temperature dependent, saturable, carrier mediated and operates against a substantial concentration gradient (Seiler, N. et al. Polyamine transport in mammalian cells. Int. J. Biochem. 1990, 22, 211-218; Khan, N. A.; Quemener, V. et al. Characterization of polyamine transport pathways, in Neuropharmacology of Polyamines (Carter, C., ed), 1994, Academic, San Diego, pp. 37-60). Ample experimental proof exists that polyamine concentration homeostasis is mediated via this transport system. Changes in the requirements for polyamines in response to growth stimulation is reflected by increases in the transport activity. Stimulation of human fibroblasts to cell proliferation by serum or epidermal growth factor was followed by an 18-100 fold increase in the uptake of putrescine (DiPasquale, A. et al. Epidermal growth factor stimulates putrescine transport and omithine decarboxylase activity in cultures human fibroblasts. Exp. Cell Res. 1978, 116, 317-323; Pohjanpelto, P. Putrescine transport is greatly increased in human fibroblasts initiated to proliferate. J. Cell Biol. 1976, 68, 512-520). Tumors have been shown to have an increased rate of putrescine uptake (Volkow, N. et al. Labeled putrescine as a probe in brain tumors. Science, 1983, 221, 673-675; Moulinoux, J-P. et al. Biological significance of circulating polyamines in Oncology. Cell. Mol. Biol. 1991, 37, 773-783).
Inhibition of polyamine biosynthesis in cells in culture by α-difluoromethylomithine (DFMO), a well-studied mechanism-based inhibitor of ODC, causes a substantial depletion of intracellular putrescine and spermidine with resultant cell growth inhibition. Upon supplementing the culture media with exogenous polyamines this depletion causes transport activity to rise several-fold (Bogle, R. G. et al. Endothelial polyamine uptake: selective stimulation by L-arginine deprivation or polyamine depletion. Am. J. Physiol. 1994, 266, C776-C783; Alhonen-Hongisto, L. et al. Intracellular putrescine deprivation induces uptake of the natural polyamines and methylglyoxal bis (guanylhydrazone). Biochem. J. 1980, 192, 941-945). The cells then returned to their original rate of growth.
Genes for the polyamine transport protein or complex have been cloned from Escherichia coli and yeast (Kashiwagi, K. et al. J. Biol. Chem. 1990, 265, 20893-20897; Tomitori, H. et al. Identification of a gene for a polyamine transport protein in yeast. J. Biol. Chem. 1999, 274, 3265-3267). The genes for the mammalian transporter await identification. A subunit of the transporter from E. coli has been crystallized and its X-ray structure has been determined (Sugiyama, S. et al. Crystal structure of PotD, the primary receptor of the polyamine transport system in Escherichia Coli. J. Biol. Chem. 1996, 271, 9519-9525). This structure represents one of a few but growing number solved for spermidine-binding proteins. Since this structure was determined on a prokaryotic species its use in the design of mammalian transport inhibitors was deemed to be of limited value.
Several researchers have studied the ability of polyamine analogs to inhibit the uptake of 3H-spermidine into cells. Bergeron and coworkers studied the effect of addition of different alkyl group substitutions on the terminal nitrogen atoms of spermidine or spermine analogs (Bergeron, R. J. et al. Antiproliferative properties of polyamine analogs: a structure-activity study. J. Med. Chem. 1994, 37, 3464-3476). They showed that larger alkyl groups diminished the ability to prevent uptake of radiolabeled spermidine. They later concluded that increases in the number of methylenes between the nitrogen atoms decreased the ability to compete for 3H spermidine uptake (Bergeron, R. J. et al. A comparison of structure-activity relationships between spermidine and spermine antineoplastics. J. Med. Chem. 1997, 40, 1475-1494). They also concluded that the polyamine transport apparatus requires only three cationic centers for polyamine recognition and transport (Porter, C. W. et al. J. Cancer Res. 1984, 44, 126-128). Two groups have analyzed literature examples of the polyamine analogs' ability to inhibit 3H spermidine uptake into L1210 cells by CoMFA and QSAR methods (Li, Y. et al. Comparative molecular field analysis-based predictive model of structure-function relationships of polyamine transport inhibitors in L1210 cells. Cancer Res. 1997, 57, 234-239; Xia, C. Q. et al. QSAR analysis of polyamine transport inhibitors in L1210 cells. J. Drug Target. 1998, 6, 65-77).
A radiochemical assay is used for biochemical analysis of transport and has been used to study polyamine transport in yeast and a variety of mammalian cells (Kakinuma, Y et al., Biochem. Biophys. Res. Comm. 216:985-992, 1995; Seiler, N. et al., Int. J. Biochem. Cell Biol. 28:843-861, 1996). See, for example Huber, M. et al. Cancer Res. 55:934-943, 1995.
WO 99/03823 and its corresponding U.S. patent application Ser. No. 09/341,400, filed Jul. 6, 1999, (both of which are hereby incorporated in their entireties as if fully set forth) as well as the recent publications of Burns, M. R.; Carlson, C. L.; Vanderwerf, S. M.; Ziemer, J. R.; Weeks, R. S.; Cai, F.; Webb, H. K.; Graminski, G F. Amino acid/spermine conjugates: polyamine amides as potent spermidine uptake inhibitors. J. Med. Chem. 2001, 44, 3632-44 and Graminski, G. F.; Carlson, C. L.; Ziemer, J. R.; Cai, F., Vermeulen, N. M.; Vanderwerf, S. M.; Burns, M. R. Synthesis of bis-spermine dimers that are potent polyamine transport inhibitors. Bioorg. Med. Chem. Lett. 2002, 12, 35-40 describe some extremely potent polyamine transport inhibitors.
Citation of any reference herein is not intended as an admission that any of the foregoing is pertinent prior art, nor does it constitute any admission as to the contents or date of these documents.
The present invention is directed to novel polyamine analogs and derivatives and methods for their use as drugs, as agricultural or as environmentally useful agents. These novel polyamine analogs and derivatives comprise a hydrophobic moiety covalently attached to a polyamine moiety. These novel PA analogs can be considered to have amphipathic character (hydrophobic as well as charged portions). The polyamine analogs and derivatives of the invention include those that may be viewed as a polyamine acylated with a hydrophobic acyl group, where acylation is by formation of either an amide or a sulfonamide linkage. While the linkage between the hydrophobic acyl group and the polyamine moiety may occur at any amine group within the polyamine, linkages to a primary amine functionality are preferred.
The analogs and derivatives of the invention are potent inhibitors of cellular polyamine transport. Without being bound by theory, they are inferred to bind to a cell's polyamine transporter apparatus with very high affinity. They may be used independently or in combination with the inhibition of cellular polyamine synthesis, even in the presence of exogenously supplied spermidine, to inhibit cell growth and proliferation.
The analogs and derivatives of the invention include those encompassed by the following formula I:
R—X-polyamine
R—X-polyamine
wherein R is selected from H or from the group of a straight or branched C1-50 saturated or unsaturated aliphatic, carboxyalkyl, carbalkoxyalkyl, or alkoxy; a C1-8 alicyclic; a single or multiring aryl substituted aliphatic; an aliphatic-substituted single or multiring aromatic; a single or multiring heterocyclic; a single or multiring heterocyclic aliphatic; a C1-10 alkyl; an aryl sulfonyl; or cyano;
“X” may be —CO—, —SO2—, or —CH2—, and
“polyamine” may be any naturally occurring, such as putrescine, spermine or spermidine, or synthetically produced polyamine.
Preferably, R is at least about C5, at least about C10, at least about C11, at least about C12, at least about C13, at least about C14, at least about C15, at least about C16, at least about C17, at least about C18, at least about C19, at least about C20, or at least about C22.
The linkage between X and the polyamine may be direct, wherein there are no atoms between X and the nitrogen of the amine group of the polyamine, or indirect, where there may be one or more atoms between X and the nitrogen of the amine group of the polyamine. The linkage between X and the polyamine may occur via any amino group within the polyamine, although a primary amino group is used in preferred embodiments of the invention.
In preferred embodiments of the invention where the linkage between X and the polyamine is indirect, the intervening one or more atoms are preferably those of an amino acid or a derivative thereof. In particularly preferred embodiments of this type, the intervening one or more atoms are those of lysine, aspartic acid, glutamic acid, omithine, or 2,4-diaminobutyric acid. Preferred compounds of this type may be represented as
R—X-L-polyamine
wherein R is a straight or branched C10-50 saturated or unsaturated aliphatic, carboxyalkyl, carbalkoxyalkyl, or alkoxy; a C1-8 alicyclic; a single or multiring aryl substituted or unsubstituted aliphatic; an aliphatic-substituted or unsubstituted single or multiring aromatic; a single or multiring heterocyclic; a single or multiring heterocyclic aliphatic; an aryl sulfonyl;
R—X-L-polyamine
wherein R is a straight or branched C10-50 saturated or unsaturated aliphatic, carboxyalkyl, carbalkoxyalkyl, or alkoxy; a C1-8 alicyclic; a single or multiring aryl substituted or unsubstituted aliphatic; an aliphatic-substituted or unsubstituted single or multiring aromatic; a single or multiring heterocyclic; a single or multiring heterocyclic aliphatic; an aryl sulfonyl;
X is —CO—, —SO2—, or —CH2—; and
L is a covalent bond or a naturally occurring amino acid, omithine, 2,4-diaminobutyric acid, or derivatives thereof.
The analogs and derivatives of the invention, may be optionally further substituted at one or more other positions of the polyamine. These include, but are not limited to, internal nitrogen and/or internal carbon atoms. In one aspect of the invention, preferred substituents are structures that increase polyamine transport inhibition, binding affinity or otherwise enhance the irreversibility of binding of the compound to a polyamine binding molecule, such as the polyamine transporter, an enzyme or DNA. Such additional substituents include the aziridine group and various other aliphatic, aromatic, mixed aliphatic-aromatic, or heterocyclic multi-ring structures. Reactive moieties which, like aziridine, bind covalently to a polyamine transporter or another polyamine binding molecule, are also within the scope of this invention. Examples of reactive groups that react with nucleophiles to form covalent bonds include chloro-, bromo- and iodoacetamides, sulfonylfluorides, esters, nitrogen mustards, etc. Such reactive moieties are used for affinity labeling in a diagnostic or research context, and may contribute to pharmacological activity in inhibiting polyamine transport or polyamine synthesis. The reactive group can be a reactive photoaffinity group such as an azido or benzophenone group. Chemical agents for photoaffinity labeling are well-known in the art (Flemming, S. A., Tetrahedron 1995, 51, 12479-12520).
A preferred aspect of the invention relates to a polyamine analog or derivative that is a highly specific polyamine transport inhibitor with pharmaceutical utility as an anti-cancer chemotherapeutic. One class of a polyamine analog or derivative of the invention that binds to a polyamine-binding site of a molecule and/or inhibits polyamine transport, is described by the following formula II:
wherein a, b, and c independently range from 1 to 10; d and e independently range from 0 to 30; each X is independently either a carbon (C) or sulfur (S) atom, and R1 and R2 are as described below, or each of R1X{O}n— and R2X{O}n— are independently replaced by H; and * denotes a chiral carbon position. Where if X is C, then n is 1; if X is S, then n is 2; and if X is C, then the XO group may be CH2 such that n is 0.
In the above formula, R1 and R2 are independently selected from H or from the group of a straight or branched C1-50 saturated or unsaturated aliphatic, carboxyalkyl, carbalkoxyalkyl, or alkoxy; a C1-8 alicyclic; a single or multiring aryl substituted aliphatic; an aliphatic-substituted single or multiring aromatic; a single or multiring aromatic or saturated heterocyclic; a single or multiring heterocyclic aliphatic; a C1-10 alkyl; an aryl sulfonyl; or cyano.
Examples of heterocyclic rings as used herein include, but are not limited to, pyrrole, furan, thiophene, imidazole, oxazole, thiazole, pyrazole, 3-pyrroline, pyrrolidine, pyridine, pyrimidine, purine, quinoline, isoquinoline, and carbazole.
All of the above described aliphatic, carboxyalkyl, carbalkoxyalkyl, alkoxy, alicyclic, aryl, aromatic, and heterocyclic moieties may, of course, also be optionally substituted with 1-3 substituents independently selected from halo (fluoro, chloro, bromo or iodo), lower alkyl (1-6C) and lower alkoxy (1-6C).
As used herein, carboxyalkyl refers to the substituent —R′—COOH wherein R′ is alkylene; and carbalkoxyalkyl refers to —R′—COOR wherein R′ and R are alkylene and alkyl respectively. In preferred embodiments, alkyl refers to a saturated straight- or branched-chain hydrocarbyl radical of 1-6 carbon atoms such as methyl, ethyl, n-propyl, isopropyl, n-butyl, t-butyl, n-pentyl, 2-methylpentyl, n-hexyl, and so forth. Alkylene is the same as alkyl except that the group is divalent. Aryl or alkyl sulfonyl moieties have the formula —SO2R, and alkoxy moieties have the formula —O—R, wherein R is alkyl, as defined above, or is aryl wherein aryl is phenyl, optionally substituted with 1-3 substituents independently selected from halo (fluoro, chloro, bromo or iodo), lower alkyl (1-6C) and lower alkoxy (1-6C).
A preferred group of compounds encompassed by the above is where d is 4 and e is 0.
An additional class of a polyamine analog or derivative of the invention that binds to a polyamine-binding site of a molecule and/or inhibits polyamine transport, is described by the following formula III:
wherein a, b, and c independently range from 1 to 10 and d and e independently range from 0 to 30. R1 and R2 are defined as above for formula II and R3 and R4 are independently selected from organic substituents including —CH3 and as defined above for R1 and R2 in formula II above. This grouping of analogs is produced by reductive amination of the free amino precursor with a ketone. Some members of this group of analogs are shown in Series V (see
In one preferred embodiment of the invention, R1 and R2 are identical and as described for formula II. Positions R3 and R4 may also be identical, and all of R1 through R4 may also be identical. Additionally, each of positions R1, R2, R3 and R4 in formula III may also be independently H.
In an additional aspect of the invention the proximal and/or the distal amino group relative to the polyamine (such as spermine) can be di-alkylated to form tertiary amines. These materials can be synthesized by reductive amination with a large excess of the carbonyl component. Additionally, these materials may be produced by a conjugate addition of the amine precursor to an α,β-unsaturated carbonyl or α,β-unsaturated nitrile. Each of R1, R2, R3 and R4 can be independently varied and are as defined as above for formula III. Each of R1, R2, R3 and R4 may also be independently H. The values of a, b, c, d and e are as described above for formula III. This aspect of the invention is depicted in the following formula IV:
In a further aspect of the invention, compounds which lack the proximal or distal amino group on the acyl portion of the molecule are also provided. These are represented by formula V:
where Z1 is NR1R3 and Z2 is selected from —R1, —CHR1R2 or —CR1R2R3 (wherein R1, R2, and R3 are as defined above for formula III) or Z2 is NR2R4 and Z1 is selected from —R1, —CHR1R2 or —CR1R2R3 (wherein R1, R2, and R3 are as defined above for formula III) Values for a, b, and c independently range from 1 to 10; d and e independently range from 0 to 30. Compounds encompassed by formula V may be prepared by first coupling amino acid derivatives (modified to contain the non-amine containing Z group) to a polyamine followed by appropriate derivatization of the amine containing Z group. Chemistries for such reactions are known in the art and disclosed herein.
In preferred embodiments of the invention, positions R1, R2, R3 and R4 of all the formulas set forth above are independently selected from the following, where each of g, h, i, j, and k are independently selected from 0 to 15:
The present invention includes the free base or acid forms, as well as salts thereof, of the polyamine analogs and derivatives described by the above formulas. The invention also includes the optical isomers of the above described analogs and derivatives, especially those resulting from the chiral center indicated above with a *. In a further embodiment of the invention, mixtures of enantiomers and/or diastereoisomers, resulting from a single preparative step, combination, or interconversion are encompassed.
The invention also provides prodrug forms of the above described analogs and derivatives, wherein the prodrug is metabolized in vivo to produce an analog or derivative as set forth above. Indeed, some of the above described analogs or derivatives may be a prodrug for another analog or derivative.
In another aspect of the invention, compositions containing the above described analogs and derivatives are provided. Preferably, the compositions are formulated to be suitable for pharmaceutical or agricultural use by the inclusion of appropriate carriers or excipients.
In a further aspect of the invention, methods for the use of the above described analogs and derivatives, as well as compositions, are provided. These methods include uses of the invention's polyamine compounds to inhibit polyamine transport, as well as treat human and agricultural diseases and conditions. Examples of human diseases and conditions include, but are not limited to, cancer, osteoporosis, asthma, autoimmune diseases, rheumatoid arthritis, systemic lupus erythematosus, Type I insulin-dependent diabetes, tissue transplantation, African sleeping sickness, psoriasis, restenosis, inhibition of unwanted hair growth as cosmetic suppression, hyperparathyroidism, inflammation, treatment of peptic ulcer, glaucoma, Alzheimer's disease, suppression of atrial tachycardias, stimulation or inhibition of intestinal motility, Crohn's disease and other inflammatory bowel diseases, high blood pressure (vasodilation), stroke, epilepsy, anxiety, neurodegenerative diseases, hyperalgesic states, protection against hearing loss (especially cancer chemotherapy induced hearing loss), and pharmacological manipulation of cocaine reinforcement and craving in treating cocaine addiction and overdose and other fungal bacterial, viral, and parasitic diseases. These compounds also find use as agents for use in the trans-cellular delivery of nucleic acids used in anti-sense DNA therapies for numerous disease states. The invention's polyamine compounds may be utilized as, but not limited to being, a soil additive or conditioner in agricultural applications.
The present inventors have designed novel polyamine analogs and derivatives for the inhibition of polyamine transport and other uses. These analogs and derivatives are inferred to bind polyamine transporters with high affinity and inhibit polyamine transport, either competitively or non-competitively. Thus these compounds can alter polyamine metabolism in cells by reducing or preventing polyamine uptake.
In particularly preferred embodiments of the invention, one or more polyamine analogs and derivatives are used in combination with polyamine synthesis inhibitors to inhibit cell growth and proliferation. As such, they are useful as drugs in a number of diseases, particularly cancer and other conditions involving cellular proliferation, including, but not limited to, inflammatory diseases or conditions where components of the immune system undergo undesired proliferation. Non-limiting examples include asthma, autoimmune diseases, rheumatoid arthritis, systemic lupus erythematosus, Type I insulin dependent diabetes, psoriasis, restenosis, inhibition of unwanted proliferation of hair on skin, tissue transplantation, African sleeping sickness, osteoporosis, hyperparathyroidism, treatment of peptic ulcer, glaucoma, Alzheimer's disease, suppression of atrial tachycardias, stimulation or inhibition of intestinal motility, Crohn's disease and other inflammatory bowel diseases, high blood pressure (vasodilation), stroke, epilepsy, anxiety, neurodegenerative diseases, hyperalgesic states, the protection of hair cells from chemotherapy induced loss of hearing, and pharmacological manipulation of cocaine reinforcement and craving in treating cocaine addiction and overdose, and other fungal, bacterial, viral, and parasitic diseases.
As used herein, the term “polyamine” includes putrescine, spermine or spermidine, as well as longer linear polyamines, branched polyamines, and the like, which may have between 2 and about 10 nitrogens. Also included in this definition are polyamine derivatives or analogs comprising a basic polyamine chain with any of a number of functional groups bound to a C atom or a terminal or internal N atom. For modification at a primary amino group, a polyamine must, of course, contain such a group.
Polyamine “analogs” and/or “derivatives” generally refer to any modified polyamine molecule disclosed or described herein. These molecules are generally modifications of existing polyamines, whether naturally occurring or synthetically produced, and may also be referred to as “polyamine agents”, “PA” or “agents” of the invention. Preferred PAs bind and/or inhibit cellular polyamine transport, and as such may also be referred to as “transport binding molecules” or “polyamine transport inhibitors”. The scope of this definition includes any modification to produce a PA from an existing polyamine or the isolation of a structurally identical PA from a naturally occurring source. Preferably, the modification is the addition of one or more chemical moieties to the polyamine.
APA that is an “inhibitor” polyamine analog or derivative (a) binds to polyamine transporters better than a native polyamine and/or (b) by some means blocks the uptake of a polyamine into a cell or a subcellular polyamine transporter preparation. The invention includes PAs that efficiently inhibit polyamine transporters in different eukaryotic cell types as well as inhibit cellular growth and proliferation when used in combination with a polyamine synthesis inhibitor.
The PAs of the invention generally have an acylated primary amine functionality and are expected to bind to a cell's polyamine transporter apparatus with a very high affinity. Measurements of Ki were determined by using an assay that shows the inhibition of polyamine uptake, such as uptake of 3H-spermidine.
The PAs were also analyzed with a secondary assay to show inhibition of cellular polyamine uptake based on a measurement of cellular growth inhibition in combination with a potent inhibitor of polyamine biosynthesis. This assay was conducted in the presence of polyamines, such as spermidine, to determine a PA's ability to prevent the uptake of polyamines thereby overcoming the polyamine biosynthesis inhibition with DFMO (difluoromethylomithine). Due to the trend that polyamine mono-anides give high potency in both of these assays, it has been inferred, without limiting the invention thereto, that there is a site on the transporter protein for tight binding of the inhibitor's amide functionality.
Preferred embodiments of these PAs are the result of acylation at a polyamine molecule with two or more primary amine groups. The linkage between the acyl group and the primary amine group is preferably an amide linkage (indicated below as the bond between “CO” and “NH”) and results in a molecule with the following general formula.
rest of acyl group-CO—NH-rest of polyamine
rest of acyl group-CO—NH-rest of polyamine
As noted above, other linkages, whether direct or indirect, may also be used. The “polyamine” in the above formula may be any polyamine with at least one primary amine group, but more preferably with two or more primary groups, for linkage to the acyl group.
One preferred class of acyl groups for inclusion in the above formula contains two primary amines for further acylation. The resultant class of PAs may be described by the following formula (formula II).
as defined above. Non-limiting examples of alkyl moieties as present in these compounds include straight or branched chains of at least about 8 carbon atoms for increased hydrophobicity (or lipophilicity), such as at least about 10, at least about 12, at least about 14, at least about 16, at least about 18, at least about 20, at least about 22, at least about 24, at least about 26, at least about 28, and at least about 30. In yet another set of preferred embodiments, the chain is of at least about 19, 21, 23, 25, or 27 carbon atoms, with at least about 20 to at least about 24 or 26 as even more preferred.
A particularly preferred group of PAs encompassed by the above formula is where d is 4 and e is 0, although generally excluded from this group are PAs where R2X{O}n— is an H and R1X{O}n— is R1SO2— wherein R1 is a thiophene moiety linked to the S atom via the 2 position, and substituted at the 5 position, of the thiophene. Preferably excluded are such PAs wherein the substitution at the 5 position includes an amide linkage. Also preferably excluded are such PAs wherein the amide linkage is attached to a chlorinated aromatic group, such as the compound identified as ORI 1340 in U.S. patent application Ser. No. 09/396,523, filed Sep. 15, 1999.
Other classes of PAs as encompassed by the invention are set forth as formulas I, III, IV, and V as described above. In all of the formulas of the invention, the term “single or multiring alicyclic” includes adamantyl type structures. Moreover, the term “substituted” used in conjunction with the description of any chemical moiety for a formula of the invention includes the attachment of the moiety to the rest of the formula by way of the “substitution”. The term also indicates that “unsubstituted” forms of the described chemical moiety is also within the scope of the invention.
By analyzing the relationship between a polyamine analog's structure and its ability to act as a polyamine transport inhibitor, it was discovered that increases in the lipophilic character of the hydrophobic substituent on the polyamine may increase transport inhibition. While the nature of the interaction between a lipophilic polyamine analog and the polyamine transport apparatus remains unclear at this time, the invention includes, but is not limited to, situations where the hydrophobic (lipophilic) moiety may serve as an anchor to some hydrophobic pocket on the transporter or in a region nearby. This may result in the interaction of the polyamine portion of the analog with the polyamine transporter.
There are a number of ways one might analyze the hydrophobic character of compounds described in the present invention. The following two scales describe ways to measure relative degrees of lipophilicity.
The logP coefficient is the logarithm of the ratio of distribution of a compound in a mixture of 1-octanol and H2O. Compounds with logP values greater than 1 are considered lipophilic (greater solubility in 1-octanol versus H2O). The presence of ionizable groups in the compound has a dramatic effect on this parameter. Ionization will greatly increase a compound's H2O solubility. For this reason, a compound's ionization potential must be taken into consideration when correlating lipophilicity with activity. One can use a variety of computerized protocols to perform calculated estimates of the logP value. One such computer program is ChemDraw Pro Version 5.0 from Cambridge-SoftCorporation. One of the several methods that this program uses to calculate the logP coefficient is through Crippen's fragmentation method (Crippen et. al., J. Chem. Inf. Comput. Sci. 1987, 27, 21). The present invention used this method to calculate logP values for fragments of the described molecules. These fragments were generated in the fashion depicted in FIG. 5 . The results of these calculations are provided in Table 1 for the D-stereoisomers of the ε-acyl substituted Lys-spm conjugates (FIG. 2 , Series I) and in Table 2 for the D-stereoisomers of the ε-alkyl substituted Lys-spm conjugates (FIG. 2 , Series IV and V).
| TABLE 1 |
| Chemical structure (with ID relative to FIG. 2), logP Calculations, HPLC data and average EC50 |
| values for D-stereoisomerS of ε-acyl-substituted spermine based analogs (FIG. 2, Series I). |
| |
| Ret | Ave | |||
| Time- | EC50 | |||
| ID | Structure | LogP | Std | value |
| IB38 |
|
1.73 | 9.63 | 13 |
| IB37 |
|
1.03 | 6.33 | 41 |
| IB2 |
|
6.59 | 21.1 | 0.083 |
| IB4 |
|
5.68 | 15.82 | 0.084 |
| IB8 |
|
1.57 | 6.07 | 3.5 |
| IB26 |
|
2.01 | 6.34 | 1.1 |
| IB36 |
|
1.21 | 4.91 | 27 |
| IB34 |
|
0.75 | 4.6 | 8.5 |
| IB6 |
|
2.58 | 10.48 | 2.2 |
| IB7 |
|
2.03 | 6.83 | 13 |
| IB9 |
|
1.12 | 5.16 | 12 |
| IB33 |
|
−0.05 | 3.56 | 8.4 |
| IB10 |
|
0.2 | 3.46 | 12 |
| IB32 |
|
0.97 | 5.29 | 3.6 |
| IB30 |
|
1.68 | 7.4 | 2 |
| IB29 |
|
1.99 | 6.08 | 2.1 |
| IB25 |
|
−0.44 | |
10 |
| IB24 |
|
0.58 | 4.23 | 30 |
| VA- 21 |
|
1.04 | 10.11 | 0.65 |
| 1426 |
|
Not calc'd | 6.68 | 3.7 |
| IA4 |
|
5.68 | 15.79 | 0.13 |
Preferred PAs of the invention with respect to Series I type compounds are those with low EC50 values, such as those with below about 5, about 6, about 7, about 8, about 9, about 10, about 15, about 20 or about 25 minute HPLC retention times.
| TABLE 2 |
| Chemical structure (with ID relative to FIG. 2), calculated logP value, HPLC retention time, and average |
| EC50 value for ε-alkylated spermine based analogs (FIG. 2, Series IV and V). |
| Series I compound are included for comparison. |
| Ret | Ave | |||
| Time- | EC50 | |||
| ID | Structure | LogP | Std | value |
| VB28 |
|
2.01 | 13.89 | 1.45 |
| IVB28 |
|
2.21 | 9.4 | 12.8 |
| VA22 |
|
1.84 | 10 | 2.42 |
| VA27 |
|
2.31 | 12.71 | 26.8 |
| VA26 |
|
1.74 | 10.84 | 4.14 |
| IVB23 |
|
0.66 | 9.05 | 1.79 |
| IVB3 |
|
0.91 | 9.16 | 2.19 |
| IVB21 |
|
1.12 | 9.62 | 1.32 |
| IVB24 |
|
1.46 | 9.35 | 1.32 |
| IVB22 |
|
1.92 | 9.85 | 0.68 |
| IVB6 |
|
2.28 | 10.87 | 0.89 |
| IVB5 |
|
1.83 | 10.27 | 0.71 |
| IVB33 |
|
2.45 | 10.01 | 1.38 |
| IVB27 |
|
1.68 | 10.31 | 0.61 |
| IVB25 |
|
0.57 | 9.89 | 0.89 |
| VA21 |
|
1.04 | 10.11 | 0.65 |
| 1426 |
|
Not calc'd | 6.68 | 3.68 |
| IA4 |
|
5.68 | 15.79 | 0.13 |
Preferred PAs of the invention with respect to Series IV and V type compounds are those with low EC50 values, such as those with below about 5, about 6, about 7, about 8, about 9, about 10, about 12, about 14, about 16, about 18, or about 20 minute HPLC retention times.
Another way to measure relative hydrophobicity would be chromatographic techniques such as comparison of HPLC retention times on C18 reverse phase columns, longer retention times would represent greater relative hydrophobicity. The present invention utilized a dansylation protocol to form dansyl derivatives of the described analogs and analyzing these derivatives by fluorescence detection on C18 reverse phase HPLC. The difference between the elution of the peak due to the analog and the peak due to an internal standard (1,7-diaminoheptane) is shown for several representative analogs in Tables 1 and 2 above.
The relationship between calculated logP values and the HPLC retention time of the dansylated derivatives are plotted in FIGS. 6 and 9 for Series I and IV type compounds, respectively. The relationship between calculated logP and average EC50 values are plotted in FIGS. 7 and 10 for Series I and IV type compounds, respectively. The relationship between HPLC retention times and average EC50 values are plotted in FIGS. 8 and 11 for Series I and IV type compounds, respectively.
An additional compound hydrophobicity scale, specific for amino acids, was devised and measured by R. Wolfenden (Wolfenden, R.; Andersson, L.; Cullis, P. M.; Southgate, C. C. B. Affinities of amino acid side chains for solvent water Biochemistry, 1981, 20, 849-855.). They measured the equilibria of distribution of amino acid side chains between their dilute aqueous solutions and the vapor phase. They describe a scale of “hydration potentials” whereby buffered H2O-vapor phase distribution measurements were made on the side-chain portions of the amino acids (e.g. methane for alanine, methanol for serine, n-butylamine for lysine or n-propylguanidine for arginine). If a side-chain had the potential for ionization a correction was made such that only the un-ionized fraction was considered. This was based on calculation of the un-ionized fraction using literature pKa values. The side chains for the twenty naturally occurring amino acids span a range of free energy values for the transfer from the vapor phase to H2O from 2.39 kcal/mol for hydrogen (glycine) or 1.94 kcal/mol for methane (alanine) to −7.00 kcal/mol for n-butylamine (lysine) or −14.6 kcal/mol for n-propylguanidine (arginine).
These values form a “hydration potential” scale, which is correlated with the potential that a given amino acid would be present on the outside, or hydrophilic portion of a protein versus the more hydrophobic interior of a protein. The authors state “that the energetic cost of removing hydrophilic side chains from water is much greater than the cost of pulling hydrophobic side chains into water, and, indeed, it has been observed that hydrophobic residues occur rather often at the surfaces of proteins.” The present invention could use this scale to describe the lipophilicity of the substituent attached to the polyamine. The polyamine portion is removed before this analysis. As an example, it is also required that the α-amino and α-carboxylate groups of any analogs containing an α-amino acid be removed before analysis. By using this scale, any substituent with a free energy of transfer from the vapor phase to H2O less than that determined for n-butylamine (and thus correlated to lysine) of −7.00 kcal/mol would be expected to be a preferred polyamine transport inhibitor in comparison to the lysine-spermine conjugate (ORI 1202). This means any substituent that gives a hydration potential greater (more positive) than −7.00 kcal/mol, as defined in this scale, results in polyamine transport inhibitors with significant activity (values of free energy of transfer which are more negative mean a given compound would have a greater solubility in H2O than the vapor phase).
The preferred group of PAs wherein d is 4 and e is 0 includes both the L and D-stereoisomers due to the chiral carbon indicated by * in the above formula. Exemplary PAs such as ORI 1202 (L-Lys-spm), 1426 (D-Lys-spm), and those containing IA4 (FIG. 2 ) demonstrated potency in both the transporter inhibition and cell growth inhibition assays described below. PA ORI 1202 also displayed effectiveness in several anti-cancer mouse xenograft models. See Weeks, R. S., Vanderwerf, S. M., Carlson, C. L., Burns, M. R., O'Day, C. L., Cai, C. F., Devens, B. H., and Webb, H. K. Exp. Cell Res. 2000, 261, 293-302. and Devens, B. H., Weeks, R. S., Burns, M. R., Carlson, C. L., and Brawer, M. K. Prostate Cancer and Prostatic Diseases 2000, 3, 275-279.
Additional modification of the two primary amine groups in the acyl group in the above formula is readily accomplished by the availability of the primary amine groups for selective functionalization together with the commercial availability of orthogonally di-protected versions of H2N (CH2)nCH(NH2)COOH type molecules (where n ranges from 1 to 50 for example), such as lysine and omithine.
Without being bound by theory, increases in the lipophilicity of the substituent at the above R1 and R2 positions may dramatically increase the affinity for the polyamine transporter. Increases in lipophilicity in the PAs of the invention may improve the inhibition of polyamine transport due to the presence of both hydrophilic and hydrophobic domains. Biological systems have a significant chemical problem when they attempt to move a very hydrophilic substance, such as polycationic polyamines, across their very hydrophobic outer membrane barriers. If the transporter moves the polyamines in their polycationic forms across this barrier, the transporter may do so via some mechanism for masking or minimizing their hydrophilicity. Mechanisms for this may include the formation of specific salt bridges between the polyamine and negatively charged residues on the protein or formation of a charged interior in the intermembrane pore. Because polyamine transport is known to be an energy dependant process, the transporter may have the task of providing a very specific polyamine shaped hydrophilic pore in the presence of the very hydrophobic environment of the membrane. For these reasons the transporter likely has hydrophobic residues for interactions with the membrane in close proximity to hydrophilic residues specific for interactions with the polyamine.
By designing PAs that contain both hydrophobic and hydrophilic domains, the present invention exploits the likely characteristics of a polyamine transporter to improve transport inhibition. Thus the present invention provides several series of PAs that contain both a polyamine-mimicking portion and a hydrophobic membrane-mimicking portion. These PAs have been inferred to have great affinity for the transporter, and they show substantially increased growth inhibition (in combination with a polyamine synthesis inhibitor) in comparison to PAs lacking a significantly hydrophobic domain. Probably for very similar reasons, the present PAs are also expected to show improved bioavailability through oral administration. Increases in lipophilicity are expected to enhance absorption after oral uptake.
It is also expected that the introduction of both hydrophilic and hydrophobic domains in the same molecule, as shown by those in the present invention, will also enable them to facilitate the transfer of nucleic acids through biological membranes. This property gives the analogs usefulness as transfer agents for anti-sense DNA for a number of scientific, analytical, diagnostic and therapeutic applications.
The above is supported by analysis of the results of extending a straight-chain aliphatic saturated hydrocarbon at position R (see FIG. 2 , Series I) results in increases in cell growth inhibition in the presence of a polyamine synthesis inhibitor. The clear trend that longer hydrocarbon chains on this amide position increase potency is indicated by a comparison of spermine based compounds IA4, IA8, and IA11 as well as IB4, IB7, and IB8 (see Table 3). FIG. 4 shows the relationship between the length of the hydrocarbon substituent at the R position and the resulting EC50 value in the presence of a polyamine synthesis inhibitor.
Table 3 shows the results from analysis of various exemplary PAs for their ability to inhibit cellular growth in combination with DFMO relative to control cells left untreated. EC50 refers to the concentration of PA resulting in 50% of maximum cell growth inhibition in the presence of both DFMO and the PA. Ki refers to the inhibition constant for polyamine transport based on double reciprocal Lineweaver-Burke plot analyses of four radioactive substrate concentrations (0.3–3 μM) and five inhibitor concentrations (0.01–1.0 μM) and a control. Compounds ORI 1202 and 1426 are included for comparison. See the Examples below.
| TABLE 3 |
| EC50 values (μM) of representative polyamine analogs (see FIG. 2) determined in the presence |
| of DFMO (1-5 mM). Also shown are the IC50 results from analyses of various exemplary PAs. |
| IC50 refers to the concentration of PA that results in 50% of maximum cell growth inhibition |
| in the presence of PA alone. |
| Cell Line EC50 (μ M) | AVG. EC50 | Cell Line IC50 (μ M) | Ki |
| Analog | A375 | MDA-MB-231 | PC-3 | SK-OV-3 | (μM) | A375 | MDA-MB-231 | PC-3 | SK-OV-3 | (μM) |
| IA40 | 29.8 | 7.87 | >300 | >300 | 0.039 | |||||
| 41.3 | 8.51 | >300 | >300 | |||||||
| IC41 | 36.9 | 16.9 | >300 | 430 | 0.191 | |||||
| 1202 | 1.49 | 4.75 | 5.3 | 0.5 | 4.542 | >300 | 560 | 0.031 | ||
| 2.5 | 1.7 | 0.51 | ||||||||
| 2.5 | 1.24 | |||||||||
| 13.5 | 1.24 | |||||||||
| 6.9 | 10.3 | |||||||||
| 8.7 | 0.822 | |||||||||
| 8.4 | 7.78 | |||||||||
| 4.35 | 4.1 | |||||||||
| 6.2 | ||||||||||
| 2.6 | ||||||||||
| IVE30 | 4.2 | 1.7 | ||||||||
| IIA21 | 1.4 | 0.46 | ||||||||
| IB41 | 31.9 | 6.73 | ||||||||
| 1426 | 1.91 | 4.5 | 5 | 0.51 | 2.254 | 1620 | 1840 | 1840 | 2530 | 0.034 |
| 1.29 | 1.5 | 8.02 | 0.93 | >100 | >100 | >100 | >100 | |||
| 2.2 | 1.27 | 0.55 | 6.09 | >300 | >300 | >300 | >300 | |||
| 1.75 | 4.25 | 2.12 | 1.36 | >100 | >300 | >300 | >300 | |||
| 0.829 | 2.02 | 0.704 | 1.41 | >100 | >100 | >100 | >300 | |||
| 2.7 | 1.27 | 0.52 | 0.53 | >100 | >100 | >100 | >100 | |||
| 2.1 | 0.26 | 2.7 | >100 | >100 | >100 | |||||
| 3.99 | 0.89 | >100 | >100 | >100 | ||||||
| 3.1 | 2.98 | 0.68 | >100 | |||||||
| 4 | 2.7 | |||||||||
| IIA20 | 0.405 | 1.61 | 0.463 | 2.65 | 1.282 | >30 | >30 | >30 | >30 | |
| IA4 | 0.049 | 0.194 | 0.129 | 0.273 | 0.077 | >30 | >30 | >30 | >30 | 0.0015 |
| 0.049 | 0.057 | 0.028 | 0.069 | 61.5 | 62.4 | >3 | >3 | |||
| 0.008 | 0.017 | <0.001 | 0.252 | >3 | >3 | >3 | >3 | |||
| 0.005 | 0.005 | 0.001 | 0.049 | >3 | >3 | >3 | >3 | |||
| 0.004 | 0.009 | <0.1 | <0.1 | >3 | >3 | 18.1 | 18.6 | |||
| <0.1 | <0.1 | 0.182 | 58.3 | 62 | >3 | |||||
| IA28 | 1.66 | >30 | 0.982 | >30 | >30 | >30 | >30 | >30 | ||
| IA19 | 0.214 | >30 | >30 | >30 | >30 | >30 | >30 | >30 | ||
| IA11 | >30 | >30 | 2.3 | >30 | >30 | >30 | >30 | >30 | ||
| IB4 | 0.071 | 0.168 | 0.197 | 0.297 | 0.105 | >30 | >30 | >30 | >30 | 0.017 |
| <0.01 | <1 | 0.044 | 0.121 | 23.1 | 58.9 | 26.1 | 27.7 | |||
| 0.026 | 0.031 | 0.177 | 0.175 | >3 | >30 | >3 | >3 | |||
| 0.015 | 0.072 | 0.09 | 0.121 | >3 | >3 | >3 | >3 | |||
| <0.1 | 0.051 | <0.1 | <0.1 | 55.4 | >3 | 15.8 | 12.6 | |||
| 0.011 | <0.1 | 0.116 | 0.157 | >3 | 56 | >3 | >3 | |||
| 0.06 | >3 | |||||||||
| IIA17 | 0.629 | <1 | 0.18 | 2.59 | >30 | 605 | >30 | >30 | ||
| IIA2 | >30 | >30 | >30 | >30 | >30 | >30 | ||||
| IA7 | 2.3 | 1.12 | 1.35 | >30 | 6.229 | >30 | >30 | >30 | >30 | |
| 1.75 | 0.853 | 30 | >30 | >30 | >30 | |||||
| IA24 | 1.56 | >30 | >30 | >30 | ||||||
| IB24 | >30 | >30 | >30 | >30 | >30 | >30 | >30 | >30 | >30 | |
| IB7 | 2.61 | >30 | 1.27 | >30 | 11.210 | >30 | >30 | >30 | >30 | |
| 4.87 | 19 | 28.3 | >30 | >30 | >30 | |||||
| IIB2 | 7.25 | 3.64 | >30 | >30 | >30 | >30 | ||||
| ID24 | 5.98 | 4.75 | 3.3 | >30 | >30 | >30 | >30 | >30 | ||
| ID7 | 5.29 | 8.25 | 7.42 | 17.2 | 9.540 | >30 | >30 | >30 | >30 | |
| IID17 | 5.87 | 5.1 | 4.09 | 23.9 | 9.740 | >30 | >30 | >30 | >30 | |
| IID2 | >30 | >30 | >30 | >30 | >30 | >30 | >30 | >30 | >30 | |
| ID25 | 8.78 | 8.76 | 5.27 | >30 | >30 | >30 | >30 | >30 | ||
| ID4 | 0.44 | 0.636 | 1.33 | 2.64 | 1.262 | 17.9 | 18.6 | 18.7 | 18.1 | |
| IB25 | 4.27 | 3 | 22.9 | >30 | >30 | >30 | ||||
| IIB10 | 0.026 | 0.169 | 0.099 | 0.134 | 0.110 | 18 | >30 | 22 | 18.1 | 0.002 |
| 0.044 | 0.074 | 0.224 | 17.7 | 19.9 | 23.1 | |||||
| IB6 | 1.85 | 1.93 | 2.84 | >30 | >30 | >30 | 0.075 | |||
| IIB17 | 1.52 | 0.919 | 26.2 | >30 | >30 | >30 | ||||
| IIA10 | 0.016 | 0.364 | 0.024 | 0.098 | 0.072 | 18.3 | >30 | 19 | 26.7 | 0.004 |
| 0.01 | 0.052 | 0.039 | 0.083 | 18.4 | >30 | 17.1 | 24.1 | |||
| 0.009 | 0.022 | 0.071 | 0.08 | >3 | >3 | >3 | >3 | |||
| IIIA1 | 0.076 | 0.197 | 0.386 | 0.398 | 0.264 | >30 | >30 | >30 | >30 | |
| IIIB1 | 0.17 | 0.491 | 0.099 | 1.57 | 0.583 | >30 | >30 | >30 | >30 | 0.054 |
| IVA18 | 0.05 | 0.107 | 0.075 | 0.14 | 0.079 | >30 | >30 | >3 | >3 | |
| 0.061 | 0.038 | >3 | >3 | |||||||
| IA1 | 0.01 | 0.016 | 0.014 | 0.083 | 0.017 | 18.5 | 15.3 | >3 | >3 | 0.015 |
| 0.004 | 0.012 | 0.005 | 0.02 | >3 | >3 | >3 | >3 | |||
| 0.002 | 0.003 | >3 | >3 | |||||||
| IIIA5 | 0.084 | 0.207 | >30 | >30 | ||||||
| IA3 | <0.01 | 0.032 | 0.022 | 0.097 | 0.053 | 23 | >30 | 18.3 | >30 | |
| 0.01 | 0.018 | 0.022 | 0.167 | >3 | >3 | >3 | >3 | |||
| IIIA4 | 0.014 | 0.039 | 0.056 | 0.134 | 0.061 | 17.3 | >30 | 23.1 | >30 | |
| IA2 | <0.01 | 0.019 | 0.016 | 0.027 | 0.014 | 13 | 27.3 | 13.3 | 16.8 | 0.0014 |
| 0.002 | 0.006 | 0.007 | 0.021 | >3 | >3 | >3 | >3 | |||
| IA5 | 0.025 | 0.208 | 0.189 | 4.6 | 1.256 | >30 | >30 | 9.87 | 21.5 | |
| IIA16 | 1.21 | 2.57 | 0.72 | >30 | >30 | >30 | >30 | >30 | ||
| IIIA3 | 0.017 | 0.03 | 0.029 | 0.082 | 0.040 | >30 | >30 | >30 | >30 | |
| IIIA6 | 0.018 | 0.047 | 0.06 | 0.095 | 0.055 | 22.3 | >30 | 25.8 | >30 | |
| IIIA2 | 0.01 | 0.029 | 0.022 | 0.076 | 0.034 | >30 | >30 | >30 | >30 | |
| IVA11 | 0.01 | 0.019 | 0.046 | 0.081 | 0.039 | >3 | >3 | >3 | >3 | |
| IIE10 | 0.392 | 0.152 | 0.272 | 24.5 | 14.3 | 20.1 | ||||
| IE4 | 0.267 | 0.2 | 0.132 | 17.9 | 21.5 | 7.25 | ||||
| IB2 | 0.016 | 0.028 | 0.091 | 0.198 | 0.083 | >3 | >3 | >3 | >3 | |
| IIIA7 | 0.087 | 0.215 | 0.255 | 2.94 | 0.874 | >3 | >3 | >3 | >3 | |
| VA21 | 0.167 | 0.392 | 0.83 | 1.86 | 1.296 | >300 | >300 | >100 | >300 | |
| 0.141 | 0.85 | 0.654 | 2.3 | >300 | >100 | >100 | >100 | |||
| 0.63 | 1.377 | 0.6 | 3.669 | >100 | >100 | >100 | >100 | |||
| 0.498 | 1.3 | 2.5 | 2.3 | >100 | >100 | >100 | >100 | |||
| 0.48 | 1.6 | 3.1 | >100 | >100 | >100 | |||||
| 0.67 | >100 | >100 | ||||||||
| IVB25 | 0.32 | 0.59 | 0.33 | 1.75 | 0.939 | >300 | >100 | >100 | 19 | |
| 0.4 | 0.93 | 0.59 | 2.6 | 61 | 61 | >100 | >100 | |||
| IVB27 | 0.14 | 0.39 | 0.58 | 0.87 | 0.414 | >300 | >100 | >100 | 33.9 | |
| 0.17 | 0.14 | 0.12 | 0.9 | >100 | >100 | >100 | >100 | |||
| IVB33 | 1.46 | 0.77 | 1.91 | >100 | >100 | 72.9 | ||||
| IB29 | 3.38 | 0.56 | 2.41 | >100 | >100 | >70 | ||||
| IVB5 | 0.53 | 0.224 | 0.295 | 1.65 | 0.868 | >100 | >100 | >100 | >100 | |
| 0.9 | 0.58 | 1.9 | >100 | >100 | >100 | |||||
| IVB6 | 0.17 | 0.193 | <0.1 | 0.478 | 0.365 | >100 | >100 | >100 | >100 | |
| 0.34 | 0.18 | 0.83 | >100 | >100 | >100 | |||||
| IVB22 | 1.2 | 0.194 | 0.25 | 1.553 | 1.335 | >100 | >100 | >100 | >100 | |
| 1.95 | 0.56 | 1.2 | 2.6 | >100 | >100 | >100 | >100 | |||
| 2.08 | 0.57 | 2.53 | >100 | >100 | >100 | |||||
| IB30 | 0.35 | 2.4 | 0.58 | 4.7 | 1.244 | >100 | >100 | >100 | >100 | |
| 0.21 | 0.55 | 0.7 | 0.46 | 7.4 | 84.4 | 18.8 | 17.8 | |||
| IB32 | 0.67 | 4.4 | 5.6 | >100 | >100 | >100 | >100 | |||
| XXX* | 2.76 | 6.761 | 6.218 | 24.1 | 9.960 | >100 | >100 | >100 | >100 | |
| IB10 | 3.633 | 5.962 | 8 | 29.091 | 11.672 | >100 | >100 | >100 | >100 | |
| IVB24 | 0.625 | 0.961 | 0.975 | 2.732 | 3.138 | >100 | >100 | >100 | >100 | |
| 0.51 | 1.4 | 15.6 | 2.3 | 84.4 | >100 | 18.8 | >100 | |||
| IVB21 | 0.526 | 0.653 | 1.454 | 2.7 | 1.522 | >100 | >100 | >100 | >100 | |
| 0.5 | 0.87 | 0.87 | 4.6 | 71 | >100 | >100 | >100 | |||
| IVB3 | 0.753 | 1.615 | 1.657 | 4.791 | 2.204 | >100 | >100 | >100 | >100 | |
| IVB23 | 0.636 | 1.636 | 1.139 | 3.788 | 1.787 | >100 | >100 | >100 | >100 | |
| 0.7 | 1.8 | 2 | 2.6 | >100 | >100 | >100 | ||||
| IB33 | 2.649 | 4.726 | 6.408 | 20.526 | 8.577 | >100 | >100 | >100 | >100 | |
| IB9 | 4.4 | 14.1 | 3.92 | 23.5 | 11.480 | >100 | >100 | >100 | >100 | |
| IB34 | 6.25 | 11.4 | 1.93 | 13.6 | 8.295 | >100 | >100 | >100 | >100 | |
| IB36 | 6.69 | 25 | 2.24 | 73.7 | 26.908 | >100 | >100 | >100 | >100 | |
| IB26 | 0.51 | 0.93 | 0.46 | 2.32 | 0.955 | >100 | >100 | >100 | >100 | |
| 0.22 | 0.6 | 0.8 | 1.8 | 24.6 | >100 | >100 | >100 | |||
| IB8 | 2.6 | 1.25 | 2.16 | 8.18 | 3.548 | >100 | >100 | >100 | >100 | |
| IB35 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | |
| VA26 | 1.44 | 4.5 | 1.9 | 7.8 | 3.910 | >100 | >100 | >100 | >100 | |
| VA27 | 3.7 | 12 | 1.6 | 8.5 | 6.450 | >100 | >100 | >100 | >100 | |
| VA22 | 0.79 | 1.3 | 0.67 | 4.7 | 6.983 | >100 | >100 | >100 | >100 | |
| 0.9 | 2.4 | 2 | 43.1 | 83.5 | >100 | >100 | >100 | |||
| IVB28 | 4.9 | 6.4 | 13.5 | 18.1 | 10.725 | 5.6 | 18.9 | 17.8 | 19.8 | |
| IB37 | 18.3 | 17.8 | 39.3 | 65 | 35.100 | 19.8 | >100 | >100 | 18.3 | |
| IB38 | 1.08 | 17.3 | 2.4 | 32.7 | 13.370 | 21.3 | 63.9 | 28 | 60.1 | |
| VB28 | 0.45 | 0.41 | 0.75 | 2.4 | 0.905 | >100 | >100 | >100 | >100 | |
| 0.3 | 0.43 | 0.8 | 1.7 | 64.5 | >100 | >100 | >100 | |||
| IA25 | ||||||||||
| VIA21 | 0.68 | 0.19 | >100 | >100 | >100 | >100 | ||||
| VIB22 | 0.38 | 5.49 | >100 | 30 | >100 | >100 | ||||
| IB39 | 52.5 | >100 | >100 | 4.26 | >100 | >100 | ||||
| IVA6 | ||||||||||
| IVB26 | 2.4 | 1.99 | 0.91 | 7.56 | 3.410 | >100 | >100 | >100 | >100 | |
| 1.53 | 6.07 | >100 | >100 | |||||||
| VIB26 | 4.43 | 8.04 | 1.58 | 17.32 | 7.843 | >100 | >100 | >100 | >100 | |
| IVF27 | 2.18 | 2.34 | 0.5 | 2.16 | 1.795 | >100 | >100 | >100 | >100 | |
| IVF6 | 0.94 | 8.03 | 1.88 | 9.5 | 5.088 | 67.89 | >100 | >100 | 67.69 | |
| IVA25 | 1.04 | 3.55 | 0.71 | 2.3 | 1.900 | >100 | >100 | >100 | >100 | |
| IVA27 | 0.94 | 1.32 | 0.62 | 0.71 | 4.691 | >100 | >100 | >100 | >100 | |
| 5.06 | 8 | 1.88 | 19 | >100 | >100 | >100 | >100 | |||
| IVA6 | 0.54 | 0.51 | 0.29 | 0.24 | 0.395 | >100 | >100 | >100 | >100 | |
| IVA22 | 0.739 | 1.66 | 0.711 | 0.937 | 1.012 | >100 | >100 | >100 | >100 | |
| *shown in FIG. 12. | ||||||||||
A set of PAs wherein positions R1 and R2 of formula I are substituted by an aliphatic chain with varying degrees of unsaturation in the hydrocarbon chain are represented in FIG. 2 , Series III. These compounds include those with internal geometrically cis (zusammen or Z-form) and trans (entgegen or E-form) isomers are also presented in this series.
In addition to lipophilicity effects, the invention incorporates considerations based on the charge character of the PA. As obvious from the above general formula II for PAs of the invention, the introduction of the R1X{O}n— and R2X{O}n— moieties reduces the number of positive charges in the analog or derivative by one. At physiological pH of 7.2 the vast majority of amine groups will be in their positively charged ammonium state. The importance of positive charges for inhibiting polyamine transport is suggested by the observation that a PA with acetamide (IA11) showed a higher EC50 in comparison to analogous PAs wherein both R1X{O}n— and R2X{O}n— are replaced by hydrogen atoms (see IA11 versus ORI 1202 and ORI 1426 in Table 3).
Series IV (see FIG. 2 ) incorporates the above considerations for both lipophilicity and positive charges by incorporating both a long hydrocarbon chain and retaining the positively charged ammonium function. The reductive amination used to produce these structures results in alkylated (instead of acylated) amines. These compounds are inferred to have great affinity for the polyamine transporter. PAs with a dimerized spermine structure, represented by structures such as IA19, showed no improvement over the original lysine-spermine conjugate.
An alternative group of PAs, based on the long-chain hydrocarbon containing carboamides (FIG. 2 , Series I), may be prepared by incorporating the lipophilic and biologically stable sulfonamide group. These PAs are shown in FIG. 2 , Series II. Without being bound by theory, it may be that the addition of an additional carbonyl-like oxygen atom in the sulfonamide series increases the interactions at an amide-binding domain of polyamine transporters. An additional factor which may be playing a role is the increased lipophilicity in sulfonamides versus carboxamides. Additionally sulfonamides are known to be more biologically stable in comparison to carboxamides.
The present invention also provides additional ways to increase the lipophilicity of the substituents on the PA molecule. Alternatives with additional alkyl groups on the acyl portion of the molecule will increase the lipophilicity of this group and thus give an analog with higher activity. One additional method to increase this lipophilicity is through attachment of an additional alkyl chain alpha to the amino group (substituent which is attached to the carbon atom attached to the nitrogen). These analogs are produced by reductive amination of the free amino precursor with one of the ketone reagents shown in Series V. An additional advantage provided by inclusion of a methyl, or other substituent, at the alpha position of the amine group is decreased rate of biological metabolism.
An additional method to increase the lipophilicity of the analogs is through the production of a tertiary amine at the proximal or distal, or both, nitrogen atoms of the molecule. These molecules, which are shown in Series VI, are produced via the reductive amination reaction using a free mono- or di-amine precursor and an excess of the carbonyl containing reagent shown in Series VI. An alternative method to produce these di-substituted tertiary amine containing molecules is the conjugate addition of the selectively protected amine precursor to an α,β-unsaturated carbonyl compound or an α,β-unsaturated nitrile compound.
The present invention further provides methods for the synthesis of the disclosed PAs. In general, an orthogonally protected diamine containing compound, such as, but not limited to, certain amino acids, is coupled to a primary amine group of a polyamine followed by deprotection of one or both of the protected amine groups followed optionally by further derivatization of the amine. Without limiting the scope of the invention, an exemplary scheme for the production of spermine based PAs according to the above formula wherein d is 4, e is 0, X is C, and either R1X{O}n— or R2X{O}n— is H is shown in FIG. 1 , where the 4-nitrophenyl activated ester Boc-L-Lys-(Cbz)-ONP is used in combination with spermine. This scheme is for illustrative purposes only, and any other diamino containing amino acid including, but not limited to, D-lysine, L-ornithine, D-omithine, L-2,4-diaminobutyric acid, D-2,4-diaminobutyric acid, L-2,3-diaminopropionic acid and D-2,3-diaminopropionic acid can be likewise orthogonally di-protected and coupled to spermine. Any appropriate protecting group(s) may be used in the practice of the invention, and the indication of Boc-(butoxycarbonyl-) and Cbz-(carbobenzoxy-) protecting groups are for illustrative purposes only. Other protective group strategies are known in the art (see, for example, “Protective Groups in Organic Synthesis—Third Ed. 1999, eds. T. W. Greene and P. G. M. Wuts. John Wiley and Sons, Inc. New York).
In another aspect of the invention, polyamine analogs may be prepared via the coupling of distal carboxylic acid containing amino acids with suitable protecting groups on this distal carboxylic acid (e.g. methyl or benzyl ester) such as N-tBoc-Asp(OCH3)—OH or N-tBoc-Glu(OCH3)—OH with a primary amine group of a polyamine (such as, but not limited to, spermine) followed by exhaustive protection of the remaining amino groups. After purification by silica gel chromatography the distal carboxylic acid is deprotected and reacted with long chain hydrocarbon containing amines or alcohols to give amides or esters respectively. Such polyamine analogs can be represented by the following structure
wherein n can also be greater than 2, preferably up to about 10 (including 3, 4, 5, 6, 7, 8 and 9) and R is defined as provided for R1 and R2 in formula II above. The alpha amino group of the distal carboxylic acid containing amino acid may also be derivatized as described above in Formula II. Such compounds may be described as “inverted” amide or ester derivatives of the compounds described in FIG. 2 .
Similar hydrophobic PAs can be prepared by the use of cysteine, serine, or homo serine to link the hydrophobic and polyamine moieties indirectly. The hydrophobic PAs may also be linked via an ester linkage (like that possible via serine), a thioester linkage (like that possible via cysteine), a urea linkage (—N—CO—N—), a carbamate linkage (—O—CO—N— or —N—CO—O—), or an extended sulfonamide linkage (—NH—SO2—),
As shown in FIG. 1 , the active ester is added to an excess of polyamine to produce a mixture of substituted and unsubstituted acyl polyamines. The remaining free amino groups of the polyamines can then be protected, such as via their tBoc or Cbz carbamates, and the desired orthogonally-protected products can be isolated. Full protection of the amino groups produces a more lipophilic product mixture which facilitates purification of the desired compound. The exemplary reaction scheme in FIG. 1 results in two synthetic intermediates, one with 4 Boc and 1 Cbz carbamates and the other with 4 Cbz and 1 Boc carbamates. These intermediates allow the exposure of selectively either the distal or proximal (relative to the starting spermine polyamine) amino groups to be selectively deprotected by catalytic hydrogenation (see left branch of scheme) or acid treatment (see right branch of scheme), respectively. When viewed relative to the lysine moiety, the distal and proximal amino groups may be considered the ε- or α-amino positions, respectively.
The deprotected amino groups may then be further modified via conventional amide chemistry. For example, and without limiting the invention, the deprotected amino groups may be acylated or alkylated with either an acyl chloride or sulfonyl chloride to produce PAs shown in FIG. 2 as Series I and II, respectively. The positions may also be carboxylic acid activated with standard peptide coupling reagents such as DCC, PyPOP or HBTU (to produce Series III PAs) or aldehydes using reductive amination conditions (to produce Series IV PAs). Additional analogs are produced by reductive amination of the free amino precursor with one of the ketone reagents shown in Series V. Series VI analogs are produced via the reductive amination reaction using a free mono- or di-amine precursor and an excess of the carbonyl containing reagent shown in the Series VI portion of FIG. 2 . An alternative method to produce these di-substituted tertiary amine-containing molecules is the conjugate addition of the selectively protected amine precursor to an α,β-unsaturated carbonyl compound or an α,β-unsaturated nitrile compound.
The above described synthetic schemes may be conducted in a parallel fashion to permit the simultaneous production of multiple PAs. For example, the reaction scheme shown in FIG. 1 may be started with a mixture of L- and D-forms of Boc-Lys-(Cbz)-ONP and spermine. This results in a possible 4 different amino groups (two based on each of the L- and D-forms, and two based on each of the distal and proximal amino groups) deprotection and subsequent modification. There are also two additional possible modifications where both amino groups are simultaneously deprotected for subsequent modification. This results in a total of 6 possible routes for modification.
Parallel acylation with just two acyl chlorides, such as by solution phase methods, would produce twelve different PAs. Each individual PA may then be purified and the protective groups on the polyamine portion removed before further characterization and use.
The invention also provides compositions containing one or more PAs, as well as acceptable salts thereof, in combination with an excipient, diluent or vehicle to facilitate its use or administration to a subject. Preferably, the compositions are formulated for pharmaceutical, therapeutic or agricultural uses. Pharmaceutically acceptable salts of the invention (which contain basic groups) are formed where appropriate with strong or moderately strong, non-toxic, organic or inorganic acids in the presence of the basic amine by methods known in the art. Exemplary salts include, but are not limited to, maleate, fumarate, lactate, oxalate, methanesulfonate, ethanesulfonate, benzenesulfonate, tartrate, citrate, hydrochloride, hydrobromide, sulfate, phosphate and nitrate salts.
As stated above, the PAs of the invention possess the ability to inhibit polyamine transport, a property that is exploited in the treatment of any of a number of diseases or conditions, most notably cancer. A composition of this invention may be active per se, or may act as a “pro-drug” that is converted in vivo to active form.
The PAs of the invention, as well as the pharmaceutically acceptable salts thereof, may be incorporated into convenient dosage forms, such as capsules, impregnated wafers, tablets or injectable preparations. Solid or liquid pharmaceutically acceptable carriers may also be employed. Pharmaceutical compositions designed for timed or delayed release may also be formulated.
Optionally, the compositions contain anti-oxidants, surfactants and/or glycerides. Examples of anti-oxidants include, but not limited to, BHT, vitamin E and/or C. Examples of glycerides include, but are not limited to, one or more selected from acetylated or unsubstituted monoglycerides; medium chain triglycerides, such as those found in oils; and caprylocaproyl macrogol-8 glycerides.
Preferably, the compounds of the invention are administered systemically, e.g., by injection or oral administration. When used, injection may be by any known route, preferably intravenous, subcutaneous, intramuscular, intracranial or intraperitoneal. Injectables can be prepared in conventional forms, either as solutions or suspensions, solid forms suitable for solution or suspension in liquid prior to injection, or as emulsions.
Solid carriers include starch, lactose, calcium sulfate dihydrate, terra alba, sucrose, talc, gelatin, agar, pectin, acacia, magnesium stearate and stearic acid. Liquid carriers include syrup, peanut oil, olive oil, saline, water, dextrose, glycerol and the like. Similarly, the carrier or diluent may include any prolonged release material, such as glyceryl monostearate or glyceryl distearate, alone or with a wax. When a liquid carrier is used, the preparation may be in the form of a syrup, elixir, emulsion, soft gelatin capsule, liquid containing capsule, sterile injectable liquid (e.g., a solution), such as an ampule, or an aqueous or nonaqueous liquid suspension. A summary of such pharmaceutical compositions may be found, for example, in Remington's Pharmaceutical Sciences, Mack Publishing Company, Easton Pa. (Gennaro 18th ed. 1990).
The pharmaceutical preparations are made following conventional techniques of pharmaceutical chemistry involving such steps as mixing, granulating and compressing, when necessary for tablet forms, or mixing, filling and dissolving the ingredients, as appropriate, to give the desired products for oral or parenteral administration. Other preparations for topical, transdermal, intravaginal, intranasal, intrabronchial, intracranial, intraocular, intraaural and rectal administration may also be prepared. The pharmaceutical compositions may also contain minor amounts of nontoxic auxiliary substances such as wetting or emulsifying agents, pH buffering agents and so forth.
Although the preferred routes of administration are systemic, the pharmaceutical composition may be administered topically or transdermally, e.g., as an ointment, cream or gel; orally; rectally; e.g., as a suppository, parenterally, by injection or continuously by infusion; intravaginally; intranasally; intrabronchially; intracranially; intraaurally; or intraocularly.
Intraaural formulations are particularly preferred for the treatment or alleviation of hearing loss due to chemotherapy.
For topical application, the compound may be incorporated into topically applied vehicles such as a salve or ointment. The carrier for the active ingredient may be either in sprayable or nonsprayable form. Non-sprayable forms can be semi-solid or solid forms comprising a carrier indigenous to topical application and having a dynamic viscosity preferably greater than that of water. Suitable formulations include, but are not limited to, solution, suspensions, emulsions, creams, ointments, powders, liniments, salves, and the like. If desired, these may be sterilized or mixed with auxiliary agents, e.g., preservatives, stabilizers, wetting agents, buffers, or salts for influencing osmotic pressure and the like. Preferred vehicles for non-sprayable topical preparations include ointment bases, e.g., polyethylene glycol-1000 (PEG-1000); conventional creams; gels; as well as petroleum jelly and the like.
Topical preparations are particularly preferred for the application of the present invention to the control of unwanted hair growth on skin.
Also suitable for topical application are sprayable aerosol preparations wherein the compound, preferably in combination with a solid or liquid inert carrier material, is packaged in a squeeze bottle or in admixture with a pressurized volatile, normally gaseous propellant. The aerosol preparations can contain solvents, buffers, surfactants, perfumes, and/or antioxidants in addition to the compounds of the invention.
For the preferred topical applications, especially for humans, it is preferred to administer an effective amount of the compound to a target area, e.g., skin surface, mucous membrane, eyes, etc. This amount will generally range from about 0.001 mg to about 1 g per application, depending upon the area to be treated, the severity of the symptoms, and the nature of the topical vehicle employed.
The compositions of the invention may be administered alone or in combination with one or more additional compounds that are used to treat the disease or condition. For treating cancer, the PAs are given in combination with anti-tumor agents, such as mitotic inhibitors, e.g., vinblastine; alkylating agents, e.g., cyclophosphamide; folate inhibitors, e.g., methotrexate, pritrexim or trimetrexate; antimetabolites, e.g., 5-fluorouracil and cytosine arabinoside; intercalating antibiotics, e.g., adriamycin and bleomycin; enzymes or enzyme inhibitors, e.g., asparaginase; topoisomerase inhibitors, e.g., etoposide; or biological response modifiers, e.g., interferon and interleukin-2. In fact, pharmaceutical compositions comprising any known cancer therapeutic in combination with the PAs disclosed herein are within the scope of this invention. Such combinations may be utilized either by combining the components into a single composition for administration or by administering the components separately as part of one therapeutic protocol.
Most preferably, the present compounds are administered in combination with one or more polyamine synthesis inhibitors such as, but not limited to, inhibitors of ornithine decarboxylase such as DFMO, aceylenic putrescine, 1-aminooxy-3-aminopropane, antizyme, 2-butylputrescine, cadaverine, L-canaline, 5′-deoxy-5′-[N-methyl-N-[3-(aminooxy)ethyl]amino]adenosine, 5′-deoxy-5′-N-methyl-N-[3-(hydrazinopropyl)amino]adenosine, diaminopropane, 1,3-diamino-2-propanol, 2-difluoromethyl putrescine, difluorophenylethyl(4-aminopropylamidinohydrazone), 2,3-dimethylputrescine, N-dimethylputrescine, 2-ethylputrescine, (+or −)-alpha-fluoromethylomithine, 2-fluoro methylputrescine, 2-hexylputrescine, 2-hydrazinoomithine, ibuprofen, D-methyl acetylenic putrescine, methylglyoxal bis(3-aminopropyl-amidinohydrazone), 2-methylomithine, 2-methylputrescine, 2-monofluoromethyl-trans-dehydoromithine, 2-monofluoromethyl dehydroputrescine, monofluoromethylomithine, 2-monofluoromethyl putrescine, neomycin, D-omithine, 2-pentylputrescine, p-phenylenediamine, phosphopeptide MG 25000, phosphothreonine, phosphotyrosine, 2-propylputrescine, putrescine, allo-S-adenosyl-L-methionine, S-ethylthioadenosine, methylthioadenosine, and 5′-methylthioadenosine as discussed in Zollner H. (1993) Handbook of Enzyme Inhibitors, 2nd Ed. Weinheim:Basel (Switzerland); inhibitors of S-adenosylmethionine decarboxylase, such as SAM486A (4-aminoindanon-1-(2′amidino)hydrazone dihydrochloride monohydrate), S-adenosyl-1,8-diamino-3-thiooctane, S-(5′-adenosyl) methylthio-2-aminooxyethan, S-adenosyl-3-methylthio-1-propylamine, 5′-{[(Z)-4-amino-2-butenyl]methylamino}-5′-deoxyadenosine , 5′-amino-5′-deoxyadenosine, 5′-[(aminoiminomethyl)amino]-5′deoxyadenosine dihydrogensulphate, 1-aminooxy-3-aminopropane, [2-(aminooxy)ethyl](5′-deoxyadenosine-5′-yl)(methyl) sulphonium, 5′-[(3-aminopropyl]-amino)-5′-deoxyadenosine, 5′-[(3-aminopropyl]-nethylamino)-5′-deoxyadenosine, 9-[6(RS)-amino-5,6,7-trideoxy-beta-D-ribo-octofuranosyl]-9H-purin-6-amine, borohydride, n-butylglyoxal bis(guanylhydrazone), 9-[6(RS)-c-carboxamido-5,6,7-trideoxy-beta-D-ribo-octofuranosyl]-9H-purin-6-amine, cyanide, cyanoborohydride, S-(5′deoxy-5′adenosyl)methionylethylhydroxylamine, S-(5′deoxy-5′adenosyl)methionylthiohydroxylamine, 5′-deoxy-5′-[N-methyl-N-[2-(aminooxy)ethyl]amino]adenosine, 9-[6(S)-diamino-5,6,7,8,9-pentadeoxy-beta-D-ribo-nanofuranosyl]-9H-purin-6-amine, diethylglyoxal bis(guanylhydrazone), difluorophynylethyl (4-aminopropylamidinohydrazone), dimethyl(5′-adenosyl)sulfonium, dimethylglyoxal bis (guanylhydrazone), ethylglyoxal bis(guanylhydrazone), hydroxylamine, 4-hydroxypenenal, MDL 73811, 5′[[3-methylamino)propyl]amino]-5′-deoxyadenosine(1,1′-(methylethanediylidine)dinitro)bis(3aminoguanididne), methylglyoxal bis(3-aminopropylamidinohydrazone), methylglyoxal bis(cyclohexylamidinohydrazone), methylglyoxal bis(guanylhydrazone), pentanedialdehyde bis guanylhydrazone), phenylbydrazine, propanedialdehyde bis (guanylhydrazone), semicarbazide, sodium borohydride, sodium cyanoborohydride, and spermine as discussed in Zollner H. (1993) Handbook of Enzyme Inhibitors, 2nd Ed.
The PAs of the invention may also be used in combination with monoclonal antibodies and tumor vaccines as well as with cellular therapy in subjects undergoing treatment for human diseases such as cancer. The PAs may also be used for chemoprevention in subjects at risk for developing cancer wherein one or more PAs are taken alone or in combination with a polyamine synthesis inhibitor to prevent the onset or recurrence of cancer.
The pharmaceutical compositions of the invention may also comprise one or more other medicaments such as anti-infectives including antibacterial, anti-fungal, anti-parasitic, anti-viral, and anti-coccidial agents.
Typical single dosages of the compounds of this invention are between about 1 ng and about 10 g/kg body weight. The dose is preferably between about 0.01 mg and about 1 g/kg body wt. and, most preferably, between about 0.1 mg and about 100 mg/kg body wt. For topical administration, dosages in the range of about 0.01-20% concentration of the compound, preferably 1-5%, are suggested. A total daily dosage in the range of about 1-500 mg is preferred for oral administration. The foregoing ranges are, however, suggestive, as the number of variables in regard to an individual treatment regime is large, and considerable excursions from these recommended values are expected and may be routinely made by those skilled in the art.
Effective amounts or doses of the compound for treating a disease or condition can be determined using recognized in vitro systems or in vivo animal models for the particular disease or condition. In the case of cancer, many art-recognized models are known and are representative of a broad spectrum of human tumors. The compounds may be tested for inhibition of tumor cell growth in culture using standard assays with any of a multitude of tumor cell lines of human or nonhuman animal origin. Many of these approaches, including animal models, are described in detail in Geran, R. I. et al., “Protocols for Screening Chemical Agents and Natural Products Against Animal Tumors and Other Biological Systems (Third Edition)”, Canc. Chemother Reports, Part 3, 3:1-112.
The present invention also provides methods of using the PAs, whether formulated in compositions or not, to inhibit cell growth and proliferation when used alone or in combination with a polyamine synthesis inhibitor. Such methods may be readily conducted by systemic or local administration of the PAs. Local delivery of a PA provides a high local concentration while reducing the likelihood of systemic effects on polyamine metabolism that may result from systemic PA administration.
The inhibition of cellular growth and proliferation is advantageously conducted with the contemporaneous administration of one or more inhibitors of polyamine synthesis. Such inhibition may be applied toward a variety of cell types, including, but not limited to, bacterial cells, fungal cells, and the eukaryotic cells of higher multicellular organisms. In one application of the invention, one or more PAs may be used to inhibit bacterial or fungal cell growth. This embodiment may be advantageously used in both the clinic and agriculture to control bacteria or fungi.
In another embodiment of the invention, one or more PAs may be used in combination with an inhibitor of polyamine synthesis to inhibit the growth and/or proliferation of cancer cells, including those of solid tumors. While this latter application may be performed in any multicellular organism, most preferred are applications of the invention for use in human subjects.
Additionally, the invention provides for the use of one or more PAs for analytical and/or preparative methods relating to polyamine transport. For example, and without limiting the invention, a PA may be used to identify and/or localize a polyamine transporter by virtue of physical binding between the PA and the transporter and the presence of a label linked to the PA. Suitable labels are well known in the art, and they permit the identification or localization of the PA either because the label itself emits a detectable signal, or by virtue of its affinity for a label-specific partner which is detectable or becomes so by binding to, or otherwise reacting with, the label. Examples of labels include, but are not limited to, radioactive isotopes, fluorescent tags, and proteinaceous tags. The methods of identification and /or localization provided by the invention may be used in whole or as part of a diagnostic or research protocol.
The invention also provides preparative uses of the PAs. For example, one or more PAs can be used to bind and isolate proteins or other cellular factors that interact with polyamines. An exemplar of such a method is the use of a PA to bind to a polyamine transporter and permit its isolation or purification. These methods can be performed in solution, where interaction between a PA and a PA binding protein or factor results in a complex that may be subsequently isolated or purified from solution, or in solid phase, where a PA is immobilized and interactions between the PA and a PA binding protein or factor results in a complex of the protein or factor with the immobilized PA.
Having now generally described the invention, the same will be more readily understood through reference to the following examples which are provided by way of illustration, and are not intended to be limiting of the present invention, unless specified.
PAs analogs were synthesized in a parallel fashion starting from the orthogonally protected diamino containing amino acid starting materials. The use of the 4-nitrophenyl activated ester L-Boc-Lys-(Cbz)-ONP in FIG. 1 provides an exemplary illustration of the synthetic process. The active ester is added dropwise to a solution of 1.5 equivalents of polyamine in methanol to give a statistical mixture of unsubstituted, mono-substituted and di-substituted acyl polyamines. Following evaporation of the solvent, the remaining free amino groups in the polyamine moiety are protected either as their tBoc or Cbz carbamates. Standard workup results in a completely protected crude product mixture. The desired orthogonally-protected product is isolated in pure form by silica gel chromatography using standard organic solvents. This purification process is based on separation of polyamine molecules with the remaining amino groups being fully protected, which provides a much more lipophilic product mixture that greatly facilitates the purification process. Thus the exemplary intermediates containing either 4 Boc groups or 4 Cbz groups in addition to the acyl functionality remained lipophilic enough to purify using standard solvents including a one to one mixture of ethyl acetate and hexanes containing various proportions of methanol (0 to 10%).
As shown in FIG. 1 , the approach provides two synthetic intermediates, one with 4 Boc and 1 Cbz carbamates and the other with 4 Cbz and 1 Boc carbamates. These intermediates allow the exposure of only one amino group, either the proximal (α-) or distal (ε-), in a selective manner. It is also possible to modify this approach such that both amino groups are exposed for further modification. The selective deprotection of either the proximal (α-) or distal (ε-) amino group as shown in FIG. 1 may occur via catalytic hydrogenation or acid treatment, respectively. The exposed amino groups were then acylated or alkylated with either an acyl chloride or sulfonyl chloride to produce Series I and II (see FIG. 2 ) type PAs, respectively. The exposed amino groups may also be carboxylic acid activated with standard peptide coupling reagents such as DCC, PyPOP or HBTU (to produce Series III type PAs) or aldehydes under reductive amination conditions (to produce Series IV type PAs). Additional analogs are produced by reductive amination of the free amino precursor with one of the ketone reagents shown in Series V. Series VI analogs are produced via the reductive amination reaction using a free mono- or di-amine precursor and an excess of the carbonyl reagent that are shown in the Series VI chart. An alternative method to produce these di-substituted tertiary amine-containing molecules is the conjugate addition of the selectively protected amine precursor to an α,β-unsaturated carbonyl compound or an α,β-unsaturated nitrile compound.
Deprotections of isolated PAs using standard conditions gave the desired products in pure form. The PAs were characterized by thin layer chromatography (TLC) analysis (using iPrOH/HOAc/pyr/H2O, 4:1:1:2); high performance liquid chromatography (HPLC) analysis (dansylation followed by HPLC using fluorescent detection); liquid chromatography-mass spectroscopy (LC-MS) by electrospray ionization; and 1H and 13C NMR analysis. All PAs were estimated to be 90 to 98% pure following synthesis.
All cell lines were obtained from ATCC (Manassas, Va.) and cultured in the recommended media, serum, and CO2 concentration. Medias were obtained from Mediatech, Inc. (Herdon, Va.) and serums from Gibco BRL (Gaithersburg, Md.). 50 U/ml penicillin, 50 μg/ml streptomycin and 2 mM L-glutamine (all from Bio Whittaker, Walkersville, Md.) were included in all cultures. DFMO was obtained from Marion Merrell Dow (Cinncinati, Ohio). When cells were cultured with polyamines or ORI compounds, 1 mM aminoguanidine (AG; Sigma) was included to inhibit serum amine oxidase activity. IC50 refers to the concentration of PA that results in 50% of maximum cell growth inhibition in the presence of PA alone.
[2,9-3H]spermidine (SPD) from DuPont NEN, Boston, Mass. was added alone or simultaneously with PAs to 24-well plates containing MDA-MB-23 1 cells in log growth. The cells were incubated at 37° C. for 15 min to determine initial rate polyamine uptake. The cells were then washed three times with cold PBS, lysed with 0.1% SDS, and the amount of polyamine incorporation into the cells was determined by scintillation counting of the cell lysates. To determine a Ki, four radioactive substrate concentrations (0.3-3 μM) and five inhibitor concentrations (0.01-1.0 μM) and a control were tested. The Ki values were determined using double reciprocal Lineweaver-Burke plot analyses. Ki values were determined from linear equations derived from graphing the slopes of Lineweaver-Burke plot vs. inhibitor concentration, with Ki=y-intercept/slope. Results of these analyses are shown in Table 3 above.
Cells were plated in 96-well plates such that they would be in log growth for the duration of the assay. The day after plating, PAs were added to the cells, and growth, if any, permitted to continue for six days in the presence of 1 mM AG and 0.5 μM SPD to insure that any growth inhibition was not the result of depletion of external polyamines in the media. At the end of the six days, cell growth was measured by MTS/PMS dye assay (Cell Titer 96 Aqueous Non-Radioactive Cell Proliferation Assay; Promega, Madison, Wis.). EC50 represents the concentration of PA that resulted in 50% of maximum growth inhibition achievable in the presence of both DFMO (5 mM in all cell lines except MDA) and PA (at different concentrations depending in part on the cell line used) compared to controls. IC50 represents the concentration of PA that resulted in 50% maximum growth inhibition when used alone. Results are shown in Table 3 above.
Sample handling for Polyamine Analysis (see Kabra, Pokar M., Hsian K. Lee, Warren P Lubich and Laurence J. Marton: Solid-Phase Extraction and Determination of Dansyl Derivatives of Unconjugated and Acetylated Polyamines by Reverse-Phase Liquid Chromatography: Improved Separation Systems for Polyamines in Cerebrospinal Fluid, Urine and Tissue. Journal of Chromatography 380 (1986) 19-32)
Plasma samples (from blood)—remove 125–150 μl sample (optimally) into a microfuge tube and mix 1:1 with 0.4M perchloric acid. Vortex and spin down sample at 13000 rpm for 10 minutes in 5° C. centrifuge. Remove 200 μl supernatant for dansylation as described in dansylation protocol. Plasma samples as small as 25 μl may be analyzed (for this and the following discussion, any sample that does not yield 200 μl supernatant for dansylation may have its volume increased to 200 μl with perchloric acid for the dansylation protocol).
Cell Culture Samples
Media—remove 1.5 ml into 1.7 ml microfuge tube and spin at 3000 rpm for 5 minutes in 5° C. centrifuge. Remove 300 μl supernatant and mix 1:1 with cold 0.4M perchloric acid. Vortex and spin down sample at 13000 rpm for 10 minutes in 5° C. centrifuge. Remove 200 μl supernatant for dansylation as described in dansylation protocol.
Cells—Trypsinize as usual and spin in 15 ml tube 6 min at 4° at 1500 rpm. Pour off supernatant and resuspend pellet in 1.5 ml 1× PBS. Transfer to large microfuge tube. Spin at 3000 rpm at 4° for 5 minutes. Remove supernatant. Resuspend pellet in 1.0 ml 1× PBS. Remove 20 μl for counting and spin @ 3000 rpm @4° for 5 minutes. Remove supernatant. To the dry pellet, add 200 μl 0.4M perchloric acid per 106 cells. Pipette up and down to mix. Vortex and spin down sample at 13000 rpm for 10 minutes in 5° C. centrifuge. Remove 200 μl supernatant for dansylation as described in dansylation protocol. Remainder of supernatant can be stored at −70° C.
Tissues—Keep samples on ice during preparation. Cut an approximately 100 mg piece from tissue sample and place into 15 ml conical tube. Add 1.2M perchloric acid in a 20:1 vol/weight ratio (i.e. 2 ml/100 mg). Homogenize tissue using a tissue grinder. Vortex sample and remove 1 ml into a microfuge tube. Spin at 13000 rpm for 10 minutes in 5° C. centrifuge. Remove 200 μl supernatant for dansylation as described in dansylation protocol.
Dansylation Protocol for Polyamine Analysis
- 200 μl sample in Perchloric acid
- 10 μl Internal Standard (IS) (1,7-diaminoheptane, 100 μM stock);
use 20 μl for 25 min and 1483 HPLC - 120 μl saturated sodium carbonate solution (360 μl is used for tissue samples)
- 400 μl dansyl chloride solution (made fresh, 10 mg/ml in acetone)
Add all ingredients to a 4 ml screw cap glass vial and vortex for 30 seconds. Float vials in 70° C. water bath for 10 minutes. Remove and allow cooling to room temp in dark, as samples are light sensitive. Proceed to sample prep protocol once samples have cooled.
Sample Prep Protocol
Alltech C-18 maxi-prep cartridges are used, one for each sample dansylated, to clean any interfering reactions from the samples. This process also places the samples in methanol for application to the HPLC system.
Each cartridge is placed on a vacuum manifold and washed once with 3 ml MeOH followed by 3 ml H2O. Samples are then removed by 1 ml syringe from the glass vials and applied to the Alltech cartridges. Each cartridge is then washed with 10 ml H2O and dried 2× with 30 cc syringe of air.
All steps to this point are allowed discarded. The cartridges are placed with a tube rack with labeled 1.7 ml microfuge tubes for elution. Samples are eluted with 1 ml MeOH into the microfuge tubes. Samples are now ready for injection onto HPLC or can be stored at −70° C. for up to several months if necessary.
The solvents used in the above are as follows:
- Solvent A:
HPLC grade Acetonitrile 60 - Solvent B: 10 mM Na acetate pH 4.5/10% acetonitrile (8.9L H2O, 1L Acetonitrile, 100 ml 1M Na acetate pH 4.5, mix well, filter and store at room temp).
Sample Injection: loop overfill is achieved by injecting 100 μl onto a 20 μl loop. Samples are kept at 4° C. until injection by a water cooled storage rack on the 231XL auto injector.
40 Minute PA Analysis:
| Gradient: | time | % | % B | |
| 0 | 48 | 52 | ||
| 25 | 90 | 10 | ||
| 30 | 100 | 0 | ||
| 35 | 48 | 52 | ||
| 40 | 48 | 52 | ||
| Flow rate is 3 ml/minute |
| Solutions and Sources are as follows: |
| Internal Standard: |
| 1,7-Diaminoheptane (Sigma D-3266) |
| Made up 20 mM in H2O, and stored at −70° C. Diluted to 100 μM |
| working stock in H2O and also stored at −70° C. |
| Perchloric acid: |
| 70% ACS reagent (Aldrich 244252) |
| For 0.4 M, mix 3.4 ml in a total of 100 ml H2O. Store at room temp. |
| For 1.2 M, mix 10.2 ml in a total of 100 ml H2O. Store at room temp. |
| Sodium carbonate: |
| anhydrous (Acros 42428-5000) |
| Make a saturated solution in H2O. |
| Sodium acetate: |
| anhydrous (Sigma S-2889) |
| Make up 1 M in H2O, then pH to 4.5 with glacial acetic acid. |
| Filter and store at room temp. |
| Dansyl chloride: |
| 95% (Sigma D-2625) |
| Acetonitrile: |
| HPLC grade (Fisher A998-4) |
| Methanol: |
| HPLC grade (Fisher A452-4) |
| Acetone: |
| HPLC grade (Fisher A949-1) |
| Glacial acetic acid: |
| ACS reagent (Fisher A38212) |
All references cited herein, including patents, patent applications, and publications, are hereby incorporated by reference in their entireties, whether previously specifically incorporated or not. As used herein, the terms “a”, “an”, and “any” are each intended to include both the singular and plural forms.
Having now fully described this invention, it will be appreciated by those skilled in the art that the same can be performed within a wide range of equivalent parameters, concentrations, and conditions without departing from the spirit and scope of the invention and without undue experimentation. While this invention has been described in connection with specific embodiments thereof, it will be understood that it is capable of further modifications. This application is intended to cover any variations, uses, or adaptations of the invention following, in general, the principles of the invention and including such departures from the present disclosure as come within known or customary practice within the art to which the invention pertains and as may be applied to the essential features hereinbefore set forth.
Claims (24)
1. A polyamine analog or derivative represented by formula II:
wherein a, b, and c independently range from 1 to 10; d and e independently range from 0 to 30; each X is independently either a carbon (C) or sulfur (S) atom, and R1 and R2 are independently selected from H or from the group of a straight or branched C1-50 saturated or unsaturated aliphatic, carboxyalkyl, carbalkoxyalkyl, or alkoxy; a C1-8 alicyclic; a single or multiring aryl substituted or unsubstituted aliphatic; an aliphatic-substituted or unsubstituted single or multiring aromatic; a single or multiring heterocyclic; a single or multiring heterocyclic aliphatic; a C1-10 alkyl; an aryl sulfonyl; or cyano; or
each of R1X{O}n— and R2X{O}n— are independently is replaced by H;
wherein * denotes a chiral carbon position; and
wherein if X is C, then n is 1; if X is S, then n is 2; and if X is C, then the XO group may be CH2 such that n is 0.
2. A polyamine analog or derivative represented by formula III:
wherein a, b, and c independently range from 1 to 10 and d and e independently range from 0 to 30; and R1 , R2, and R3 , and R4 may be the same or different and are independently selected from H or from the group of a straight or branched C1-50 saturated or unsaturated aliphatic, carboxyalkyl, carbalkoxyalkyl, or alkoxy; a C1-8 alicyclic; a single or multiring aryl substituted or unsubstituted aliphatic; an aliphatic-substituted or unsubstituted single or multiring aromatic; a single or multiring heterocyclic; a single or multiring heterocyclic aliphatic; a C1-10 alkyl; an aryl sulfonyl; or cyano; and R2 and R4 may be the same or different and are independently selected from the group of a straight or branched C1-50 saturated or unsaturated aliphatic, carboxyalkyl, carbalkoxyalkyl, or alkoxy; a C1-8 alicyclic; a single or multiring aryl substituted or unsubstituted aliphatic; an aliphatic-substituted or unsubstituted single or multiring aromatic; a single or multiring heterocyclic; a single or multiring heterocyclic aliphatic; a C1-10 alkyl; an aryl sulfonyl; or cyano.
3. A polyamine analog or derivative represented by formula IV:
wherein a, b, and c independently range from 1 to 10 and d and e independently range from 0 to 30; and R1 , R2, and R3 , and R4 may be the same or different and are independently selected from H or from the group of a straight or branched C1-50 saturated or unsaturated aliphatic, carboxyalkyl, carbalkoxyalkyl, or alkoxy; a C1-8 alicyclic; a single or multiring aryl substituted or unsubstituted aliphatic; an aliphatic-substituted or unsubstituted single or multiring aromatic; a single or multiring heterocyclic; a single or multiring heterocyclic aliphatic; a C1-10 alkyl; an aryl sulfonyl; or cyano; and R2 and R4 may be the same or different and are independently selected from the group of a straight or branched C1-50 saturated or unsaturated aliphatic, carboxyalkyl, carbalkoxyalkyl, or alkoxy; a C1-8 alicyclic; a single or multiring aryl substituted or unsubstituted aliphatic; an aliphatic-substituted or unsubstituted single or multiring aromatic; a single or multiring heterocyclic; a single or multiring heterocyclic aliphatic; a C1-10 alkyl; an aryl sulfonyl; or cyano.
4. A polyamine analog or derivative represented by formula V:
wherein a, b, and c independently range from 1 to 10 and d and C e independently range from 0 to 30; and wherein Z1 is NR1R3 and Z2 is selected from —R1, —CHR1R2 or —CR1R2R3 or Z2 is NR2R4 NR1R3 and Z1 is selected from —R1, —CHR1R2 or —CR1R2R3, wherein R1, and R2, and R3 may be the same or different and are independently selected from H or from the group of a straight or branched C1-50 saturated or unsaturated aliphatic, carboxyalkyl, carbalkoxyalkyl, or alkoxy; a C1-8 alicyclic; a single or multiring aryl substituted or unsubstituted aliphatic; an aliphatic-substituted or unsubstituted single or multiring aromatic; a single or multiring heterocyclic; a single or multiring heterocyclic aliphatic; a C1-10 alkyl; an aryl sulfonyl; or cyano;
and wherein R3 is selected from the group of a straight or branched C1-50 saturated or unsaturated aliphatic, carboxyalkyl, carbalkoxyalkyl, or alkoxy; a C1-8 alicyclic; a single or multiring aryl substituted or unsubstituted aliphatic; an aliphatic-substituted or unsubstituted single or multiring aromatic; a single or multiring heterocyclic; a single or multiring heterocyclic aliphatic; a C1-10 alkyl; an aryl sulfonyl; or cyano.
5. The polyamine analog or derivative of claims 1 –4 wherein said a, b, and c are such that the analog or derivative is putrescine, spermine or spermidine based.
6. The polyamine analog or derivative of claims 1 –4 wherein each of R1, R2, R3, and R4 is independently selected from H or a straight or branched C10-50 saturated or unsaturated aliphatic, carboxyalkyl, carbalkoxyalkyl, or alkoxy.
7. A polyamine analog or derivative selected from spermine based compounds IA4, IB4, IA7, IVB22 or IVA22 as illustrated in FIG. 2 .
8. A polyamine analog or derivative selected from the compounds depicted in FIG. 12 .
9. The polyamine analog or derivative of claims 1 –4 wherein d is 4 and e is 0.
11. A composition comprising a the polyamine analog or derivative according to claims 1 –4 and an excipient, diluent or vehicle.
12. The composition of claim 11 wherein said excipient, diluent or vehicle is pharmaceutically or cosmetically acceptable.
13. The composition of claim 11 wherein said excipient, diluent or vehicle is for topical or intra-aural administration.
14. The composition of claim 11 further comprising a polyamine biosynthesis inhibitor.
15. The composition of claim 14 wherein said inhibitor is DFMO difluoromethylornithine (DFMO).
16. The composition of claim 11 formulated for intravenous, subcutaneous, intramuscular, intracranial, intraperitoneal, topical, transdermal, intravaginal, intranasal, intrabronchial, intracranial, intraocular, intraaural, rectal, or parenteral administration.
21. A composition comprising the polyamine analog or derivative according to claims 17-20 and an excipient, diluent or vehicle.
22. The composition of claim 21 further comprising a polyamine biosynthesis inhibitor.
23. The composition of claim 22 wherein said inhibitor is difluoromethylornithine (DFMO).
24. The composition of claim 21 formulated for intravenous, subcutaneous, intramuscular, intracranial, intraperitoneal, topical, transdermal, intravaginal, intranasal, intrabronchial, intraocular, intraaural, rectal, or parenteral administration.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/047,297 USRE43327E1 (en) | 2001-01-08 | 2002-01-08 | Hydrophobic polyamine analogs and methods for their use |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US26041501P | 2001-01-08 | 2001-01-08 | |
| US10/296,259 US6963010B2 (en) | 2001-01-08 | 2002-01-08 | Hydrophobic polyamine analogs and methods for their use |
| US13/047,297 USRE43327E1 (en) | 2001-01-08 | 2002-01-08 | Hydrophobic polyamine analogs and methods for their use |
| PCT/US2002/000347 WO2002053519A2 (en) | 2001-01-08 | 2002-01-08 | Hydrophobic polyamine analogs and methods for their use |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/296,259 Reissue US6963010B2 (en) | 2001-01-08 | 2002-01-08 | Hydrophobic polyamine analogs and methods for their use |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| USRE43327E1 true USRE43327E1 (en) | 2012-04-24 |
Family
ID=29218555
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/047,297 Expired - Lifetime USRE43327E1 (en) | 2001-01-08 | 2002-01-08 | Hydrophobic polyamine analogs and methods for their use |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | USRE43327E1 (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10632145B2 (en) | 2016-03-25 | 2020-04-28 | Aminex Therapeutics, Inc. | Bioavailable polyamines |
| US20220096412A1 (en) * | 2020-09-30 | 2022-03-31 | Aminex Therapeutics, Inc. | Combination Drug Substance of Polyamine Transport Inhibitor and DFMO |
| US20230321012A1 (en) * | 2021-12-01 | 2023-10-12 | Aminex Therapeutics, Inc. | Methods To Treat Cancer With AMXT 1501 and DFMO |
Citations (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4309442A (en) | 1977-07-11 | 1982-01-05 | Merrell Toraude Et Compagnie | Method for controlling fertility in mammals |
| WO1985002769A1 (en) | 1983-12-20 | 1985-07-04 | Kovacs Adam | Antitumour pharmaceutical compositions and process for preparing the same |
| US4590288A (en) | 1983-06-29 | 1986-05-20 | Exxon Research And Engineering Co. | Bi- or polymetallic coordination complex |
| US4774339A (en) | 1987-08-10 | 1988-09-27 | Molecular Probes, Inc. | Chemically reactive dipyrrometheneboron difluoride dyes |
| US4818770A (en) | 1986-10-22 | 1989-04-04 | Boyce Thompson Institute For Plant Research | Prevention of a plant disease by specific inhibition of fungal polyamine biosynthesis |
| US4950744A (en) | 1985-01-10 | 1990-08-21 | Molecular Diagnostics, Inc. | Photochemical nucleic acid-labeling reagent having a polyalkylamine spacer |
| JPH02256656A (en) | 1988-12-09 | 1990-10-17 | Terumi Nakajima | New polyamine compound and glutamic acid receptor blocker |
| WO1991000853A1 (en) | 1989-07-03 | 1991-01-24 | New York University | Use of polyamines as ionic-channel regulating agents |
| WO1992014709A2 (en) | 1991-02-11 | 1992-09-03 | Cambridge Neuroscience, Inc. | Calcium channel antagonists and methodology for their identification |
| US5187288A (en) | 1991-05-22 | 1993-02-16 | Molecular Probes, Inc. | Ethenyl-substituted dipyrrometheneboron difluoride dyes and their synthesis |
| US5248782A (en) | 1990-12-18 | 1993-09-28 | Molecular Probes, Inc. | Long wavelength heteroaryl-substituted dipyrrometheneboron difluoride dyes |
| US5252714A (en) | 1990-11-28 | 1993-10-12 | The University Of Alabama In Huntsville | Preparation and use of polyethylene glycol propionaldehyde |
| US5274113A (en) | 1991-11-01 | 1993-12-28 | Molecular Probes, Inc. | Long wavelength chemically reactive dipyrrometheneboron difluoride dyes and conjugates |
| US5433896A (en) | 1994-05-20 | 1995-07-18 | Molecular Probes, Inc. | Dibenzopyrrometheneboron difluoride dyes |
| WO1995021612A2 (en) | 1993-02-08 | 1995-08-17 | Nps Pharmaceuticals, Inc. | Compounds active at a novel site on receptor-operated calcium channels useful for treatment of neurological disorders and diseases |
| US5541230A (en) * | 1993-11-05 | 1996-07-30 | Us Health | Therapeutic polyamines |
| WO1996022926A1 (en) | 1995-01-25 | 1996-08-01 | Sealed Air Corporation | Inflatable cushion and method of making same |
| WO1996038464A1 (en) | 1995-05-30 | 1996-12-05 | Lehigh University | Antimicrobial sterol conjugates |
| US5648394A (en) | 1993-05-27 | 1997-07-15 | Boxall; Brian Alfred | Topical composition for inhibiting hair growth |
| US5654287A (en) * | 1990-12-13 | 1997-08-05 | Merrell Phamaceuticals Inc. | Method of treating cancer by conjunctive therapy with N,N1 -bis[3-(ethylamino)propyl]-1,7-heptanediamine and a cytotoxic agent |
| US5656671A (en) | 1986-12-02 | 1997-08-12 | University Of Florida Research Foundation, Inc. | Sterically hindered tetraamines and method for their production |
| JPH09235271A (en) | 1996-02-29 | 1997-09-09 | Suntory Ltd | Polyamine derivative |
| WO1997033560A1 (en) | 1996-03-14 | 1997-09-18 | Schering-Plough Healthcare Products, Inc. | Sunless tanning composition and method |
| WO1999003823A2 (en) | 1997-07-15 | 1999-01-28 | Oridigm Corporation | Novel polyamine analogues as therapeutic and diagnostic agents |
| WO1999054283A1 (en) | 1998-04-21 | 1999-10-28 | Universite Laval | Polyamine transport inhibitors |
| WO2000034226A1 (en) | 1998-12-10 | 2000-06-15 | Universite Laval | Polyamine transport inhibitors |
| WO2000046187A2 (en) | 1999-02-05 | 2000-08-10 | Oridigm Corporation | Antizyme modulators and their use |
| EP1085011A1 (en) | 1999-09-15 | 2001-03-21 | Oridigm Corporation | Novel polyamine analogues as therapeutic and diagnostic agents |
| US6235737B1 (en) | 2000-01-25 | 2001-05-22 | Peter Styczynski | Reduction of hair growth |
| WO2001072685A2 (en) | 2000-03-24 | 2001-10-04 | Oridigm Corporation | Polyamine analogues as cytotoxic agents |
| US7208528B1 (en) | 1997-07-15 | 2007-04-24 | Mediquest Therapeutics, Inc. | Polyamine analogues as therapeutic and diagnostic agents |
-
2002
- 2002-01-08 US US13/047,297 patent/USRE43327E1/en not_active Expired - Lifetime
Patent Citations (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4309442A (en) | 1977-07-11 | 1982-01-05 | Merrell Toraude Et Compagnie | Method for controlling fertility in mammals |
| US4590288A (en) | 1983-06-29 | 1986-05-20 | Exxon Research And Engineering Co. | Bi- or polymetallic coordination complex |
| WO1985002769A1 (en) | 1983-12-20 | 1985-07-04 | Kovacs Adam | Antitumour pharmaceutical compositions and process for preparing the same |
| US4950744A (en) | 1985-01-10 | 1990-08-21 | Molecular Diagnostics, Inc. | Photochemical nucleic acid-labeling reagent having a polyalkylamine spacer |
| US4818770A (en) | 1986-10-22 | 1989-04-04 | Boyce Thompson Institute For Plant Research | Prevention of a plant disease by specific inhibition of fungal polyamine biosynthesis |
| US5656671A (en) | 1986-12-02 | 1997-08-12 | University Of Florida Research Foundation, Inc. | Sterically hindered tetraamines and method for their production |
| US4774339A (en) | 1987-08-10 | 1988-09-27 | Molecular Probes, Inc. | Chemically reactive dipyrrometheneboron difluoride dyes |
| JPH02256656A (en) | 1988-12-09 | 1990-10-17 | Terumi Nakajima | New polyamine compound and glutamic acid receptor blocker |
| WO1991000853A1 (en) | 1989-07-03 | 1991-01-24 | New York University | Use of polyamines as ionic-channel regulating agents |
| US5252714A (en) | 1990-11-28 | 1993-10-12 | The University Of Alabama In Huntsville | Preparation and use of polyethylene glycol propionaldehyde |
| US5654287A (en) * | 1990-12-13 | 1997-08-05 | Merrell Phamaceuticals Inc. | Method of treating cancer by conjunctive therapy with N,N1 -bis[3-(ethylamino)propyl]-1,7-heptanediamine and a cytotoxic agent |
| US5248782A (en) | 1990-12-18 | 1993-09-28 | Molecular Probes, Inc. | Long wavelength heteroaryl-substituted dipyrrometheneboron difluoride dyes |
| WO1992014709A2 (en) | 1991-02-11 | 1992-09-03 | Cambridge Neuroscience, Inc. | Calcium channel antagonists and methodology for their identification |
| US5187288A (en) | 1991-05-22 | 1993-02-16 | Molecular Probes, Inc. | Ethenyl-substituted dipyrrometheneboron difluoride dyes and their synthesis |
| US5274113A (en) | 1991-11-01 | 1993-12-28 | Molecular Probes, Inc. | Long wavelength chemically reactive dipyrrometheneboron difluoride dyes and conjugates |
| US5451663A (en) | 1991-11-01 | 1995-09-19 | Molecular Probes, Inc. | Long wavelength chemically reactive dipyrrometheneboron difluoride dyes and conjugates |
| WO1995021612A2 (en) | 1993-02-08 | 1995-08-17 | Nps Pharmaceuticals, Inc. | Compounds active at a novel site on receptor-operated calcium channels useful for treatment of neurological disorders and diseases |
| US5648394A (en) | 1993-05-27 | 1997-07-15 | Boxall; Brian Alfred | Topical composition for inhibiting hair growth |
| US5541230A (en) * | 1993-11-05 | 1996-07-30 | Us Health | Therapeutic polyamines |
| US5433896A (en) | 1994-05-20 | 1995-07-18 | Molecular Probes, Inc. | Dibenzopyrrometheneboron difluoride dyes |
| WO1996022926A1 (en) | 1995-01-25 | 1996-08-01 | Sealed Air Corporation | Inflatable cushion and method of making same |
| WO1996038464A1 (en) | 1995-05-30 | 1996-12-05 | Lehigh University | Antimicrobial sterol conjugates |
| JPH09235271A (en) | 1996-02-29 | 1997-09-09 | Suntory Ltd | Polyamine derivative |
| WO1997033560A1 (en) | 1996-03-14 | 1997-09-18 | Schering-Plough Healthcare Products, Inc. | Sunless tanning composition and method |
| US6172261B1 (en) * | 1997-07-15 | 2001-01-09 | Oridigm Corporation | Polyamine analogues as therapeutic and diagnostic agents |
| WO1999003823A2 (en) | 1997-07-15 | 1999-01-28 | Oridigm Corporation | Novel polyamine analogues as therapeutic and diagnostic agents |
| US7160923B1 (en) | 1997-07-15 | 2007-01-09 | Mediquest Therapeutics, Inc. | Polyamine analogues as therapeutic and diagnostic agents |
| US7208528B1 (en) | 1997-07-15 | 2007-04-24 | Mediquest Therapeutics, Inc. | Polyamine analogues as therapeutic and diagnostic agents |
| WO1999054283A1 (en) | 1998-04-21 | 1999-10-28 | Universite Laval | Polyamine transport inhibitors |
| WO2000034226A1 (en) | 1998-12-10 | 2000-06-15 | Universite Laval | Polyamine transport inhibitors |
| WO2000046187A2 (en) | 1999-02-05 | 2000-08-10 | Oridigm Corporation | Antizyme modulators and their use |
| EP1085011A1 (en) | 1999-09-15 | 2001-03-21 | Oridigm Corporation | Novel polyamine analogues as therapeutic and diagnostic agents |
| US6235737B1 (en) | 2000-01-25 | 2001-05-22 | Peter Styczynski | Reduction of hair growth |
| WO2001072685A2 (en) | 2000-03-24 | 2001-10-04 | Oridigm Corporation | Polyamine analogues as cytotoxic agents |
Non-Patent Citations (167)
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10632145B2 (en) | 2016-03-25 | 2020-04-28 | Aminex Therapeutics, Inc. | Bioavailable polyamines |
| EP3785706A1 (en) | 2016-03-25 | 2021-03-03 | Aminex Therapeutics, Inc. | Bioavailable polyamines |
| US11395834B2 (en) | 2016-03-25 | 2022-07-26 | Aminex Therapeutics, Inc. | Bioavailable polyamines |
| US20220096412A1 (en) * | 2020-09-30 | 2022-03-31 | Aminex Therapeutics, Inc. | Combination Drug Substance of Polyamine Transport Inhibitor and DFMO |
| US11865095B2 (en) * | 2020-09-30 | 2024-01-09 | Aminex Therapeutics, Inc. | Combination drug substance of polyamine transport inhibitor and DFMO |
| US20230321012A1 (en) * | 2021-12-01 | 2023-10-12 | Aminex Therapeutics, Inc. | Methods To Treat Cancer With AMXT 1501 and DFMO |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20050176828A1 (en) | Hydrophobic polyamine analogs and methods for their use | |
| US7160923B1 (en) | Polyamine analogues as therapeutic and diagnostic agents | |
| US7235695B2 (en) | Cycloalkyl substituted polyamines for cancer therapy and methods of synthesis therefor | |
| US20070066646A1 (en) | Compounds for Inhibiting Copper-Containing Amine Oxidases and Uses Thereof | |
| US20080269282A1 (en) | Compounds for Inhibiting Copper-Containing Amine Oxidases and Uses Thereof | |
| EP1159261B1 (en) | Antizyme modulators and their use | |
| US20090124832A1 (en) | Oligoamine compounds and derivatives thereof for cancer therapy | |
| AU781525B2 (en) | Novel polyamine analogues as therapeutic and diagnostic agents | |
| KR100851452B1 (en) | Novel polyamine analogues as therapeutic and diagnostic agents | |
| CZ296122B6 (en) | Derivative of isoxazole carboxylic and crotonic acid amides, process of their preparation, a medicament containing thereof and use of such derivative | |
| JP2004500406A (en) | Polyamine analogs as cytotoxic agents | |
| USRE43327E1 (en) | Hydrophobic polyamine analogs and methods for their use | |
| US7208528B1 (en) | Polyamine analogues as therapeutic and diagnostic agents | |
| AU2008201086B2 (en) | Hydrophobic Polyamine Analogs and Methods for their Use | |
| AU2002248311A1 (en) | Hydrophobic polyamine analogs and methods for their use | |
| AU2003200048B2 (en) | Novel polyamine analogues as therapeutic and diagnostic agents |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| FPAY | Fee payment |
Year of fee payment: 8 |
|
| FPAY | Fee payment |
Year of fee payment: 12 |