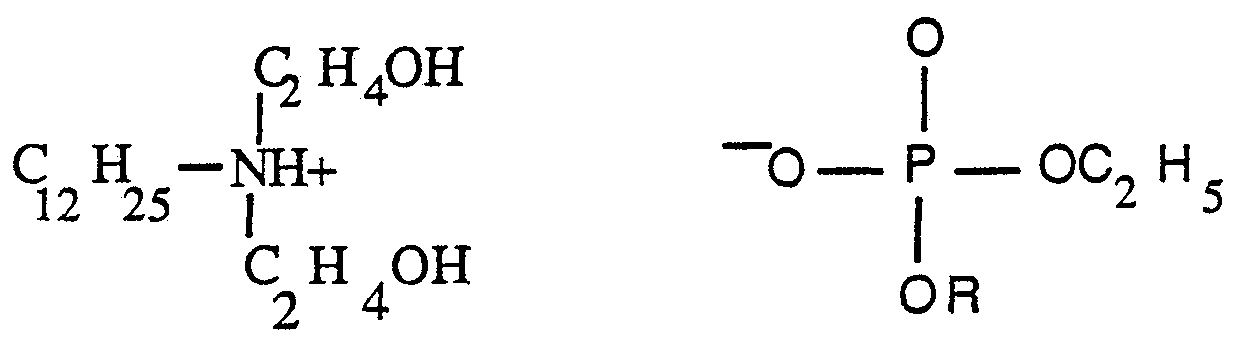WO1989001023A1 - Microbiocidal cleansing or disinfecting formulations and preparation thereof - Google Patents
Microbiocidal cleansing or disinfecting formulations and preparation thereof Download PDFInfo
- Publication number
- WO1989001023A1 WO1989001023A1 PCT/US1988/002647 US8802647W WO8901023A1 WO 1989001023 A1 WO1989001023 A1 WO 1989001023A1 US 8802647 W US8802647 W US 8802647W WO 8901023 A1 WO8901023 A1 WO 8901023A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- carbon atoms
- alkyl group
- approximately
- microbiocidal
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/48—Medical, disinfecting agents, disinfecting, antibacterial, germicidal or antimicrobial compositions
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N33/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic nitrogen compounds
- A01N33/02—Amines; Quaternary ammonium compounds
- A01N33/08—Amines; Quaternary ammonium compounds containing oxygen or sulfur
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N57/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic phosphorus compounds
- A01N57/10—Biocides, pest repellants or attractants, or plant growth regulators containing organic phosphorus compounds having phosphorus-to-oxygen bonds or phosphorus-to-sulfur bonds
- A01N57/12—Biocides, pest repellants or attractants, or plant growth regulators containing organic phosphorus compounds having phosphorus-to-oxygen bonds or phosphorus-to-sulfur bonds containing acyclic or cycloaliphatic radicals
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N57/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic phosphorus compounds
- A01N57/10—Biocides, pest repellants or attractants, or plant growth regulators containing organic phosphorus compounds having phosphorus-to-oxygen bonds or phosphorus-to-sulfur bonds
- A01N57/14—Biocides, pest repellants or attractants, or plant growth regulators containing organic phosphorus compounds having phosphorus-to-oxygen bonds or phosphorus-to-sulfur bonds containing aromatic radicals
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/36—Organic compounds containing phosphorus
- C11D3/362—Phosphates or phosphites
Definitions
- the present invention relates to microbiocidal compositions and methods for the preparation and use of such compositions.
- these microbiocidal compositions are effective in killing or inhibiting a wide variety of harmful, destructive, or offensive microorganisms including viruses, bacteria, algae, yeasts, and molds.
- microorganism means any organism that cannot easily be seen with the naked eye and includes organisms such as bacteria, molds, yeasts, fungi, algae and viruses.
- Antimicrobial and “microbiocidal” describe the killing of, as well as the inhibition of the growth of, bacteria, yeasts, fungi, algae, and molds.
- Bacicidal describes the killing or inhibition of the growth of bacteria.
- Fungicidal describes the killing of, as well as the inhibition of the growth of, fungi, yeasts and molds..
- viralicidal is used to describe the inactivation of virus particles so that they are unable to infect host cells.
- plastic includes both thermosetting and thermoplastic materials.
- plastic materials include, but are not limited to, polyolefins (such as polyethylenes, polypropylenes, polybutylenes) polystyrenes, vinyl phenolics, vinyl acetates, polymeric vinyl chlorides, ureas, melamines, acrylics, polyesters, epoxies and nylons.
- molded as used in this application is used in its broad sense to include any technique for forming plastic or other materials. Molding is generally, but not always, accomplished with elevated temperature and includes, but is not limited to, forming methods such as potting, extruding, sheeting, calendering, pulltruding, casting, vacuum forming, blow molding, and the like.
- cleaning agent includes any substance capable of cleaning, emulsifying, or removing unwanted material from a surface.
- detergent describes any substance or product which is capable of dislodging, removing, or dispersing solid and liquid soils from a surface being cleansed.
- detergent also includes soaps comprising metal salts of long chain fatty acids.
- disinfectant includes any liquid that is capable of killing or inhibiting microorganisms.
- free hydroxyl as used herein means an oxygen that is bonded to a phosphorus in a phosphate group.
- microorganisms Bacteria, fungi, viruses, algae and other microorganisms are always present in our environment. Such microorganisms are frequently an essential part of ecological systems, industrial processes, and healthy human and animal bodily functions, such as digestion. In other instances, however, the presence of microorganisms is highly undesirable because they may cause illnesses or death of humans and animals, create odors and damage or destroy a wide variety of materials.
- the species and numbers of microorganisms present vary depending on the general environment, on the nutrients and the moisture available for the growth of the microorganisms, and on humidity and temperature of the local environment. Nutrients for microorganisms abound in the normal environment. Any protein matter such as dried skin, discarded foods, plants, and animal wastes all are excellent nutrient media for many types of potentially harmful microorganisms. Furthermore, many organic synthetic and natural materials like plastic coatings and objects, and wood, paper and natural fibers can serve as nutrients for microorganisms which will degrade those materials. In addition, certain bacteria are capable of remaining viable in a dormant state on floors or on objects for long periods of time until they are deposited in the proper media for growth. Consequently, potentially harmful microorganisms can be transported merely by walking on floors, brushing against walls or furniture or by handling objects.
- Potentially dangerous microorganisms are spread in health care facilities and elsewhere by a variety of vectors.
- One of the most common vectors is health care personnel. For example, a nurse or doctor may administer care to one patient and then be called upon to treat a second patient. Even though he or she may carefully wash his or her hands before treating the second patient, potentially dangerous microorganisms may be transferred from the first patient to the second patient. The microorganism can then cause a serious infection in the second patient.
- bacteria which are classified as procaryotes, can be killed or inhibited by many different types of antibiotics.
- antibiotics that are effective against procaryotic organisms are usually ineffective against eucaryotic microorganisms, such as fungi and yeasts.
- Gram-positive and Gram- negative bacteria Even within the family of Bacteriaceae, there are two broad categories of bacteria known as Gram-positive and Gram- negative bacteria. These classifications stem from the ability or non-ability of bacteria to absorb certain vital stains, and the two groups of bacteria generally respond differently to the same microbiocidal agent. A particular agent that may be effective against one group may not be effective against the other group.
- One conventional method of inhibiting the growth of both eucaryotes and procaryotes or both Gram-negative and Gram- positive bacteria is to combine two or more microbiocidal inhibitors, each designed to inhibit or kill a specific organism or class of organisms.
- various problems arise when introducing two or more additives into a material such as a detergent.
- the multiple additive system may alter the physical properties of the detergent into which it is mixed.
- the multiple components must be tested to insure compatibility and continued microbiocidal effectiveness when combined with the detergent.
- the relative microbiocidal or microbiostatic strength of each of the components in the multiple system must be determined.
- microbiocidal additives it is not uncommon for the combination of microbiocidal additives to initially have effective inhibiting or killing properties for both Gram-positive and Gram-negative organisms whereupon, with the passage of time, one or the other of the inhibiting additives will deteriorate and lose its effectiveness while the other inhibiting additive remains effective. In addition, one additive may have an unexpected inhibitory effect on the other additive. In addition, the requirement of adding two or more additives can become prohibitively expensive.
- microbiocidal additive must be non-toxic to humans and animals around which the additive is used. Such an additive should not cause an allergic reaction and must have no long term detrimental health effects on humans or animals. Finally, such an microbiocidal additive should be compatible with the material with which it is being used and not cause the material to deteriorate or lose its desired properties.
- the present invention solves the problems described above by providing a composition including a broad spectrum, safe, microbiocidal additive that is effective in killing or inhibiting a wide variety of microorganisms including viruses, bacteria, yeasts, molds and fungi and algae.
- the microbiocidal additive comprises a phosphate derivative having microbiocidal activity.
- the microbiocidal phosphate derivative has at least one free hydroxyl group thereon.
- the present invention solves the above problems by providing a safe cleansing solution containing a microbiocidal additive.
- the additive can be added to water to provide a microbiocidal agent or can be added to a conventional detergent to provide a microbiocidal cleansing agent.
- the detergents that can be used in the present invention include, but are not limited to, linear alkyl sulfonates and alkyl benzene sulfonates. These detergents also include, but are not limited to, metal salts of long chain fatty acids.
- the microbiocidal cleansing agent of the present invention is effective in killing or significantly inhibiting the growth of a wide spectrum of both procaryotic and eucaryotic microorganisms which may reside on surfaces to be cleaned or treated with the microbiocidal detergent.
- certain alkyl phosphate additives provide unique and unexpected fungicidal, viricidal and bactericidal properties to a conventional detergent.
- the microbiocidal cleansing agent of the present invention has the capacity to kill or inhibit the growth of many types of bacteria, fungi, viruses, yeasts and other destructive or disease-producing microorganisms which might be on a surface cleaned with the microbiocidal detergent of the present invention.
- the microbiocidal cleansing agent of the present invention is particularly effective against both Gram-positive bacteria, such as Staphylococcus aureus, and Gram-negative bacteria, such as Pseudomonas aeruginosa.
- the microbiocidal cleansing agent of the present invention also comprises a method for the preparation of and the incorporation into detergents of the alkyl phosphate additives that give the detergents the ability to kill or significantly reduce bacterial growth, fungal growth and yeast growth on surfaces that are cleaned with the detergent.
- the microbiocidal cleansing agent of the present invention also inactivates virus particles that may be on the surface that is cleaned with the microbiocidal detergent of the present invention.
- the concentration of the reactants in the preparation of the alkyl phosphate additive the bactericidal activity of the resulting alkyl phosphate additive can be selected.
- the alkyl phosphate additive can be prepared so that it is effective primarily against Gram-negative bacteria, against Gram-positive bacteria or both.
- the microbiocidal additive of the present invention is a phosphate derivative with at least one free hydroxyl group.
- the phosphate derivative has the following general formula:
- R and R' may be alkyl, aryl, aralkyl and alkaryl groups including, but not limited to, straight chain, branched chain or cyclic alkyl groups having from 1 to 24 carbon atoms, polyoxyethylene or polyoxypropylene having from 2 to 32 ethylene oxide or propylene oxide units respectively, alkyl phenoxy polyoxyethylene containing 2 to 32 ethylene oxide units, alkyl phenoxy polyoxyethylene containing ethylene oxide units and 1 to 24 carbon atoms in the phenolic alkyl chain, and polyhydroxy compounds, including but not limited to, ethylene glycol, glycerol, or sorbitol, wherein R and R' include at least one free hydroxyl group.
- X is selected from the group consisting of Group IA metals, Group IIA metals, transition metals, hydrogen, and an organic ion.
- the positively charged ion is not necessary for microbiocidal activity.
- microbiocidal additive of the present invention can be added to a wide variety of materials in accordance with the present invention to impart microbiocidal activity to those materials.
- the microbiocidal additive of the present invention can be added to aqueous solutions of detergents in accordance with the present invention to provide microbiocidal cleansing agents, hi addition, the present invention can be added to water or other solvents to provide an effective disinfectant.
- Th microbiocidal additive of the present invention is capable of killing the causitive organism of Legionaires' disease, Legionella pneumophilia.
- the present invention embraces addition of the identified compound to cooling tower water to control the growth of this pathological organism.
- Another object of the present invention is to provide for the use of microbiocidally effective phosphate derivatives which can be added to an aqueous detergent to impart microbiocidal activity to the detergent.
- Yet another object of the present invention is to provide for the addition of microbiocidally effective phosphate derivatives to water or other solvents to provide effective disinfectants.
- Another object of the present invention is to provide for incorporation of microbiocidally effective phosphate derivatives into detergents to provide a cleansing agent with microbiocidal activity against a wide variety of organisms including bacteria, fungi, molds, algae and viruses.
- Another object of the present invention is to provide for incorporation into products or application onto products of microbiocidally effective phosphate derivatives to preserve the products from degradation by microorganisms.
- Another object of the present invention is to provide an effective insecticidal phosphate derivatives which can be mixed with an aqueous detergent solution and used to wash an animal or object to kill or repel insects or related organisms.
- Another object of the present invention is to provide a microbiocidal phosphate derivative.
- the present invention relates to microbiocidal and insecticidal compositions comprising certain derivatives of phosphates.
- the phosphate derivatives are capable of killing or inhibiting the growth of a wide variety of microorganisms including fungi, yeasts, viruses, algae and bacteria.
- the phosphate derivative utilized in accordance with the present invention inhibits the growth of the following representative Gram-negative and Gram-positive bacteria: Sarcina lutea, Staphylococcus species, Pseudomonas aeruginosa, Pseudomonas cepacia, Escherichia coli, Escherichia communior, Bacillus subtilis, Klebsiella species, Salmonella species, Legionella pneumophilia, Enterobacter aerogenes and Streptococcus species.
- the phosphate derivative also inhibits the growth of the following representative fungi and yeasts: Candida albicans, Trichophyton metagrophytes, Trichophyton rubrum, Trichophyton interdigitale and Aspergillus niger.
- the phosphate derivative also inactivates Herpes simplex virus.
- the foregoing microorganisms are representative of those organisms that are responsible for infections in hospitals and other health care facilities.
- the microbiocidal additive of the present invention can be added to water or other solvents to provide a disinfectant formulation or can be added to a conventional detergent to provide a microbiocidal cleansing agent.
- the detergents that can be used in the present invention include, but are not limited to, linear alkyl sulfonates and alkyl benzene sulfonates. These detergents also include, but are not limited to, metal salts of long chain fatty acids.
- microbiocidal cleansing agent is effective in killing or significantly inhibiting the growth of a wide spectrum of both procaryotic and eucaryotic microorganisms which may reside on surfaces to be cleaned or treated with the microbiocidal detergent.
- certain phosphate derivatives impart fungicidal, algaecidal, viricidal and bactericidal properties to a conventional detergent.
- the microbiocidal additive of the present invention can be mixed in water at various concentrations and be used as a disinfectant formulation to kill or inhibit microorganisms that may reside on that surface.
- a solution of the additive of the present invention containing from approximately 500 to 1000 parts per million (PPM) of the phosphate derivative makes an excellent disinfectant formulation for light duty such as mopping and cleaning of hard surfaces such as vinyl walls, floors, counters and table tops.
- the phosphate derivative can be mixed in with a conventional detergent at a concentration of between approximately 15% and 70% by weight.
- microbiocidal cleansing agent prepared by the addition of the microbiocidal additive of the present invention to a conventional cleansing agent has the capacity to kill or inhibit the growth of many types of bacteria, fungi, viruses, yeasts and other destructive or disease-producing microorganisms which might be on a surface.
- a microbiocidal cleansing agent is particularly effective against both Gram-positive bacteria, such as Staphylococcus aureus, and Gram-negative bacteria, such as Pseudomonas aeruginosa.
- the phosphate derivative can be added to the water in cooling towers or can be included in a coating that is used to coat the surfaces in cooling towers to kill or inhibit the growth of the pathogen that causes Legionaire's disease, Legionella pneumophilia.
- the phosphate derivative has also been found to be an effective deodorant.
- the phosphate derivative will no longer exhibit microbiocidal activity. It is believed that, in general, the more hydroxyl groups that are free, the greater the gram negative microbiocidal activity will be exhibited by the phosphate derivative.
- microbiocidal additive of the present invention can be more specifically defined as a phosphate ester with at least one free hydroxyl group with the following general formula:
- R and R' may be alkyl, aryl, aralkyl and alkaryl groups including, but not limited to, straight chain, branched chain or cyclic alkyl groups having from 1 to 24 carbon atoms, polyoxyethylene or polyoxypropylene having from 2 to 32 ethylene oxide or propylene oxide units respectively, alkyl phenoxy polyoxyethylene containing 2 to 32 ethylene oxide units, alkyl phenoxy polyoxyethylene containing ethylene oxide units and 1 to 24 carbon atoms in the phenolic alkyl chain, and polyhydroxy compounds, including but not limited to, ethylene glycol, glycerol, or sorbitol, wherein R and R' include at least one free hydroxyl group.
- R may also be hydrogen, but only if X is a quaternary amine.
- X is selected from the group consisting of Group IA metals, Group IIA metals, transition metals, hydrogen, and an organic ion.
- a preferred structure of the phosphate derivative comprises the following formula:
- R and R * an alkyl group of from 6 to 18 carbon atoms and one group can be H.
- X is selected from the group consisting of Group IA metals, Group HA metals, transition metals, hydrogen, and an organic ion.
- the microbiocidal additive defined by this formula is water insoluble or only slightly soluble in water and is especially useful for addition to non-aqueous products such as plastics, fibers and non-aqueous coatings.
- An aqueous suspension of the phosphates with the alkyl group of greater than 6 carbon atoms in the R position can be prepared by adding a surfactant such as Tween 80 (Sigma Chemical Company, St. Louis, MO) or other suitable nonionic surfactant..
- An especially preferred structure of the microbiocidal additive of the present invention comprises a phosphate with the following formula: O
- R and R' an alkyl group of from 1 to 5 carbon atoms and one group can be H.
- X is selected from the group consisting of Group IA metals, Group IIA metals, transition metals, hydrogen, and an organic ion.
- microbiocidal additive defined by this formula is water soluble and is especially useful as an additive for a disinfectant or for a detergent.
- An additional preferred structure of the microbiocidal additive of the present invention has the following formula:
- X is selected from the group consisting of Group IA metals, Group HA metals, transition metals, hydrogen, and an organic ion.
- R H or C 2 H 5 ; and X is selected from the group consisting of Group IA metals, Group HA metals, transition metals, hydrogen, and an organic ion.
- This embodiment of the microbiocidal additive of the present invention is soluble in water.
- the microbiocidal activity of the product can be improved by substituting for "X" a large organic ion such as a quaternary amine.
- X a large organic ion such as a quaternary amine.
- An example of such a compound is a tertiary amine with the following general formula:
- Ri an alkyl group of from 4 to 18 carbon atoms or a hydroxy alkyl group of from 1 to 18 carbon atoms.
- the Ri groups are not necessarily identical.
- R 2 an alkyl group of from 8 to 18 carbon atoms.
- This especially preferred embodiment can be prepared by partially neutralizing phosphoric acid with between 1 to 2 moles of the tertiary amine. If more tertiary amine is used to neutralize the phosphoric acid, the free hydroxyls on the phosphate group will be e___minated and it is believed that the microbiocidal activity of the compound will be diminished.
- the type of component to be used as the "X" substituent will depend largely upon the compatibility of the base material for the "X" substituent.
- the microbiocidal additive of the present invention may be prepared as follows: One mole of phosphorous pentoxide is reacted with between approximately 1 to 3 moles of an alcohol.
- the alcohol can be an alkyl or an aryl compound.
- the alkyl alcohol can be straight chained, branched chain or cyclic. It is believed that the important aspect of the phosphate derivative is that the compound have at least one free hydroxyl.
- the alcohol should be heated to a temperature of between approximately 60° and 120° C depending upon the boiling point of the alcohol used.
- the phosphorous pentoxide is slowly added to the alcohol while the mixture is vigorously agitated. The reaction is complete two to four hours after the addition of phosphorous pentoxide is completed.
- the product formed in this reaction is a mixture of mono-ester phosphate and di-ester phosphate.
- the reaction equation is as follows:
- R is the alkyl or aryl group depending upon the substituent used.
- the phosphate derivative is an effective microbiocidal compound and is capable of killing or inhibiting a wide variety of microorganisms including bacteria, yeasts, fungi, algae, molds and viruses.
- the phosphate can be partially neutralized with a tertiary amine as shown in the following reaction equation:
- the preferable range for X is between approximately 1 and 1.5 moles with the especially preferable range of approximately 1.3 moles.
- R a straight chain or a branched chain alkyl group of from 1 to 18 carbon atoms
- Ri an alkyl group of from 4 to 18 carbon atoms or a hydroxyalkyl group of from 1 to 1 8 carbon atoms .
- R a straight chain or a branched chain alkyl group of from 8 to 18 carbon atoms .
- the microbiocidal activity of the phosphate derivative of the present invention is evaluated as follows. Petri dishes are prepared using appropriate nutrient agar as a food source for the microorganism to be tested. The microorganism is seeded into the agar as is well known to one or ordinary skill in the art. A hole 6mm in diameter and 5mm deep is cut into the agar. 0.05 ml. of each of the indicated test compounds is placed in the hole and the inoculated petri dish is incubated for 24 hours at 37°C.
- the relative susceptibility of the test organisms to the phosphate derivative of the present invention is demonstrated by a clear zone of growth inhibition around the test solution.
- This zone of inhibition is the result of two processes: (1) the diffusion of the compound and (2) growth of the bacteria.
- the area of the suppressed microbial growth, the zone of inhibition is determined by the concentration of the phosphate derivative present in the area. Therefore, within the limitations of the test, the area of the inhibition zone is proportional to the relative susceptibility of the microorganisms to the phosphate derivative of the present invention.
- each plate is examined and the diameters of the complete inhibition zones are noted and measured using either reflected light and a measuring device such as sliding calipers, a ruler, or a template prepared for this purpose and held on the bottom of the plate.
- the end point measured to the nearest millimeter, is the point at which no visible growth that can be detected with the unaided eye minus the diameter of the test drop or sample.
- the area of the zone of inhibition is then calculated.
- the mono-ethyl alkyl phosphate derivative is prepared as follows: One mole of phosphorous pentoxide is reacted with three moles of ethanol at a temperature of 60°C. The phosphorous pentoxide is slowly added to the ethanol while the mixture is vigorously agitated. At the reaction temperature of 60° C the reaction is complete in about two hours. The progress of the reaction is determined by titrating the acid that is produced with a solution of potassium hydroxide. The reaction products include approximately equimolar quantities of the mono-ethyl alkyl phosphate and the di-ethyl alkyl phosphate. The mono-ethyl alkyl phosphate is the more microbiocidally active species.
- the mono-(2-ethylhexyl) phosphate derivative is prepared as follows. One mole of phosphorous pentoxide is reacted with three moles of 2-ethylhexanol at a temperature of 100°C. The phosphorous pentoxide is slowly added to the ethanol while the mixture is vigorously agitated. At the reaction temperature of 100°C the reaction is complete in about two hours. The progress of the reaction is dete ⁇ nined by titrating the acid that is produced with a solution of potassium hydroxide. The reaction products include approximately equimolar quantities of the mono-(2-ethylhexyl) alkyl phosphate and the di(2-ethylhexyl) alkyl phosphate. The mono-(2-ethylhexyl) alkyl phosphate is the more microbiocidally active species.
- Petri dishes are prepared using trypticase soy nutrient agar (Baltimore Biological Laboratory, Cockeysville, MD).
- the microorganisms used in this test are the Gram-positive Staphylococcus aureus and the Gram-negative Pseudomonas aeruginosa.
- Each microorganism is seeded into the agar as is well known to one of ordinary skill in the art.
- a hole 6mm in diameter and 5mm deep is cut into the agar.
- 0.05 ml. of each of the indicated test compounds is placed in the hole, and the inoculated petri dish is incubated for 24 hours at 37°C. After the 24 hour incubation period, the relative susceptibility of the test organisms to the phosphate derivative of the present invention is demonstrated by a clear zone of growth inhibition around the test solution.
- each plate After the 24 hour incubation period, each plate is examined and the diameters of the complete inhibition zones are noted and measured as described above. Each test is performed at least 6 times. The areas shown in Table A are the average of the 6 separate tests.
- the reaction product of Example II is partially neutralized with bis(hydroxyethyl) cocoamine. 1.3 moles of bis(hydroxyethyl) cocoamine per mole each of the reaction products from Example I is slowly added to the reaction product from Example I until the pH is between approximately 3.2 and 3.8 in a 75% ethanol solution. This reaction is carried out at a temperature of 100°C. The reaction mixture is vigorously agitated during the reaction.
- An aqueous mixture of the microbiocidal alkyl phosphate is prepared by mixing the alkyl phosphate derivative from Example II with an aqueous detergent solution. The concentration of alkyl phosphate derivative is .05%.
- the microbiocidal detergent is heated to 85°C. Cotton fabric is then introduced and remains in the heated solution for 15 minutes. The fabric is then rinsed in water at 40° C, removed, and dried.
- Example V To produce a microbiocidal alkyl phosphate derivative that can be used with a detergent to make a microbiocidal detergent, the reaction product of Example II is neutralized with bis(hydroxy ethyl) cocoamine. 1.3 moles of bis(hydroxyethyl) cocoamine per mole each of the reaction products from Example I is slowly added to the reaction product from Example II until the pH is between approximately 3.2 and 3.8 in a 75% ethanol solution. This reaction is carried out at a temperature of 100°C. The reaction mixture is vigorously agitated during the reaction.
- a dry free-flowing mixture comprising the microbiocidal cleansing agent of the present invention is prepared by mixing 0.3 grams of the above alkyl phosphate amine derivative with 138.5 grams of "All" detergent as purchased over the counter. One gram of the cleansing agent mixture is then placed in the center of appropriately inoculated petri dishes and incubated for 24 hours at 37°C. Control plates are also prepared with one gram samples of the detergent without any phosphate derivative. After this period of incubation, each plate is examined and the diameters of the inhibition zones are measured. The results are shown in Table B.
- the detergent alone exhibits some microbiocidal activity probably because of the presence of sodium hypochlorite which would be washed out of fabrics during the rinsing process.
- the detergent plus additive demonstrates a significant increase in microbiocidal activity over the detergent alone.
- microbiocidal capability of an alkyl phosphate amine is demonstrated by the following example. Between 0.5 moles and 3.0 moles of bis (hydroxy ethyl) cocoamine per mole of the reaction products from Example II is slowly added to the reaction products from Example II until the pH of the solution is between approximately 2.5 and 6 in a 75% ethanol solution. This reaction is carried out at a temperature of 100°C. The reaction mixture is vigorously agitated during the reaction. The resulting compounds are tested for microbiocidal activity.
- Petri dishes are prepared using trypticase soy nutrient agar (Baltimore Biological Laboratory, Cockeysville, MD).
- the microorganisms used in this test are the Gram-positive Staphylococcus aureus and the Gram-negative Pseudomonas aeruginosa.
- the microorganisms are seeded into nutrient agar as is well known to one of ordinary skill in the art.
- a hole 6mm in diameter and 5mm deep is cut into the agar.
- 0.05 ml. of each of the indicated test compounds is placed in the hole and the inoculated petri dish is incubated for 24 hours at 37°C. After the 24 hour incubation period, the relative susceptibility of the test organisms to the phosphate derivative of the present invention is demonstrated by a clear zone of growth inhibition around the test solution.
- each plate is examined and the diameters of the complete inhibition zones are noted and measured.
- sample A which is the reaction product from Example II, has excellent microbiocidal activity against both the Gram positive Staphylococcus aureus and the Gram negative Pseudomonas aeruginosa.
- the reaction product from Example II retains its microbiocidal activity against both these organisms even when reacted with up to 2 moles of the bis- hydroxyethyl cocoamine. When one mole each of the reaction product from Example II is reacted with more than 2 moles of the cocoamine, the microbiocidal activity is diminished.
Abstract
The present invention relates to microbiocidal compositions and methods for the preparation and use of such compositions. Properly used accordance with the present invention, these microbiocidal compositions are effective in killing or inhibiting a wide variety of harmful, destructive or offensive microorganisms including viruses, bacteria, yeasts, algae and molds. The microbiocidal compositions of the present invention are suitable for use with conventional detergents to provide microbiocidal cleansing agents. The microbiocidal compositions can also be mixed with a liquid to provide an effective disinfectant.
Description
"MICROBIOCIDAL CLEANSING OR DISINFECTING FORMULATIONS AND PREPARATION THEREOF"
Cross-Reference to Related Cases
This is a continuation-in-part of application Serial No. 047,561, filed on April 27, 1987; 781,710 filed on October 2, 1985; 635,728 filed on July 30, 1984, now abandoned; application Serial No.. 658,695 filed on October 9, 1984, now abandoned; application Serial No. 713,445 filed on March 19, 1985, now abandoned; application Serial No. 736,652 filed on May 21, 1985; application Serial No. 744,916 filed on June 13, 1985; and application Serial No. 744,730 filed on June 13, 1985, now abandoned; all of which are continuations -in-part of application Serial No. 570,952 filed March 8, 1984, which in turn was a continuation of application Serial No. 523,734 filed August 16, 1983, now abandoned, which was a continuation of application Serial No. 226,006 filed January 19, 1981, now abandoned, which was a continuation of application Serial No. 930,879 filed August 4, 1978, also now abandoned.
Technical Field
The present invention relates to microbiocidal compositions and methods for the preparation and use of such
compositions. Properly used in accordance with the present invention, these microbiocidal compositions are effective in killing or inhibiting a wide variety of harmful, destructive, or offensive microorganisms including viruses, bacteria, algae, yeasts, and molds.
Background
Discussion of the present invention and its background will be facilitated by definition of several terms.
As used herein, the term "microorganism" means any organism that cannot easily be seen with the naked eye and includes organisms such as bacteria, molds, yeasts, fungi, algae and viruses. "Antimicrobial" and "microbiocidal" describe the killing of, as well as the inhibition of the growth of, bacteria, yeasts, fungi, algae, and molds. "Bactericidal" describes the killing or inhibition of the growth of bacteria. "Fungicidal" describes the killing of, as well as the inhibition of the growth of, fungi, yeasts and molds.. The term "viricidal" is used to describe the inactivation of virus particles so that they are unable to infect host cells. The term "plastic," as used herein, includes both thermosetting and thermoplastic materials. Examples of "plastic" materials include, but are not limited to, polyolefins (such as polyethylenes, polypropylenes, polybutylenes) polystyrenes, vinyl phenolics, vinyl acetates, polymeric vinyl chlorides, ureas, melamines, acrylics, polyesters, epoxies and nylons. The term "molded" as used in this application is used in its broad sense to include any technique for forming plastic or other materials. Molding is generally, but not always, accomplished with elevated temperature and includes, but is not limited to, forming methods such as potting, extruding, sheeting, calendering, pulltruding, casting, vacuum forming, blow molding, and the like.
The term "cleansing agent" includes any substance capable of cleaning, emulsifying, or removing unwanted material from a surface. The term "detergent" describes any substance or product which is capable of dislodging, removing, or dispersing
solid and liquid soils from a surface being cleansed. The term "detergent" also includes soaps comprising metal salts of long chain fatty acids. The term "disinfectant" includes any liquid that is capable of killing or inhibiting microorganisms.
The term "free hydroxyl" as used herein means an oxygen that is bonded to a phosphorus in a phosphate group.
Bacteria, fungi, viruses, algae and other microorganisms are always present in our environment. Such microorganisms are frequently an essential part of ecological systems, industrial processes, and healthy human and animal bodily functions, such as digestion. In other instances, however, the presence of microorganisms is highly undesirable because they may cause illnesses or death of humans and animals, create odors and damage or destroy a wide variety of materials.
The species and numbers of microorganisms present vary depending on the general environment, on the nutrients and the moisture available for the growth of the microorganisms, and on humidity and temperature of the local environment. Nutrients for microorganisms abound in the normal environment. Any protein matter such as dried skin, discarded foods, plants, and animal wastes all are excellent nutrient media for many types of potentially harmful microorganisms. Furthermore, many organic synthetic and natural materials like plastic coatings and objects, and wood, paper and natural fibers can serve as nutrients for microorganisms which will degrade those materials. In addition, certain bacteria are capable of remaining viable in a dormant state on floors or on objects for long periods of time until they are deposited in the proper media for growth. Consequently, potentially harmful microorganisms can be transported merely by walking on floors, brushing against walls or furniture or by handling objects.
It is well recognized that a major difficulty in health care facilities, such as hospitals and nursing homes, is the spread of dangerous infectious diseases caused by a wide variety of microorganisms. The problem is exacerbated in these facilities
because many of the patients are in a weakened condition due to their primary health care problem. A microorganism that would not be a major threat to a healthy person could be fatal to a patient with a diininished capacity to defend himself from infection.
Potentially dangerous microorganisms are spread in health care facilities and elsewhere by a variety of vectors. One of the most common vectors is health care personnel. For example, a nurse or doctor may administer care to one patient and then be called upon to treat a second patient. Even though he or she may carefully wash his or her hands before treating the second patient, potentially dangerous microorganisms may be transferred from the first patient to the second patient. The microorganism can then cause a serious infection in the second patient.
In short, the control of microbial contamination and infection has been a major problem throughout history in both industry and the home, and such infection and contamination continues to cause disease, death, and destruction of property. It has proved difficult, however, to develop a microbiocidal additive that is effective in controlling the growth of a wide variety of unwanted microorganisms and is, at the same time, safe for use around human beings and animals. Accordingly, there is an acute need, both in industry and in the home, for a safe and effective microbiocidal additive that can be used in or on a wide variety of substances to impart microbiocidal activity to the product from which the substance is made.
One of the sources of difficulty in the control of potentially harmful microorganisms is the extreme variability of response of various microorganisms to conventional microbiocidal agents. For example, bacteria, which are classified as procaryotes, can be killed or inhibited by many different types of antibiotics. However, these same antibiotics that are effective against procaryotic organisms are usually ineffective against eucaryotic microorganisms, such as fungi and yeasts.
Even within the family of Bacteriaceae, there are two broad categories of bacteria known as Gram-positive and Gram-
negative bacteria. These classifications stem from the ability or non-ability of bacteria to absorb certain vital stains, and the two groups of bacteria generally respond differently to the same microbiocidal agent. A particular agent that may be effective against one group may not be effective against the other group.
One conventional method of inhibiting the growth of both eucaryotes and procaryotes or both Gram-negative and Gram- positive bacteria is to combine two or more microbiocidal inhibitors, each designed to inhibit or kill a specific organism or class of organisms. However, various problems arise when introducing two or more additives into a material such as a detergent. The multiple additive system may alter the physical properties of the detergent into which it is mixed. In addition, the multiple components must be tested to insure compatibility and continued microbiocidal effectiveness when combined with the detergent. The relative microbiocidal or microbiostatic strength of each of the components in the multiple system must be determined. It is not uncommon for the combination of microbiocidal additives to initially have effective inhibiting or killing properties for both Gram-positive and Gram-negative organisms whereupon, with the passage of time, one or the other of the inhibiting additives will deteriorate and lose its effectiveness while the other inhibiting additive remains effective. In addition, one additive may have an unexpected inhibitory effect on the other additive. In addition, the requirement of adding two or more additives can become prohibitively expensive.
The ideal microbiocidal additive must be non-toxic to humans and animals around which the additive is used. Such an additive should not cause an allergic reaction and must have no long term detrimental health effects on humans or animals. Finally, such an microbiocidal additive should be compatible with the material with which it is being used and not cause the material to deteriorate or lose its desired properties.
Summary of the Invention
The present invention solves the problems described above by providing a composition including a broad spectrum, safe, microbiocidal additive that is effective in killing or inhibiting a wide variety of microorganisms including viruses, bacteria, yeasts, molds and fungi and algae. The microbiocidal additive comprises a phosphate derivative having microbiocidal activity. The microbiocidal phosphate derivative has at least one free hydroxyl group thereon.
The present invention solves the above problems by providing a safe cleansing solution containing a microbiocidal additive. The additive can be added to water to provide a microbiocidal agent or can be added to a conventional detergent to provide a microbiocidal cleansing agent. The detergents that can be used in the present invention include, but are not limited to, linear alkyl sulfonates and alkyl benzene sulfonates. These detergents also include, but are not limited to, metal salts of long chain fatty acids. The microbiocidal cleansing agent of the present invention is effective in killing or significantly inhibiting the growth of a wide spectrum of both procaryotic and eucaryotic microorganisms which may reside on surfaces to be cleaned or treated with the microbiocidal detergent. Thus, in accordance with the present invention, it has been determined that certain alkyl phosphate additives provide unique and unexpected fungicidal, viricidal and bactericidal properties to a conventional detergent.
The microbiocidal cleansing agent of the present invention has the capacity to kill or inhibit the growth of many types of bacteria, fungi, viruses, yeasts and other destructive or disease-producing microorganisms which might be on a surface cleaned with the microbiocidal detergent of the present invention. The microbiocidal cleansing agent of the present invention is particularly effective against both Gram-positive bacteria, such as Staphylococcus aureus, and Gram-negative bacteria, such as Pseudomonas aeruginosa.
The microbiocidal cleansing agent of the present invention also comprises a method for the preparation of and the
incorporation into detergents of the alkyl phosphate additives that give the detergents the ability to kill or significantly reduce bacterial growth, fungal growth and yeast growth on surfaces that are cleaned with the detergent. The microbiocidal cleansing agent of the present invention also inactivates virus particles that may be on the surface that is cleaned with the microbiocidal detergent of the present invention. In addition, by adjusting the concentration of the reactants in the preparation of the alkyl phosphate additive, the bactericidal activity of the resulting alkyl phosphate additive can be selected. The alkyl phosphate additive can be prepared so that it is effective primarily against Gram-negative bacteria, against Gram-positive bacteria or both.
The microbiocidal additive of the present invention is a phosphate derivative with at least one free hydroxyl group. The phosphate derivative has the following general formula:
OR'
wherein:
R and R' may be alkyl, aryl, aralkyl and alkaryl groups including, but not limited to, straight chain, branched chain or cyclic alkyl groups having from 1 to 24 carbon atoms, polyoxyethylene or polyoxypropylene having from 2 to 32 ethylene oxide or propylene oxide units respectively, alkyl phenoxy polyoxyethylene containing 2 to 32 ethylene oxide units, alkyl phenoxy polyoxyethylene containing ethylene oxide units and 1 to 24 carbon atoms in the phenolic alkyl chain, and polyhydroxy compounds, including but not limited to, ethylene glycol, glycerol, or sorbitol, wherein R and R' include at least one free hydroxyl group.
X is selected from the group consisting of Group IA metals, Group IIA metals, transition metals, hydrogen, and an
organic ion. The positively charged ion is not necessary for microbiocidal activity.
The microbiocidal additive of the present invention can be added to a wide variety of materials in accordance with the present invention to impart microbiocidal activity to those materials.
For example, the microbiocidal additive of the present invention can be added to aqueous solutions of detergents in accordance with the present invention to provide microbiocidal cleansing agents, hi addition, the present invention can be added to water or other solvents to provide an effective disinfectant.
Th microbiocidal additive of the present invention is capable of killing the causitive organism of Legionaires' disease, Legionella pneumophilia. Thus, the present invention embraces addition of the identified compound to cooling tower water to control the growth of this pathological organism.
Accordingly, it is an object of the present invention to provide for the use of microbiocidally effective phosphate derivatives in and on a variety of products.
Another object of the present invention is to provide for the use of microbiocidally effective phosphate derivatives which can be added to an aqueous detergent to impart microbiocidal activity to the detergent.
Yet another object of the present invention is to provide for the addition of microbiocidally effective phosphate derivatives to water or other solvents to provide effective disinfectants.
Another object of the present invention is to provide for incorporation of microbiocidally effective phosphate derivatives into detergents to provide a cleansing agent with microbiocidal activity against a wide variety of organisms including bacteria, fungi, molds, algae and viruses.
Another object of the present invention is to provide for incorporation into products or application onto products of microbiocidally effective phosphate derivatives to preserve the products from degradation by microorganisms.
Another object of the present invention is to provide an effective insecticidal phosphate derivatives which can be mixed with an aqueous detergent solution and used to wash an animal or object to kill or repel insects or related organisms.
Another object of the present invention is to provide a microbiocidal phosphate derivative.
These and other objects, features and advantages of the present invention will become apparent after a review of the following detailed description of the disclosed embodiment and the appended claims.
Detailed Description of the Preferred Embodiment
The present invention relates to microbiocidal and insecticidal compositions comprising certain derivatives of phosphates. When used in accordance with the present invention, the phosphate derivatives are capable of killing or inhibiting the growth of a wide variety of microorganisms including fungi, yeasts, viruses, algae and bacteria.
The phosphate derivative utilized in accordance with the present invention inhibits the growth of the following representative Gram-negative and Gram-positive bacteria: Sarcina lutea, Staphylococcus species, Pseudomonas aeruginosa, Pseudomonas cepacia, Escherichia coli, Escherichia communior, Bacillus subtilis, Klebsiella species, Salmonella species, Legionella pneumophilia, Enterobacter aerogenes and Streptococcus species. The phosphate derivative also inhibits the growth of the following representative fungi and yeasts: Candida albicans, Trichophyton metagrophytes, Trichophyton rubrum, Trichophyton interdigitale and Aspergillus niger. In addition, the phosphate derivative also inactivates Herpes simplex virus. The foregoing microorganisms are representative of those organisms that are responsible for infections in hospitals and other health care facilities.
The microbiocidal additive of the present invention can be added to water or other solvents to provide a disinfectant formulation or can be added to a conventional detergent to provide
a microbiocidal cleansing agent. The detergents that can be used in the present invention include, but are not limited to, linear alkyl sulfonates and alkyl benzene sulfonates. These detergents also include, but are not limited to, metal salts of long chain fatty acids.
Such a microbiocidal cleansing agent is effective in killing or significantly inhibiting the growth of a wide spectrum of both procaryotic and eucaryotic microorganisms which may reside on surfaces to be cleaned or treated with the microbiocidal detergent. Thus, in accordance with the present invention, it has been determined that certain phosphate derivatives impart fungicidal, algaecidal, viricidal and bactericidal properties to a conventional detergent.
The microbiocidal additive of the present invention can be mixed in water at various concentrations and be used as a disinfectant formulation to kill or inhibit microorganisms that may reside on that surface. For example, a solution of the additive of the present invention containing from approximately 500 to 1000 parts per million (PPM) of the phosphate derivative makes an excellent disinfectant formulation for light duty such as mopping and cleaning of hard surfaces such as vinyl walls, floors, counters and table tops.
For more demanding microbiocidal activity such as that required for a surgical scrub, the phosphate derivative can be mixed in with a conventional detergent at a concentration of between approximately 15% and 70% by weight.
The microbiocidal cleansing agent prepared by the addition of the microbiocidal additive of the present invention to a conventional cleansing agent has the capacity to kill or inhibit the growth of many types of bacteria, fungi, viruses, yeasts and other destructive or disease-producing microorganisms which might be on a surface. Such a microbiocidal cleansing agent is particularly effective against both Gram-positive bacteria, such as Staphylococcus aureus, and Gram-negative bacteria, such as Pseudomonas aeruginosa.
In accordance with the present invention, the phosphate
derivative can be added to the water in cooling towers or can be included in a coating that is used to coat the surfaces in cooling towers to kill or inhibit the growth of the pathogen that causes Legionaire's disease, Legionella pneumophilia.
The phosphate derivative has also been found to be an effective deodorant.
Although not wanting to be bound by the following description of the mechanism of the microbiocidal additive of the present invention, it is believed that at least one free hydroxyl group on the phosphate group is necessary for microbiocidal activity. Thus, if all of the hydroxyl groups are replaced with alkyl or other organic groups, the phosphate derivative will no longer exhibit microbiocidal activity. It is believed that, in general, the more hydroxyl groups that are free, the greater the gram negative microbiocidal activity will be exhibited by the phosphate derivative.
The microbiocidal additive of the present invention can be more specifically defined as a phosphate ester with at least one free hydroxyl group with the following general formula:
O
X — O— P — OR
O R'
wherein:
R and R' may be alkyl, aryl, aralkyl and alkaryl groups including, but not limited to, straight chain, branched chain or cyclic alkyl groups having from 1 to 24 carbon atoms, polyoxyethylene or polyoxypropylene having from 2 to 32 ethylene oxide or propylene oxide units respectively, alkyl phenoxy polyoxyethylene containing 2 to 32 ethylene oxide units, alkyl phenoxy polyoxyethylene containing ethylene oxide units and 1 to 24 carbon atoms in the phenolic alkyl chain, and polyhydroxy
compounds, including but not limited to, ethylene glycol, glycerol, or sorbitol, wherein R and R' include at least one free hydroxyl group.
R may also be hydrogen, but only if X is a quaternary amine.
X is selected from the group consisting of Group IA metals, Group IIA metals, transition metals, hydrogen, and an organic ion.
A preferred structure of the phosphate derivative comprises the following formula:
O
+ - ' I χ o — P OR
I
OR' wherein:
R and R* = an alkyl group of from 6 to 18 carbon atoms and one group can be H.
X is selected from the group consisting of Group IA metals, Group HA metals, transition metals, hydrogen, and an organic ion. The microbiocidal additive defined by this formula is water insoluble or only slightly soluble in water and is especially useful for addition to non-aqueous products such as plastics, fibers and non-aqueous coatings. An aqueous suspension of the phosphates with the alkyl group of greater than 6 carbon atoms in the R position can be prepared by adding a surfactant such as Tween 80 (Sigma Chemical Company, St. Louis, MO) or other suitable nonionic surfactant..
An especially preferred structure of the microbiocidal additive of the present invention comprises a phosphate with the following formula:
O
+ - il
X 0 — P — OR
I
OR' wherein:
R and R'= an alkyl group of from 1 to 5 carbon atoms and one group can be H.
X is selected from the group consisting of Group IA metals, Group IIA metals, transition metals, hydrogen, and an organic ion.
The microbiocidal additive defined by this formula is water soluble and is especially useful as an additive for a disinfectant or for a detergent.
An additional preferred structure of the microbiocidal additive of the present invention has the following formula:
O
X -Q— • P — OCg H 17
X is selected from the group consisting of Group IA metals, Group HA metals, transition metals, hydrogen, and an organic ion.
Another especially preferred embodiment of the present invention has the following formula:
O
+ II
X -o— P — O C „ H
I 2 5
OH wherein :
R = H or C2H5; and
X is selected from the group consisting of Group IA metals, Group HA metals, transition metals, hydrogen, and an organic ion.
This embodiment of the microbiocidal additive of the present invention is soluble in water.
When the additive utilized in accordance with the present invention is incorporated into a non-aqueous material such as a plastic or fiber, the microbiocidal activity of the product can be improved by substituting for "X" a large organic ion such as a quaternary amine. An example of such a compound is a tertiary amine with the following general formula:
R.
/
N
Ri = an alkyl group of from 4 to 18 carbon atoms or a hydroxy alkyl group of from 1 to 18 carbon atoms. The Ri groups are not necessarily identical.
R2 = an alkyl group of from 8 to 18 carbon atoms.
Another especially preferred embodiment of the present invention is an alkyl phosphate derivative with the following formula:
CH OH O
I + - II
This especially preferred embodiment can be prepared by partially neutralizing phosphoric acid with between 1 to 2 moles of the tertiary amine. If more tertiary amine is used to neutralize the phosphoric acid, the free hydroxyls on the phosphate group will be e___minated and it is believed that the microbiocidal activity of the compound will be diminished.
The type of component to be used as the "X" substituent will depend largely upon the compatibility of the base material for the "X" substituent.
The microbiocidal additive of the present invention may be prepared as follows: One mole of phosphorous pentoxide is reacted with between approximately 1 to 3 moles of an alcohol. The alcohol can be an alkyl or an aryl compound. The alkyl alcohol can be straight chained, branched chain or cyclic. It is believed that the important aspect of the phosphate derivative is that the compound have at least one free hydroxyl. The alcohol should be heated to a temperature of between approximately 60° and 120° C depending upon the boiling point of the alcohol used.
The phosphorous pentoxide is slowly added to the alcohol while the mixture is vigorously agitated. The reaction is complete two to four hours after the addition of phosphorous pentoxide is completed.
The product formed in this reaction is a mixture of mono-ester phosphate and di-ester phosphate. The reaction equation is as follows:
O O
II
P„ 2 o 5 + 3ROH II
H O- ■P - OR + H O— P P—' OR I I
OH OR
where R is the alkyl or aryl group depending upon the substituent used.
The phosphate derivative is an effective microbiocidal compound and is capable of killing or inhibiting a wide variety of microorganisms including bacteria, yeasts, fungi, algae, molds and viruses.
The phosphate can be partially neutralized with a tertiary amine as shown in the following reaction equation:
The preferable range for X is between approximately 1 and 1.5 moles with the especially preferable range of approximately 1.3 moles.
This reaction results in a mixture of the following mono-alkyl phosphate amine product:
Ri O l + — II
R; N O " OR I \ H
R OR
and the following monoalkyl phosphate:
O
II
H O- ■P - OR
OH wherein:
R = a straight chain or a branched chain alkyl group of from 1 to 18 carbon atoms;
Ri = an alkyl group of from 4 to 18 carbon atoms or a hydroxyalkyl group of from 1 to 1 8 carbon atoms .
R = a straight chain or a branched chain alkyl group of from 8 to 18 carbon atoms .
The microbiocidal activity of the phosphate derivative of the present invention is evaluated as follows. Petri dishes are prepared using appropriate nutrient agar as a food source for the microorganism to be tested. The microorganism is seeded into the agar as is well known to one or ordinary skill in the art. A hole 6mm in diameter and 5mm deep is cut into the agar. 0.05 ml. of each of the indicated test compounds is placed in the hole and the inoculated petri dish is incubated for 24 hours at 37°C. After the 24 hour incubation period, the relative susceptibility of the test organisms to the phosphate derivative of the present invention is demonstrated by a clear zone of growth inhibition around the test solution. This zone of inhibition is the result of two processes: (1) the diffusion of the compound and (2) growth of the bacteria. As the phosphate derivative diffuses through the agar medium from the hole, its concentration progressively diminishes to a point where it is no longer inhibitory for the test organism. The area of the suppressed microbial growth, the zone of inhibition, is determined by the concentration of the phosphate derivative present in the area. Therefore, within the limitations of the test, the area of the inhibition zone is proportional to the relative susceptibility of the microorganisms to the phosphate derivative of the present invention.
After the 24 hour incubation period, each plate is examined and the diameters of the complete inhibition zones are noted and measured using either reflected light and a measuring device such as sliding calipers, a ruler, or a template prepared for this purpose and held on the bottom of the plate. The end point, measured to the nearest millimeter, is the point at which no visible growth that can be detected with the unaided eye minus the diameter of the test drop or sample. The area of the zone of inhibition is then calculated.
The following examples will serve to further illustrate the present invention without, at the same time, however, constituting any limitation thereof.
Example I
The mono-ethyl alkyl phosphate derivative is prepared as follows: One mole of phosphorous pentoxide is reacted with three moles of ethanol at a temperature of 60°C. The phosphorous pentoxide is slowly added to the ethanol while the mixture is vigorously agitated. At the reaction temperature of 60° C the reaction is complete in about two hours. The progress of the reaction is determined by titrating the acid that is produced with a solution of potassium hydroxide. The reaction products include approximately equimolar quantities of the mono-ethyl alkyl phosphate and the di-ethyl alkyl phosphate. The mono-ethyl alkyl phosphate is the more microbiocidally active species.
Example II
The mono-(2-ethylhexyl) phosphate derivative is prepared as follows. One mole of phosphorous pentoxide is reacted with three moles of 2-ethylhexanol at a temperature of 100°C. The phosphorous pentoxide is slowly added to the ethanol while the mixture is vigorously agitated. At the reaction temperature of 100°C the reaction is complete in about two hours. The progress of the reaction is deteπnined by titrating the acid that is produced with a solution of potassium hydroxide. The reaction products include approximately equimolar quantities of the mono-(2-ethylhexyl) alkyl phosphate and the di(2-ethylhexyl) alkyl phosphate. The mono-(2-ethylhexyl) alkyl phosphate is the more microbiocidally active species.
Example III
Since the preferred method of preparing the phosphate of Examples I and II results in two reaction products, the mono- alkyl phosphate and the di-alkyl phosphate, the relative microbiocidal activity of each of the products is evaluated.
Three samples are tested:
1. 91% mono-(2-ethylhexyl) phosphate, 9% di-(2-
ethylhexyl) phosphate
2. 55% mono-(2-ethylhexyl)phosphate and 45% di- (2-ethylhexyl) phosphate
3. 95% di-(2-ethylhexyl) phosphate, 5% mono-(2- ethylhexyl) phosphate.
Petri dishes are prepared using trypticase soy nutrient agar (Baltimore Biological Laboratory, Cockeysville, MD). The microorganisms used in this test are the Gram-positive Staphylococcus aureus and the Gram-negative Pseudomonas aeruginosa. Each microorganism is seeded into the agar as is well known to one of ordinary skill in the art. A hole 6mm in diameter and 5mm deep is cut into the agar. 0.05 ml. of each of the indicated test compounds is placed in the hole, and the inoculated petri dish is incubated for 24 hours at 37°C. After the 24 hour incubation period, the relative susceptibility of the test organisms to the phosphate derivative of the present invention is demonstrated by a clear zone of growth inhibition around the test solution.
After the 24 hour incubation period, each plate is examined and the diameters of the complete inhibition zones are noted and measured as described above. Each test is performed at least 6 times. The areas shown in Table A are the average of the 6 separate tests.
Table A
The results of Table A indicate that the mono-alkyl phosphate is the compound which has the significant amount of the microbiocidal activity.
Example IV
To produce a microbiocidal alkyl phosphate derivative that can be used with a detergent to make a microbiocidal detergent, the reaction product of Example II is partially neutralized with bis(hydroxyethyl) cocoamine. 1.3 moles of bis(hydroxyethyl) cocoamine per mole each of the reaction products from Example I is slowly added to the reaction product from Example I until the pH is between approximately 3.2 and 3.8 in a 75% ethanol solution. This reaction is carried out at a temperature of 100°C. The reaction mixture is vigorously agitated during the reaction.
An aqueous mixture of the microbiocidal alkyl phosphate is prepared by mixing the alkyl phosphate derivative from Example II with an aqueous detergent solution. The concentration of alkyl phosphate derivative is .05%. The microbiocidal detergent is heated to 85°C. Cotton fabric is then introduced and remains in the heated solution for 15 minutes. The fabric is then rinsed in water at 40° C, removed, and dried.
Square samples of the treated fabric of approximately 400 mm2 are cut and placed on agar plates which have previously been inoculated with Staphylococcus aureus and Pseudomonas aeruginosa:', the plates are then incubated at 35°C for 24 hours.
After the 24 hour incubation, neither Staphylococcus aureus nor Pseudomonas aeruginosa are found to be present in or on the squares. Microscopic examination shows a zone of inhibition around the individual threads.
Example V To produce a microbiocidal alkyl phosphate derivative that can be used with a detergent to make a microbiocidal detergent, the reaction product of Example II is neutralized with bis(hydroxy ethyl) cocoamine. 1.3 moles of bis(hydroxyethyl) cocoamine per mole each of the reaction products from Example I is slowly added to the reaction product from Example II until the pH is between approximately 3.2 and 3.8 in a 75% ethanol solution.
This reaction is carried out at a temperature of 100°C. The reaction mixture is vigorously agitated during the reaction.
A dry free-flowing mixture comprising the microbiocidal cleansing agent of the present invention is prepared by mixing 0.3 grams of the above alkyl phosphate amine derivative with 138.5 grams of "All" detergent as purchased over the counter. One gram of the cleansing agent mixture is then placed in the center of appropriately inoculated petri dishes and incubated for 24 hours at 37°C. Control plates are also prepared with one gram samples of the detergent without any phosphate derivative. After this period of incubation, each plate is examined and the diameters of the inhibition zones are measured. The results are shown in Table B.
Table B
The detergent alone exhibits some microbiocidal activity probably because of the presence of sodium hypochlorite which would be washed out of fabrics during the rinsing process. In any event, the detergent plus additive demonstrates a significant increase in microbiocidal activity over the detergent alone.
Example VII
The microbiocidal capability of an alkyl phosphate amine is demonstrated by the following example. Between 0.5 moles and 3.0 moles of bis (hydroxy ethyl) cocoamine per mole of the reaction products from Example II is slowly added to the reaction products from Example II until the pH of the solution is between approximately 2.5 and 6 in a 75% ethanol solution. This
reaction is carried out at a temperature of 100°C. The reaction mixture is vigorously agitated during the reaction. The resulting compounds are tested for microbiocidal activity.
Petri dishes are prepared using trypticase soy nutrient agar (Baltimore Biological Laboratory, Cockeysville, MD). The microorganisms used in this test are the Gram-positive Staphylococcus aureus and the Gram-negative Pseudomonas aeruginosa. The microorganisms are seeded into nutrient agar as is well known to one of ordinary skill in the art. A hole 6mm in diameter and 5mm deep is cut into the agar. 0.05 ml. of each of the indicated test compounds is placed in the hole and the inoculated petri dish is incubated for 24 hours at 37°C. After the 24 hour incubation period, the relative susceptibility of the test organisms to the phosphate derivative of the present invention is demonstrated by a clear zone of growth inhibition around the test solution.
After the 24 hour incubation period, each plate is examined and the diameters of the complete inhibition zones are noted and measured.
The results are summarized in Table C.
Table C
Molar Ratio S. aureus P. aeruginosa of reactants Area of Inhibition measured in m 2
a Moles of cocoamine reacted with one mole each of the diester and monoester produced in Example II.
As can be seen in Table C, sample A, which is the reaction product from Example II, has excellent microbiocidal activity against both the Gram positive Staphylococcus aureus and the Gram negative Pseudomonas aeruginosa. The reaction product from Example II retains its microbiocidal activity against both these organisms even when reacted with up to 2 moles of the bis- hydroxyethyl cocoamine. When one mole each of the reaction product from Example II is reacted with more than 2 moles of the cocoamine, the microbiocidal activity is diminished.
While this invention has been described in detail with particular reference to preferred embodiments thereof, it will be understood that variations and modifications can be effected within the spirit and scope of the invention as described hereinbefore and as defined in the appended claims.
Claims
1. (Amended) A biocidal cleansing composition comprising a detergent with a biocidally effective amount of a phosphate ester having the general formula:
ED — P OR
O R' wherein R and R' are selected from the group consisting of alkyl, aryl, aralkyl and alkaryl groups, one of R or R' can be H, and there is at least one free hydroxyl group.
2. The biocidal cleansing composition of Claim 1, wherein the phosphate ester is a partially neutralized acid having the general formula:
O
XT" O — P — OR
O R'
wherein:
R and R' are selected from the group consisting of an alkyl, aryl, aralkyl and alkaryl group and either R or R' can be H; and
X is a positive ion selected from the group consisting of organic ions, Group IA metals, Group HA metals and transition metals.
3. The biocidal cleansing composition of Claim 2, wherein R or R is an alkyl group of from 1 to 18 carbon atoms.
4. The biocidal cleansing composition of Claim 2, wherein X has the following formula:
Ri
R2— N— H
I R.
wherein:
Ri is selected from the group consisting of an alkyl group of from 4 to 18 carbon atoms and a hydroxy alkyl group of from 1 to 18 carbon atoms; and
R2 is an alkyl group of from 8 to 18 carbon atoms.
5. The biocidal cleansing composition of Claim 4, wherein said biocidal phosphate ester has the following formula:
R is H or C2H5.
6. The biocidal cleansing composition of Claim 2, wherein the X is a quatemary amine and R is hydrogen.
7. The biocidal cleansing composition of Claim 6, wherein the X in the microbiocidal phosphate derivative is a quaternary amine with the following general formula:
Ri l+
R— N H
i wherein:
Ri is selected from the group consisting of an alkyl group of from 4 to 18 carbon atoms and a hydroxy alkyl group of from 1 to 18 carbon atoms; and
R2 is an alkyl group of from 8 to 18 carbon atoms..
8. The biocidal cleansing composition of Claim 7, wherein said microbiocidal phosphate derivative has the following formula:
CH« OH O ι +2 II
C1 2 H25- O— P — OH
P H CH2 CH OH
9. A method of preparing a biocidal cleansing composition comprising the steps of: a. neutralizing phosphoric acid with between approximately 1 and 2 moles of bis(hydroxyethyl) cocoamine; and b. mixing the mixture from step (a) at a concentration of between approximately 0.01% and 70% by weight with a detergent.
10. A method of preparing a microbiocidal plastic material comprising the steps of: a. reacting phosphorous pentoxide with between approximately 1 mole to 3 moles of a hydroxy alkyl, aryl, alaryl or alkaryl compound at a temperature between approximately 60°C and 120°C to form a phosphate derivative with at least one free hydroxyl group; and b. mixing the mixture from step (a) at a concentration of between approximately 0.01% and 70% by weight with a detergent.
11. The method of Claim 10, wherein said hydroxy alkyl group comprises 1 to 18 carbon atoms.
12. The method of Claim 1 o , further comprising the step of reacting the product of step (a) with between approximately 0.5 to 1.5 moles of a tertiary amine, said tertiary amine having one substituent comprising an alkyl group of 8 to 18 carbon atoms, and two substituents being selected from the group consisting of an alkyl group of from 4 to 18 carbon atoms and a hydroxy alkyl group of from 1 to 18 carbon atoms.
13. The method of Claim 12, wherein the tertiary amine is bis(hydroxyethyl) cocoamine.
14. A microbiocidal cleansing agent prepared by a process comprising: a. reacting phosphorous pentoxide with between approximately 1 moles to 3 moles of a hydroxy alkyl, aryl, alaryl or alkaryl compound per mole of phosphorus pentoxide at a temperature between approximately 60° C and 120° C to form a phosphate derivative with at least one free hydroxyl group; b. reacting the product of step (a) with between approximately 0.5 to 1.5 moles of a tertiary amine, said tertiary amine having one substituent comprising an alkyl group of 8 to 18 carbon atoms, and two substituents being selected from the group consisting of an alkyl group of from 1 to 18 carbon atoms and a hydroxy alkyl group of from 1 to 18 carbon atoms; and c. mixing the mixture from step (a) at a concentration of between approximately 0.01% and 70% by weight with a detergent.
15. A disinfectant formulation comprising a biocidally effective amount of a phosphate ester having the general formula:
O
HD — P — OR
I O R'
wherein:
R and R' are selected from the group consisting of alkyl, aryl, aralkyl and alkaryl groups, one of R or R' can be H, and thereis at least one free hydroxyl group.
16. The disinfectant formulation of Claim 15, wherein said liquid is water.
17. The disinfectant formulation of Claim 1 5 , wherein the phosphate ester is a partially neutralized acid having the general formula:
O
X4" ~~0 — P — OR
I O R'
wherein!
R and R' are selected from the group consisting of an alkyl, aryl, aralkyl and alkaryl group and either R or R' can be H; and
X is a positive ion selected from the group consisting of organic ions, Group IA metals, Group HA metals and transition metals.
18. The disinfectant formulation of Claim 17, wherein R or R' is an alkyl group of from 1 to 18 carbon atoms.
19. The disinfectant formulation of Claim 16. wherein X has the following formula:
wherein:
Ri is selected from the group consisting of an alkyl group of from 4 to 18 carbon atoms and a hydroxy alkyl group of from 1 to 18 carbon atoms; and
R2 is an alkyl group of from 8 to 18 carbon atoms.
20. The disinfectant formulation of Claim 19, wherein said biocidal phosphate ester has the following formula:
21. The disinfectant formulation of Claim 16, wherein the X is a quatemary amine and R is hydrogen.
22. The disinfectant formulation of Claim 21 , wherein the X in the microbiocidal phosphate derivative is a quaternary amine with the following general formula:
N H
R. wherein:
Ri is selected from the group consisting of an alkyl group of from 4 to 18 carbon atoms and a hydroxy alkyl group of from 1 to 18 carbon atoms; and
R2 is an alkyl group of from 8 to 18 carbon atoms.
23. The disinfectant formulation of Claim 22, wherein said biocidal phosphate ester has the following formula:
24. A method of preparing a disinfectant formulation comprising the steps of: a. neutralizing phosphoric acid with between approximately 1 and 2 moles of bis(hydroxyethyl) cocoamine; and b. mixing the mixture from step (a) at a concentration of between approximately 0.01% and 70% by weight with water.
25. A method of preparing a disinfectant formulation comprising the steps of: a. reacting phosphorous pentoxide with between approximately 1 mole to 3 moles of hydroxy alkyl, aryl, alaryl or alkaryl compound at a temperature between approximately 60°C and 120°C to form a phosphate derivative with at least one free hydroxyl group; and b. mixing the mixture from step (a) at a concentration of between approximately 0.01% and 70% by weight with water.
26. The method of Claim 25, wherein said hydroxy alkyl group comprises 1 to 18 carbon atoms.
27. The method of Claim 25, further comprising the step of reacting the product of step (a) with between approximately 0.5 to 1.5 moles of a tertiary amine, said tertiary amine having one substituent comprising an alkyl group of 8 to 18 carbon atoms, and two substituents being selected from the group consisting of an alkyl group of from 4 to 18 carbon atoms and a hydroxy alkyl group of from 1 to 18 carbon atoms.
28. The method of Claim 27, wherein the tertiary amine is bis(hydroxyethyl) cocoamine.
29. A disinfectant formulation prepared by a process comprising: a. reacting phosphorous pentoxide with between approximately 1 moles to 3 moles of a hydroxy alkyl, aryl, alaryl or alkaryl compound per mole of phosphorus pentoxide at a temperature between approximately 60°C and 120°C to form a phosphate derivative; b. reacting the product of step (a) with between approximately 0.5 to 1.5 moles of a tertiary amine, said tertiary amine having one substituent comprising an alkyl group of 8 to 18 carbon atoms, and two substituents being selected from the group consisting of an alkyl group of from 1 to 18 carbon atoms and a hydroxy alkyl group of from 1 to 18 carbon atoms; and c. mixing the mixture from step (a) at a concentration of between approximately 0.01% and 70% by weight with a liquid.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1019890700490A KR910005713B1 (en) | 1987-08-03 | 1988-08-03 | Microbiocidal cleansing or disinfecting formulations and preparation thereof |
| DK160589A DK160589D0 (en) | 1987-08-03 | 1989-04-03 | MICROBIOCIDE CLEANING OR DISINFECTING PREPARATIONS AND MANUFACTURING THEREOF |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US080,787 | 1987-08-03 | ||
| US8078787A | 1987-09-03 | 1987-09-03 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1989001023A1 true WO1989001023A1 (en) | 1989-02-09 |
Family
ID=22159606
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US1988/002647 WO1989001023A1 (en) | 1987-08-03 | 1988-08-03 | Microbiocidal cleansing or disinfecting formulations and preparation thereof |
Country Status (6)
| Country | Link |
|---|---|
| EP (1) | EP0486484A1 (en) |
| JP (1) | JPH02504401A (en) |
| KR (1) | KR910005713B1 (en) |
| AU (1) | AU2426688A (en) |
| DK (1) | DK160589D0 (en) |
| WO (1) | WO1989001023A1 (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0371802A1 (en) * | 1988-12-01 | 1990-06-06 | Unilever Plc | Topical composition |
| EP0371804A1 (en) * | 1988-12-01 | 1990-06-06 | Unilever Plc | Topical composition |
| EP0371803A1 (en) * | 1988-12-01 | 1990-06-06 | Unilever Plc | Topical composition |
| EP0371542A2 (en) * | 1988-12-01 | 1990-06-06 | Unilever N.V. | Oral composition |
| WO1995001414A1 (en) * | 1993-07-01 | 1995-01-12 | Allergan, Inc. | Contact lens cleaning solution based on quaternary ammonium phosphate esters |
| FR2727289A1 (en) * | 1994-11-30 | 1996-05-31 | Derives Resiniques Terpenique | DISINFECTING COMPOSITION CONSISTING OF AT LEAST ONE TERPENIC ALCOHOL AND AT LEAST ONE BACTERICIDAL ACID SURFACTANT, AND USE OF SUCH SURFACTANTS |
| EP1389909A1 (en) * | 2001-05-03 | 2004-02-25 | Oligos Etc. Inc. | Antimicrobial compounds and methods for their use |
| US7868162B2 (en) | 1998-12-30 | 2011-01-11 | Lakewood-Amedex, Inc. | Antimicrobial and antiviral compounds and methods for their use |
| US8435960B2 (en) | 1998-12-30 | 2013-05-07 | Lakewood-Amedex, Inc. | Devices for improved wound management |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010202817A (en) * | 2009-03-05 | 2010-09-16 | Seiren Co Ltd | Allergen reducing composition and allergen reduced cloth |
| JP7265097B1 (en) * | 2021-08-05 | 2023-04-25 | 日華化学株式会社 | Antibacterial/Antiviral Agent Composition, Antibacterial/Antiviral Structure, and Method for Producing Antibacterial/Antiviral Structure |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4165369A (en) * | 1975-09-11 | 1979-08-21 | Kao Soap Co., Ltd. | Liquid hair rinse containing quaternary ammonium salts and a synthetic secondary alcohol |
| US4235733A (en) * | 1978-07-13 | 1980-11-25 | Kao Soap Co., Ltd. | Antibacterial soap containing trichlorohydroxy diphenyl ether bactericide and an organic phosphoric ester as a stabilizer therefor |
| US4272395A (en) * | 1978-05-30 | 1981-06-09 | Lever Brothers Company | Germicidal compositions |
| US4661477A (en) * | 1980-08-27 | 1987-04-28 | Borsodi Vegyi Kombinat | Phosphoric acid monoester salts, process for their preparation, and fungicidal compositions containing them as active ingredient |
| GB2157952B (en) * | 1984-04-23 | 1988-06-08 | Kao Corp | Phosphate-activated biocides |
| US4770694A (en) * | 1981-03-27 | 1988-09-13 | Kao Soap Co., Ltd. | Aqueous biocide suspension |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR910001409B1 (en) * | 1984-10-09 | 1991-03-05 | 인터훼이스 리서어치 코오포레이숀 | Microbiocidal composition and method of preparation thereof |
-
1988
- 1988-08-03 AU AU24266/88A patent/AU2426688A/en not_active Abandoned
- 1988-08-03 JP JP63507703A patent/JPH02504401A/en active Pending
- 1988-08-03 KR KR1019890700490A patent/KR910005713B1/en not_active IP Right Cessation
- 1988-08-03 WO PCT/US1988/002647 patent/WO1989001023A1/en not_active Application Discontinuation
- 1988-08-03 EP EP88908477A patent/EP0486484A1/en not_active Withdrawn
-
1989
- 1989-04-03 DK DK160589A patent/DK160589D0/en not_active Application Discontinuation
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4165369A (en) * | 1975-09-11 | 1979-08-21 | Kao Soap Co., Ltd. | Liquid hair rinse containing quaternary ammonium salts and a synthetic secondary alcohol |
| US4272395A (en) * | 1978-05-30 | 1981-06-09 | Lever Brothers Company | Germicidal compositions |
| US4235733A (en) * | 1978-07-13 | 1980-11-25 | Kao Soap Co., Ltd. | Antibacterial soap containing trichlorohydroxy diphenyl ether bactericide and an organic phosphoric ester as a stabilizer therefor |
| US4661477A (en) * | 1980-08-27 | 1987-04-28 | Borsodi Vegyi Kombinat | Phosphoric acid monoester salts, process for their preparation, and fungicidal compositions containing them as active ingredient |
| US4770694A (en) * | 1981-03-27 | 1988-09-13 | Kao Soap Co., Ltd. | Aqueous biocide suspension |
| GB2157952B (en) * | 1984-04-23 | 1988-06-08 | Kao Corp | Phosphate-activated biocides |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP0486484A4 * |
Cited By (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5180579A (en) * | 1988-12-01 | 1993-01-19 | Chesebrough-Pond's Usa Co., Division Of Conopco, Inc. | Topical composition comprising a mixture of particular di-alkyl or alkenyl phosphate esters and mono-alkyl or alkenyl phosphate esters |
| EP0371802A1 (en) * | 1988-12-01 | 1990-06-06 | Unilever Plc | Topical composition |
| EP0371542A2 (en) * | 1988-12-01 | 1990-06-06 | Unilever N.V. | Oral composition |
| EP0371542A3 (en) * | 1988-12-01 | 1990-09-26 | Unilever Nv | Oral composition |
| US5015471A (en) * | 1988-12-01 | 1991-05-14 | Chesebrough-Pond's Usa Co., Division Of Conopco, Inc. | Topical composition |
| US5019373A (en) * | 1988-12-01 | 1991-05-28 | Chesebrough-Pond's Usa Co., Division Of Conopco, Inc. | Oral composition |
| US5078991A (en) * | 1988-12-01 | 1992-01-07 | Chesebrough-Pond's Usa Co., Division Of Conopco, Inc. | Topical composition |
| EP0371803A1 (en) * | 1988-12-01 | 1990-06-06 | Unilever Plc | Topical composition |
| EP0371804A1 (en) * | 1988-12-01 | 1990-06-06 | Unilever Plc | Topical composition |
| WO1995001414A1 (en) * | 1993-07-01 | 1995-01-12 | Allergan, Inc. | Contact lens cleaning solution based on quaternary ammonium phosphate esters |
| WO1996016548A1 (en) * | 1994-11-30 | 1996-06-06 | Action Pin | Disinfecting or antiseptic composition including at least one terpene alcohol and at least one bactericidal acidic surfactant, and use thereof |
| FR2727289A1 (en) * | 1994-11-30 | 1996-05-31 | Derives Resiniques Terpenique | DISINFECTING COMPOSITION CONSISTING OF AT LEAST ONE TERPENIC ALCOHOL AND AT LEAST ONE BACTERICIDAL ACID SURFACTANT, AND USE OF SUCH SURFACTANTS |
| US5763468A (en) * | 1994-11-30 | 1998-06-09 | Action Pin | Disinfectant or antiseptic composition comprising at least one terpene alcohol and at least one bactericidal acidic surfactant, and use of such a mixture |
| US8435960B2 (en) | 1998-12-30 | 2013-05-07 | Lakewood-Amedex, Inc. | Devices for improved wound management |
| US7176191B2 (en) | 1998-12-30 | 2007-02-13 | Oligos Etc. Inc. | Antimicrobial compounds and methods for their use |
| US7868162B2 (en) | 1998-12-30 | 2011-01-11 | Lakewood-Amedex, Inc. | Antimicrobial and antiviral compounds and methods for their use |
| EP1389909A4 (en) * | 2001-05-03 | 2005-03-30 | Oligos Etc Inc | Antimicrobial compounds and methods for their use |
| EP2311466A3 (en) * | 2001-05-03 | 2012-04-25 | Lakewood-Amedex, Inc | Antimicrobial compounds and methods for their use |
| EP1389909A1 (en) * | 2001-05-03 | 2004-02-25 | Oligos Etc. Inc. | Antimicrobial compounds and methods for their use |
Also Published As
| Publication number | Publication date |
|---|---|
| DK160589A (en) | 1989-04-03 |
| EP0486484A1 (en) | 1992-05-27 |
| DK160589D0 (en) | 1989-04-03 |
| EP0486484A4 (en) | 1991-02-01 |
| AU2426688A (en) | 1989-03-01 |
| JPH02504401A (en) | 1990-12-13 |
| KR890701720A (en) | 1989-12-21 |
| KR910005713B1 (en) | 1991-08-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4935232A (en) | Microbiocidal composition and method of preparation thereof | |
| EP0371103B1 (en) | Biocidal delivery system and method of preparation thereof | |
| CN109169653B (en) | Cation composite disinfectant and application thereof | |
| EP0252310B1 (en) | Antimicrobial agents | |
| CN102088858A (en) | Use of cationic surfactants as sporicidal agents | |
| KR20100031505A (en) | Method for treating microorganisms and/or infectious agents | |
| KR910005713B1 (en) | Microbiocidal cleansing or disinfecting formulations and preparation thereof | |
| EP0197128B1 (en) | Microbiocidal composition and method of preparation thereof | |
| US4996052A (en) | Microbiocidal fabric having phosphate derivatives and method of preparation thereof | |
| US5474739A (en) | Microbiocidal composition | |
| FI83586C (en) | Use of an ester compound as a temporary microbicide | |
| US5032310A (en) | Microbiocidal cleansing and disinfecting formulations and preparation thereof | |
| US5133933A (en) | Microbiocidal preservative | |
| US4753749A (en) | Microbiocidal cleaning agent and preparation thereof | |
| US20230056710A1 (en) | Antimicrobial bedding product for pets and animals | |
| RU2316355C2 (en) | Using dialkylketone peroxide as biocide: sterilizing, antiseptic, disinfecting and antiparasitic agent | |
| JP2023550769A (en) | biocide | |
| KR960004500B1 (en) | A sterilizing detergent composition | |
| AU589142B2 (en) | Microbiocidal composition and method of preparation thereof | |
| WO2008154210A2 (en) | Surface cleaning method and composition | |
| RU2796846C1 (en) | Antiseptic and disinfectant | |
| DE2723118C3 (en) | Liquid microbicidal agents | |
| US9980490B1 (en) | Method of disinfecting bacterial contaminated surfaces or removing bacteria from contaminated surfaces | |
| IE852455L (en) | Micorbicocidal composition | |
| RU2130320C1 (en) | Disinfectant agent "selodez" and method of disinfection using this agent |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AU BR DK JP KR |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): AT BE CH DE FR GB IT LU NL SE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1988908477 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 1988908477 Country of ref document: EP |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: 1988908477 Country of ref document: EP |