WO1995031979A1 - Solutions of aryl or heteroaryl substituted alkanoic acids in lipophilic solvents and soft gelatin capsules containing such solutions - Google Patents
Solutions of aryl or heteroaryl substituted alkanoic acids in lipophilic solvents and soft gelatin capsules containing such solutions Download PDFInfo
- Publication number
- WO1995031979A1 WO1995031979A1 PCT/US1995/006183 US9506183W WO9531979A1 WO 1995031979 A1 WO1995031979 A1 WO 1995031979A1 US 9506183 W US9506183 W US 9506183W WO 9531979 A1 WO9531979 A1 WO 9531979A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- ketoprofen
- weight
- pharmaceutical composition
- formula
- Prior art date
Links
- YXFVVABEGXRONW-UHFFFAOYSA-N Cc1ccccc1 Chemical compound Cc1ccccc1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/42—Oxazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/14—Esters of carboxylic acids, e.g. fatty acid monoglycerides, medium-chain triglycerides, parabens or PEG fatty acid esters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/4858—Organic compounds
Definitions
- the present invention relates to solutions containing therapeutically useful substituted alkanoic acids in combination with at least one lipophilic solvent for encapsulation in soft gelatin capsules (softgel capsules).
- Hydrophilic softgels are well known for the oral administration of pharmaceutical agents.
- softgel capsules consist of an outer shell bt gelatin containing a pla ⁇ ticizer and an inner filling of hydrophilic liquid containing a dissolved hydrophobic pharmaceutical agent.
- the plasticizer is chosen so that the solubility in the fill liquid is as low as possible. If the plasticizer is soluble in the fill liquid, it can migrate out of the shell over time into the fill, leaving the shell brittle and subject to rupture.
- softgel capsules can pose problems for the pharmaceutical fonnulator.
- a given pharmaceutical agent may need a relatively large volume of solution in order to deliver a pharmaceutically acceptable unit dose.
- the practical limitations on the size of capsules suitable for conventional oral administration to human patierits could well preclude pharmaceutical use of the resulting softgel.
- a large volume of solution may again be a necessity for delivery of the require dosage.
- Softgel encapsulation of such a large solution volume may be impractical because the size of the needed softgel would likely exceed the maximum limit for conventional oral administration to huiaan patients.
- U.S. Patent No. 5,071,643 discloses the use of polyethylene glycol based solutions for acidic, basic and amphoteric pharmaceutical agents. These polyethylene glycol based solutions contain either an hydroxide species or a hydrogen ion species that causes the appropriate pharmaceutical agent to partially ionize, i.e., the pharmaceutical agent is present in both the free form and the salt form. The partial ionization described in Yu et al. results in enhanced solubillity for the acidic, basic or amphoteric pharmaceutical agent.
- enhanced solubility in turn, nay permit the preparation of a solution of pharmaceutical agent that is highly concentrated enough to be encapsulated in a capsule acceptably sized for oral administration to human patients.
- the Yu et al. patent discloses that enhanced solubility solutions can be prepared using polyethylene glycol and contemplated ecnaivalents of polyethylene glycol, such as polyethylene glycol e ⁇ hers or various alcohols and copolymers of polyethylene glycol.
- Softgel encapsulation is sometimes the preferred delivery system for many pharmaceutical agents that are administered orally to human patients.
- a pharmaceutical formulation should be in the form of a clear, stable solution.
- the present inventors have discovered that the enhanced solubility solutions disclosed by the Yu et al. patent are not as effective with various substituted alkanoic acid pharmaceutical agents.
- Therapeutically useful 2- or 3-aryl or 2- or 3-heteroaryl substituted alkanoic acids function as anti-inflammatory and analgesic agents and may be administered orally. They are also essentially insoluble in water.
- An example of such a useful alkanoic acid suitable for use in the present invention is ketoprofen which is 2-(3-benzoylphenyl) propionic acid.
- Ketoprofen is an anti-inflammatory, analgesic agent that is principally indicated for the acute and long-term management of rheumatoid arthritis and osteoarthritils. Additionally it is a nonsteroidal compound and poorly water soluble. Some gastrointestinal irritation is ordinarily associated with oral do ⁇ age forms of ketoprofen. The properties of ketoprofen render it a good candidate for formulation with the enhanced solubility solutions disclosed in the Yu et al. patent. In a number of experiments, the present inventors applied the Yu et al. enhanced solubility solutions in formulations of ketoprofen for softgel encapsulation.
- polyethylene glycol 400 and potassium hydroxide were used to solubilize the ketoprofen, with the mole ratio of potassium hydroxide to ketoprofen being in the range of 0.4 to 1. It was surprisingly found that the resulting formulation was not sufficiently stable for softgel encapsulation due to the undesirable formation of ketoprofen esters.
- the potassium hydroxide to ketoprofen mole ratio was adjusted to range from 1.1 to 1.
- the ketoprofen salt thus formed and/or the high pH caused by the excess potassium hydroxide used could affect the physical stability of the softgel capsule when the formulation was encapsulated.
- an equilibrium amount of the ketoprofen Ifree acid remained in the solution, it could form ketoprofen esters that could drive the reaction to form more ketoprofen free acid species, which could eventually result in a chemically unstable formulation.
- non-hydroxyl containing solvents may be used to form pharmaceutically acceptable solutions of 2- or 3-aryl or 3-heteroaryl substituted alkanoic acids that are stable and suitable for softgel encapsulation.
- the present invention provides enhanced solubility pharmaceutically acceptable solutions of therapeutically useful substituted alkanoic acids, preferably 2- or 3-aryl or 2- or 3- heteroaryl alkanoic acids, that can be encapsulated in softgel capsules of a size suitable for subsequent oral administration to human patients, having improved chemical stability compared with polyethylene glycol water miscible formulations of the alkanoic acids.
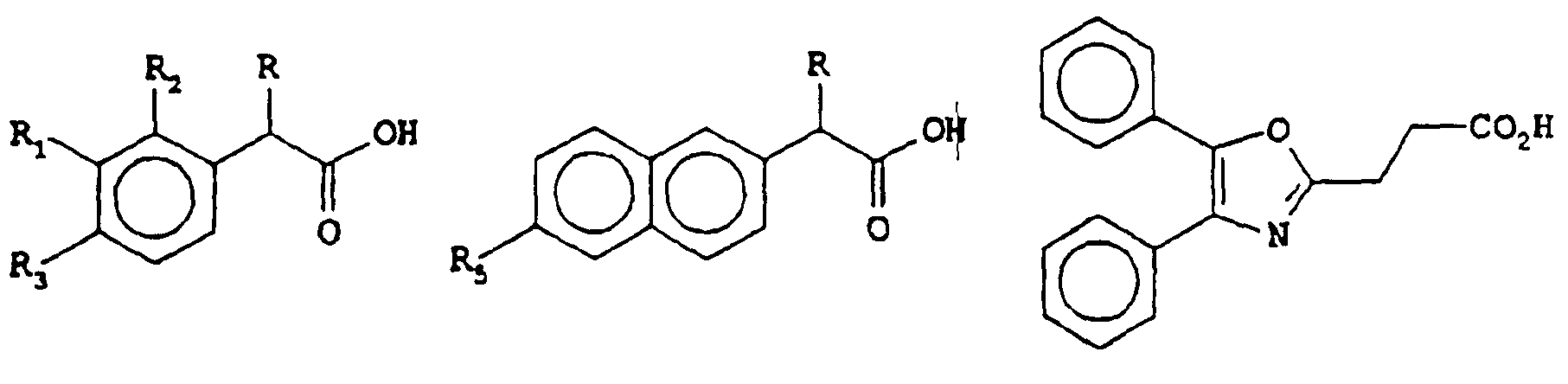
- the therapeutically useful active agents i.e., substituted alkanoic acids, preferred for use in the present invention have general formulas I, la or lb:
- R represents a hydrogen atom or an alkyl group containing
- R 1 represents hydrogen, halogen, C 1 -C 8 alkyl, phenylalkyl where the alkyl is C 1 -C 6 alkyl, a benzoyl group of the formula:
- R 4 represents hydrogen, C 1 -C 6 alkyl, or an alkylthio group having 1 to 4 carbon atoms;
- R 1 represents a group of the formula: where n is 0, 1 or 2;
- R 2 represents hydrogen, hydroxy or C 1 -C 6 alkoxy
- R 3 represents hydrogen, C 1 -C 6 alkyl or phenyl
- R 5 is C 1 -C 6 alkoxy.
- the enhanced solubility pharmaceutically acceptable solutions of therapeutically useful alkanoic acids can be encapsulated in softgel capsules of a size suitable for subsequent oral administration to human patients, which improves the physical stability of the softgel capsules used to encapsulate the pharmaceutical solutions compared with polyethylene glycol water miscible formulations of the alkanoic acids.
- the present invention also provides enhanced solubility pharmaceutically acceptable solutions of alkanoic acids that unexpectedly can be encapsulated in a softgel capsule of a size smaller than what is required to encapsulate the same dose of the acid in polyethylene glycol water miscible formulations.
- the enhanced solubility pharmaceutically acceptable solutions of 2- or 3-aryl or 3-heteroaryl alkanoic acids provided by the present invention may reduce or eliminate the gastrointestinal irritation associated with oral dosage forms of these agents.
- the lipophilic solvent and the hydroxyl containing softgel capsule plasticizers are immiscible, thereby improving both the chemical stability of the acid solution and improving the physical stability of the softgel capsule by greatly reducing the migration of capsule plasticizers into the encapsulated pharmaceutical formulation. Additionally, the use of the lipophilic solvent prevents the formation of esters which can decrease the chemical stability of the alkanoic acid solution.
- Suitable lipophilic solvents are polyol esters of fatty acids.
- the polyol esters of fatty acids may be mono-, di-, tri-, etc, esters of the polyols.
- thare may be free hydroxyl groups present in the polyol esters of fatty acids useful as lipophilic solvents of the invention.
- the lipophilic solvent preferred for use in the present invention is an alkylene glycol derivative of formula II:
- A represents C 1 -C 4 alkylene optionally substituted with alkyl or a group of the formula
- R" groups are the same of different and represent C 1 -C 12 alkyl.
- the present invention is useful for providing pharmaceutically acceptable solutions of substituted alkanoic acids dissolved in at least one lipophilic solvent, which are chemically stable and suitable for softgel encapsulation.
- the therapeutically useful active agents I.e., substituted alkanoic acids, preferred for use in the present invention have general formulas I, la or lb:
- R represents a hydrogen atom or an alkyl group containing
- R 1 represents hydrogen, halogen, C 1 -C 6 alkyl, phenylalkyl where the alkyl is C 1 -C 6 alkyl, a benzoyl group of the formula:
- R 4 represents hydrogen, C 1 -C 6 alkyl, or an alkylthio group having 1 to 4 carbon atoms; or R 1 represents a group of the formula:
- n 0, 1 or 2;
- R 2 represents hydrogen, hydroxy or C 1 -C 6 alkoxy
- R 3 represents hydrogen, C 1 -C 6 alkyl or phenyl
- R 5 is C 1 -C 6 alkoxy.
- Suitable pharmaceutically acceptable, non-toxic salts include salts such as, for example, alkali metal, alkaline earth metal, ammonium and amine salts.
- Compounds of general formulas I, la, and lb in which R represents an alkyl group can exist in optically active forms, including isomers and racemates thereof.
- Preferred alkanoic acids suitable for use in the present invention include ketoprofen (formula l where R is methyl, R 1 is benzoyl, and R 1 and R 3 are hydrogen, i.e..
- 2-(3- benzoylphenyl)propionic acid 2-(3- benzoylphenyl)propionic acid); ibuprofen (formula I where R is methyl, R 1 and R 2 are hydrogen, and R 3 is isobutyl, i.e., 2-(4- isobutylphenyl)propionic acid); naproxen (formula la where R is methyl and R 5 is methoxy, i.e., 2-(6-methoxy naphthyl)propionic acid); and oxaprozin, (formula lb, i.e., 4,5-diphenyl-2- oxazolepropionic acid).
- ibuprofen formula I where R is methyl, R 1 and R 2 are hydrogen, and R 3 is isobutyl, i.e., 2-(4- isobutylphenyl)propionic acid
- naproxen formula la where R is methyl and R 5
- the enhanced solubility pharmaceutically acceptable solutions of therapeutically useful substituted alkanoic acids can be encapsulated in softgel capsules of a size suitable for eubsequent oral administration to human patients, which improves the physical stability of the softgel capsules used to encapsulate the pharmaceutical solutions compared with polyethylene glycol water miscible formulations of the alkanoic acids.
- the present invention also provides enhanced solubility pharmaceutically acceptable solutions of ketoprofen that can be encapsulated in a softgel capsule of a size smaller than what is required to encapsulate the same dose of the acids in polyethylene glycol water miscible formulations.
- the present invention provides pharmaceutically acceptable solutions containing from about 0.1 to 1000 mg, preferably about 5 to 200 mg, and most preferably about 10 to 100 mg, of an alkanoic acid dissolved in at least one lipophilic solvent, resulting in a clear solution suitable for softgel encapsulation.
- the lipophilic solvent and the hydroxyl containing softgel capsule plasticizers, such as glycerin, are immiscible, thereby improving both the chemical stability of the alkanoic acid solution and improving the physical stability of the softgel capsule by greatly reducing the migration of capsule plasticizers into the encapsulated pharmaceutical formulation. Additionally, the use of the lipophilic solvent prevents the formation of esters which can decrease the chemical stability of the alkanoic acid solution.
- Suitable lipophilic solvents are polyol esters of fatty acids.
- the polyol esters of fatty acids may be mono-, di-, tri-, etc, esters of the polyols. Thus, there may be free hydroxyl groups present in the polyol esters of fatty acids useful as lipophilic solvents of the invention.
- the lipophilic solvent preferred for use in the present invention is an alkylene glycol derivative of formula II:
- A represents C 1 -C 4 alkylene optionally substituted with alkyl or
- R" groups are the same or different and represent C 1 -C 12 alkyl.
- Suitable lipophilic solvents include those of formula III:
- R" groups are the same or different and represent C 1 -
- Suitable lipophilic solvents also include those of formula IV:
- R H groups are the same or different and represent C 1 - C 12 alkyl and R' is C 1 -C 6 alkyl.
- Suitable lipophilic solvents are those of formula III where the R" groups are the same and represent C 1 -C 4 alkyl and R''' is
- Still other suitable lipophilic solvents are those of formula IV where the R" groups are the same or different and represent C 1 -C 4 alkyl and R' is methyl.
- Most preferred lipophilic solvents of formula III are those where R" is methyl.
- Most preferred lipophilic solvents of formula IV are those where the R" groups are the ⁇ arne or different and represent CH 3 (CH 2 ) 6 or CH 3 (CH 2 ) 8 .
- Particularly preferred solvents are selected from the group consisting of propylene glycol dicaprylate/dicaprate, 1,2,3- propanetriol triacetate and mixtures thereof.
- the solvents suitable for use in the present invention include propyl ⁇ ne glycol dicaprylate/dicapxfate, 1,2,3-propanetriol triacetate and mixtures thereof.
- Propylene glycol dicaprylate/dicaprate is available under the trade name Captex 200 from Karlshamn Lipid Specialties and 1,2,3-propanetriol triacetate is available under the trade name Triacetin from Eastman Chemicals.
- inventive solutions may also contain optional, additional ingredients to improve the dispersivity and dissolution of the substituted alkanoic acid.
- additional components include surfactants such as, for example, polyglyceryl esters of fatty acids, polyglycolyzed glycerides, propylene glycol esters, mono- and di-glycerides, sorbitan fatty acid esters, polyoxyethylene sorbitan fatty acid esters, polyoxyethylene sorbitol esters, polyoxyethylene adds, polyoxyethylene alcohols, and mixtures thereof.
- a preferred class of surfactants for use in combination with the lipophilic ⁇ olvents is the polyoxyethylene sorbitan fatty acid esters. Suitable sorbitan esters are sold under the trade name Tween. A particularly useful Tween is polyoxyethylene (20) sorbitan mono-oleate (Tween 80).
- the active substituted alkanoic acid pharmaceutical agent may be present in the solution in amounts ranging up to about 30% by weight of the solution. Preferred concentrations of the active agent are from about 5-20%, more preferably about 10-15%, by weight of the final solution. Combinations of lipophilic solvents may be used to obtain a desired final concentration.
- ketoprofen may be present in the solution in amounts ranging up to about 5% by weight of the solution when dissolved only in propylene glycol dicaprylate/dicaprat ⁇ .
- Ketoprofen may be present in the solution in amounts ranging up to about 14% by weight of the solution when dissolved only in 1,2,3-propanetriol triacetate.
- ketoprofen pharmaceutical agent When dissolved In a mixture of propylene glycol dicaprylate/dicaprate, 1,2,3-propan ⁇ triol triacetate and Tween, the ketoprofen pharmaceutical agent may be present in solution in amounts ranging Up to about 22% by weight of solution.
- ketoprofen pharmaceutical agent In addition to the ketoprofen pharmaceutical agent and the lipophilic solvents, other adjuncts may optionally be present.
- Polyoxyethylene (20) sorbitan mono-oleate (Tween 80) may be included in the solution up to about 50% by weight of the solution.
- the appropriate pharmaceutically acceptable solution of the substituted alkanoic acid is formulated, it can be encapsulated into conventional softgel capsules using any suitable encapsulation method, such as for example, the rotary die process.
- ketoprofen is prepared in the following manner. First, mix the following until homogeneous:
- ketoprofen solution can be encapsulated in suitable softgel capsules, such as 4 oval softgel. The filled softgel capsules are thereafter dry finished to the appropriate hardness.
- ketoprofen is prepared in the following manner. First, mix the following until homogeneous: (1) about 112 mg of propylene glycol dicaprylate/dicaprate;
- ketoprofen tlo the homogeneous mixture of propylene glycol dicaprylate, 1,2,3-propanetriol acetate and polyoxyethylene (20) sorbitan mono-oleate, and mix again. While mixing in the ketoprofen, heat the mixture and maintain the temperature between 110-125°F until the ketoprofen is dissolved. Once the ketoprofen is fully dissolved, the solution is then cooled and deaerated. After being cooled and deaerated, the ketoprofen solution can be encapsulated in suitable softgel capsules, such as 4 oval softgel. The filled softgel capsules are thereafter dry finished to the appropriate hardness.
- ketoprofen by weight of solution are prepared in the following manner, which provides a self-emulsifying system. First, mix the following until homogeneous:
- propylene glycol dicaprylate/dicaprate in an amount ranging from about 40% to about 98% by weight
- ketoprofen to the homogeneous mixture of propylene glycol dicaprylate, 1,2,3-propanetriol triacetate and polyoxyethylene (20) sorbitan mono-oleate, and mix again. While mixing in the ketoprofen, heat the mixture and maintain the temperature between 110-125°F until the ketoprofen is dissolved.
- the solution is then cooled and deaerated. After being cooled and deaerated, the ketoprofen solution can be encapsulated in suitable softgel capsules. The filled softgel capsules are thereafter dry finished to the appropriate hardness.
- ketoprofen by weight of solution are prepared in the following manner. First, mix the following until homogeneous:
- propylene glycol dicaprylate/dicaprate in an amount ranging from about 1% to about 50% by weight
- ketoprofen to the homogeneous mixture of propylene glycol dicaprylate and 1,2,3-propanetriol acetate and mix again. While mixing in the ketoprofen, heat the mixture and maintain the temperature between 110-125°F until the ketoprofen is dissolved. Once the ketoprofen is fully dissolved, the solution is then cooled and deaerated. After being cooled and deaerated, the ketoprofen solution can be encapsulated in suitable softgel capsules. The filled softgel capsujles are thereafter dry finished to the appropriate hardness.
- ketoprofen solution containing up to about 5% ketoprofen by weight of solution are prepared by mixing the ketoprofen with propylene glycol dicaprylate/dicaprate while heating the mixture. The temperature of the mixture should be maintained between 110-125°F until the ketoprofen is dissolved. Once the ketoprofen is fully dissolved, the solution is then cooled and deaerated. After being cooled and deaerated, the ketoprofen solution can be encapsulated in suitable softgel capsules. The filled softgel capeules are thereafter dry finished to the appropriate hardness.
- ketoprofen solution containing up to about 14% ketoprofen by weight of solution are prepared by mixing the ketoprofen with 1,2,3-propanetriol acetate while heating the mixture. The temperature of the mixture should be maintained between 110-125°F until the ketoprofen is dissolved. Once the ketoprofen is fully dissolved, the solution is then cooled and deaerated. After being cooled and deaerated, the ketoprofen solution can be encapsulated in suitable softgel capsules. The filled softgel capsules are thereafter dry finished to the appropriate hardness.
Abstract
Methods and compositions are disclosed for preparing liquid mixtures of aryl or heteroaryl alkanoic acids suitable for encapsulation in soft gelatin capsules. The compositions comprise alkanoic acids of formulas (I), (Ia), (Ib) or pharmaceutically acceptable salts thereof, wherein R, R1, R2, R3, and R5 represent hydrogen or various organic substituents, and an effective solubilizing amount of at least one lipophilic solvent.
Description
SOLUTIONS OF ARYL OR HETEROARYL SUBSTITUTED
ALKANOIC ACIDS IN LIPOPHILIC SOLVENTS AND
SORT GELATIN CAPSULES CONTAINING SUCH SOLUTIONS BACKGROUND OF THE INVENTION
Field of the Invation
The present invention relates to solutions containing therapeutically useful substituted alkanoic acids in combination with at least one lipophilic solvent for encapsulation in soft gelatin capsules (softgel capsules).
Description of the Related Art
Hydrophilic softgels are well known for the oral administration of pharmaceutical agents. Typically, softgel capsules consist of an outer shell bt gelatin containing a plaβticizer and an inner filling of hydrophilic liquid containing a dissolved hydrophobic pharmaceutical agent. The plasticizer is chosen so that the solubility in the fill liquid is as low as possible. If the plasticizer is soluble in the fill liquid, it can migrate out of the shell over time into the fill, leaving the shell brittle and subject to rupture.
With respect to pharmaceutical atøents of relatively low solubility and/or relatively high dosage amount, softgel capsules can pose problems for the pharmaceutical fonnulator. For example, if a given pharmaceutical agent has a relatively low solubility, it may need a relatively large volume of solution in order to deliver a pharmaceutically acceptable unit dose. While theoretically possible to encapsulate such a large volume of solution in a softgel capsule, for example, the practical
limitations on the size of capsules suitable for conventional oral administration to human patierits could well preclude pharmaceutical use of the resulting softgel.
Similarly, if a pharmaceutical agent requires a relatively high dose, a large volume of solution may again be a necessity for delivery of the require dosage. Softgel encapsulation of such a large solution volume may be impractical because the size of the needed softgel would likely exceed the maximum limit for conventional oral administration to huiaan patients.
As one approach to handling the problems of encapsulating low solubility or high dose pharmaceutical agents, U.S. Patent No. 5,071,643 (Yu et. al.) discloses the use of polyethylene glycol based solutions for acidic, basic and amphoteric pharmaceutical agents. These polyethylene glycol based solutions contain either an hydroxide species or a hydrogen ion species that causes the appropriate pharmaceutical agent to partially ionize, i.e., the pharmaceutical agent is present in both the free form and the salt form. The partial ionization described in Yu et al. results in enhanced solubillity for the acidic, basic or amphoteric pharmaceutical agent. This enhanced solubility, in turn, nay permit the preparation of a solution of pharmaceutical agent that is highly concentrated enough to be encapsulated in a capsule acceptably sized for oral administration to human patients. The Yu et al. patent discloses that enhanced solubility solutions can be prepared using polyethylene glycol and contemplated ecnaivalents of polyethylene
glycol, such as polyethylene glycol eφhers or various alcohols and copolymers of polyethylene glycol.
Softgel encapsulation is sometimes the preferred delivery system for many pharmaceutical agents that are administered orally to human patients. Generally, to be suitable for softgel encapsulation, a pharmaceutical formulation should be in the form of a clear, stable solution. The present inventors have discovered that the enhanced solubility solutions disclosed by the Yu et al. patent are not as effective with various substituted alkanoic acid pharmaceutical agents.
Therapeutically useful 2- or 3-aryl or 2- or 3-heteroaryl substituted alkanoic acids function as anti-inflammatory and analgesic agents and may be administered orally. They are also essentially insoluble in water. An example of such a useful alkanoic acid suitable for use in the present invention is ketoprofen which is 2-(3-benzoylphenyl) propionic acid.
Ketoprofen is an anti-inflammatory, analgesic agent that is principally indicated for the acute and long-term management of rheumatoid arthritis and osteoarthritils. Additionally it is a nonsteroidal compound and poorly water soluble. Some gastrointestinal irritation is ordinarily associated with oral doβage forms of ketoprofen. The properties of ketoprofen render it a good candidate for formulation with the enhanced solubility solutions disclosed in the Yu et al. patent. In a number of experiments, the present inventors applied the Yu et al. enhanced solubility solutions in formulations of ketoprofen for softgel encapsulation.
In one formulation, polyethylene glycol 400 and potassium hydroxide were used to solubilize the ketoprofen, with the mole ratio of potassium hydroxide to ketoprofen being in the range of 0.4 to 1. It was surprisingly found that the resulting formulation was not sufficiently stable for softgel encapsulation due to the undesirable formation of ketoprofen esters.
In an attempt to completely ionize the ketoprofen to prevent the formation of undesirable esters, the potassium hydroxide to ketoprofen mole ratio was adjusted to range from 1.1 to 1. With this second formulation, concerns arose that the ketoprofen salt thus formed and/or the high pH caused by the excess potassium hydroxide used could affect the physical stability of the softgel capsule when the formulation was encapsulated. Additionally, if an equilibrium amount of the ketoprofen Ifree acid remained in the solution, it could form ketoprofen esters that could drive the reaction to form more ketoprofen free acid species, which could eventually result in a chemically unstable formulation.
The present inventors have discovered that non-hydroxyl containing solvents may be used to form pharmaceutically acceptable solutions of 2- or 3-aryl or 3-heteroaryl substituted alkanoic acids that are stable and suitable for softgel encapsulation.
SUMMARY OF THE INVENTION
The present invention provides enhanced solubility pharmaceutically acceptable solutions of therapeutically useful substituted alkanoic acids, preferably 2- or 3-aryl or 2- or 3- heteroaryl alkanoic acids, that can be encapsulated in softgel capsules of a size suitable for subsequent oral administration to human patients, having improved chemical stability compared with polyethylene glycol water miscible formulations of the alkanoic acids.
The therapeutically useful active agents, i.e., substituted alkanoic acids, preferred for use in the present invention have general formulas I, la or lb:
or pharmaceutically acceptable salts thereof, wherein
R represents a hydrogen atom or an alkyl group containing
1 to 4 carbon atoms;
R1 represents hydrogen, halogen, C1-C8 alkyl, phenylalkyl where the alkyl is C1-C6 alkyl, a benzoyl group of the formula:
R2 represents hydrogen, hydroxy or C1-C6 alkoxy;
R3 represents hydrogen, C1-C6 alkyl or phenyl; and
R5 is C1-C6 alkoxy.
The enhanced solubility pharmaceutically acceptable solutions of therapeutically useful alkanoic acids can be encapsulated in softgel capsules of a size suitable for subsequent oral administration to human patients, which improves the physical stability of the softgel capsules used to encapsulate the pharmaceutical solutions compared with polyethylene glycol water miscible formulations of the alkanoic acids.
The present invention also provides enhanced solubility pharmaceutically acceptable solutions of alkanoic acids that unexpectedly can be encapsulated in a softgel capsule of a size smaller than what is required to encapsulate the same dose of the acid in polyethylene glycol water miscible formulations.
The enhanced solubility pharmaceutically acceptable solutions of 2- or 3-aryl or 3-heteroaryl alkanoic acids provided
by the present invention may reduce or eliminate the gastrointestinal irritation associated with oral dosage forms of these agents.
The lipophilic solvent and the hydroxyl containing softgel capsule plasticizers, such as glycerin, are immiscible, thereby improving both the chemical stability of the acid solution and improving the physical stability of the softgel capsule by greatly reducing the migration of capsule plasticizers into the encapsulated pharmaceutical formulation. Additionally, the use of the lipophilic solvent prevents the formation of esters which can decrease the chemical stability of the alkanoic acid solution.
Suitable lipophilic solvents are polyol esters of fatty acids. The polyol esters of fatty acids may be mono-, di-, tri-, etc, esters of the polyols. Thus, thare may be free hydroxyl groups present in the polyol esters of fatty acids useful as lipophilic solvents of the invention.
The lipophilic solvent preferred for use in the present invention is an alkylene glycol derivative of formula II:
wherein
A represents C1-C4 alkylene optionally substituted with alkyl or a group of the formula
and
the R" groups are the same of different and represent C1-C12 alkyl.
Further objects and embodiments of the present invention will be described in the following description of the preferred embodiments.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention is useful for providing pharmaceutically acceptable solutions of substituted alkanoic acids dissolved in at least one lipophilic solvent, which are chemically stable and suitable for softgel encapsulation.
The therapeutically useful active agents, I.e., substituted alkanoic acids, preferred for use in the present invention have general formulas I, la or lb:
or pharmaceutically acceptable salts thereof,
wherein
R represents a hydrogen atom or an alkyl group containing
1 to 4 carbon atoms;
R1 represents hydrogen, halogen, C1-C6 alkyl, phenylalkyl where the alkyl is C1-C6 alkyl, a benzoyl group of the formula:
where R4 represents hydrogen, C1-C6 alkyl, or an alkylthio group having 1 to 4 carbon atoms; or
R1 represents a group of the formula:
R2 represents hydrogen, hydroxy or C1-C6 alkoxy;
R3 represents hydrogen, C1-C6 alkyl or phenyl; and
R5 is C1-C6 alkoxy.
Suitable pharmaceutically acceptable, non-toxic salts include salts such as, for example, alkali metal, alkaline earth metal, ammonium and amine salts. Compounds of general formulas I, la, and lb in which R represents an alkyl group can exist in optically active forms, including isomers and racemates thereof. Preferred alkanoic acids suitable for use in the present invention include ketoprofen (formula l where R is methyl, R1 is benzoyl, and R1 and R3 are hydrogen, i.e.. 2-(3- benzoylphenyl)propionic acid); ibuprofen (formula I where R is methyl, R1 and R2 are hydrogen, and R3 is isobutyl, i.e., 2-(4- isobutylphenyl)propionic acid); naproxen (formula la where R is methyl and R5 is methoxy, i.e., 2-(6-methoxy naphthyl)propionic acid); and oxaprozin, (formula lb, i.e., 4,5-diphenyl-2- oxazolepropionic acid).
The enhanced solubility pharmaceutically acceptable solutions of therapeutically useful substituted alkanoic acids can be encapsulated in softgel capsules of a size suitable for
eubsequent oral administration to human patients, which improves the physical stability of the softgel capsules used to encapsulate the pharmaceutical solutions compared with polyethylene glycol water miscible formulations of the alkanoic acids.
The present invention also provides enhanced solubility pharmaceutically acceptable solutions of ketoprofen that can be encapsulated in a softgel capsule of a size smaller than what is required to encapsulate the same dose of the acids in polyethylene glycol water miscible formulations.
The present invention provides pharmaceutically acceptable solutions containing from about 0.1 to 1000 mg, preferably about 5 to 200 mg, and most preferably about 10 to 100 mg, of an alkanoic acid dissolved in at least one lipophilic solvent, resulting in a clear solution suitable for softgel encapsulation. The lipophilic solvent and the hydroxyl containing softgel capsule plasticizers, such as glycerin, are immiscible, thereby improving both the chemical stability of the alkanoic acid solution and improving the physical stability of the softgel capsule by greatly reducing the migration of capsule plasticizers into the encapsulated pharmaceutical formulation. Additionally, the use of the lipophilic solvent prevents the formation of esters which can decrease the chemical stability of the alkanoic acid solution.
Suitable lipophilic solvents are polyol esters of fatty acids. The polyol esters of fatty acids may be mono-, di-, tri-, etc, esters of the polyols. Thus, there may be free hydroxyl
groups present in the polyol esters of fatty acids useful as lipophilic solvents of the invention.
The lipophilic solvent preferred for use in the present invention is an alkylene glycol derivative of formula II:
the R" groups are the same or different and represent C1-C12 alkyl.
Suitable lipophilic solvents include those of formula III:
Other suitable lipophilic solvents are those of formula III where the R" groups are the same and represent C1-C4 alkyl and R''' is
Still other suitable lipophilic solvents are those of formula IV where the R" groups are the same or different and represent C1-C4 alkyl and R' is methyl.
Most preferred lipophilic solvents of formula III are those where R" is methyl. Most preferred lipophilic solvents of formula IV are those where the R" groups are the βarne or different and represent CH3(CH2)6 or CH3(CH2)8.
Particularly preferred solvents are selected from the group consisting of propylene glycol dicaprylate/dicaprate, 1,2,3- propanetriol triacetate and mixtures thereof. Most preferably the solvents suitable for use in the present invention include
propylβne glycol dicaprylate/dicapxfate, 1,2,3-propanetriol triacetate and mixtures thereof. Propylene glycol dicaprylate/dicaprate is available under the trade name Captex 200 from Karlshamn Lipid Specialties and 1,2,3-propanetriol triacetate is available under the trade name Triacetin from Eastman Chemicals.
The inventive solutions may also contain optional, additional ingredients to improve the dispersivity and dissolution of the substituted alkanoic acid. Suitable additional components include surfactants such as, for example, polyglyceryl esters of fatty acids, polyglycolyzed glycerides, propylene glycol esters, mono- and di-glycerides, sorbitan fatty acid esters, polyoxyethylene sorbitan fatty acid esters, polyoxyethylene sorbitol esters, polyoxyethylene adds, polyoxyethylene alcohols, and mixtures thereof. A preferred class of surfactants for use in combination with the lipophilic βolvents is the polyoxyethylene sorbitan fatty acid esters. Suitable sorbitan esters are sold under the trade name Tween. A particularly useful Tween is polyoxyethylene (20) sorbitan mono-oleate (Tween 80).
The active substituted alkanoic acid pharmaceutical agent may be present in the solution in amounts ranging up to about 30% by weight of the solution. Preferred concentrations of the active agent are from about 5-20%, more preferably about 10-15%, by weight of the final solution. Combinations of lipophilic solvents may be used to obtain a desired final concentration.
For example, ketoprofen may be present in the solution in amounts ranging up to about 5% by weight of the solution when dissolved only in propylene glycol dicaprylate/dicapratβ. Ketoprofen may be present in the solution in amounts ranging up to about 14% by weight of the solution when dissolved only in 1,2,3-propanetriol triacetate. When dissolved In a mixture of propylene glycol dicaprylate/dicaprate, 1,2,3-propanβtriol triacetate and Tween, the ketoprofen pharmaceutical agent may be present in solution in amounts ranging Up to about 22% by weight of solution.
In addition to the ketoprofen pharmaceutical agent and the lipophilic solvents, other adjuncts may optionally be present.
Polyoxyethylene (20) sorbitan mono-oleate (Tween 80) may be included in the solution up to about 50% by weight of the solution.
Once the appropriate pharmaceutically acceptable solution of the substituted alkanoic acid is formulated, it can be encapsulated into conventional softgel capsules using any suitable encapsulation method, such as for example, the rotary die process.
All documents, e.g., patents and journal articles, cited above or below are hereby incorporated by reference in their entirety.
One skilled in the art will recognize that modifications may be made in the present invention without deviating from the spirit or scope of the invention. The invention is illustrated further by the following examples which are not to be construed
as limiting the invention or scope of the specific procedures described herein.
Example 1
Pharmaceutically acceptable solutions containing ketoprofen are prepared in the following manner. First, mix the following until homogeneous:
(1) about 92 mg of propylene glycol dicaprylate/dicaprate;
(2) about 92mg of 1,2,3-propanetriol acetate; and
(3) about 10 mg of polyoxyethylene (20) sorbitan mono- oleate.
Second, add about 25 mg of ketoprofen to the homogeneous mixture of propylene glycol dicaprylate, 1,2,3-propanetriol acetate and polyoxyethylene (20) sorbitan mono-oleate, and mix again. While mixing in the ketoprofen, heat the mixture and maintain the temperature between 110-125°F until the ketoprofen is dissolved. Once the ketoprofen is fully dissolved, the solution is then cooled and deaerated. After being cooled and deaerated, the ketoprofen solution can be encapsulated in suitable softgel capsules, such as 4 oval softgel. The filled softgel capsules are thereafter dry finished to the appropriate hardness.
Example 2
Pharmaceutically acceptable solutions containing ketoprofen are prepared in the following manner. First, mix the following until homogeneous:
(1) about 112 mg of propylene glycol dicaprylate/dicaprate;
(2) about 72 mg of 1,2,3-propanetriol acetate; and
(3) about 14 mg of polyoxyethylene (20) sorbitan mono- oleate.
Second, add about 25 mg of ketoprofen tlo the homogeneous mixture of propylene glycol dicaprylate, 1,2,3-propanetriol acetate and polyoxyethylene (20) sorbitan mono-oleate, and mix again. While mixing in the ketoprofen, heat the mixture and maintain the temperature between 110-125°F until the ketoprofen is dissolved. Once the ketoprofen is fully dissolved, the solution is then cooled and deaerated. After being cooled and deaerated, the ketoprofen solution can be encapsulated in suitable softgel capsules, such as 4 oval softgel. The filled softgel capsules are thereafter dry finished to the appropriate hardness.
Example 3
Pharmaceutically acceptable solutions containing up to about 22% ketoprofen by weight of solution are prepared in the following manner, which provides a self-emulsifying system. First, mix the following until homogeneous:
(1) propylene glycol dicaprylate/dicaprate in an amount ranging from about 40% to about 98% by weight;
(2) 1,2,3-ρropanβtriol acetate in an amount ranging from about 1% to about 55% by weight; and
(3) polyoxyethylene (20) sorbitan mono-oleate in an amount ranging from about 1% to about 50% by weight.
Second, add ketoprofen to the homogeneous mixture of propylene glycol dicaprylate, 1,2,3-propanetriol triacetate and polyoxyethylene (20) sorbitan mono-oleate, and mix again. While mixing in the ketoprofen, heat the mixture and maintain the temperature between 110-125°F until the ketoprofen is dissolved.
Once the ketoprofen is fully dissolved, the solution is then cooled and deaerated. After being cooled and deaerated, the ketoprofen solution can be encapsulated in suitable softgel capsules. The filled softgel capsules are thereafter dry finished to the appropriate hardness.
Example 4
Pharmaceutically acceptable solutions containing up to about 14% ketoprofen by weight of solution are prepared in the following manner. First, mix the following until homogeneous:
(1) propylene glycol dicaprylate/dicaprate in an amount ranging from about 1% to about 50% by weight; and
(2) 1,2,3-propanetriol acetate in an amount ranging from about 50% to about 99% by weight.
Second, add ketoprofen to the homogeneous mixture of propylene glycol dicaprylate and 1,2,3-propanetriol acetate and mix again. While mixing in the ketoprofen, heat the mixture and maintain the temperature between 110-125°F until the ketoprofen is dissolved. Once the ketoprofen is fully dissolved, the solution is then cooled and deaerated. After being cooled and deaerated, the ketoprofen solution can be encapsulated in suitable softgel
capsules. The filled softgel capsujles are thereafter dry finished to the appropriate hardness.
Example 5
Pharmaceutically acceptable solutions containing up to about 5% ketoprofen by weight of solution are prepared by mixing the ketoprofen with propylene glycol dicaprylate/dicaprate while heating the mixture. The temperature of the mixture should be maintained between 110-125°F until the ketoprofen is dissolved. Once the ketoprofen is fully dissolved, the solution is then cooled and deaerated. After being cooled and deaerated, the ketoprofen solution can be encapsulated in suitable softgel capsules. The filled softgel capeules are thereafter dry finished to the appropriate hardness.
Example 6
Pharmaceutically acceptable solutions containing up to about 14% ketoprofen by weight of solution are prepared by mixing the ketoprofen with 1,2,3-propanetriol acetate while heating the mixture. The temperature of the mixture should be maintained between 110-125°F until the ketoprofen is dissolved. Once the ketoprofen is fully dissolved, the solution is then cooled and deaerated. After being cooled and deaerated, the ketoprofen solution can be encapsulated in suitable softgel capsules. The filled softgel capsules are thereafter dry finished to the appropriate hardness.
Example 7
The following formulations are prepared according to the invention using the procedure set fortth above in Example 1.
Example 8
The following comparative formulations are prepared essentially as in the procedure set forth above in Example 1 but do not include the lipophilic solvent according to the invention.
Certain specific embodiments of the present invention have been discussed and disclosed in detail. Many other embodiments
that have not been disclosed or described are nevertheless the equivalent of and fall within the scope of the present invention and/or the following claims.
Claims
1. A pharmaceutical composition comprising alkanoic acids selected from the group consisting of alkanoic acids of the formulas:
wherein
R represents a hydrogen atom or aln alkyl group containing
1 to 4 carbon atoms;
R1 represents hydrogen, halogen, C1-C6 alkyl, phenylalkyl where the alkyl is C1-C6 alkyl, a benzoyl group of the formula:
where n is 0, 1 or 2;
R2 represents hydrogen, hydroxy or C1-C6 alkoxy;
R3 represents hydrogen, C1-C6 alkyl or phenyl; and
R5 is C1-C6 alkoxy.
the 2-phenyl or naphthyl alkanoic acid being solubilized in a lipophilic solvent.
2. A pharmaceutical composition according to Claim 1 wherein the lipophilic solvent has the formula:
wherein
A represents C1-C4 alkylene optionally substituted with alkyl or and
3. A pharmaceutical composition according to Claim 1 wherein the lipophilic solvent has the formula:
4. A pharmaceutical composition according to Claim 1 wherein the lipophilic solvent has the formula:
6. A pharmaceutical composition accoriding to Claim 4, where the R" groups are the same or different and represent C1-C4 alkyl and R' is methyl.
7. A pharmaceutical composition accoriding to claim 1, wherein the lipophilic solvent comprises a mixture of a alkylene glycol derivative of the formula:
where the R" groups are the same or different and represent C1- C12 alkyl and R''' is hydrogen or and
a alkylene glycol derivative of the formula:
where the R" groups are the same or different and represent C1- C12 alkyl and R' is C1-C6 alkyl.
8. A pharmaceutical composition! of Claim 1 wherein at least one lipophilic solvent has no free hydroxy1 groups.
9. A pharmaceutical composition comprising ketoprofen, naproxen, oxaprozin or ibuprofen solubilized up to 14% by weight in 1,2,3-propanetriol triacetate.
10. A pharmaceutical composition comprising ketoprofen, ibuprofen, oxaprozin or naproxen solubilized up to 5% by weight in propylene glycol dicaprylatβ/dicaprate.
11. The pharmaceutical composition of claim 9, wherein the ketoprofen, naproxen, oxaprozin or ibuprofen is solubilized in a mixture of 1 to 50% by weight of propylene glycol dicaprylate/dicaprata and 50 to 99% by weight of 1,2,3- propanetriol triacetate.
12. A pharmaceutical composition comprising ketoprofen, oxaprozin, naproxen, oxaprozin or ibuprcjfen solubilized up to 22% by weight in a mixture of 40 to 98% by weight of propylene glycol dicaprylate/dicaprate, 1 to 55% by weight of 1,2,3-propanetriol triacetate, and 1 to 50% by weight of a surfactant.
13. A solution comprising from about 0.1 to about 30% by weight of Ibuprofen, naproxen, oxaprozin or ketoprofen in a lipophilic solvent.
14. A solution according to Claim 13, comprising from about 5 to about 20% by weight of ibuprofen, naproxen, oxaprozin or ketoprofen in a lipophilic solvent.
15. A solution according to Claim 13, comprising from about 10 to about 15% by weight of ibuprofen!, naproxen, oxaprozin or ketoprofen in a lipophilic solvent.
16. A soft gelatin capsule comprising a solution of ketoprofen, naproxen, or ibuprofen in 4 lipophilic solvent.
17. A soft gelatin capsule according to claim 16, wherein the amount of ketoprofen, naproxen, oxaprozin or ibuprofen in the solution is from about 10 to 15% by weight of the solution.
18. A solution according to Claim 13, wherein the lipophilic solvent is suitable for encapsulation by a gelatin shell.
19. A pharmaceutical composition comprising an amount of ketoprofen, ibuprofen, oxaprozin or naproxen effective to produce analgesia in a patient, the ketoprofen, ibuprofen, oxaprozin or naproxen being present as a solution in a pharmaceutically acceptable lipophilic solvent.
20. A method for preparing a liquid mixture of a 2- or 3- aryl or 3-heteroaryl alkanoic acid suitable for encapsulation in a soft gelatin capsule comprising mixing a 2- or 3-aryl or 3- heteroaryl alkanoic acid of the formula:
wherein
R represents a hydrogen atom or an alkyl group containing
1 to 4 carbon atoms;
R1 represents hydrogen, halogen, C1-C6 alkyl, phenylalkyl where the alkyl is C1-C6 alkyl, a benzoyl group of the formula:
where R4 represents hydrogen, C1-C8 alkyl, or an alkylthio group having 1 to 4 carbon atoms; or
R2 represents hydrogen, hydroxy or C1-C6 alkoxy;
R3 represents hydrogen, C1-C8 alkyl or phenyl; and R5 is C1-C6 alkoxy,
wherein
A represents C1-C4 alkylene optionally substituted with alkyl or
and
the R" groups are the same of different and represent C1-C12 alkyl.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU25170/95A AU2517095A (en) | 1994-05-19 | 1995-05-19 | Solutions of aryl or heteroaryl substituted alkanoic acids in lipophilic solvents and soft gelatin capsules containing such solutions |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US24702894A | 1994-05-19 | 1994-05-19 | |
| US08/247,028 | 1994-05-19 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1995031979A1 true WO1995031979A1 (en) | 1995-11-30 |
Family
ID=22933249
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US1995/006183 WO1995031979A1 (en) | 1994-05-19 | 1995-05-19 | Solutions of aryl or heteroaryl substituted alkanoic acids in lipophilic solvents and soft gelatin capsules containing such solutions |
Country Status (2)
| Country | Link |
|---|---|
| AU (1) | AU2517095A (en) |
| WO (1) | WO1995031979A1 (en) |
Cited By (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6962931B2 (en) | 2001-06-21 | 2005-11-08 | Pfizer Inc. | Self-emulsifying formulations of cholesteryl ester transfer protein inhibitors |
| WO2006096580A1 (en) * | 2005-03-08 | 2006-09-14 | Banner Pharmacaps, Inc. | Solvent system for enhancing the solubility of pharmaceutical agents |
| US8933023B2 (en) | 2004-03-12 | 2015-01-13 | Biodel Inc. | Rapid acting injectable insulin compositions |
| US9006175B2 (en) | 1999-06-29 | 2015-04-14 | Mannkind Corporation | Potentiation of glucose elimination |
| US9060927B2 (en) | 2009-03-03 | 2015-06-23 | Biodel Inc. | Insulin formulations for rapid uptake |
| US9192675B2 (en) | 2008-06-13 | 2015-11-24 | Mankind Corporation | Dry powder inhaler and system for drug delivery |
| US9220687B2 (en) | 2008-12-29 | 2015-12-29 | Mannkind Corporation | Substituted diketopiperazine analogs for use as drug delivery agents |
| US9233159B2 (en) | 2011-10-24 | 2016-01-12 | Mannkind Corporation | Methods and compositions for treating pain |
| US9241903B2 (en) | 2006-02-22 | 2016-01-26 | Mannkind Corporation | Method for improving the pharmaceutic properties of microparticles comprising diketopiperazine and an active agent |
| US9283193B2 (en) | 2005-09-14 | 2016-03-15 | Mannkind Corporation | Method of drug formulation based on increasing the affinity of crystalline microparticle surfaces for active agents |
| US9346766B2 (en) | 2004-08-20 | 2016-05-24 | Mannkind Corporation | Catalysis of diketopiperazine synthesis |
| US9364619B2 (en) | 2008-06-20 | 2016-06-14 | Mannkind Corporation | Interactive apparatus and method for real-time profiling of inhalation efforts |
| US9364436B2 (en) | 2011-06-17 | 2016-06-14 | Mannkind Corporation | High capacity diketopiperazine microparticles and methods |
| US9630930B2 (en) | 2009-06-12 | 2017-04-25 | Mannkind Corporation | Diketopiperazine microparticles with defined specific surface areas |
| US9662461B2 (en) | 2008-06-13 | 2017-05-30 | Mannkind Corporation | Dry powder drug delivery system and methods |
| US9675674B2 (en) | 2004-08-23 | 2017-06-13 | Mannkind Corporation | Diketopiperazine salts for drug delivery and related methods |
| US9700690B2 (en) | 2002-03-20 | 2017-07-11 | Mannkind Corporation | Inhalation apparatus |
| US9706944B2 (en) | 2009-11-03 | 2017-07-18 | Mannkind Corporation | Apparatus and method for simulating inhalation efforts |
| US9802012B2 (en) | 2012-07-12 | 2017-10-31 | Mannkind Corporation | Dry powder drug delivery system and methods |
| US9925144B2 (en) | 2013-07-18 | 2018-03-27 | Mannkind Corporation | Heat-stable dry powder pharmaceutical compositions and methods |
| US9943571B2 (en) | 2008-08-11 | 2018-04-17 | Mannkind Corporation | Use of ultrarapid acting insulin |
| US9983108B2 (en) | 2009-03-11 | 2018-05-29 | Mannkind Corporation | Apparatus, system and method for measuring resistance of an inhaler |
| US10159644B2 (en) | 2012-10-26 | 2018-12-25 | Mannkind Corporation | Inhalable vaccine compositions and methods |
| US10307464B2 (en) | 2014-03-28 | 2019-06-04 | Mannkind Corporation | Use of ultrarapid acting insulin |
| US10342938B2 (en) | 2008-06-13 | 2019-07-09 | Mannkind Corporation | Dry powder drug delivery system |
| US10421729B2 (en) | 2013-03-15 | 2019-09-24 | Mannkind Corporation | Microcrystalline diketopiperazine compositions and methods |
| US10561806B2 (en) | 2014-10-02 | 2020-02-18 | Mannkind Corporation | Mouthpiece cover for an inhaler |
| US10625034B2 (en) | 2011-04-01 | 2020-04-21 | Mannkind Corporation | Blister package for pharmaceutical cartridges |
| US11446127B2 (en) | 2013-08-05 | 2022-09-20 | Mannkind Corporation | Insufflation apparatus and methods |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4727109A (en) * | 1985-01-04 | 1988-02-23 | R. P. Scherer Corporation | Pharmaceutical preparation with an active substance of low solubility in water and gastric juices |
| US5059626A (en) * | 1988-07-25 | 1991-10-22 | Applied Analytical Industries, Inc. | Liquid oral pharmaceutical compositions of non-steroidal anti-inflammatory drugs |
| US5071643A (en) * | 1986-10-17 | 1991-12-10 | R. P. Scherer Corporation | Solvent system enhancing the solubility of pharmaceuticals for encapsulation |
| WO1992008445A1 (en) * | 1990-11-13 | 1992-05-29 | Affinity Biotech, Inc. | Non-aqueous microemulsion for drug delivery |
| WO1992010996A1 (en) * | 1990-12-18 | 1992-07-09 | Merrell Dow Pharmaceuticals Inc. | Enhanced bioavailability pharmaceutical composition containing probucol |
| WO1994007488A1 (en) * | 1992-10-07 | 1994-04-14 | Pfizer Inc. | 3-substituted 2-oxindole-1-carboxamide pharmaceutical compositions |
-
1995
- 1995-05-19 AU AU25170/95A patent/AU2517095A/en not_active Abandoned
- 1995-05-19 WO PCT/US1995/006183 patent/WO1995031979A1/en active Application Filing
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4727109A (en) * | 1985-01-04 | 1988-02-23 | R. P. Scherer Corporation | Pharmaceutical preparation with an active substance of low solubility in water and gastric juices |
| US5071643A (en) * | 1986-10-17 | 1991-12-10 | R. P. Scherer Corporation | Solvent system enhancing the solubility of pharmaceuticals for encapsulation |
| US5059626A (en) * | 1988-07-25 | 1991-10-22 | Applied Analytical Industries, Inc. | Liquid oral pharmaceutical compositions of non-steroidal anti-inflammatory drugs |
| WO1992008445A1 (en) * | 1990-11-13 | 1992-05-29 | Affinity Biotech, Inc. | Non-aqueous microemulsion for drug delivery |
| WO1992010996A1 (en) * | 1990-12-18 | 1992-07-09 | Merrell Dow Pharmaceuticals Inc. | Enhanced bioavailability pharmaceutical composition containing probucol |
| WO1994007488A1 (en) * | 1992-10-07 | 1994-04-14 | Pfizer Inc. | 3-substituted 2-oxindole-1-carboxamide pharmaceutical compositions |
Cited By (53)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9801925B2 (en) | 1999-06-29 | 2017-10-31 | Mannkind Corporation | Potentiation of glucose elimination |
| US9006175B2 (en) | 1999-06-29 | 2015-04-14 | Mannkind Corporation | Potentiation of glucose elimination |
| US6962931B2 (en) | 2001-06-21 | 2005-11-08 | Pfizer Inc. | Self-emulsifying formulations of cholesteryl ester transfer protein inhibitors |
| US9700690B2 (en) | 2002-03-20 | 2017-07-11 | Mannkind Corporation | Inhalation apparatus |
| US8933023B2 (en) | 2004-03-12 | 2015-01-13 | Biodel Inc. | Rapid acting injectable insulin compositions |
| US9346766B2 (en) | 2004-08-20 | 2016-05-24 | Mannkind Corporation | Catalysis of diketopiperazine synthesis |
| US9796688B2 (en) | 2004-08-20 | 2017-10-24 | Mannkind Corporation | Catalysis of diketopiperazine synthesis |
| US10130685B2 (en) | 2004-08-23 | 2018-11-20 | Mannkind Corporation | Diketopiperazine salts for drug delivery and related methods |
| US9675674B2 (en) | 2004-08-23 | 2017-06-13 | Mannkind Corporation | Diketopiperazine salts for drug delivery and related methods |
| US10028925B2 (en) | 2005-03-08 | 2018-07-24 | Patheon Softgels, Inc. | Liquid dosage forms of sodium naproxen |
| US10022344B2 (en) | 2005-03-08 | 2018-07-17 | Patheon Softgels, Inc. | Liquid dosage forms of sodium naproxen |
| CN101166520B (en) * | 2005-03-08 | 2013-01-09 | 班纳制药公司 | Solvent system for enhancing the solubility of pharmaceutical agents |
| WO2006096580A1 (en) * | 2005-03-08 | 2006-09-14 | Banner Pharmacaps, Inc. | Solvent system for enhancing the solubility of pharmaceutical agents |
| US9693978B2 (en) | 2005-03-08 | 2017-07-04 | Banner Life Sciences Llc | Solvent system for enhancing the solubility of pharmaceutical agents |
| US9693979B2 (en) | 2005-03-08 | 2017-07-04 | Banner Life Sciences Llc | Liquid dosage forms of sodium naproxen |
| EP3061447A1 (en) * | 2005-03-08 | 2016-08-31 | Banner Life Sciences LLC | Solvent system for enhancing the solubility of pharmaceutical agents |
| US10143655B2 (en) | 2005-09-14 | 2018-12-04 | Mannkind Corporation | Method of drug formulation |
| US9283193B2 (en) | 2005-09-14 | 2016-03-15 | Mannkind Corporation | Method of drug formulation based on increasing the affinity of crystalline microparticle surfaces for active agents |
| US9717689B2 (en) | 2005-09-14 | 2017-08-01 | Mannkind Corporation | Method of drug formulation based on increasing the affinity of crystalline microparticle surfaces for active agents |
| US9446001B2 (en) | 2005-09-14 | 2016-09-20 | Mannkind Corporation | Increasing drug affinity for crystalline microparticle surfaces |
| US9241903B2 (en) | 2006-02-22 | 2016-01-26 | Mannkind Corporation | Method for improving the pharmaceutic properties of microparticles comprising diketopiperazine and an active agent |
| US10130581B2 (en) | 2006-02-22 | 2018-11-20 | Mannkind Corporation | Method for improving the pharmaceutic properties of microparticles comprising diketopiperazine and an active agent |
| US10342938B2 (en) | 2008-06-13 | 2019-07-09 | Mannkind Corporation | Dry powder drug delivery system |
| US9662461B2 (en) | 2008-06-13 | 2017-05-30 | Mannkind Corporation | Dry powder drug delivery system and methods |
| US10751488B2 (en) | 2008-06-13 | 2020-08-25 | Mannkind Corporation | Dry powder inhaler and system for drug delivery |
| US9192675B2 (en) | 2008-06-13 | 2015-11-24 | Mankind Corporation | Dry powder inhaler and system for drug delivery |
| US9339615B2 (en) | 2008-06-13 | 2016-05-17 | Mannkind Corporation | Dry powder inhaler and system for drug delivery |
| US9446133B2 (en) | 2008-06-13 | 2016-09-20 | Mannkind Corporation | Dry powder inhaler and system for drug delivery |
| US9511198B2 (en) | 2008-06-13 | 2016-12-06 | Mannkind Corporation | Dry powder inhaler and system for drug delivery |
| US10201672B2 (en) | 2008-06-13 | 2019-02-12 | Mannkind Corporation | Dry powder inhaler and system for drug delivery |
| US9364619B2 (en) | 2008-06-20 | 2016-06-14 | Mannkind Corporation | Interactive apparatus and method for real-time profiling of inhalation efforts |
| US10675421B2 (en) | 2008-06-20 | 2020-06-09 | Mannkind Corporation | Interactive apparatus and method for real-time profiling of inhalation efforts |
| US9943571B2 (en) | 2008-08-11 | 2018-04-17 | Mannkind Corporation | Use of ultrarapid acting insulin |
| US9220687B2 (en) | 2008-12-29 | 2015-12-29 | Mannkind Corporation | Substituted diketopiperazine analogs for use as drug delivery agents |
| US9655850B2 (en) | 2008-12-29 | 2017-05-23 | Mannkind Corporation | Substituted diketopiperazine analogs for use as drug delivery agents |
| US10172850B2 (en) | 2008-12-29 | 2019-01-08 | Mannkind Corporation | Substituted diketopiperazine analogs for use as drug delivery agents |
| US9060927B2 (en) | 2009-03-03 | 2015-06-23 | Biodel Inc. | Insulin formulations for rapid uptake |
| US9983108B2 (en) | 2009-03-11 | 2018-05-29 | Mannkind Corporation | Apparatus, system and method for measuring resistance of an inhaler |
| US9630930B2 (en) | 2009-06-12 | 2017-04-25 | Mannkind Corporation | Diketopiperazine microparticles with defined specific surface areas |
| US9706944B2 (en) | 2009-11-03 | 2017-07-18 | Mannkind Corporation | Apparatus and method for simulating inhalation efforts |
| US10625034B2 (en) | 2011-04-01 | 2020-04-21 | Mannkind Corporation | Blister package for pharmaceutical cartridges |
| US9364436B2 (en) | 2011-06-17 | 2016-06-14 | Mannkind Corporation | High capacity diketopiperazine microparticles and methods |
| US10130709B2 (en) | 2011-06-17 | 2018-11-20 | Mannkind Corporation | High capacity diketopiperazine microparticles and methods |
| US9233159B2 (en) | 2011-10-24 | 2016-01-12 | Mannkind Corporation | Methods and compositions for treating pain |
| US10258664B2 (en) | 2011-10-24 | 2019-04-16 | Mannkind Corporation | Methods and compositions for treating pain |
| US9610351B2 (en) | 2011-10-24 | 2017-04-04 | Mannkind Corporation | Methods and compositions for treating pain |
| US9802012B2 (en) | 2012-07-12 | 2017-10-31 | Mannkind Corporation | Dry powder drug delivery system and methods |
| US10159644B2 (en) | 2012-10-26 | 2018-12-25 | Mannkind Corporation | Inhalable vaccine compositions and methods |
| US10421729B2 (en) | 2013-03-15 | 2019-09-24 | Mannkind Corporation | Microcrystalline diketopiperazine compositions and methods |
| US9925144B2 (en) | 2013-07-18 | 2018-03-27 | Mannkind Corporation | Heat-stable dry powder pharmaceutical compositions and methods |
| US11446127B2 (en) | 2013-08-05 | 2022-09-20 | Mannkind Corporation | Insufflation apparatus and methods |
| US10307464B2 (en) | 2014-03-28 | 2019-06-04 | Mannkind Corporation | Use of ultrarapid acting insulin |
| US10561806B2 (en) | 2014-10-02 | 2020-02-18 | Mannkind Corporation | Mouthpiece cover for an inhaler |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2517095A (en) | 1995-12-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO1995031979A1 (en) | Solutions of aryl or heteroaryl substituted alkanoic acids in lipophilic solvents and soft gelatin capsules containing such solutions | |
| JP3678745B2 (en) | Gelatin capsule containing high concentration acetaminophen solution | |
| JP4049805B2 (en) | Pharmaceutical composition containing polyvinylpyrrolidone and triester and process for producing the same | |
| US5641512A (en) | Soft gelatin capsule compositions | |
| US4690823A (en) | Ibuprofen-containing soft gelatin capsules and process for preparing same | |
| CA1275930C (en) | Therapeutic agents containing 2-(2-fluoro-4-biphenylyl)-propionic acid | |
| EP1207872B1 (en) | Ibuprofen-containing softgels | |
| WO2009066146A2 (en) | Stable solutions of sparingly soluble actives | |
| WO2000030619A1 (en) | Self-emulsifying ibuprofen solution and soft gelatin capsule for use therewith | |
| US5665384A (en) | Oily capsules of ketoprofen | |
| JPH09501150A (en) | Capsule formulation | |
| EP1485081B1 (en) | Ibuprofen solution for hard shell capsules | |
| WO2005046727A2 (en) | Ibuprofen-containing soft gelatin capsules | |
| US6383515B2 (en) | Solvent system for enhancing solubility | |
| EP1453489B1 (en) | Acetaminophen compositions | |
| RU2084226C1 (en) | Composition exhibiting antitumor action | |
| KR101859200B1 (en) | Pharmaceutical composition of monoacetyldiacylglycerol compound for oral administration and solid pharmaceutical preparation | |
| DK169566B1 (en) | Liquid pharmaceutical composition containing a 4-aroyl-imidazol-2-one for use in dosage forms for oral administration and method of preparation of the composition | |
| JP2764581B2 (en) | A novel sodium picosulfate formulation with fast intestinal diffusion | |
| JPS621925B2 (en) | ||
| US5281421A (en) | Gemfibrozil formulations | |
| KR100201907B1 (en) | A softcapsule containing biphenyldimethyldicarboxylate (pmc) solution |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AM AT AU BB BG BR BY CA CH CN CZ DE DK EE ES FI GB GE HU IS JP KE KG KP KR KZ LK LR LT LU LV MD MG MN MW MX NO NZ PL PT RO RU SD SE SG SI SK TJ TM TT UA UG US UZ VN |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): KE MW SD SZ UG AT BE CH DE DK ES FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN ML MR NE SN TD TG |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |
|
| 122 | Ep: pct application non-entry in european phase | ||
| NENP | Non-entry into the national phase |
Ref country code: CA |