ELECTROCHEMILUMINESCENT METAL CHELATE LABELS AND MEANS FOR DETECTION
Background of the Invention
1. Field of the Invention
The present invention relates to ruthenium-containing and osmium-containing compounds and methods of use for labelling. More particularly, the present invention relates to ruthenium and phenantoolme-containing compounds which also contain osmium or phenanthroline and methods of using same for labelling by elecfrocheπώuminescence.
2. Discussion of Background Art
The technology of electrocheirdluminescence (ECL) has provided a new and highly specific way to detect the presence of selected chemical, biochemical or biological substances. ECL works by having a chelate compound attached to a target biochemical or biological substance to form a biomolecular conjugate. Non-attached chelates are then removed and the remainder is exposed to an electrical potential causing the chelates attached to the target substance to luminesce. This provides a highly accurate method for determining small quantities of pharmaceuticals, metabolites, microorganisms and other materials of diagnostic value.
Electrc hemiluminescence is not the same as other kinds of luminescence such as photochemical or chemical luminescence. "Photoluminescence" is the process whereby a material is induced to luminesce when it absorbs electromagnetic radiation. Fluorescence and phosphorescence are types of photoluminescence. "Chemiluminescence" is a process whereby a luminescent species is created by a chemical transfer of energy. In contrast, ''electroche__ilu__inescence'' is the process wherein the luminescent species is created electrochemically. ECL may be performed by a variety of methods as disclosed by U.S. Patent Nos. 5,310,687 to Bard et al and 5,068,088 to Hall et al incorporated herein by reference.
In ECL, the biomolecular conjugates are detected by their light emission resulting from an energetic electron transfer reaction of two reactants in solution. One or both of the reactants, or reagents, may be created through an electrochemical reaction which is started at an electrode.
Electrogeneration of reagents allows highly reactive reagents to be produced at a known time, position, and concentration, from stable precursors. By comparison with simple chemiliiminescence, the procedures of storing, handling, and mixing of reactive reagents are circumvented. (Such reagents are inactivated through exposure to light or air, so that they have limited shelf life and require much care in handling). With electrochemical excitation to trigger formation of reactive species, the time of observation of the light can be set based on foreknowledge of the time and position of the reaction to take place. This allows use of small samples in flowing streams, with a resulting enhancement in speed and efficiency of analysis. Clinical immunoassays developed using ECL include cancer markers such as CEA
(carcinoembryonic antigen), AFP (alpha-fetoprotein), and PSA (prostate-specific antigen); hormones such as TSH (thyroid-stimulating hormone); therapeutic drugs such as digoxin and theophylline; and infectious disease markers such as hepatitis B surface antigen. IGEN, Inc., Rockville, Maryland is also developing miniaturized instruments that use microdisposable electrodes for clinical immunoassays that can be used for near patient testing (otherwise known as point-of-care testing).
Nucleic acid hybridization-based assays, similar to the formats described for immunoassays, have also been developed to detect HIV (human immunodeficiency virus), cystic fibrosis, and human papilloma virus. In each of these cases, nucleic acids were amplified by using polymerase chain reaction (PCR) or nucleic-acid sequence-based amplification (NASBA). The PCR amplification system proved so successful that Perkin- Elmer (Norwalk, Connecticut) licensed the technology from IGEN and has made the system commercially available as the QPCR-5000™. This product is capable of measuring PCR products at attomole levels (10"18 mole).
While extremely useful organometallic compounds such as ruthenium tris (2,2'-bipyridyl), also known as ruthenium tris (2,2'-bipyridine), and methods of use of these compounds in ECL are known, it would be useful to provide new labels working in aqueous systems that give a high sensitivity.
Objects of the Present Invention
It is an object of the present invention to provide a biomolecular conjugate comprising a target substance and an electtrchemiluminescent metal chelate label comprising a phenanthroline ligand (also known as a phenanthrolyl ligand).
It is another object of the present invention to provide methods for deteπnining the presence of the target substance with the electrochemttuminescent metal chelate label which comprises the phenanthroline ligand.
It is another object of the present invention to provide apparatus which employs electrochemiliiminescence to determine the presence of the target substance attached to the metal chelate label comprising the phenanthroline ligand.
Summary of the Invention
The present invention provides a biomolecular conjugate of formula I:
(A)k:(B)u (I)
A is an organometallic chelate comprising osmium or ruthenium and at least one phenanthroline ligand, B is a target substance attached to A, k is an integer equal to or greater than 1 and u is an integer equal to or greater than 1. These conjugates form a chelate- target complex. In particular, the present invention provides a biomolecular conjugate having a formula II
In formula π, M is ruthenium or osmium. P is an unsubstituted or substituted phenanthroline ligand. L1 and L2 are ligands of M. Each of L1 and L2 may be the same or different and are selected from the group of bidentate ligands. Preferably, L1 and L2 are 1,10-phenanthroline. The parameter m is an integer equal to or greater than 1. Parameter n is 1 and o is zero or 1. Parameter t is an integer equal to or greater than 1. Ligands P, L1 and L2 as well as substance B are of such composition and number that the biomolecular conjugate can be induced to emit electromagnetic radiation. The total number of bonds to M provided by P, L1 and L2 equals the coordination number of M. The present invention also provides chelate compounds, comprising at least one phenanthroline ligand, suitable for attaching a luminescent ruthenium- or osmium-containing label to target substances.
The method of the present invention comprises:
a) forming a reagent mixture under suitable conditions containing the biomolecular conjugate of formula II; b) inducing the biomolecular conjugate to emit electromagnetic radiation by exposing the reagent mixture to electrochemical energy; and c) detecting the emitted electromagnetic radiation and thereby determining the presence of the biomolecular conjugate.
The present invention also provides for using the phe__mt_roline-containing chelate labels to deteraiine analytes of interest bound to the conjugates, or to use the biomolecular conjugates to determine analytes of interest in competitive binding assays. These binding methods may be homogeneous or heterogeneous binding methods.
The present invention also provides apparatus for determining the presence of the phenanthroline-containing biomolecular conjugates. These apparatus comprise a means for inducing the biomolecular conjugate to emit electromagnetic radiation.
A particular embodiment of the present invention employs multiwavelength ECL technology. Under such technology two or more different biochemical conjugates are present. The different biochemical conjugates can be induced to emit electromagnetic radiation of different wavelengths, respectively. In another embodiment of the invention, the biochemical conjugates may be chemical species, each of which is induced to emit electromagnetic radiation by exposure to different sources or values of energy. A different substance or analyte of interest may then be specifically attached to each biochemical conjugate. By using these compositions and methods, it is possible to determine two or more different substances or analytes of interest in a single sample under examination.
Brief Description of the Drawings
Fig. 1 discloses a reaction sequence for electrochemttuminescence.
Fig. 2 shows an apparatus for performing electrochemiluminescence.
Fig. 3 shows the fluorescence spectrum of osmium tris(l,10-phenanthroline)2+ with excitation at a wavelength equal to 413 nanometers.
Fig. 4 displays a voltamogram and a plot of ECL counts (via an ECLD method) versus applied potential for osmium (l,10-phenathroline)3 2+.
Fig. 5 shows the fluorescence spectrum of ruthenium (l,10-phenanthroline)3 2+.
Fig. 6 shows a cyclic voltamogram of ruthenium (l,10-phenanthroline)3 2+.
Fig. 7 shows a comparison of ECL measurements (via the ECLD method) of ruthenium (2,2'-bipyridine)3 2+, osmium (2,2'-bipyridine)3 2+ and osmium (1,10- phenanthroline)3 2+ .
Fig. 8A compares ECL results (via the ECLD method) for ruthenium (2,2'-bipyridine)3 2+ with osmium (l,10-phenanthroline)3 2+.
Fig. 8B plots the ECLD data of Fig. 8A on a logarithmic scale.
Fig. 9A illustrates ECL results (via an ECLS method) from testing the samples employed for generating the data of Fig. 8A.
Fig. 9B is the data of Fig. 9A plotted on a logarithmic scale. Fig. 10 lists ECLD results for ruthenium (1 , 10-phenanthroline)3 2+ .
Detailed Description of the Invention
1. Biomolecular Conjugates of the Invention
The present invention relates to biomolecular conjugates as well as methods and apparatus for using such biochemical conjugates for identifying target substances. These conjugates have the general formula I
(A)k:(B)u
(D
A is an organometallic chelate comprising osmium or ruthenium and at least one phenanthroline ligand, B is a target substance attached to A, k is an integer equal to or greater than 1 and u is an integer equal to or greater than 1. These conjugates form a chelate- target complex. In particular, the present invention provides a biochemical conjugate having a formula II
In formula π, M is a ruthenium or osmium atom; P is a phenanthroline ligand of M; L1 and L2 are ligands of M, each of which may be the same as, or different from, each other ligand. Target substance B is a biological or non-biological substance; m is an integer equal to or greater than 1. Parameter n is 1 and o is zero or 1. Parameter t is an integer equal to or greater than 1. P, L1, L2 and B are of such composition and number that the biomolecular
conjugate can be induced to emit electromagnetic radiation. The total number of bonds to M provided by P, L1 and L2 equals the coordination number of M.
B may be inco orated into the biomolecular conjugates by coordination directly to M or by attachment to a ligand of M. Attachment may be through covalent bonding, or by electrostatic or hydrogen bonding. Many diverse means of effecting covalent bonding of substances (B) to ligands of M are available. The attaching linkage may be, for example, an amide or amine bond, an ester or thioester, an ether or thioether, or any of many other means known to the art. Preferably substance B is covalently bound to one or more of P, L1 or L2 through one or more amide, amine or phosphodiester linkages. In the case of amide linkages, the linkages may be oriented so that material (B) is bonded directly either to the carbonyl or to the nitrogen of the amide linkage. The type of linkage will be determined by the substituents of the ligand and the suitable chemical groups available for binding with the ligand on the substance that is to be labeled. For example, where substance B includes DNA, the bonding may occur by a phosphodiester bond.
Suitable target substances B include many biological substances, for example, whole cells, viruses, subcellular particles, proteins, lipoproteins, glycoproteins, polypeptides, nucleic acids, polysaccharides, lipopolysaccharides, lipids, fatty acids, cellular metabolites, hormones, pharmacological agents, tranquilizers, barbiturates, alkaloids, steroids, vitamins, amino acids and sugars. Whole cell may be animal, plant, or bacterial, and may be viable or dead. Examples include plant pathogens such as fungi and nematodes. Within this application the term "subcellular particles" means subcellular organelles, membrane particles as from disrupted cells, fragments of cell walls, ribosomes, multienzyme complexes, and other particles which can be derived from living organisms. Polypeptides include, for example, enzymes, antibodies, transport proteins, receptor proteins, and structural proteins such as viral coat proteins. Preferred polypeptides are enzymes and serum-derived antibodies. Particularly preferred polypeptides are monoclonal antibodies. Hormones include, for example, insulin and T4 thyroid hormone. Pharmacological agents include, for example, cardiac glycosides. It is also within the scope of this invention to include synthetic substances which chemically resemble biological materials, such as synthetic peptides, synthetic nucleic acids, and synthetic membranes, vesicles and liposomes.
It is within the scope of this invention to include labeled nonbiological target substances, including polymeric materials. These substances may be in the form of soluble
polymeric molecules, or any of the large variety of known macroscopic forms such as, for example, beads, or containers such as test tubes, bottles, assay wells or the like.
P, L1 and L2 may be unsubstituted, or substituted by any of a large number of substituents known to the art. Suitable substituents include for example, alkyl, substituted alkyl, aryl, substituted aryl, aralkyl, substituted aralkyl, carboxylate, carboxaldehyde, carboxamide, cyano, amino, hydroxy, imino, hydroxycarbonyl, aminocarbonyl, amidine, guanidinium, ureide, maleimide sulfiir-containing groups, phosphorus containing groups, and the carboxylate ester of N-hydroxysuccir mide.
A preferred unsubstituted phenanthroline is 1,10 phenanthroline which has the formula HI
A preferred substituted phenanthroline is tetramethyl 1,10-phenanthroline.
At least one of L1 and L2 may be a polydentate aromatic heterocyclic ligand. Furthermore, at least one of these polydentate aromatic heterocyclic ligands may contain nitrogen. Suitable polydentate ligands include, but are not limited to, bipyridyl, bipyrazyl, terpyridyl, phenanthroline, substituted bipyridyl, substituted bipyrazyl, substituted terpyridyl or a substituted phenanthroline.
In one embodiment of the invention n is 1 and o is zero.
In another embodiment of the invention n is 1 and o is 1.
Particularly preferred embodiments of this biochemical conjugate comprise bis(l,10- phenanthroline) ruthenium(II), tris (1,10-phenanthroline) ruthenium(II), bis (1,10- phenanthroline) osmium (II) and tris (1,10-phenanthroline) osmium π. The tris versions are especially preferred.
The present invention also provides chelates particularly suitable as intermediates for attaching a luminescent ruthenium-containing or osmium-containing label to amino groups of chemical, biochemical and biological substances. These intermediates are thus particularly suitable for synthesizing biomolecular conjugates according to the present invention. The inventive intermediates include the ruthenium or osmium-chelate having the following formula IV
The inventive intermediates also include the mono- and di-N-hydroxysuccinimide esters of ruthenium and osmium 4,7-(dicarboxy)-l,10-phenanthroline, bis(l,10-phenanthroline) and their salts; and ruthenium and osmium 4,4'-(dichloromethyl)-2,2'-l,10-phenanthroline, bis(l,10-phenanthroline) and their salts.
One of these intermediates is mono-N-hydroxysurcinimide esters of ruthenium or osmium 4,4'-(dicarboxy)-l,10-phenanthroline, bis(l,10-phenanthroline) having the formula V
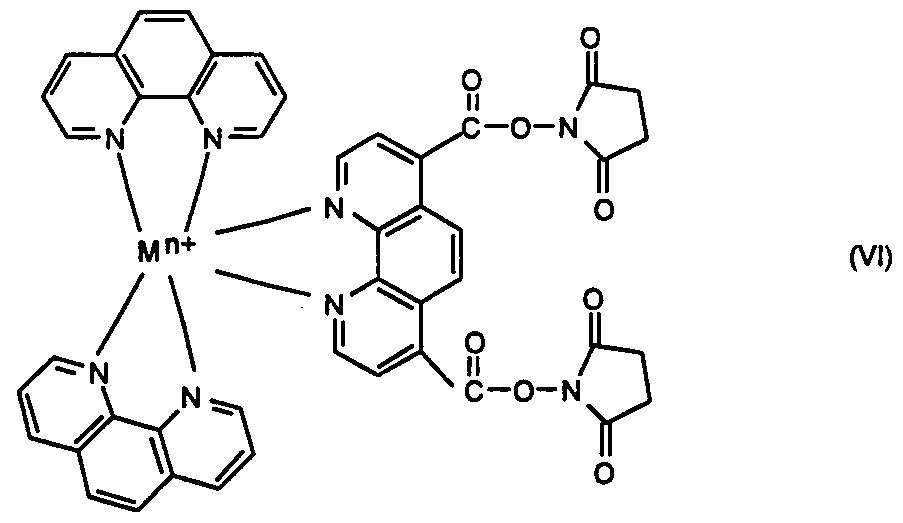
wherein M is Ru or Os, n is the integer 1, 2, or 3, and salts and stereoisomers thereof. Another intermediate is the di-N-hydroxysuccinimide ester of ruthenium- or osmium 4,7- (dicarboxyl)-l,10-phenanthroline, bis(l,10-phenanthroline) having the formula VI
wherein M is Ru or Os, n is the integer 1, 2, or 3, and salts and stereoisomers thereof. Yet another intermediate is ruthenium or osmium 4,7-(dichloromethyl)-l,10-phe___ιthroline, bis(l,10-phenanthroline) having the formula NIL
wherein M is Ru or Os, n is the integer 1, 2, or 3, and salts and stereoisomers thereof. These compounds may be synthesized by means known to the art.
A preferred method of synthesizing the mthenium-containing Ν-hydroxysuccinimide esters is to first react ruthemum dichlorobis 1,10-phenanthroline with 1,10-phenanthroline- 4,7-dicarboxylic acid in a hot aqueous methanol solution of sodium bicarbonate. After acidification, an aqueous solution of ΝaPF6 is added to the solution of carboxylated ruthenium compound. The isolated phosphonium hexafluride salt of the ruthenium complex is then esterified by reaction with N-hydroxysuccinimide in the presence of dicyclohexylcarbodiimide in dimemylformamide.
These intermediates (as well as the biomolecular conjugates) may be ionized. If so, it is understood in the art that many different counterions will serve to neutralize the charge and form a salt. Suitable cations for forming these salts include for example NH4 +, guanidinium, Ag+, Li+, Na+, K+, Ca2+, Mg2+, Mn2+, and Cd2+. Suitable anions for forming these salts
include, for example, halides, carbonate, SO4 2", PF6 (phosphonium hexafluoride), and tetrafluoroborate. An especially preferred salt is the phosphonium hexafluoride salt of Os or Ru tris (1,10-phenanthroline).
These intermediates of Formulas V, VI and VII are useful for labeling substances containing a free amino group capable of attacking the carboxylate ester, and thereby displacing N-hydroxysuccinimide, or of attacking the chloromethyl group, and thereby displacing chloride. Also, the intermediates of the present invention are easily stored and handled.
2. Methods and Apparatus of the Invention
The present invention provides methods and apparatus for determining the presence of the biomolecular conjugates of the invention. The methods includes forming a reagent mixture which comprises the biomolecular conjugates. The biomolecular conjugates are then induced to emit electromagnetic radiation by exposing the reagent mixture to electrochemical energy. Then, the emitted electromagnetic radiation is detected to determine the presence of the biochemical conjugates.
The reagent mixture may be in the form of an aqueous or nonaqueous solution, a suspension or emulsion, a solid or semisolid, or a gas. Suitable conditions for forming the reagent mixture will be known to those skilled in the art and will depend on the type of ' reagent mixture involved. For example, suitable conditions for an aqueous reagent mixture may include appropriate concentrations of biochemical conjugates and other reagents such as oxidants, pH, salt concentrations and the like. For a solid sample, suitable conditions for forming a reagent mixture may include addition of a conducting liquid. For a non-water soluble substance such as osmium (l,10-phenanthroline)3 (PF6)2, employing a 1:49 volume ratio of acetonitrile:water to dissolve the chelate prior to mixing with buffer is suitable.
The conjugates are detected by elecfrochemiluminescence methods well known in the art. Methods for ECL in general are disclosed by numerous references including U.S. Patent Nos. 5,310,687 to Bard et al, 5,147,806 to Kamin et al, 5,093,268 to Leventis et al, and 5,068,088 to Hall et al, all of which are incorporated herein by reference. Other literature references disclosing ECL methods include A.J. Bard and L.R. Faulkner, Electrochemical Methods, Ch 14.4, John Wiley & Sons (1980); H. Tachikawa and L.R. Faulkner, Laboratory Techniques In Electroanatytical Chemistry, pages 660-74 (Marcel Dekker - BAS Press, P.T. Kissinger and W.R. Heineman eds., 1994); Hongjun Yang et al,
Electrochemiluminescence: A New Diagnostic and Research Tool, 12 Bio/Technology 193- 94 (Feb. 1994); J. DiCesare et al, A High-Sensitivity Electrochemiluminescence-Based Detection System for Automated PCR Product Quantitation, 15 BioTechniques 1, 152-57 (1993); Jonathan K. Leland and Michael J. Powell, Electrogenerated Chemiluminescence: An Oxidative-Reduction Type ECL Reaction Sequence Using Tripropyl Amine, 137 J. Electrochemical Soc. 10, 3127-31 (Oct. 1990); and Gary F. Blackburn et al, Electrochemiluminescence Detection for Development of Immunoassays and DNA Probe Assays for Clinical Diagnostics, 37 Clin. Chem. 9, 1534-39 (1991).
In general, ECL involves the measurement of the interaction of two chemical reagents in an electrochemical flow cell by a photomultiplier tube (PMT). (Film or a photodiode may be employed in place of the PMT). Typically, one of the reagents is a biomolecular conjugate of the present invention and the other reagent is a compound such as tripropylamine (TPA) which oxidizes to convert to a strong reductant.
Under the ECL method the biomolecular conjugate is oxidized at an electrode, and subsequently reacts with an energetic reductant to form an electronically excited state. Then it will luminesce (emit light while relaxing to its ground state). After luminescing, the biomolecular conjugate may be repeatedly oxidized and reduced during sample measurement (normally a few seconds). Advantageously, the biomolecular conjugate is not destroyed in the ECL reaction. The repetitive nature of the detectable event distinguishes these labels from radioactive isotopes or bound chemUuminescent molecules such as luminol. The latter labels produce a detectable event only once per molecule (or atom) of label, thereby limiting their detectability.
In detail, the ECL method involves a series of electrochemical and chemical steps shown in Fig. 1 and described as follows for a reagent mixture containing Ru(l,10- phenanthroline)3 2+ and TPA.
(1) The biomolecular conjugate is oxidized at a working electrode 10 to a higher oxidation state: B:Ru(l,10-phenanthroline)3 +2 → B:Ru(l,10-phenanthroline)3 +3 + e-
(2) The tripropylamine (TPA) is also oxidized at the electrode to be active. Then rapid loss of a proton follows to give a very strong reductant; the TPA radical.
TPA → TPA +- + e → TPA • + H+
(3) The energetic electron transfer between Ru(l , 10-phenanthroline)3 +3 and TPA results in production of an electronically excited state* of Ru(l,10- phenanthroline)3 2+. The production of an electronically excited state occurs because a large amount of energy is transferred in a very short time (< 10*15 sec), and the luminescent molecule is unable to disperse all the energy through mechanical interactions.
B B::RRuu((Ll,10-phenanthroline)3 +3+TPA • → • B:Ru(l,10-phenan_ιroline)3 2+* +
TPA +
(4) The electronically excited state of Ru(l, 10-phenanthroline)3 2+ emits light in the process of relaxing to its ground state:
B:Ru(l , 10-phenanthroline)3 2+* → B:Ru(l , 10-phenanthroline)3 2+ + Light
(5) The process of steps 1-4 repeats as long as the potential is correct and sufficient concentrations of the reactants are present.
The TPA is present at a concentration in excess of the oxidized ruthenium (1,10- phenanthroline)3 2+. The desired result is that every oxidized ruthenium (1,10- phenanthroline)3 2+ undergoes d e energetic (light-producing) reaction. After the electron transfer reaction, the radical which was produced from TPA is destroyed. It does not return to its original form, so subsequent analyses of the same sample will be occurring with a smaller TPA concentration, and will give diminished signals. The label emits light at a long visible wavelength. This is an advantage because few naturally occurring species absorb or luminesce at such a wavelength. Typically the conjugate luminesces at wavelengths between 200 nanometers and 900 nanometers at ambient temperatures. The present invention envisions osmium-containing conjugates as well as rathemum-containing conjugates and encompasses the wide variety of luminescent conjugates which can be made by varying the chemical structure of the ligands. Each of these variations in the metal and the ligands can change the precise value of the energy input required to create the luminescent excited state. Similarly, the wavelength of the emitted electromagnetic radiation will be dependent upon the nature and environment of the ruthenium-containing or osmium-contairiing material. The methods of this invention also include applying the above method to determine the biochemical conjugate wherein the conjugate is capable of binding to an analyte, i.e. forming a specific complex with the analyte. The method comprises:
a) forming a reagent mixture under suitable conditions containing the biochemical conjugate and analyte of interest; b) inducing the conjugate to emit electromagnetic radiation by exposing the reagent mixture to electrochemical energy; and c) detecting the emitted electromagnetic radiation and thereby determining the presence of the analyte of interest.
Suitable analytes include, but are not limited to, whole cells, viruses, subcellular particles, nucleic acids, polysaccharides, proteins, glycoproteins, lipoproteins, lipopolysaccharides, lipids, fatty acids, peptides, cellular metabolites, hormones, pharmacological agents, tranquilizers, barbiturates, alkaloids, steroids, vitamins, amino acids, sugars or non-biological polymers.
Of particular interest are antibody-antigen pairs. Of especially particular interest are those pairs including a monoclonal antibody or a serum-derived antibody.
Also provided are methods of determining the presence of an analyte of interest wherein the analyte and the biomolecular conjugate of formula π bind competitively to a complementary material. The method comprises: a) contacting the complementary material, the biochemical conjugate and the analyte under suitable conditions so as to form a reagent mixture; b) inducing the biochemical conduit to emit electromagnetic radiation by exposing the reagent mixture to electrochemical energy; and c) detecting the emitted electromagnetic radiation and thereby determining the analyte of interest.
Within this application "complementary material" means any substance capable of foπning complexes with both an analyte of interest and a labeled analyte of interest or a labeled analogue of an analyte of interest.
In competitive binding assays, B may be the same substance as the analyte of interest or an analogue of the analyte, and capable of participating in the formation of a specific complex with a complementary material. Of particular interest are analytes and complementary materials such as insulin, digoxin, digitoxin, T4 thyroid hormone, a fungus or nematode, a serum-derived antibody or a monoclonal antibody, a DNA fragment or an RNA fragment of especially particular interest are antibody-antigen-based methods. These methods are analogous to the well known radioimmunoassay, wherein an analyte of interest is detected when it displaces a radioactive analogue of the analyte from an antibody.
The present invention further provides heterogeneous and homogeneous binding methods which utilize the biomolecular conjugates provided herein. In heterogeneous binding methods, the bound labeled substance must be physically separated from the unbound labeled substance before measurement of the presence of label. This is frequently accomplished in antibody-antigen systems by immobilizing one component, the antibody for example, by attachment to an insoluble matrix such as a filter or to the surface of beads or reaction vessels such as test tubes. The antigen-containing solution is poured through the filter or into the reaction vessel, and then washed away from the filter or sides of the reaction vessel. Only antigen specifically bound to antibody will remain to be determined. In homogeneous methods, by contrast, the bound and unbound labeled material are present in the same reaction mixture when the presence of label is measured.
In its apparatus aspects, the present invention also provides a system for determining the presence of (i) the biomolecular conjugate of formula π, (ii) the analyte of interest which is bound to the biomolecular conjugate or (iii) the analyte which competes with the biomolecular conjugate to bind to a complementary material.
The system for determining the presence of the biomolecular conjugate of formula II comprises: a) a reagent mixture comprising the biomolecular conjugate; b) a means for inducing the biomolecular conjugate to emit electromagnetic radiation; and c) a means for detecting the emitted electromagnetic radiation.
A means for contacting the biomolecular conjugate with (i) the analyte of interest, or (ii) the analyte and complementary material, to form the reagent mixture and then perform methods described above may also be provided.
Fig. 2 illustrates an example of a system for making ECL measurements. The system includes a carousel 20 for holding sample tubes 25. An assay buffer tank 30 and a cleaning solution tank 40 communicate with a valve 60. A sample probe 50 in communication with the valve 60 removes sample from the respective sample tube 25, mixes the sample with assay buffer solution from buffer tank 30 and sends the mixed sample and buffer to an electrochemical flowcell 70. The cell 70 includes the working electrode 10, a coimter electrode 74, and a reference electrode 76 provided with a gasket 78. Typically a gold working electrode, a gold counter electrode and a silver/silver chloride reference electrode are employed. A photomultiplier tube (PMT) 80 is provided. Typically, a magnet 90 is also
provided to hold the sample in place when the sample to be tested is captured on magnetic beads. The magnet 90 may be movable from a first position away from the working electrode 10 to a second position closer to electrode 10. The magnetic beads are held in place when the magnet 90 is in the second position.
The PMT tube 80 is a transducer for the detection of light and measurement of its intensity. A transducer is a device which converts a physical quantity into an electrical signal. A PMT is a transducer which converts light intensity (photons) into an electrical signal. The electrical signal can then be processed by the appropriate electronic circuit or computer. The PMT works by converting the photons which strike the PMT face (called a photocathode) to electrons (called photoelectrons). These events are greatly amplified by the large applied voltage to produce the signal at the output. Thus, photons are counted. The photon counting method is used because of its insensitivity to temperature changes and power supply drift.
The photomultiplier tube is placed just above the working electrode 10 of the flowcell to achieve efficient light detection as disclosed by Hongjan Yang et al, Electrochemiluminescence: A New Diagnostic and Research Tool, 12 Bio/Technology 193- 94 (Feb. 1994) incorporated herein by reference. However, the actual number of counts registered by die electronic counter is not the actual number of photons reaching the PMT, but only proportional. The PMT is not 100% efficient in converting light intensity to an electrical quantity. For example, PMTs manufactured by Hamamatsu Inc. have a quantum efficiency near 10%. This is typical of PMT's. No two PMTs are completely identical. The small variations in PMTs between instruments is compensated by instrument calibration. A typical buffer employed as part of the reagent of the present invention is the ORIGEN® Assay Buffer produced by IGEN, Inc., Rockville, Maryland. ORIGEN® Assay Buffer is composed of a pH-adjusted phosphate buffer, with added surfactant and other compounds, e.g. preservatives or inert salts, and TPA, the second precursor to the ECL reaction. The assay buffer is used as a diluent to prepare the sample for analysis. It also supports both the chemistry of me immunoreaction and the electrochemical reactions which lead to ECL. The buffer also generates a small quantity of light in the absence of biomolecular conjugates under conditions which normally produce ECL. The level and reproducibility of this background light emission sets the lower limit of detection of the biomolecular conjugates. The detection limit for the biomolecular conjugates takes the background light
emission into account, so that reported detection limits are not degraded by the observation of this addition to the light output.
The ORIGEN® Conditioning Buffer, produced by IGEN, Inc., serves to prepare the electrodes before introduction of the sample. In combination with electrochemical pulsing, the ORIGEN® Cell Cleaner, produced by IGEN, Inc., removes proteins and other adsorbed species from the electrodes and cell so that these species do not impede or carry over to the next sample.
Nonionic surfactants may be added to the ORIGEN® Assay Buffer in support of die immunoreaction and to aid in wetting surfaces in the device for bubble-free flow. Preservatives in the ORIGEN® Assay Buffer discourage bacterial and fungal growth and/or natural breakdown of buffer components on exposure to light or air. Many preservatives in common use for biological preparations are deleterious to the ECL reaction, and the choice of preservative in the ORIGEN® Assay Buffer has been made by determining its effect on ECL. Where the reagent will be used during oxidation of the biomolecular conjugate, it may also include oxalate, pyruvate, lactate, malonate, tartrate or citrate. Where the reagent will be used during reduction, it may also include peroxydisulfate and/or acetonitrile.
The apparatus of Fig. 2 is typically used to detect an antibody-antigen pair as follows. In a typical immunoassay, anti-target antibodies are bound to magnetic beads. Next, anti-target antibodies recognizing a different epitope on the same target are made into reporter molecules by attaching an ECL label. Incubating the target molecule with both antibodies results in a "sandwich" — the two antibodies attaching to the antigen at different sites. This antibody-antigen sandwich is then drawn into the flow cell 70 and mixed with a buffer solution containing precursor (e.g. TPA). The magnet 90 applies magnetic force to capture the magnetic beads on a surface of the electrode 10. This stabilizes the target molecule and its attached reporter for maximum detection by die PMT 80. Unbound reagents from the sample mix are washed away by continued flow of buffer solution.
After sample capture, me pump 100 is stopped and ECL measurement is performed by application of the electrical potential to die working electrode 10 to oxidize the biomolecular conjugate. Typical ways to apply such potential include sweep and step memods. The sweep method (ECLD) pulses the voltage through the electrodes as a triangular wave. The step method (ECLS) pulses the voltage through the electrodes as a square wave. A hybrid memod employing a truncated triangular wave may also be employed. These triangular, square and truncated waves for ECL are disclosed in U.S.
Patent No. 5,147,806 to Kaimin et al. The electrode 10 may be run to oxidize the biomolecular conjugate by having the electrode 10 oscillate above and below a potential sufficient to oxidize the biomolecular conjugate. In the alternative, the biomolecular conjugate may be oxidized by having electrode 10 maintain a potential which is constant and sufficient to oxidize the conjugate.
Only those labels bound to die "sandwich" that are surrounded by precursors (e.g.
TPA) and in contact with d e electrode emit light witii little interference from the buffer background. After measurement, the magnet 90 is released, a cleaning solution is drawn into the system to flush it, and more precursor containing solution is pumped into me flow cell 70 to flush die cleaner. The instrument is then ready for the next sample.
Instead of employing oxidation to excite a biomolecular conjugate, the biomolecular conjugate may be reduced by exposure to an electrode whose potential oscillates above and below a potential sufficient to reduce it. In the alternative, die biomolecular conjugate may be reduced by exposure to an electrode whose potential is constant and sufficient to reduce it.
It is within the scope of the present invention to use a combination of means for determining me presence of labeled compounds. For example, it may be desirable to measure die total amount of labeled substances by a means which does not distinguish between bound and unbound labeled substances such as photoluminescence or chemiluminescence, and to determine me amount of bound labeled substance by a means which does distinguish between bound and unbound labeled substances, such as electrocherrdluminescence, for example. Such a combination of metiiods could be performed on me same sample, and mus provide a richer source of information about the sample than could any method when used individually.
3. Inducing the Biomolecular Conjugate to Repeatedly Luminesce
The above methods and apparatus can be adapted to cause d e biochemical conjugate to emit light repeatedly. This provides more, and hence more accurate, light measurements.
4. Employing the Present Invention to Determine More Than One Target This invention also concerns compositions which comprise two or more different biomolecular conjugates, wherein at least a first of the conjugates is die ruthenium-containing or osmium-containing biomolecular conjugate of formula II . Each additional conjugate can
be any conjugate which luminesces by ECL at a different wavelengm or due to a different voltage ti an does any odier conjugate in me mixture.
The present invention also concerns methods and apparatus for determining me presence of two or more such biomolecular conjugates. The conjugates may be employed with (i) analytes of interest which bind selectively to the different biomolecular conjugates, and/or (ii) analytes of interest which bind selectively to different complementary materials in competition wim different biomolecular conjugates, present in me same samples. Methods and apparatus, described previously for detemύning die rud enium-containing and osmium- containing luminescent labels, can be adapted to determine the presence of two or more biomolecular conjugates (and/or analytes) simultaneously present in the same sample. U.S. Patent No. 5,093,268 to Leventis et al discloses an apparatus for conducting a plurality of simultaneous ECL measurements.
Methods for determining the presence of these biomolecular conjugates and/or analytes of interest comprise forming a reagent mixture, under suitable conditions, containing the biomolecular conjugates and/or analytes. Then the biomolecular conjugates are induced to emit electromagnetic radiation by exposing die reagent mixture to electrochemical energy. The presence of each of the biomolecular conjugates and/or analytes is determined by detecting d e electromagnetic radiation of different wavelengdis emitted by each of me conjugates. The apparatus for deterrmning me presence of die biomolecular conjugates and/or analytes of interest comprises means for contacting the biomolecular conjugates and/or analytes under suitable conditions to form a reagent mixture. The apparatus also comprises means for inducing the biomolecular conjugates to emit electromagnetic radiation by exposing die reagent mixture to electrochemical energy and means for detecting me electromagnetic radiation of different wavelengths emitted by each of me biomolecular conjugates and/or analytes.
Also provided are methods and apparatus for deterrnining the presence of one or more different biomolecular conjugates or analytes of interest which bind to me biomolecular conjugates, each of which may be induced to emit electromagnetic radiation by exposure to energy of different values or different sources. At least one of die conjugates is a conjugate of formula π. The methods and apparatus for deterrnining these different biomolecular conjugates are essentially the same as those for deterrnining die biomolecular conjugates which emit different wavelengths of electromagnetic radiation, except for induction. The
sample containing die biomolecular conjugates is exposed to each of die different energy values or sources to induce emittance of electromagnetic radiation at a different time. The electromagnetic radiation emitted by the biomolecular conjugates is detected and this determines the presence of the conjugate.
This invention is illustrated in the examples which follow. The examples are set forth to aid in an understanding of die invention but are not intended to, and should not be construed to, limit in any way d e invention as set forth in the claims which follow diereafter.
EXAMPLE 1 ECL of Osmium tris-phenanthroline
Os(l,10-phenandιroline)3 (PF6)2 was syndiesized and purified. It is not soluble in water, but is soluble in organic solvents. Thus, a solution was made by dissolving the compound in a 1:49 mixture of acetonitrile and water, and then diluting die mixture with ORIGEN® Assay Buffer solution manufactured by IGEN, Inc. The buffer is composed of a pH-adjusted phosphate buffer wid added surfactant and other compounds, such as preservatives or inert salts and tripropyl amine.
Fig. 3 shows the fluorescence spectrum of Os(l,10-phenand_roline)3 2+ with excitation at a wavelengtii of 413 nanometers obtained witii an LS-5 Fluorescence Spectr .photometer. The spectrophotometer was made by Perkin-Elmer, Norwalk, Connecticut. The emission peak occurs at a wavelengtii of 715 nanometers. This is basically the same as that of osmium
(bipyridine)3 2+. This emission peak suggests that the phenanthroline ligand field possesses d e same strength and symmetry as the bipyridine ligand field. Thus, the energy level split (between T2g and Eg) of die d-orbital of die osmium2+ atom in its octahedral field stays the same.
Fig. 4 shows a voltamogram and a plot of the ECL signal of a 50 nanomolar solution of tiie osmium tris(l , 10-phenanthroline)3 2+ versus potential. The signal was generated by d e ECLD process with an uplimit potential of 1.8 volts, a PMT set at 750 volts, and die solution was maintained at a temperature of 35°C. The ECL signal was measured on an ORIGEN® 1.5 Instrument #141, manufactured by IGEN, Inc. Botii die voltamogram and plot are similar to that known for the ruthenium tris(bipyridine)2+ label.
While not limiting the present invention, it is theorized that the present invention changes d e hydrophobicity of die ligand to change d e duration of the lifetime of excited states. This is especially true for some compounds with small energy bandgaps. As already
mentioned with regards to this EXAMPLE, ECL signals of osmium (phenand roline)3 2+* show almost the same absorption and emission spectra as that of osmium (bipyridine)3 2+*. This indicates they were of the similar ligand field and therefore, the energy bandgaps were almost the same. However, ECL from osmium (phenanthroline)3 2+* was stronger than tiiat from osmium (bipyridine)3 2+* (See EXAMPLE 3). This suggests that osmium (phenanthroline)3 2+* has a longer lifetime. The ligand phenanthroline has a more rigorous aromatic structure than the ligand bipyridine. Thus, osmium (phenanthroline)3 2+* is more hydropholic than osmium (bipyridine)3 2+*. Accordingly, this confirms the theory that increasing tiie hydrophobicity of die structure is beneficial to reduce die quenching effects of excited states.
EXAMPLE 2 ECL of Ruthenium 2+ tris(l,10-phenanthroline) Ruthenium2"1" tris(l,10-phenanthroline)(PF6)2 was synthesized and purified. The salt was light-orange crystalline material, slightly soluble in water, but easily dissolved in acetonitrile. Thus, d e material was mixed witii acetonitrile and water to make a first solution. The first solution was then diluted with ORIGEN® Assay Buffer solution manufactured by IGEN, Inc. The fluorescence spectrum of ruthemum tris(l,10- phenanthroline)2+ is shown in Fig. 5. The emission peak appears at a wavelength of 595 nanometers, which was blue-shifted 25 nanometers compared with that of ruthenium tris(bipyridine)2+. Consequentiy, at the wavelength of 700 nanometers, tiiere was almost no emission. Thus, tiiere is litde overlap with the emission spectrum of osmium tris(l,10- phenanthroline)2"1" of Example 1. This has the advantage tiiat there would be very good signal separation in the event tiiese two labels are employed together.
A cyclic voltamogram of ruthenium tris(l,10-phenanthroline)2+ is shown in Fig. 6. To obtain this cyclic voltamogram, an indium tin oxide (ITO) glass was used as a working electrode. The oxidation peak of ruthenium tris(l,10-phenanthroline)2+ occurred at a potential of 1.1 volts. However, the oxidation was not reversible.
EXAMPLE 3 ECLD of Osmium tris( 1 , 10-phenanthroline)2+
Samples of solutions of 1000 picomolar ruthenium tris(2,2'-bipyridine)2+, 50,000 picomolar osmium tris(2,2'-bipyridine)2+ and 50,000 picomolar osmium tris(l,10- phenandιroline)2+ were measured by the ECLD method. The results are shown in Fig. 7.
The ECLD measurements were conducted on an ORIGEN® 1.5 instrument No. 141 with an uplimit potential of 1.8 volts and die solutions were maintained at 35°C. The test cell employed a gold working electrode, a gold coimter electrode and a silver/silver chloride reference electrode.
The ECLD metiiod involved applying a potential perturbation, which is of a triangular waveform, to the working electrode immersed in the solution containing the osmium or ruthenium labels, acetonitrile (in the case of the 1,10-phenanthroline-containing label) and ORIGEN® Assay Buffer. ORIGEN® Conditioning Buffer was employed to prepare die electrodes before introduction of the sample. After testing, the ORIGEN® Cell Cleaner, in combination with electrochemical pulsing, was employed to clean the electrodes so that samples from a prior test would not carry over to the next test. Typically, a triangular wave form was pulsed over a period of about 5 to 6 seconds. The triangular wave form rose from 565 to 1800 mV vs Ag/AgCl at a rate of 1600 mV per second and then decreased at the same rate to 1000 mV vs Ag/AgCl. Oxidation of both the TPA and the labeled substance became evident when the sample produced luminescence. The intensity of die observed luminescence was measured with a single photomultiplier tube set at 750 volts.
As shown in Fig. 7, the ECLD signals from 50,000 pM osmium tris(l,10- phenanthroline)2+ were about four times that of 50,000 pM osmium tris(2,2'-bipyridine)2+. Also, signals from 50,000 pM osmium tris(2,2'-bipyridine)2+ were more than twice that of 1000 pM ruthenium tris(2,2'-bipyridine)2+. Thus, taking the concentration differences into account, tiie signal ratio of osmium tris(l,10-phenanthroline)2+ to ruthenium tris(2,2'- bipyridine)2+ under d e same concentrations would be about 1:6. This is significantly better than the signal ratio of osmium tris(2,2'-bipyridine) to ruthenium tris(2,2'-bipyridine).
EXAMPLE 4 Linearity Measurements of Osmium tris(l,10-phenanthroline)2+ The 24 solution samples listed in Table 1 were measured by ECLD. The solutions all include ORIGEN® Assay Buffer solution. In Table 1, Ru(bpy)3 2+ is ruthenium tris(2,2'- bipyridine)2"1" and Os(phen)3 2+ is osmium tris(l,10-phenanthroline)2+.
Table 1
Tube # Content
1,3 assay buffer
2 lOOOpM Ru(bpy)3 2+
4-6 500pM Os(phen)3 2+
7-9 lOOOpM Os(phen)3 2+
10-12 2500pM Os(phen)3 2+
13-15 5000pM Os(phen)3 2+
16-18 lOOOOpM Os(phen)3 2+
19-21 25000pM Os(phen)3 2+
22-24 50000pM Os(ρhen)3 2+
The counts were measured using the same equipment and ECLD sequence employed in Example 3. Figs. 8A and 8B show the results of these ECLD measurements. Figs. 8A and 8B are die same except that a logarithmic scale was used in Fig. 8B. When employing the ECLD sequence, ECL signals of 5000 picomolar osmium tris(l,10-phenanthroline)2+ can almost reach the same level as tiiat of 1000 picomolar ruthenium tris(2,2'-bipyridine)2+, shown as a dashed line in Figs. 8A and 8B. The data of Figs. 8A and 8B indicates die number of ECL counts is directiy proportional to the amount of osmium tris(l,10- phenanthroline)2"1".
Using the same protocol as employed to measure linearity, the sensitivity of osmium tris(l,10-phenanthroline)2+ was measured. It was found that the reliable detection limit was 50 picomolar.
EXAMPLE 5
ECLS of Osmium tris(l,10-phenanthroline)
Figs. 9 A and 9B show the results of measuring the samples of Example 4 using the
ECL potential step method (ECLS). ECLS was measured with the same equipment as
Example 4. However, it was programmed for the ECLS method and the PMT was set at 600 volts. In this Example, a square wave form was pulsed over a period of about 5 to 6 seconds. The square wave form rose from 565 to 1800 mV, was held at 1800 mV for 5 seconds, and then pulsed to 1000 mV vs. Ag/AgCl. Oxidation of both the TPA and die
labeled substance became evident when the sample produced luminescence. As shown in Figs. 9A and 9B, the ECLS signals of 2500 picomolar osmium tris(l,10-phenanthroline)2+ reach the signal level of 1000 picomolar ruthenium tris(2,2'-bipyridine)2+. This suggests that the potential step method enhances the ECL signals of osmium tris(l,10-phenanthroline)2+ more effectively than that of ruthenium tris(2,2'-bipyridine)2+. The data of Figs. 9A and 9B indicates the number of ECL counts is proportional to the concentration of the osmium tris(l,10-phenanthroline)2+ label. Using this ECLS protocol as used to measure linearity, the sensitivity of osmium tris(l,10-phenanthroline)2+ was found to have a reliable detection limit of 25 picomolar.
The above examples indicate tiiat the change of ligands from bipyridine to phenanthroline does not alter the energy gap but significantiy increases the ECLD efficiency of an osmium-containing label by a factor of 4.
EXAMPLE 6 ECLD of Ruthenium tris(l,10-phenanthroline \)2''+ The ruthenium tris(l,10-phenanthroline)2+ of Example 2 was subjected to ECLD measurements employing the equipment and the protocol of Example 3. The samples contained ruthenium tris(l,10-phenanthroline)2+ in a solution of acetonitrile, water and ORIGEN® Assay Buffer solution. The photomultiplier tube was set at 750 volts and die cell was heated at 35°C. The samples were arranged such that tube 1 contained assay buffer, tubes 2-8 contained 1000 picomolar ruthenium tris(2,2'-bipyridine)2+ solution, while tubes 9- 15 contained 1000 picomolar ruthenium tris(l,10-phenand roline)2+ solution. The results of these ECLD measurements are listed by Table 2 below:
Table2
Tube Dark Led Ep ECL
1 19304 833682 1794 41914
2 19215 909636 1256 316485
3 19470 937400 1238 313219
4 19722 950843 1238 316959
5 19675 965002 1229 314250
6 19910 972918 1229 311530
7 19978 978370 1238 312928
8 19492 983874 1238 311362
9 19879 988757 1247 425178
10 20034 993729 1256 425545
11 19914 992362 1256 425873
12 20500 997969 1256 424775
13 20158 999748 1238 424191
14 19989 1003376 1247 430739
15 20067 1002010 1247 424315
Dark is measured as counts at die dark and without any electrochemical reaction. Led is measured as counts when a light emitting diode (LED) is turned on. Ep is measured as peak potential of ECL, mV vs. Ag/AgCl. ECL is measured as counts
The ECLD measurements of a typical 1000 picomolar ruthenium tris(l,10- phenanthroline)2+ sample are shown by Figure 10.
EXAMPLE 7 ECLS of Ruthenium tris(l,10-phenantiιroline)2+ The solutions in tubes 1-15 of EXAMPLE 6 were also subjected to sequence ECLS. Tables 3 and 4 show ECLS data using die same protocol used for generating die data of Table 2 (except for using sequence ECLS).
As shown in Table 3, the ECLS intensity of ruthemum tris(l,10-phenantiιroline)2+ was slighdy higher than that of ruthenium tris(2,2'-bipyridine)2+. In contrast, Table 4 shows that the ECLS intensity of ruthenium tris(l,10-phenanthroline)2+ was slighdy lower than that of ruthenium tris (2,2'-bipyridine)2+.
Table 3
Tube Dark Led Ep ECL
1 20500 957928 1802 40252
2 20412 983069 1802 953453
3 20359 987688 1802 952057
4 20445 996083 1802 944837
5 20596 1002844 1794 954758
6 20471 1002312 1802 955088
7 20888 1005647 1802 958307
8 20873 1006870 1802 958017
9 20857 1010340 1794 987998
10 20932 1010879 1794 961183
11 21125 1010331 1802 971306
12 21003 1013335 1802 972880
13 20997 1017255 1794 969491
14 20950 1018133 1802 969798
15 21299 1017786 1802 984486
Table4
Tube Dark Led Ep ECL
1 21040 968541 1802 42969
2 20617 986033 1802 1421553
3 20543 991737 1799 1428979
4 20990 994353 1794 1456623
5 21015 1000053 1802 1421155
6 21012 998545 1794 1454112
7 21323 1003714 1802 1456290
8 21012 1004665 1794 1456601
9 21278 1007303 1800 1276535
10 21510 1004804 1802 1240961
11 21508 1007856 1797 1301729
12 21325 1012231 1802 1276588
13 21669 1009280 1794 1274486
14 21762 1010989 1794 1278640
15 21359 1013849 1802 1279043
EXAMPLE 8 Signal Discrimination With Optical Filters The present invention may be employed when different analytes are simultaneously monitored by different labels which have different emission spectra. An approach for discriminating die ECL signals in terms of wavelength without a spectrometer is to employ filters which transmit essentially only the wavelength of a particular label. Thus, transmission through filters as well as transmission without a filter was compared as follows. Two groups of labels were used in this example: one was the osmium tris(l,10- phenanthroline)2"1" label which had a wavelengtii of about 715 nanometers. The other group was ruthenium complex labels that had emission peaks of about 600 nanometers. This group includes rudienium tris(bipyridine)2+ (at 620 nanometers), ruthenium tris(l,10- phenanthroline)2+ (at 605 nanometers) and rudienium tris(tetramethyl-l,10-phenanthroline)2+ (at 585 nanometers). The light was transmitted eitiier through a 655 EFSP filter or a 702 EFLP filter made by Omega Opticals, Brattleboro, Vermont. The signals were generated both by sequence ECLD and sequence ECLS. Table 5 simimarizes die experimental results of four labels with and without filters using sequences ECLD and ECLS.
Table 5
702 EFLP Filter 655 EFSP Filter No Filter
ECLD ECLS ECLD ECLS ECLD ECLS
1000 pM a.b. 25599 32849 17717 18390 74963 79644 Ru-bpy sample 56906 261748 139205 691299 447233 2278823
1000 pM a.b. 26088 36975 14303 28183 73304 76678 Ru-phen sample 54116 231969 284071 967989 648499 2085545
5000 pM a.b. 23006 16250 77113 Ru-tmph sample 101385 203806 697583
5000 pM a.b. 25904 33248 12712 24121 72507 80685 Os-phen
sample 289740 5133130 9508 134427 489152 9060708
Ru-bpy is Ruthenium tris (2,2'-bipyridine)2+.
Ru-phen is Rudienium tris (l,10-phenanthroline)2+.
Ru-tmph is Rudienium tris(tetramethyl l,10-phenantiιroline)2+.
Os-phen is Osmium tris(l,10-phenanthroline)2+.
In Table 5, the ECL counts of the samples are the average of five mbe measurements. To obtain the particular transmittance of different labels with the two filters, the filtered sample counts were divided by die corresponding non-filtered counts. The results of this division are listed in Table 6 below:
Table 6
655 EFSP Filter 655 EFSP Filter 702 EFLP Filter 702 EFLP Filter
ECLD ECLS ECLD ECLS
Ru-bpy 0.311258 0.303358 0.12724 0.114861
Ru-phen 0.438044 0.464142 0.083448 0.111227
Ru-tmphen 0.29216 0.145338
Os-phen 0.019438 0.014836 0.592331 0.566526
In Table 6, the factor of concentrations is eliminated and several characteristics are notable. Filter 655 EFSP effectively blocks the emission from osmium tris(l,10- phenanthroline)2"1". Filter 702 EFLP does not effectively block die ruthenium labels' emission. Among the ruthenium labels, ruthemum tris(l,10-phenanthroline)2+ has the best signal discrimination over ruthenium tris(2,2'-bipyridine) and ruthenium tris(tetramethyl- l,10-phenanthroline)2+ by a factor of greater than 50%. Moreover, although the emission peak of ruthenium tris(tetramethyl-l,10-phenanthroline) is 585 nanometers, the signal discrimination with these particular two filters is insufficient to meet the desired sensitivity of measurements. The data indicates that the emission from ruthenium tris(tetramethyl-l,10- phenanthroline) possesses a large tail in a longer wavelength range. Thus, the emission passes through both filters. Also the data indicates tiiat ECLD and ECLS are essentially the same in terms of signal discrimination.
EXAMPLE 9 Signatures of Labels in ECL Wave Forms Different labels have different physical and chemical properties which impact on their ECL waveforms. Analysis of ECL waveforms is helpful for distinguishing different labels. Such an analysis only can provide qualitative information not quantitative information. Table 7 summarizes the ECL waveform characteristics of a number of labels, in solution, measured using ECLD.
Table 7
Peak Threshold Symmetry Sharpness Post-peak
Potential Potential of Peak of Peak ECL
(mV) (mV)
Ru-bpy 1221 930 Yes Sharp Yes
Ru-phen 1238 940 Yes Sharp No
Ru-tmphen 1550 1060 No Broad No
Os-phen 1115 770 Yes Sharp Yes
The ECL peak of ruthenium tris(l,10-phenanthroline)2+ looks like a purely diffusion controlled peak. The peak of ruthenium tris(tetramethyl l,10-phenanthroline)2+ was broader due to slower electron transfer kinetics. ECL of ruthenium tris(2,2'-bipyridine)2+ and osmium tris(l , 10-phenanthroline) had a post-peak ECL behavior, which suggests that some intermediates other than TPA+ were generated at more positive potentials and they again catalyzed ECL reaction.
Altiiough embodiments of the present invention have been described in detail herein witii reference to the accompanying drawings, it will be apparent that the invention is not limited tiiereto, and tiiat various changes and modifications may be effected therein by one skilled in the art without departing from the spirit and scope of die invention as defined in the appended claims.