WO2001072317A1 - Formulation comprising thymol useful in the treatment of drug resistant bacterial infections - Google Patents
Formulation comprising thymol useful in the treatment of drug resistant bacterial infections Download PDFInfo
- Publication number
- WO2001072317A1 WO2001072317A1 PCT/IN2000/000033 IN0000033W WO0172317A1 WO 2001072317 A1 WO2001072317 A1 WO 2001072317A1 IN 0000033 W IN0000033 W IN 0000033W WO 0172317 A1 WO0172317 A1 WO 0172317A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- range
- formulation
- thymol
- present
- resistant
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/23—Apiaceae or Umbelliferae (Carrot family), e.g. dill, chervil, coriander or cumin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/53—Lamiaceae or Labiatae (Mint family), e.g. thyme, rosemary or lavender
- A61K36/534—Mentha (mint)
Definitions
- the present invention relates to a novel synergistic composition useful in the treatment of drug resistant bacterial infections, said composition comprises an effective amount of thymol, a mixture of the essential oils of Mentha arvensis and Mentha spicata or their monoterpene components m appropnate ratio and conventional additives
- the said composition is useful in the treatment of drug resistant enteric and systemic infections
- the formulation with enhanced activity of thymol action comp ⁇ ses thymol in combmation with the said oil which is a combination containing the rare mixture of carvone, hmonene and menthol
- the invention also provides methods of producing the said composition and a method of using thymol obtained from the seeds of the plant Trychyspermum ammi (Ajwam) as a fourth generation antibiotic formulation for control of drug resistant bacte ⁇ a More particularly, the invention relates to the use of a compound 'Thymol' isolated from the oil distilled from the seeds of the plant Trychyspermum ammi (A
- Microbial infections are a major cause of human health hazards and misery leading to sizeable number of human deaths globally
- the infections drastically affect human efficiency by incapacitating various metabolic functions and systems like digestive, respiratory, u ⁇ nary, circulatory, nervous systems and skm This then leads to continuous human - suffering till the patient is completel cured of the causative microbes
- Bacterial infections present a se ⁇ ous threat to the health and w ell being of people of all ages
- Antibiotics and antimicrobial dmgs ever since the discovery of Penicillin by Alexander Fleming m the 1940s have been used by the medical practitioners to eliminate infective agents and curing the diseases
- the infective microbes have always been able to fight back ev ery new drug through development of resistance against the dnig/antibiotic m use Emergence of multiple drug resistant strains has appeared as a real problem in the field of medical science
- the primary cause of the development of resistance is occurrence of random mutations Mutations may occur in genes responsible for conferring
- nalidixic acid found limited utility in treating systemic infections and subsequently marginally improved qumolones like oxolimc acid, pipemidic acid and cinoxacin were released m 1970 " s Then came a breakthrough in early 1980 ' s with the beginning of evolution of fluo ⁇ nated qumolones First to come up was norfloxacin.
- a second-generation quinolone having 6-fluo ⁇ ne and " -piperazme substituents developed by Wolfson and Hooper It had enhanced activity against both gram-negative and gram-positive bacteria like Pseudomonas aeruginosa and Staphylococcus aiireus.
- thymol in various herbal preparations ranging from mouthwashes and enteric disorders to skin infections is common.
- the parent oil and thymol itself as component of grandma ' s household recipes to treat a range of common ailments has found resident place because of being equally effective for children and adults.
- Patent 4,702.916. October, ⁇ '7 . Grohe 1989, US Patent 4,844.902.
- the applicants have taken the classes of drag res ⁇ stance(s) in bacteria as the approach to classify them into categories of endangerment to human health, which is over and above the traditional taxonomic classes
- the invention deals specifically with the drag resistant bacteria arising due to mutational events followed by selection due to continued presence of the said antibiotics
- the applicants have used Escherichia coli and Mycobacterium as the model systems to monitor the evolution of resistance to quinolone and flouroquinolone drags, mdr strain emergence and found out the novel use of the plant molecule (thymol) to kill the mdr strains and advanced generation drug resistant bacteria developed in continued presence of these drags This way this is a unique finding with great utility m the field of medicine
- the mam object of the invention is to provide a novel formulation useful in the treatment of drag resistant bacterial infections
- Another object of the invention is to provide a novel formulation comprising an effective amount of Thymol derived from the plant Tn chx spermum ammi (Ajwam) and essential oil or monoterpene combination derived from Mentha spicata and Mentha arvensis useful m the treatment of bacterial infections
- Another object of the invention is to provide an anti-bacterial agent comprising Thymol, useful in the treatment of bacterial infections
- Still another object is to provide method of using Thymol for control of drag resistant bacteria
- the present invention provides a novel formulation comprising an effective amount of Thymol obtained from the plant Trychyspermum ammi (Ajwam), appropriate mint oil combination obtained from Mentha spicata and Mentha arvensis. and conventional additives
- the mvention also provides methods for the preparation of the said novel formulation useful in the treatment of drag resistant bacterial infections Further, the mvention provides an anti-bacte ⁇ al agent comprising an effective amount of Thymol, useful in controlling drag resistant bacteria
- the invention also provides a method of using Thymol for control of drag resistant bacteria
- the invention provides a novel synergistic formulation useful m the treatment of drag resistant bacterial infections, said formulation comprising an effective amount ' Thymol ' obtained from the plant Tiychy spermum ammi, mint oil combination containing approp ⁇ ate amounts of specific monoterpene obtained from Mentha spicata and Mentha arvensis and conventional additives
- Thymol is present m the range of about 100 to 500 mg or
- mint oil combination is presented an amount of about 0 1 to 0 5 mg.
- the additives are selected from the group comprising Citric acid present m the range of about 2-10 mg.
- Citric acid present in the range of about 2-10 mg.
- Calcium carbonate present in the range of about 100 -200 mg
- Magnesium hydroxide gel present in the range of about 50-100 mg
- Lactose present in the range of about 200-600 mg
- honey in the range of about 0.1 to 1%
- sodium glutamate present in the range of about 200mg and sodium buffer.
- mint oil is diluted upto 10 trmes if desired.
- the mint oil comprises Limonene tn the range of about 6 to 25%, Menthol in the range of about 0 50 to 2.50% Carvone m the range of about 64 0 to 76%
- Yet another embodiment comprises dilution of honey upto 10 times.
- the formulation is effective against the group of bacteria selected from the genus Mycobacterinm or Escherchia.
- the formulation is effective against bacte ⁇ a resistant to arugs selected from the group comprising Ethidium bromide. Isoniazid. Chloremphemcol.
- Tetracvc ne Rifampicin. Xalidixic acid. Oxolimc acid, Spartloxicin. Ciprofloxicin and Loamtloxicin 1 )
- the synergistic formulation ox the invention exhibited surprising and unexpected anti-bacterial prope ⁇ ies
- the individual ingredients of the formulation do not have the prope ⁇ y of the composition useful in the treatment of dmg resistant bacte ⁇ al infections
- the present formulation comprising an effective amount -"Thymol " obtained from the plant Trychyspermum ammi. mint oil obtained from a hyb ⁇ d o ⁇ Mentha spicata and Mentha arvensis and conventional additives exhibited surprising and unexpected anti-bacterial properties
- the formulation of the present invention may be derived in various physical forms such as tablets, syrup, powders, injections, etc as are known in the an
- the essential ingredients of the formulation namely the compound Thymol and the essential oil combination having the desired monoterpene combination are mixed with conventional additives such as honey, sodium glutamate.
- the amounts of the respective ingredients of the formulation herein mentioned are only exemplary and appropriate amount of the respectiv e ingredients will vary and may be readily determined by a person skilled in the art
- the ratio o the amounts in the formulation of the present mv ention are not critical and vary widely
- the best results would of course be obtained employing Thymol and specific mint oil combination in the proportion aforementioned
- Optimal amounts of the ingredients of the formulation will vary with the method of administration of the formulation
- the invention also provides a method for the preparation of a formulation useful in the treatment of d ⁇ ig resistant bacterial infections, said method comprising the step of mixing an effective amount of Thymol oil obtained from the plant Tnchyspermiim ammi, combination of mint oils obtained from vtentha spicata and Mentha arvensis with conventional additives
- Thymol is present in the range of about 100 to 500 mg or 20 to 50% w/w
- mint oil is presented an amount of about 0 1 to 0 5 g
- the additives are selected from the group comprising Citric acid present in the range of about 2-10 mg, Calcium carbonate present m the range of about 100 -200 mg.
- Magnesium hydroxide gel present in the range of about 50-100 mg, Lactose present in the range of about 200-600 mg, honey m the range of about 0 1 to 1%.
- sodium glutamate present in the range of about 200mg and sodium buffer
- mint oil is diluted upto 10 times if desired
- the mint oil comprises Limonene m the range of about 6 8 to 23 2%.
- Menthol in the range of about 0 66 to 2 50% Carvone m the range of about 64 0 to 76 1%
- honey is optionally diluted upto 10 times
- the Thymol oil and the mint oil are dispersed m 0 1 to 1% honey to obtain a syrup
- Thymol oil and the mint oil are mixed with Citric acid 2 to lOmg and dissolved m a buffer containing sodium glutamate to obtain an injection
- the formulation is effectiv e against the group of bacteria selected from the genus Mycobactenum or scherchia
- the formulation is effective against bacteria resistant to drags selected from the group comprising Ethidium bromide.
- the invention also provides a method for the treatment of drag resistant bacterial infections in humans comprising the steps of administration of a therapeutically effective amount of the novel formulation to a subject in need thereof In an embodiment. the formulation is administered through oral or subcutaneous routes
- the formulation is dissolved 5 ml 0 05 M Sodium buffer (pH 7 0) containing 200mg sodium glutamate to be applied as subcutaneous injection
- the formulation is effective against bacterial infections such as enteric and systemic infections
- the treatment comprises administration of the formulation for bacterial infections caused by bacteria resistant to drags selected from the group comprising Ethidium bromide. Isomazid. Chloremphemcol. Tetracyclme. Rifampicm. Nahdixic acid. Oxolmic acid. Sparfloxicm. Ciprofloxicm and Loamfloxicin
- the formulation is used for treatment of infections caused by multi-drug resistant bacte ⁇ a selected from the genus Mxtobacienum or
- the formulation is used to kill bacte ⁇ a resistant to the group of drugs comprising Ethidium bromide, Isomazid.
- the present invention is the consequence of planned experimentation through specific activity bioevaluation assays
- the mtent of the investigation has been to ascertain and evaluate the potential of plant compound thymol from the oil of ' Ajwam " as the advanced generation antibiotic and development of a herbal antibiotic formulation with enhanced activity particularly the activity of killing drag resistant bacte ⁇ a
- the expe ⁇ ments progressed in finding this novel use following the first observation by us that the strains of Escherichia coli that had become resistant to nahdixic acid fa broad spectram quinolone drag) due to mutations m the gyr ⁇ gene rather more sensitive to the Trychyspermum ammi oil and its major component 'thymol'
- Example 1 Antibacterial activity of the Tnchyspermiim ammi oil. thymol and non thvmol fraction of the oil was determined m terms of growth inhibition zones produced on the bacterial lawn of drug resistant strains using disc diffusion assays (Table 1 ).
- CA 8000 is the wild type strain of E. coli while DH5 ⁇ and ET 8000 harbor gyr mutations i.e. the gene encoding for the Gyr A subunit of DNA gyrase enzyme responsible for DNA relaxation is modified. These mutants are resistant to the quinolone because of the altered DNA gyrase.
- the specific mutations were confirmed through genetic complementation by transferring the plasmid cloned gyr A. As indicated in the table the killing zone of thymol is more in case of gyr mutant strains which are resistant to the above mentioned drags. The activity was not observed in the non-thymol fraction of the oil.
- the nahdixic acid resistant strains isolated were grown in presence of different antibiotics as mentioned in the above table These mutants showed positive growth in presence of high concentration of nahdixic acid Some of them were also resistant to oxolmic acid and all to sparfloxacm but none of them were resistant to second generation drag ciprofloxacm and third generation drag lomefloxacm.
- the control wild type strain CA8000 as expected was sensitive to these antibiotics.
- Table 3 Disk diffusion assay for determining the activity of thymol against nalidixic acid resistant mutants of Escherichia coli.
- Tr actn irv in traces ( ⁇ 0 5 mm).
- WT Wild Type
- the compound thymol was able to kill all the nalidixic acid resistant mutants of E. coli with greater efficiency than the wild type strain CA8000
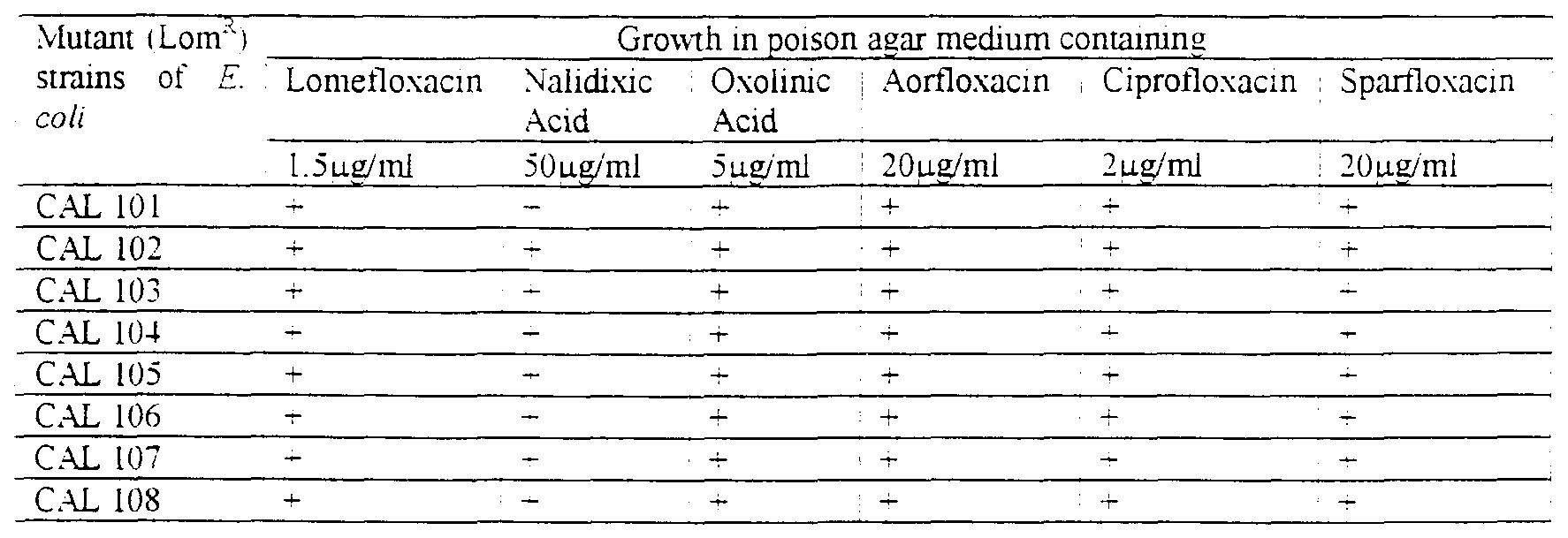
- Induced mutants were isolated after selection in the presence of lomefloxacin in the growth medium. Growth of lomefloxacin resistant strains of Escherichia coli was studied in presence of different antibiotics by poison agar method to determine the cross-resistance. The lomefloxacm (third generation ) resistant strains as expected were also resistant to first generation and second generation antibiotics (Table 4).
- the mutants resistant to lomefloxacin were tested against thymol for sesnsitivity through the disc diffusion assays. As obvious from the Table 5. thymol was effective in killing all the Lom R mutant cells of Escherichia coli indicating its direct use as the advanced generation drag against drug resistant bacteria.
- Table 5 Disk diffusion assay for determining the activity of thymol against lomefloxacin resistant mutants of Escherichia coli.
- Tr activity in traces ( ⁇ 0 5 mm).
- WT Wild Type
- Tr activ in traces ( ⁇ 0 5 mm)
- WT Wild Tvpe
- Table 7 Disk diffusion assay for determining the activity of thymol against lomefloxacin resistant mutants of Mycobacterium smegmatis.
- Example 8 Activity of thymol against bacterial mutants resistant to another frontline anti-tubercular drug isoniazid.
- Isoniazid is another drag widely used for controlling tuberculosis as the bacteria is sensitive to this drug due to absence of oxyR system.
- Wild type E. coli strain is resistant to high concentration of antibiotic (1000 ⁇ g /ml). This strain was mutagenised by N'N-methyl Nitrosoguanidine to isolate the sensitive strain which is being killed at a concentration of 250 ⁇ g /ml in broth (CA03). Further, a spontaneous mutation was detected from the sensitive mutant culture, which was resistant to 1000 ⁇ g /ml isoniazid. These cultures were tested for thymol sensitivity at a concentration of 100 ⁇ g / disc through disc difftision assay and observed that the isomazid resistant revertant strain (CAC ⁇ revert) was more sensitive.
- CAC ⁇ revert isomazid resistant revertant strain
- a mutant of Mycobacterium smegmetis was isolated by successive enrichment of the normally sensitive wild type strains upto 12 ⁇ g ethidium bromide /ml in broth. This mutant grow well at this concentration of ethidium bromide, while the wild type is killed at 3 ⁇ g ethidium bromide /ml in broth. Further, this ethidium bromide resistant strain was found to be multiply drug resistant (mdr) against antibiotics like Chloromphenicol (20 ⁇ g / ml), Tetracycline (10 ⁇ g / ml) and Rifampicin (40 ⁇ g / ml).
- Example 10 Activity of the compound of the present invention thymol at different pH in Escherchia coli strain CA8000.
- Example 11 Preparation of synergistic antibacterial composition of thymol against multidrug resistant bacteria.
- thymol is a potent bactericide agent against multidrag resistant bacteria . Further it was observed that the potency is increased by combining the oils from mints Mentha arvensis and Mentha spicata. The oil combination had Limonene ( 6.8 - 23.2%), Menthol(0.66 - 2.45%), Carvone(64.0 - 76.1%) and unidentified fractions in the essential oils totalling to 100% at different stages of growth. When this oil combination is used at a concentration of 0.1 % of thymol the antibacterial activity of thymol is increased by 45%. Beside this the oil adds the pleasant carvone flavor to the composition with a menthol tinge. Considering this we prepared different compositions as follows.
- Table 1 1 Disk diffusion assay for determining the synergistic effect of thymol with nalidixic acid against E. coli cells
- Table 12 Disk diffusion assay for determining the synergistic effect of thvmol with Mentha hvbrid oil against E. coli cells
- the ingredients are mixed properly, powdered and packed m gelatin capsule available commercially in the market
- Composition 2 The essential oil is diluted 0 to 10 times
- Honey is diluted 0 to 10 times in sterile distilled water and following ingredients added
- composition 4 Thymol 100 -500mg 2 Citric acid 2-10 mg
Abstract
Description
Claims
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/IN2000/000033 WO2001072317A1 (en) | 2000-03-28 | 2000-03-28 | Formulation comprising thymol useful in the treatment of drug resistant bacterial infections |
| BR0017198-0A BR0017198A (en) | 2000-03-28 | 2000-03-28 | Formulation comprising thymol useful in treating drug resistant bacterial infections |
| CNB008194823A CN100339069C (en) | 2000-03-28 | 2000-03-28 | Formulation comprising thymol useful in treatment of drug resistant bacterial infections |
| AU2000255628A AU2000255628A1 (en) | 2000-03-28 | 2000-03-28 | Formulation comprising thymol useful in the treatment of drug resistant bacterial infections |
| US09/536,124 US6514541B2 (en) | 2000-03-28 | 2000-03-28 | Formulation comprising thymol useful in the treatment of drug resistant bacterial infections |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/IN2000/000033 WO2001072317A1 (en) | 2000-03-28 | 2000-03-28 | Formulation comprising thymol useful in the treatment of drug resistant bacterial infections |
| US09/536,124 US6514541B2 (en) | 2000-03-28 | 2000-03-28 | Formulation comprising thymol useful in the treatment of drug resistant bacterial infections |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2001072317A1 true WO2001072317A1 (en) | 2001-10-04 |
Family
ID=26324096
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IN2000/000033 WO2001072317A1 (en) | 2000-03-28 | 2000-03-28 | Formulation comprising thymol useful in the treatment of drug resistant bacterial infections |
Country Status (3)
| Country | Link |
|---|---|
| US (1) | US6514541B2 (en) |
| AU (1) | AU2000255628A1 (en) |
| WO (1) | WO2001072317A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003083028A2 (en) * | 2002-03-28 | 2003-10-09 | Council Of Scientific And Industrial Research | Cleaning and desinfecting herbal compositions and their preparation |
| FR2925334A1 (en) * | 2007-12-21 | 2009-06-26 | Ceva Sante Animale Sa | NEW ANTIBACTERIAL COMPOSITIONS |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2231275T3 (en) | 1999-10-08 | 2005-05-16 | Affinium Pharmaceuticals, Inc. | FAB INHIBITORS I. |
| ATE420640T1 (en) * | 2001-04-06 | 2009-01-15 | Affinium Pharm Inc | FAB I INHIBITORS |
| ES2518316T3 (en) * | 2002-12-06 | 2014-11-05 | Debiopharm International Sa | Heterocyclic compounds, their manufacturing methods and their use in therapy |
| EP1608377B1 (en) | 2003-03-17 | 2008-10-01 | Affinium Pharmaceuticals, Inc. | Pharmaceutical compositions comprising inhibitors of fab i and further antibiotics |
| US20050013882A1 (en) * | 2003-04-16 | 2005-01-20 | Jacques Owen | Phyto fluid |
| JP5087406B2 (en) | 2004-06-04 | 2012-12-05 | アフィニウム ファーマシューティカルズ, インク. | Therapeutic agents and methods for making and using the same |
| US7517541B2 (en) | 2005-01-18 | 2009-04-14 | A.M. Todd Company | Oral care compositions derived from the Labiatae family |
| KR20080075027A (en) * | 2005-12-05 | 2008-08-13 | 아피늄 파마슈티컬스, 인크. | Heterocyclylacrylamide compounds as fabi inhibitors and antibacterial agents |
| WO2008009122A1 (en) | 2006-07-20 | 2008-01-24 | Affinium Pharmaceuticals, Inc. | Acrylamide derivatives as fab i inhibitors |
| EP3255045A1 (en) * | 2007-02-16 | 2017-12-13 | Debiopharm International SA | Salts, prodrugs and polymorphs of fab i inhibitors |
| WO2009049900A1 (en) * | 2007-10-18 | 2009-04-23 | Dsm Ip Assets B.V. | Novel nutraceutical compositions containing thymol and/or p-cymene or plant extracts for cognition |
| WO2010009572A1 (en) * | 2008-07-23 | 2010-01-28 | 国防教育研究基金会 | New low side effect pharmaceutical composition containing isoniazid |
| EP2861608B8 (en) | 2012-06-19 | 2019-06-19 | Debiopharm International SA | Prodrug derivatives of (e)-n-methyl-n-((3-methylbenzofuran-2-yl)methyl)-3-(7-oxo-5,6,7,8-tetrahydro-1,8-naphthyridin-3-yl)acrylamide |
| EA037121B1 (en) | 2016-02-26 | 2021-02-09 | Дебиофарм Интернэшнл C.A. | Medicament for treatment of diabetic foot infections |
| EP3793511A1 (en) | 2018-05-18 | 2021-03-24 | Kancor Ingredients Ltd | Anti-dandruff composition |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS62252718A (en) * | 1986-04-24 | 1987-11-04 | Nitto Electric Ind Co Ltd | Deodorized chemical |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2035267A (en) | 1934-02-10 | 1936-03-24 | Louis Schumacher | Effervescent sodium perborate |
| US2094671A (en) | 1936-04-04 | 1937-10-05 | Poetschke Paul | Composition of matter suitable for a dentifrice |
| US3518343A (en) | 1967-10-02 | 1970-06-30 | Miles Lab | Effervescent tablet and process for making same |
| US3821117A (en) | 1971-07-23 | 1974-06-28 | Carter Wallace | Effervescent tablet |
| US3936385A (en) | 1973-08-09 | 1976-02-03 | Colgate-Palmolive Company | Denture cleanser |
| US4307109A (en) | 1980-05-08 | 1981-12-22 | Abbott Laboratories | Biocidal chelate |
| US4383986A (en) | 1981-08-17 | 1983-05-17 | Ortho Pharmaceutical Corporation | Hemorrhoidal compositions |
| US4863725A (en) | 1982-10-27 | 1989-09-05 | Deckner George E | Novel clear oil-free moisturizer composition |
| CH643138A5 (en) | 1983-08-29 | 1984-05-30 | Mepha Ag | INDOMETHACIN CONTAINING, gelatinous OINTMENT. |
| US4702916A (en) | 1985-12-03 | 1987-10-27 | Warner-Lambert Company | Analgesic stick compositions |
| DE3704907A1 (en) | 1987-02-17 | 1988-08-25 | Bayer Ag | TOPICALLY APPLICABLE PREPARATIONS OF GYRASE INHIBITORS IN COMBINATION WITH CORTICOSTEROIDS |
| US4847071A (en) | 1987-10-22 | 1989-07-11 | The Procter & Gamble Company | Photoprotection compositions comprising tocopherol sorbate and an anti-inflammatory agent |
| US4925655A (en) | 1988-03-04 | 1990-05-15 | Robell Research | Powder composition for forming a mouthwash |
| US5073366A (en) | 1989-05-30 | 1991-12-17 | Fred Beck | Analgesic composition useful in providing a temporary relief from symptoms of arthritis |
| US5801153A (en) | 1991-09-13 | 1998-09-01 | Badaway; Mohammed A. | Method of enhancing the antimicrobial properties of antibacterial antibiotics to massively control and prevent bacterial, fungal, and viral diseases in plants |
| US5411733A (en) | 1992-04-27 | 1995-05-02 | Hozumi; Toyoharu | Antiviral agent containing crude drug |
| US5529778A (en) | 1994-09-13 | 1996-06-25 | Rohatgi; Surendra | Ayurvedic composition for the prophylaxis and treatment of AIDS, flu, TB and other immuno-deficiencies and the process for preparing the same |
| US5980903A (en) | 1997-08-27 | 1999-11-09 | Pruthi; Som C. | Composition for the treatment of viral infections including HIV |
| USPP12030P2 (en) * | 1998-06-03 | 2001-08-07 | Council Of Scientific And Industrial Research | Hybrid mint plant named ‘Neerkalka’ |
| US6127405A (en) * | 1998-07-10 | 2000-10-03 | Council Of Scientific And Industrial Research | Method for the use of alpha arteether as an anti-bacterial and anti-fungal agent |
-
2000
- 2000-03-28 WO PCT/IN2000/000033 patent/WO2001072317A1/en active Application Filing
- 2000-03-28 AU AU2000255628A patent/AU2000255628A1/en not_active Abandoned
- 2000-03-28 US US09/536,124 patent/US6514541B2/en not_active Expired - Fee Related
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS62252718A (en) * | 1986-04-24 | 1987-11-04 | Nitto Electric Ind Co Ltd | Deodorized chemical |
Non-Patent Citations (3)
| Title |
|---|
| DATABASE BIOSIS [online] BIOSCIENCES INFORMATION SERVICE, PHILADELPHIA, PA, US; June 1998 (1998-06-01), SINGH SANJAY ET AL: "Effects of active molluscicidal agents of common spices on biochemical parameters in the ovotestis of Lymnaea acuminata.", XP002154364, Database accession no. PREV199900340736 * |
| DATABASE WPI Section Ch Week 198750, Derwent World Patents Index; Class A96, AN 1987-351000, XP002154365 * |
| MALAYSIAN APPLIED BIOLOGY, vol. 27, no. 1-2, June 1998 (1998-06-01), pages 45 - 49, ISSN: 0126-8643 * |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003083028A2 (en) * | 2002-03-28 | 2003-10-09 | Council Of Scientific And Industrial Research | Cleaning and desinfecting herbal compositions and their preparation |
| WO2003083028A3 (en) * | 2002-03-28 | 2003-12-18 | Council Scient Ind Res | Cleaning and desinfecting herbal compositions and their preparation |
| FR2925334A1 (en) * | 2007-12-21 | 2009-06-26 | Ceva Sante Animale Sa | NEW ANTIBACTERIAL COMPOSITIONS |
| WO2009083521A2 (en) * | 2007-12-21 | 2009-07-09 | Ceva Sante Animale | Novel antibacterial compositions |
| WO2009083521A3 (en) * | 2007-12-21 | 2010-02-18 | Ceva Sante Animale | Antibacterial compositions comprising an antiseptic in combination with an antibiotic |
Also Published As
| Publication number | Publication date |
|---|---|
| US6514541B2 (en) | 2003-02-04 |
| AU2000255628A1 (en) | 2001-10-08 |
| US20020090380A1 (en) | 2002-07-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2001072317A1 (en) | Formulation comprising thymol useful in the treatment of drug resistant bacterial infections | |
| de Boer et al. | Anti-fungal and anti-bacterial activity of some herbal remedies from Tanzania | |
| World Health Organization | Health aspects of chemical and biological weapons: report of a WHO group of consultants | |
| Mellanby | Transmission of scabies | |
| KR100187992B1 (en) | Pharmaceutical compositions active in the therapy of neurological affections in aids patients, containing as an active principle at last one compound selected from the group consisting of s-adenosyl-l-methionine salt, 5-methyl-tetrahydrofolic acid, 5-formylterahydrofolic acid | |
| Dzoyem et al. | Antimycobacterial activity against different pathogens and selectivity index of fourteen medicinal plants used in southern Africa to treat tuberculosis and respiratory ailments | |
| Diop et al. | Survey on medicinal plants traditionally used in Senegal for the treatment of tuberculosis (TB) and assessment of their antimycobacterial activity | |
| Chhetri et al. | Formulation and evaluation of antimicrobial herbal ointment | |
| Nelson | Immunotherapy for house-dust mite allergy. | |
| Keusch | Antimicrobial therapy for enteric infections and typhoid fever | |
| Penduka et al. | Evaluation of the anti-Listeria potentials of some plant-derived triterpenes | |
| Prabhakar et al. | Effect of curry leaves, garlic and tea tree oil on Streptococcus mutans and Lactobacilli in children: A clinical and microbiological study | |
| Lenoir et al. | A double-blind randomised trial to investigate three different concentrations of a standardised fresh plant extract obtained from the shoot tips of Hypericum perforatum L. | |
| Mukanganyama et al. | Screening for anti-infective properties of selected medicinal plants from Botswana | |
| US6824795B2 (en) | Formulation comprising thymol useful in the treatment of drug resistant bacterial infections | |
| RU2245719C2 (en) | Thymol-containing composition useful in treatment of patients suffering from drug-resistant bacterial infections | |
| GB2275194A (en) | Plant Extract Disinfectant | |
| AU760967B2 (en) | Herbal composition for the prophylaxis and treatment of AIDS | |
| ZA200207674B (en) | Formulation comprising thymol useful in the treatment of drug resistant bacterial infections. | |
| JP4566562B2 (en) | Antibacterial plant composition | |
| Kumari et al. | EVALUATION OF ANTIMICROBIAL ACTIVITY OF AQUEOUS AND ALCOHOLIC EXTRACT OF BILWADI AGAD AGAINST BACTERIAL STRAINS. | |
| Biswas et al. | In Vitro Assessment of Antisecretory Activity of Root Extracts of Acacia arabica Lam. Willd against Biodiversity of E. coli Isolated from Bhilai-Durg Region | |
| Singh | Bioactive compounds from South African plants against Mycobacterium tuberculosis | |
| Mistry et al. | New drugs for tuberculosis | |
| Vivian et al. | Antibacterial Activity of Different Toothpastes and Chewing Sticks on Selected Bacteria Isolated from the Oral Cavity |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 1200200890 Country of ref document: VN |
|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AL AM AT AU AZ BA BB BG BR BY CA CH CN CR CU CZ DE DK DM EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT TZ UA UG US UZ VN YU ZA ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW SD SL SZ TZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2002/07674 Country of ref document: ZA Ref document number: 200207674 Country of ref document: ZA |
|
| ENP | Entry into the national phase |
Ref document number: 2002 2002128741 Country of ref document: RU Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 008194823 Country of ref document: CN |
|
| 122 | Ep: pct application non-entry in european phase | ||
| NENP | Non-entry into the national phase |
Ref country code: JP |