HYALURONIC ACID DERIVATIVES AND PROCESSES FOR PREPARING
THE SAME
FIELD OF THE INVENTION
The present invention relates to hyaluronic acid derivatives in which hyaluronic acid is crosslinked to glycol polymer by amide bonds and processes for preparing the same, and more particularly, to hyaluronic acid derivatives in which a hyaluronic acid is crosslinked by an amide bond directly, or via chitosan, to a glycol polymer in which a free amine group has been introduced to one or both terminals thereof, and processes for preparation of the same. The hyaluronic acid derivatives according to the present invention can be used for various applications such as biomaterials for post-operative adhesion-prevention gel, dermal augmentation, correction of facial wrinkles, osteoarthritic viscosupplement, plastic surgery, drug delivery, etc.
BACKGROUND OF THE INVENTION
Hyaluronic acid (sometimes referred to as "HA" in the present specification and drawings) is, as represented by the below Formula 1, a linear biocompatible polymer comprising linked repeating units of N-acetyl-D-glucosamine and D-glucuronic acid, which is present in high concentrations in the vitreous body of the eye, the synovial fluid of joints, rooster comb, etc. Hyaluronic acid and its derivatives are disclosed in Korean Patent No. 375.299, entitled "Cross-linking Type Amide Derivatives Of Hyaluronic Acid And Process For Preparation Of Them," which is incorporated herein as background material.
wherein, n is 1 or an integer of more than 1.
Hyaluronic acid derivatives have been widely developed to be used as post-operative adhesion-preventing films or gels, materials for wrinkle treatment, materials for plastic surgery, materials for arthritis treatment, vehicles for drug delivery systems, etc. Especially, increasing attention has been focused on hyaluronic acid derivative gels, due to peculiar rheological properties thereof, in many application fields. For example, U.S. Patent. No. 5,356,883 discloses hyaluronic acid derivative gels in which the carboxyl group of hyaluronic acid, or a salt thereof, has been modified to O-acyl or N-acyl ureas by using various kinds of carbodiimides. U.S. Patent No. 5,827,937 discloses a cross-linked polysaccharide gel obtained by a cross-linking reaction consisting of two steps. Further, U.S. Patent No. 5,399,351 discloses methods for preparing gels having various properties.
However, hyaluronic acid derivatives disclosed in these patents fail to achieve a high viscoelasticity.
SUMMARY OF THE INVENTION
Accordingly, the object of the present invention is to provide novel biocompatible hyaluronic acid derivatives capable of overcoming the problems in the prior art.
More particularly, an object of the present invention is to provide hyaluronic acid derivatives having a very high viscoelasticity and a chemical structure entirely different from that of already known hyaluronic acid derivatives.
Another object of the present invention is to provide processes of efficiently preparing
these hyaluronic acid derivatives by simple methods.
A further object of the present invention is to provide use of these hyaluronic acid derivatives as biocompatible materials.
Hyaluronic acid derivatives according to the present invention have a chemical structure in which a hyaluronic acid is crosslinked to a glycol polymer by amide bonds.
Hyaluronic acids that can be used in the present invention include hyaluronic acid itself and its salts. Accordingly, the term "hyaluronic acid" as used in the present invention means hyaluronic acid, its salts, and a mixture of hyaluronic acid and its salts. These salts of hyaluronic acid include, for example, such inorganic salts as sodium hyaluronate, potassium hyaluronate, calcium hyaluronate, magnesium hyaluronate, zinc hyaluronate and cobalt hyaluronate, and such organic salts as tetrabutylammonium hyaluronate, but are not limited thereto. In an embodiment of the present invention, two or more of the above salts may be used. The moleculars weight of hyaluronic acid and its salts are not particularly limited and are preferably in the range of 100,000 to 10,000,0000.
Glycol polymers that can be used in the present invention are polymers having hydroxyl groups (-OH) at both terminals thereof and include, for example, polyethylene glycol, and a block copolymer of polyethylene and polypropylene (so called, "Pluronic"). Polyethylene glycol and Pluronic are not toxic and have good solubility in aqueous solution, thus contributing to the stability of hyaluronic acid derivatives according to the present invention.
In hyaluronic acid derivatives according to the present invention, the hyaluronic acid is crosslinked to a glycol polymer by amide bonds. Hyaluronic acid, as seen in Formula 1, has carboxyl groups in its molecular chain and glycol polymer has hydroxyl groups at its both terminals, thus an amide bond cannot be formed between the groups. However, as will be described later in the present specification, in accordance with the present invention, free amine groups are introduced to one or both terminals of a glycol polymer by activating these terminals, and then the free amine groups react with the carboxyl groups of hyaluronic acid to form amide
bonds.
Hyaluronic acid derivatives according to the present invention can be used for various applications such biomaterials for post-operative adhesion-prevention gel, dermal augmentation, correction of facial wrinkles, osteoarthritic viscosupplement, plastic surgery, drug delivery, etc. and can also be made in various forms such as gel, film, thread, etc. according to their uses. Especially, since hyaluronic acid derivatives according to the present invention have an excellent viscoelasticity, they are very useful as materials for osteoarthritic viscosupplement. Furthermore, since hyaluronic acid derivatives according to the present invention have an amide bond (covalent bond) between hyaluronic acid and glycol polymer, they can withstand various in vivo conditions and have entirely different properties from existing hyaluronic acid derivatives.
Preferable examples of hyaluronic acid derivatives according to the present invention are derivatives represented by Formulas 2 and 3 in which hyaluronic acids are crosslinked to both terminals of a glycol polymer:
wherein m, n, x, y and z each independently are 1 or an integer of more than 1 and satisfy the condition of n>z>y>x.
wherein a, b, n, x, y and z each independently are 1 or an integer of more than 1 and satisfy the condition of n>z>y>x.
The hyaluronic acid derivative of Formula 2 has a chemical structure of "hyaluronic acid - polyethylene glycol - hyaluronic acid" including polyethylene glycol as a glycol polymer, and the hyaluronic acid derivative of Formula 3 has a chemical structure of "hyaluronic acid - (polyethylene glycol - polypropylene glycol - polyethylene glycol) - hyaluronic acid" including Pluronic as a glycol polymer. As mentioned previously, the linkage of hyaluronic acid and glycol polymer in these derivatives is accomplished by amide bonding.
Hyaluronic acid derivatives according to the present invention include a derivative in which hyaluronic acid is crosslinked to glycol polymer by amide bonding via chitosan, as well as a derivative in which hyaluronic acid is crosslinked directly to glycol polymer by amide bonding. That is, in some derivatives, chitosan is positioned between hyaluronic acid and glycol polymer. Also in these cases, chitosan crosslinked to hyaluronic acid is crosslinked to glycol
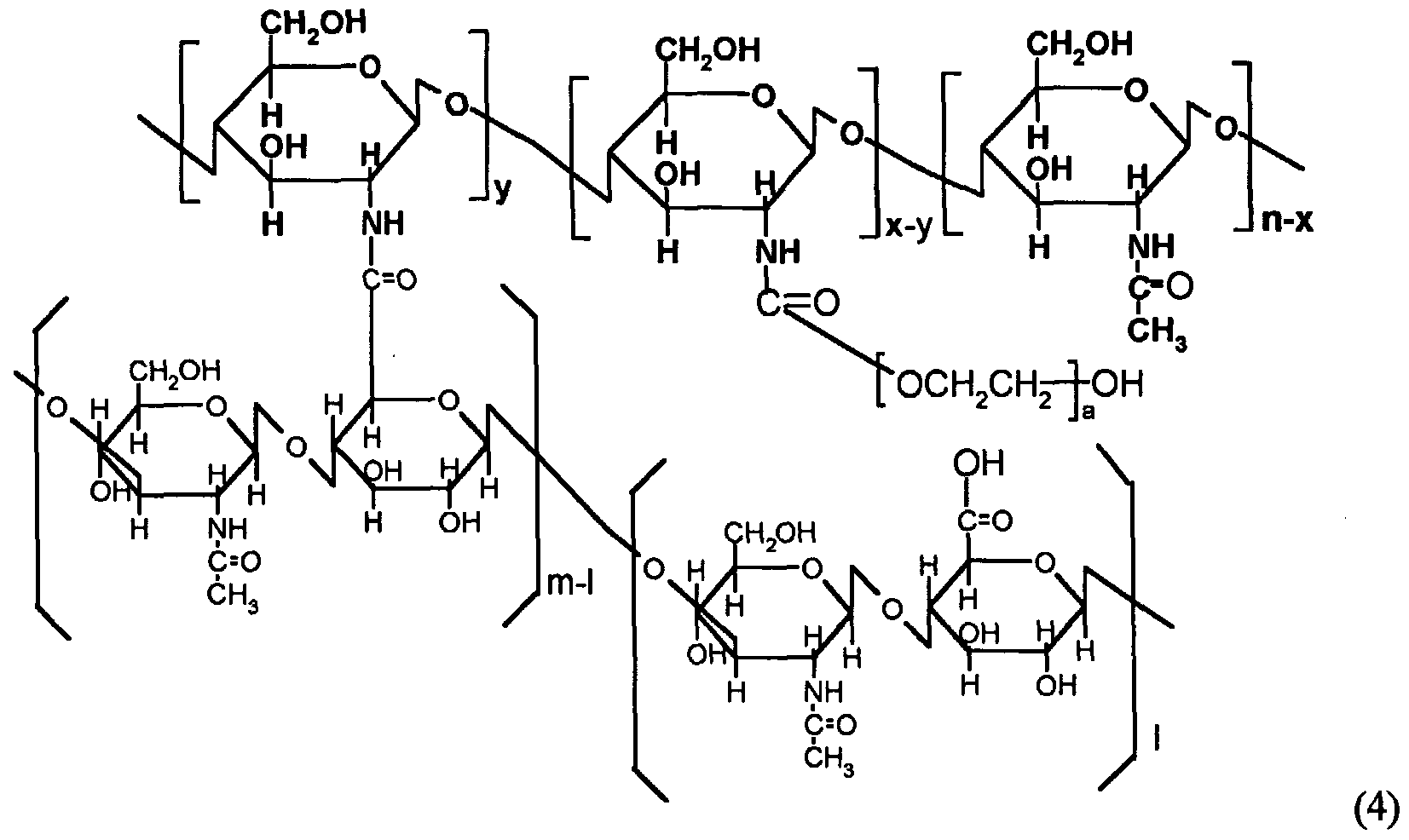
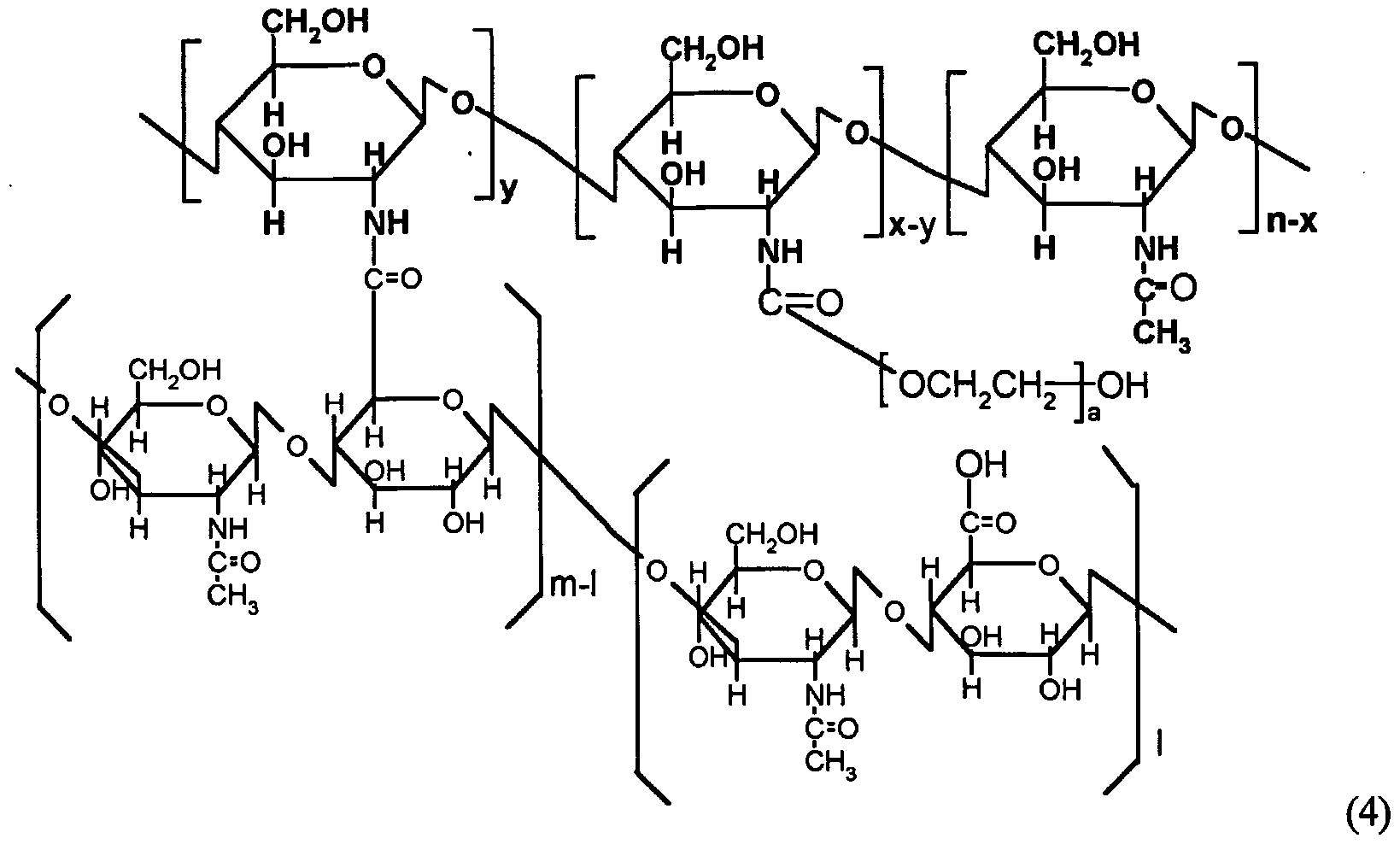
polymer by amide bonding. Preferable examples of these derivatives include hyaluronic acid derivatives represented by FORMULAS 4 and 5 as below:
wherein a, 1, m, n, x and y each independently are 1 or an integer of more than 1 and satisfy the conditions of m>l and n>x>y.
wherein a, b, 1, m, n, x and y each independently are 1 or an integer of more than 1 and satisfy
the conditions of m>l and n>x>y.
The hyaluronic acid derivative of Formula 4 has a chemical structure of "hyaluronic acid - chitosan - polyethylene glycol" including polyethylene glycol as a glycol polymer, and the hyaluronic acid derivative of Formula 5 has a chemical structure of "hyaluronic acid - chitosan - (polyethylene glycol - polypropylene glycol - polyethylene glycol)" including Pluronic as a glycol polymer. In these derivatives in which hyaluronic acid is crosslinked to glycol polymer via chitosan, the linkage of glycol polymer and chitosan is formed via a urethane bond (-NHC(=O)O-); however, the portion being ultimately crosslinked to hyaluronic acid, i.e., the crosslinkion of hyaluronic acid and chitosan, is accomplished by an amide bond (- NHC(=O)-).
Chitosan in the above derivatives acts to further increase the viscoelasticity of hyaluronic acid derivatives. The chitosan in the present invention includes chitosan oligomers as well as chitosan polymer itself, and its molecular weight is not particularly limited and, is preferably in the range of 10,000 ~ 1,000,000.
The present invention also provides processes for preparation of hyaluronic acid derivatives in which hyaluronic acid is crosslinked to glycol polymer by amide bonds.
Processes for preparation of hyaluronic acid derivatives can be divided into ones for a derivative (I) in which a glycol polymer is crosslinked directly to a hyaluronic acid, for example, derivatives of FORMULAS 2 and 3, and ones for a derivative (H) in which a hyaluronic acid is crosslinked to a glycol polymer via a chitosan, for example, derivatives of
FORMULAS 4 and 5.
A process for preparing the hyaluronic acid derivative (I) comprises:
(A) a step of introducing a free amine group to one or both terminals of glycol polymer; and
(B) a step of reacting the free amine group-introduced glycol polymer with a hyaluronic
acid to form an amide bond between them.
In the step (A), the introduction of the free amine group to the terminal of glycol polymer is carried out by a method comprising the steps of:
(a) activating one or both terminals of the glycol polymer to make an activated glycol polymer; and
(b) reacting the activated glycol polymer with a diamine compound.
The activation of the step (a) is to convert the terminal of the glycol polymer into one capable of accepting the diamine compound to provide, for example, the activated glycol polymers of FORMULAS 6 and 7 as below. Where free amine groups are introduced to both terminals of the glycol polymer in the step (b), the activated glycol polymer having a symmetrical structure of Formula 6 or 7 is produced in the activation step (a).
wherein, Rl and R2 each independently are HO-PEG-CH2CH2O-, HO-PEG-CH2CH2CH2O-, HO-PEG-CONH(CH2)5O-, HO-PEG-S-OCH2CH2O-, HO-PEG-S-CH2CH2O-, HO-PEG- NHCOCH2CH2O-, HO-PEG-CO CHz^O-, HO-PEG-COCH2CH2O-, HO-PEG-, HO-PEG- CH2O-, MeO-PEG-CH2CH2O-, MeO-PEG-CH2CH2CH2O-, MeO-PEG-CONH(CH2)5O-, MeO-PEG-S-OCH2CH2O-, MeO-PEG-S-CH2CH2O-, MeO-PEG-NHCOCH2CH2O-, MeO- PEG-CO CHz^O-, MeO-PEG-COCH2CH2O-, MeO-PEG-, MeO-PEG-CH2O-, HO-PLU- CH2CH2O-, HO-PLU-CH2CH2CH2O-, HO-PLU-CONH(CH2)5O-, HO-PLU-S-OCH2CH2O-,
HO-PLU-S-CH2CH2O-, HO-PLU-NHCOCH2CH2O-, HO-PLU-CO^Hz^O-, HO-PLU- COCH2CH2O-, HO-PLU-, HO-PLU-CH2O-, MeO-PLU-CH2CH2O-, MeO-PLU- CH2CH2CH2O-, MeO-PLU-CONH(CH2)5O-, MeO-PLU-S-OCH2CH2O-, MeO-PLU-S- CH2CH2O-, MeO-PLU-NHCOCH2CH2O-, MeO-PLU-CO(CH2)3θ-, MeO-PLU- COCH2CH2O-, MeO-PLU-, or MeO-PLU-CH2O-, in which PEG is -(CH2CH2O)n- (n is 1 or an integer of more than 1), PLU is CH2CH2)a-(CH2CH(CH3)O)b-(CH2CH2θ)c- (a, b and c each independently are 1 or an integer of more than 1), and Me is a methyl group.
Exemplary methods of synthesizing the activated glycol polymers will be later illustrated in EXAMPLES 1 to 4.
The activated glycol polymers obtained in the step (a) are exemplified as polymers of
FORMULAS 8 to 11 as below:
wherein a, b, n, x and y as repeating units of polymer chain each independently are 1 or an integer of more than 1.
The polymer of Formula 8 has one activated terminal of polyethylene glycol as a glycol polymer, and the polymer of Formula 9 has two activated terminals of polyethylene
glycol as a glycol polymer, and the polymer of Formula 10 has one activated terminal of Pluronic as a glycol polymer, and the polymer of Formula 11 has two activated terminals of Pluronic as a glycol polymer.
The molecular weight of a polyethylene glycol with the two activated terminals, like a polymer of Formula 8, is preferably in the range of 1,000 to 40,000, and the molecular weight of Pluronic with the two activated terminals, like a polymer of Formula 11, is preferably in the range of 5,000 to 50,000.
In the step (b), when the activated glycol polymer reacts with a diamine compound, a glycol polymer with one or two free amine groups at its one or both terminals is obtained by substitution reaction. The kind of diamine compounds is not particularly limited and includes, for example, ethylene diamine, propylene diamine, isopropylene cliamine, butylene diamine, etc. Among them, ethylene diamine is particularly preferred. Examples of glycol polymers are described in Formulas 12 and 13, each of which has, at its both terminals, free amine groups obtained by reacting the activated glycol polymer of Formulas 9 and 11 with ethylene diamine, respectively.
(12)
(13)
Exemplary methods of introducing free amine group to one or both terminals of the activated glycol polymer will be later illustrated in EXAMPLES 5 to 8.
When the free amine group-introduced glycol polymer reacts with a hyaluronic acid(s)
(step (B)), a hyaluronic acid derivative (I) is produced wherein the glycol polymer is crosslinked to the hyaluronic acid by amide bonding.
For induction of a proper crosslinking reaction, the concentration of a hyaluronic acid in the amidation reaction is preferably in the range of 0.01 ~ 100 mg/ml, and the mixing ratio of free amine group-introduced glycol polymer to hyaluronic acid is preferably in the range of 1 : 100 ~ 100 : 1 (carboxyl group of hyaluronic acid : free amine group of glycol polymer).
As mentioned previously, hyaluronic acid has carboxyl groups and thus can react with the free amine groups to form amide bonds. This amidation reaction may be performed by activating the carboxyl group of hyaluronic acid and this activation can be accomplished by adding a carboxyl group-activating agent thereto. Examples of preferable activating agents include carbodiimide-based compounds, such as l-alkyl-3-(3-dimethylaminopropyl) carbodiimides (alkyl herein is alkyl of 1-10 carbon atoms), l-ethyl-3-(3-(trimethylammonio) propyl) carrxκiiimide ("ETC"), and l-cyclohexyl-3-(2-mo holinoethyl) carbodiirnide ("CMC"). Among them, l-emyl-3-(3-dimethylaminopropyl) c^bodiimide ("EDC") is particularly preferred.
Preferably, an auxiliary activating agent is used together with the carboxyl group- activating agent Examples of preferable auxiliary activating agents include N- hyckoxysuccinimide ("ΝHS"), 1-hydroxybenzotriazole ("HOBt"), 3,4-dihydro-3-hydroxy-4- oxo-l,2,3-benzotriazine ("HOOBt"), l-hydroxy-7-azabenzotriazole ("HOAt"), and Ν-hydroxy-
-αilfosuccinimide ("Sulfo-ΝHS"). Among them, ΝHS is particularly preferred.
The addition amount of the carboxyl group-activating agent and auxiliary activating agent is decided by various factors such as the concentration of hyaluronic acid, the activity of both agents, etc. For example, the addition amount of EDC is preferably in the range of 0.0001 to 100 mg ml, and the addition amount of ΝHS is preferably in the range of 0.00001 to 100 mg/ml.
Reactions in the present invention can be performed at 0 ~ 40°C, preferably, at room temperature and pH 2- 8 for 0.5 ~20 hours.
Next, there is described a process for preparation of a derivative (H) in which a
hyaluronic acid is crosslinked to a glycol polymer via a chitosan, comprising:
(Al) a step of introducing a free amine group to one terminal of the glycol polymer;
(B 1) a step of reacting the free amine group-introduced glycol polymer with chitosan to make a chitosan-glycol polymer coupling product; and
(Cl) a step of reacting the chitosan-glycol polymer coupling product with hyaluronic acid to perform an amidation reaction.
A method of introducing a free amine group in the step (Al) is the same as that in the step (A) of the preparation process of a hyaluronic acid derivative (I) except that a free amine group is introduced to only one terminal of the glycol polymer.
The chitosan-glycol polymer coupling product in the step (Bl) is obtained by substitution reaction, and their examples are illustrated in Formulas 14 and 15 as below, wherein polyethylene glycol and Pluronic were used as the glycol polymer, respectively.
wherein a, b, m, n, x and y each independently are 1 or an integer of more than 1 and satisfy the condition of n>x>y.
In preparation of the chitosan-glycol polymer coupling product, the mixing ratio of chitosan and activated glycol polymer is preferably in the range of 1 : 100 ~ 100 : 1.
Exemplary methods of preparing chitosan-glycol polymer coupling products will be later illustrated in EXAMPLES 15 - 18.
The amidation reaction in the step (Cl) is the same as that in the step (B) of the preparation process of a hyaluronic acid derivative (I) except that the amidation reaction occurs between chitosan and hyaluronic acid.
In an embodiment, the hyaluronic acid derivative (H) obtained in the step (Cl) is made in a certain form of gel, film or thread and then an additional amidation reaction is performed at residual carboxyl groups and amine groups, which may be also applied to the case of a hyaluronic acid derivative (I).
The products obtained in each step of processes according to the present invention can be separated andor refined by well-known methods in the art to which the present invention pertains. These separation and refinement methods include distillation under atmospheric pressure or reduced pressure, recrystallization, column chromatography, ion-exchange chromatography, gel chromatography, affinity chromatography, thin-layer chromatography,
phase separation, solvent extraction, dialysis, washing, etc. Each refinement may be performed after each reaction step or after a series of reaction steps.
Moreover, within the range of not damaging the effect of the present invention, in order to improve the efficiency of overall process or yield, the process may be partially modified or include other steps, which should be interpreted to be included in the scope of the present invention.
Raw materials or intermediates necessary for synthesis of hyaluronic acid derivatives according to the present invention can be prepared by known methods or the above-mentioned methods, or are commercially available.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a graph showing the viscoelasticity of hyaluronic acid derivatives according to the present invention, compared to that of a hyaluronic acid according to a prior art, as measured byarheometer.
DETAILED DESCRDTTION OF PREFERRED EMBODIMENTS
Hereinafter, the present invention will be described in more detail by EXAMPLES, but the scope of the present invention is not limited thereto.
EXAMPLE 1 : Activation of polyethylene glycol - 1
Polyethylene glycol (MW: 2,000) was dissolved in 30 ml of methyl chloride as a solvent at a concentration of 100 mg/ml. 607.2 μl of triethyl amine as a neutralizer was added thereto, and then 4-nitrophenyl chloroformate was added at a concentration of 40.3 mg/ml. The reaction was allowed to proceed at room temperature for 24 hours, and then poured into n- hexane as a poor solvent A precipitate was separated and then washed several times with the poor solvent The precipitate was dried under nitrogen atmosphere for 3 days (4.0956 g; yield:
97.3%).
EXAMPLE 2: Activation of polyethylene glvcol - 2
Polyethylene glycol (MW: 5,000) was dissolved in 30 ml of methyl chloride as a solvent at a concentration of 100 mg/ml. 379.5 μl of triethyl amine as a neutralizer was added thereto, and then 4-nitrophenyl chloroformate was added at a concentration of 25.19 mg/ml. The reaction was allowed to proceed at room temperature for 24 hours, and then poured into n- hexane as a poor solvent. A precipitate was separated and then washed several times with the poor solvent The precipitate was dried under nitrogen atmosphere for 3 days (3.6881 g; yield: 98.2%).
EXAMPLE 3: Activation of polyethylene glvcol - 3
Polyethylene glycol (MW: 8,000) was dissolved in 30 ml of methyl chloride as a solvent at a concentration of 100 mg/ml. 151.8 μl of triethyl amine as a neutralizer was added thereto, and then 4-nitrophenyl chloroformate was added at a concentration of 10.08 mg/ml. The reaction was allowed to proceed at room temperature for 24 hours, and then poured into n- hexane as a poor solvent A precipitate was separated and then washed several times with the poor solvent The precipitate was dried under nitrogen atmosphere for 3 days (3.2390 g; yield: 98.08%).
EXAMPLE 4: Activation of Pluronic
Pluronic F127 (MW: 12,600) was dissolved in 30 ml of methyl chloride as a solvent at a concentration of 100 mg/ml. 192.9 μl of triethyl amine as a neutralizer was added thereto, and then 4-nitrophenyl chloroformate was added at a concentration of 12.8 mg/ml. The reaction was allowed to proceed at room temperature for 24 hours, and then poured into n-hexane as a poor solvent. A precipitate was separated and then washed several times with the poor solvent The precipitate was dried under nitrogen atmosphere for 3 days (3.190 g; yield: 94.27%).
EXAMPLE 5: Introduction of amine group into polyethylene glycol - 1
The activated polyethylene glycol (MW: 2,000) produced in EXAMPLE 1 was dissolved in 20 ml of methyl chloride as a solvent at a concentration of 50 mg/ml. 240 μl of ethylene diamine was added thereto and allowed to react at room temperature for 24 hours, then poured into n-hexane as a poor solvent, and a precipitate was separated and then washed several times with the poor solvent The precipitate was dried under nitrogen atmosphere for 3 days (912.7 mg; yield: 73.58%). After drying, the precipitate was dissolved in water and then dialyzed using a dialysis membrane of molecular weight cutoff around 1,000 for 2 days. After dialysis, the product was dried under nitrogen atmosphere for 1 day.
EXAMPLE 6: Introduction of amine group into polyethylene glvcol - 2
The activated polyethylene glycol (MW: 5,000) produced in EXAMPLE 2 was dissolved in 20 ml of methyl chloride as a solvent at a concentration of 50 mg/ml. 96.16 μl of ethylene diamine was added thereto and allowed to react at room temperature for 24 hours, then poured into n-hexane as a poor solvent, and a precipitate was separated and then washed several times with the poor solvent The precipitate was dried under nitrogen atmosphere for 3 days (899.9 mg; yield: 82.10%). After drying, the precipitate was dissolved in water and then dialyzed using a dialysis membrane of molecular weight cutoff around 3,500 for 2 days. After dialysis, the product was dried under nitrogen atmosphere for 1 day.
EXAMPLE 7: Introduction of amine group into polyethylene glycol - 3
The activated polyethylene glycol (MW: 8,000) produced in EXAMPLE 3 was dissolved in 20 ml of methyl chloride as a solvent at a concentration of 50 mg/ml. 60.1 μl of ethylene diamine was added thereto and allowed to react at room temperature for 24 hours, then poured into n-hexane as a poor solvent, and a precipitate was separated and then washed several times with the poor solvent. The precipitate was dried under nitrogen atmosphere for 3 days (906.9 mg; yield: 85.55%). After drying, the precipitate was dissolved in water and then
dialyzed using a dialysis membrane of molecular weight cutoff around 6,000 for 2 days. After dialysis, the product was dried under nitrogen atmosphere for 1 day.
EXAMPLE 8: Introduction of amine group into Pluronic
The activated polyethylene glycol (MW: 12,600) produced in EXAMPLE 4 was dissolved in 20 ml of methyl chloride as a solvent at a concentration of 50 mg/ml. 56.75 μl of ethylene ώamine was added thereto and allowed to react at room temperature for 24 hours, then poured into n-hexane as a poor solvent, and a precipitate was separated and then washed several times with the poor solvent The precipitate was dried under nitrogen atmosphere for 3 days. After drying, the precipitate was again dissolved in water and then dialyzed using a dialysis membrane of molecular weight cutoff around 12,000 ~ 14,000 for 2 days. After dialysis, the product was dried under nitrogen atmosphere for 1 day (921.3 mg; yield: 88.74%).
EXAMPLE 9: Preparation of hyaluronic acid derivative gel with hyaluronic acid crosslinked to polyethylene glycol by amide bond - 1
10 ml of an aqueous solution containing sodium hyaluronate (MW: more than 1,000,000) at a concentration of 5.0 mg/ml was prepared. Also, 5 ml of an aqueous solution containing an amine group-introduced polyethylene glycol (MW: 5,000), produced in
EXAMPLE 6, at a concentration of 1.0 mg/ml was prepared in which amine groups were introduced to both terminals thereof. While the hyaluronic acid solution was stirred, the polyethylene glycol solution was added thereto. While the mixture of both solutions was stirred, 1 ml of EDC was added to concentration of 0.0588 mg/ml and 1 ml of NHS was also added to a concentration of 0.0706 mg/ml to perform an amidation reaction at 25°C for about 2 hours.
After the reaction was completed, it was added to acetone to precipitate a hyaluronic acid derivative in which hyaluronic acids were crosslinked to a polyethylene glycol wherein amine groups had been introduced to both terminals thereof. A precipitate (52 mg; yield: 94.54%) was separated from the solution and then dried. When water was added to the dried hyaluronic acid
derivative at a concentration of 10 mg/ml, a soluble gel was formed having an increased viscoelasticity.
EXAMPLE 10: Preparation of hyaluronic acid derivative gel with hyaluronic acid crosslinked to polyethylene glycol by amide bond - 2
10 ml of an aqueous solution containing sodium hyaluronate (MW: more than
1,000,000) at a concentration of 5.0 mg/ml was prepared. Also, 5 ml of an aqueous solution containing an amine group-introduced polyethylene glycol (MW: 5,000), produced in EXAMPLE 6, at a concentration of 1.0 mg/ml was prepared in which amine groups were introduced to both terminals thereof. While the hyaluronic acid solution was stirred, the polyethylene glycol solution was added thereto. While the mixture of both solutions was stirred, 1 ml of EDC was added to a concentration of 0.1471 mg/ml and 1 ml of NHS was also added to a concentration of 0.1765 mg/ml to perform an amidation reaction at 25°C for about 2 hours. After the reaction was completed, it was added to acetone to precipitate a hyaluronic acid derivative in which hyaluronic acids were crosslinked to a polyethylene glycol in which amine groups had been introduced to both terminals thereof. A precipitate (40.3 mg; yield: 73.27%) was separated from the solution and then dried. When water was added to the dried hyaluronic acid derivative at a concentration of 10 mg/ml, a soluble gel was formed having an increased viscoelasticity.
EXAMPLE 11: Preparation of hyaluronic acid derivative gel with hvaluronic acid crosslinked to polyethylene glycol by amide bond - 3
10 ml of an aqueous solution containing sodium hyaluronate (MW: more than
1,000,000) at a concentration of 5.0 mg/ml was prepared. Also, 5 ml of an aqueous solution containing an amine group-introduced polyethylene glycol (MW: 5,000), produced in
EXAMPLE 6, at a concentration of 1.0 mg/ml was prepared in which amine groups were introduced to both terminals thereof. While the hyaluronic acid solution was stirred, the
polyethylene glycol solution was added thereto. While the mixture of both solutions was stirred, 1 ml of EDC was added to a concentration of 0.2941 mg/ml and 1 ml of NHS was also added to a concentration of 0.3529 mg/ml to perform an amidation reaction at 25°C for about 2 hours. After the reaction was completed, it was added to acetone to precipitate a hyaluronic acid derivative in which hyaluronic acids were crosslinked to a polyethylene glycol in which amine groups had been introduced to both terminals thereof. A precipitate (47.2 mg; yield: 85.82%) was separated from the solution and then dried. When the dried hyaluronic acid derivative was added to water at a concentration of 10 mg/ml, a soluble gel was formed having an increased viscoelasticity.
EXAMPLE 12: Preparation of hyaluronic acid derivative gel with hvaluronic acid crosslinked to Pluronic by amide bond - 1
10 ml of an aqueous solution containing sodium hyaluronate (MW: more than 1,000,000) at a concentration of 5.0 mg/ml was prepared. Also, 5 ml of an aqueous solution containing an amine group-introduced Pluronic (MW: 12,600), produced in EXAMPLE 8, at a concentration of 1.0 mg/ml was prepared in which amine groups were introduced to both terminals thereof. While the hyaluronic acid solution was stirred, the Pluronic solution was added thereto. While the mixture of both solutions was stirred, 1 ml of EDC was added to a concentration of 0.0588 mg/ml and 1 ml of NHS was also added to a concentration of 0.0706 mg/ml to perform an amidation reaction at 25°C for about 2 hours. After the reaction was completed, it was added to acetone to precipitate a hyaluronic acid derivative in which hyaluronic acids were crosslinked to a Pluronic in which amine groups had been introduced to both terminals thereof. A precipitate (17.9 mg; yield: 32.55%) was separated from the solution and then dried. When the dried hyaluronic acid derivative was added to water at a concentration of 10 mg/ml, a soluble gel was formed having an increased viscoelasticity.
EXAMPLE 13: Preparation of hvaluronic acid derivative gel with hvaluronic acid crosslinked to Pluronic by amide bond - 2
10 ml of an aqueous solution containing sodium hyaluronate (MW: more than 1,000,000) at a concentration of 5.0 mg/ml was prepared. Also, 5 ml of an aqueous solution containing an amine group-introduced Pluronic (MW: 12,600), produced in EXAMPLE 8, at a concentration of 1.0 mg/ml was prepared in which amine groups were introduced to both terminals thereof. While the hyaluronic acid solution was stirred, the Pluronic solution was added thereto. While the mixture of both solutions was stirred, 1 ml of EDC was added to a concentration of 0.1471 mg/ml and 1 ml of NHS was also added to a concentration of 0.1765 mg/ml to perform an amidation reaction at 25°C for about 2 hours. After the reaction was completed, it was added to acetone to precipitate a hyaluronic acid derivative in which hyaluronic acids were crosslinked to a Pluronic in which amine groups had been introduced to both terminals thereof. A precipitate (22.7 mg; yield: 41.27%) was separated from the solution and then dried. When the dried hyaluronic acid derivative was added to water at a concentration of 10 mg/ml, a soluble gel was formed having an increased viscoelasticity.
EXAMPLE 14: Preparation of hvaluronic acid derivative gel with hvaluronic acid crosslinked to Pluronic by amide bond - 3
10 ml of an aqueous solution containing sodium hyaluronate (MW: more than 1,000,000) at a concentration of 5.0 mg/ml was prepared. Also, 5 ml of an aqueous solution containing an amine group-introduced Pluronic (MW: 12,600), produced in EXAMPLE 8, at a concentration of 1.0 mg/ml was prepared in which amine groups were introduced to both terminals thereof. While the hyaluronic acid solution was stirred, the Pluronic solution was added thereto. While the mixture of both solutions was stirred, 1 ml of EDC was added to a concentration of 0.2941 mg/ml and 1 ml of NHS was also added to a concentration of 0.3529 mg/ml to perform an amidation reaction at 25°C for about 2 hours. After the reaction was completed, it was added to acetone to precipitate a hyaluronic acid derivative in which hyaluronic acids were crosslinked to a Pluronic in which amine groups had been introduced to both terminals thereof. A precipitate (28.3 mg; yield: 51.45%) was separated from the solution and then dried. When the dried hyaluronic acid derivative was added to water at a concentration
of 10 mg/ml, a soluble gel was formed having an increased viscoelasticity.
EXAMPLE 15: Preparation of chitosan-polvethylene glycol coupling product- 1
20 ml of an aqueous solution containing chitosan (MW: less than 1,600; EugenBio) was prepared at a concentration of 5.0 mg/ml. An activated polyethylene glycol (MW: 2,000) produced in EXAMPLE 1 was added to the chitosan solution at a concentration of 2.353 mg/ml, 4.706 mg/ml and 7.059 mg/ml, respectively, followed by stirring. Reaction was performed at 25°C for 24 hours, and the product was dialyzed using a dialysis membrane of molecular weight cutoff around 6,000 at room temperature for 2 days so as to remove the unreacted chitosan and unreacted polyethylene glycol. After dialysis, the product was dried under nitrogen atmosphere to obtain a chitosan-polyethylene glycol coupling product (depending upon the concentration of activated polyethylene glycols, 125.4 mg, 243.5 mg and 348.1 mg; yield: 57.62%, 72.62% and 76.85%).
EXAMPLE 16: Preparation of chitosan-polyethylene glycol coupling product - 2
20 ml of an aqueous solution containing chitosan (MW: less than 1,600) was prepared at a concentration of 5.0 mg/ml. An activated polyethylene glycol (MW: 5,000) produced in EXAMPLE 2 was added to the chitosan solution at a concentration of 5.8825 mg/ml, 11.765 mg/ml and 17.6475 mg/ml, respectively, followed by stirring. Reaction was performed at 25°C for 24 hours, and the product was dialyzed using a dialysis membrane of molecular weight cutoff around 12,000 at room temperature for 2 days so as to remove the unreacted chitosan and unreacted polyethylene glycol. After dialysis, the reactant was dried under nitrogen atmosphere to obtain a chitosan-polyethylene glycol coupling product (depending upon the concentration of activated polyethylene glycols, 118.28 mg, 218.5 mg and 329.4 mg; yield: 54.3%, 62.18% and 72.72%).
EXAMPLE 17: Preparation of chitosan-polyethylene glycol coupling product- 3
20 ml of an aqueous solution containing chitosan (MW: less than 1,600) was prepared at a concentration of 5.0 mg/ml. An activated polyethylene glycol (MW: 8,000) produced in EXAMPLE 3 was added to the chitosan solution at a concentration of 9.412mg/ml, 18.824 mg/ml and 28.236 mg/ml, respectively, followed by stirring. Reaction was performed at 25°C for 24 hours, and the product was dialyzed using a dialysis membrane of molecular weight cutoff around 12,000 - 14,000 at room temperature for 2 days so as to remove the unreacted chitosan and unreacted polyethylene glycol. After dialysis, the reactant was dried under nitrogen atmosphere to obtain a chitosan-polyethylene glycol coupling product (depending upon the concentration of activated polyethylene glycols, 110.34 mg, 204.12 mg and 301.2 mg; yield: 50.69%, 60.88% and 66.50%).
EXAMPLE 18: Preparation of chitosan-Pluronic coupling product
20 ml of an aqueous solution containing chitosan (MW: less than 1,600) was prepared at a concentration of 5.0 mg/ml. An activated Pluronic F127 (MW: 12,600) produced in EXAMPLE 4 was added to the chitosan solution at a concentration of 5 mg/ml, 10 mg/ml, 15 mg/ml and 20 mg/ml, respectively, followed by stirring. Reaction was performed at 25°C for 24 hours, and the product was dialyzed using a dialysis membrane of molecular weight cutoff around 12,000 ~ 14,000 at room temperature for 2 days so as to remove the unreacted chitosan and unreacted Pluronic. After dialysis, the reactant was dried under nitrogen atmosphere to obtain a chitosan-Pluronic product (depending upon the concentration of activated Pluronic F127, 188.5 mg, 136.4 mg, 248 mg and 339 mg; yield: 47.125%, 45.47%, 62% and 67.8%).
EXAMPLE 19: Preparation of hvaluronic acid derivative gel with hvaluronic acid crosslinked to polyethylene glycol via chitosan - 1
10 ml of an aqueous solution containing sodium hyaluronate (MW: more than
1,000,000) at a concentration of 5.0 mg/ml was prepared. Also, 5 ml of an aqueous solution containing the coupling product of chitosan (MW: less than 1,600) and polyethylene glycol
(MW: 5,000), produced at a concentration of 5.8825 mg/ml, 11.765 mg/ml and 17.6475 mg/ml in EXAMPLE 16, was prepared at a concentration of 1.0 mg/ml. While the hyaluronic acid solution was stirred, the chitosan-polyethylene glycol coupling product solution was added thereto. While the mixture of both solutions was stirred, 1 ml of EDC was added to a concentration of 0.0588 mg/ml and 1 ml of NHS was also added to a concentration of 0.0706 mg/ml to perform an amidation reaction at 25°C for about 2 hours. After the reaction was completed, it was added to acetone to precipitate a hyaluronic acid derivative in which a hyaluronic acid was crosslinked to a chitosan-polyethylene glycol coupling product. A precipitate was separated from a solution and then dried (depending upon of the concentration of activated polyethylene glycols in chitosan-polyethylene glycol coupling products, 22.3 mg, 32.6 mg and 14.4 mg; yield: 41.28%, 59.27% and 26.18%). When the dried hyaluronic acid derivative was added to water at a concentration of 10 mg/ml, a soluble gel was formed having an increased viscoelasticity.
EXAMPLE 20: Preparation of hvaluronic acid derivative gel with hvaluronic acid crosslinked to polyethylene glvcol via chitosan - 2
10 ml of an aqueous solution containing sodium hyaluronate (MW: more than 1,000,000) at a concentration of 5.0 mg/ml was prepared. Also, 5 ml of an aqueous solution containing the coupling product of chitosan (MW: less than 1,600) and polyethylene glycol (MW: 5,000), produced at a concentration of 5.8825 mg/ml, 11.765 mg/ml and 17.6475 mg/ml in EXAMPLE 16, was prepared at a concentration of 1.0 mg/ml. While the hyaluronic acid solution was stirred, the chitosan-polyethylene glycol coupling product solution was added thereto. While the mixture of both solutions was stirred, 1 ml of EDC was added to a concentration of 0.1471 mg/ml and 1 ml of NHS was also added to a concentration of 0.1765 mg/ml to perform an amidation reaction at 25°C for about 2 hours. After the reaction was completed, it was added to acetone to precipitate a hyaluronic acid derivative in which a hyaluronic acid was crosslinked to a chitosan-polyethylene glycol coupling product. A precipitate was separated from the solution and then dried (depending upon of the concentration
of activated polyethylene glycols in chitosan-polyethylene glycol coupling products, 24.4 mg, 33.7 mg and 30.8 mg; yield: 44.36%, 61.27% and 56%). When the dried hyaluronic acid derivative was added to water at a concentration of 10 mg/ml, a soluble gel was formed having an increased viscoelasticity.
EXAMPLE 21: Preparation of hvaluronic acid derivative gel with hvaluronic acid crosslinked to polyethylene glvcol via chitosan - 3
10 ml of an aqueous solution containing sodium hyaluronate (MW: more than 1,000,000) at a concentration of 5.0 mg/ml was prepared. Also, 5 ml of an aqueous solution containing the coupling product of chitosan (MW: less than 1,600) and polyethylene glycol (MW: 5,000), produced at a concentration of 5.8825 mg/ml, 11.765 mg/ml and 17.6475 mg/ml in EXAMPLE 16, was prepared at a concentration of 1.0 mg/ml. While the hyaluronic acid solution was stirred, the chitosan-polyethylene glycol coupling product solution was added thereto. While the mixture of both solutions was stirred, 1 ml of EDC was added to a concentration of 0.2941 mg/ml and 1 ml of NHS was also added to a concentration of 0.3529 mg/ml to perform an amidation reaction at 25°C for about 2 hours. After the reaction was completed, it was added to acetone to precipitate a hyaluronic acid derivative in which a hyaluronic acid had been crosslinked to a chitosan-polyethylene glycol coupling product A precipitate was separated from the solution and then dried (depending upon of the concentration of activated polyethylene glycols in chitosan-polyethylene glycol coupling products, 26.5 mg, 34.1 mg and 44.8 mg; yield: 48.18%, 62% and 81.45%). When the dried hyaluronic acid derivative was added to water at a concentration of 10 mg/ml, a soluble gel was formed having an increased viscoelasticity.
EXAMPLE 22: Preparation of hvaluronic acid derivative film with hvaluronic acid crosslinked to polyethylene glvcol via chitosan
20 ml of HC1 solution containing a chitosan (MW: more than 10,000) at a concentration
of 5.0 mg/ml was prepared. An activated polyethylene glycol (MW: 5,000) as produced in EXAMPLE 2 was added to the chitosan-HCl solution at a concentration of 5.8825 mg/ml, 11.765 mg/ml and 17.6475 mg/ml, respectively, followed by stirring. Reaction was performed at 25°C for 24 hours, and the product was dialyzed at room temperature for 2 days using a dialysis membrane of molecular weight cutoff around 12,000 so as to remove the unreacted chitosan and unreacted polyethylene glycol. After dialysis, the reactant was dried under nitrogen atmosphere to obtain a chitosan-polyethylene glycol coupling product (depending upon the concentration of activated polyethylene glycols, 187.3 mg, 310.1 mg and 391.8 mg; yield: 86.06%, 92.48% and 86.50%).
Furthermore, 20 ml of an aqueous solution containing sodium hyaluronate (MW:
200,000) at a concentration of 10.0 mg/ml was prepared. Also, 20 ml of an aqueous solution containing the chitosan-polyethylene glycol coupling product, obtained as above, at a concentration of 2.5 mg/ml, was prepared. While the hyaluronic acid solution was stirred, the chitosan-polyethylene glycol coupling product solution was added thereto. While the mixture of both solutions was stirred, EDC was added to a concentration of 1.25 mg/ml and NHS was also added to a concentration of 1.5 mg/ml. An amidation reaction was performed at 25°C for 4 hours. The product was dialyzed using a dialysis membrane of molecular weight cutoff around 12,000 ~ 14,000 for 2 days and then freeze-dried to obtain a hyaluronic acid derivative (depending upon the concentration of activated polyethylene glycols in chitosan-polyethylene glycol coupling products, 141.6 mg, 137.4 mg and 138.75 mg; yield: 56.64%, 54.96% and 55.50%).
The hyaluronic acid derivative produced thus was made to a solution of 2% and poured into a Petti dish and naturally dried for 5 days to make a film. The film was soaked in a mixed solution of ethanol and water (ethanol : water = 8 : 2) and then EDC and NHS were added thereto to a concentration of 2.0 mg/ml, respectively, to perform a second amidation reaction (second crosslinking reaction). The solubility and swelling ratio of the film obtained thus are described in the below TABLE 1.
[TABLE 1]
* [chitosan] : [PEG (MW: 5,000)] = 10 : 1 ** [chitosan] : [PEG (MW: 5,000)] = 20 : 1
EXAMPLE 23: Preparation of hvaluronic acid derivative thread with hyaluronic acid crosslinked to polyethylene glvcol via chitosan
20 ml of an aqueous solution containing sodium hyaluronate (MW: 200,000) at a concentration of 5.0 mg/ml was prepared. Also, 5 ml of an aqueous solution containing the chitosan-polyethylene glycol coupling product, obtained in EXAMPLE 16, at a concentration of 5.0 mg/ml was prepared, in which the molecular weight of chitosan was 5,000 and the concentration of the activated polyethylene glycol used for preparation of the coupling product was 5.8825 mg/ml, 11.765 mg/ml and 17.6475 mg/ml, respectively. While the hyaluronic acid solution was stirred, the chitosan-polyethylene glycol coupling product solution was added thereto. While the mixture of both solutions was stirred, EDC was added thereto to a concentration of 1.0 mg/ml and NHS was also added to a concentration of 1.2 mg/ml. An amidation reaction was performed at 25°C for 4 hours. The product was dialyzed using a dialysis membrane of molecular weight cutoff around 12,000 ~ 14,000 for 2 days and then freeze-dried to obtain a hyaluronic acid derivative (depending upon the concentration of
activated polyethylene glycols in chitosan-polyethylene glycol coupling products, 121 mg, 115.6 mg and 114.25 mg; yield: 96.8%, 92.48% and 91.40%).
The hyaluronic acid derivative produced thus was made to a solution of 10% and spun into a mixed solution of ethanol and acetone through a syringe with a needle of 23G to make a thread. After the thread was sufficiently solidified for 1 day, it was again put in a mixed solution of ethanol and water (ethanol : water = 8 : 2) and then EDC and NHS were added thereto to a concentration of 2.0 mg/ml, respectively, to perform a second amidation reaction (second crosslinking reaction). The thread obtained thus had a fine and even thickness and smooth surface.
EXAMPLE 24: Preparation of hvaluronic acid derivative gel with hyaluronic acid crosslinked to Pluronic via chitosan - 1
10 ml of an aqueous solution containing sodium hyaluronate (MW: more than 1,000,000) at a concentration of 5.0 mg/ml was prepared. Also, 5 ml of an aqueous solution containing the coupling product of chitosan (MW: less than 1 ,600) and Pluronic (MW: 12,600), produced at a concentration of 5 mg/ml, 10 mg/ml, 15 mg/ml and 20 mg/ml in EXAMPLE 18, was prepared at a concentration of 1.0 mg/ml. While the hyaluronic acid solution was stirred, the chitosan-Pluronic coupling product solution was added thereto. While the mixture of both solutions was stirred, 1 ml of EDC was added to a concentration of 0.0588 mg/ml and 1 ml of NHS was also added to a concentration of 0.0706 mg/ml to perform an amidation reaction at 25°C for about 2 hours. After the reaction was completed, it was added to acetone to precipitate a hyaluronic acid derivative in which a hyaluronic acid was crosslinked to a chitosan-Pluronic coupling product A precipitate was separated from a solution and then dried (depending upon of the concentration of Pluronic in chitosan-Pluronic coupling products, 29.7 mg, 25.8 mg, 26.8 mg and 24 mg; yield: 54%, 46.91%, 48.73% and 41.82%). When the dried hyaluronic acid derivative was added to water at a concentration of 10 mg/ml, a soluble gel was formed having an increased viscoelasticity.
EXAMPLE 25: Preparation of hyaluronic acid derivative gel with hyaluronic acid crosslinked to Pluronic via chitosan - 2
10 ml of an aqueous solution containing sodium hyaluronate (MW: more than 1,000,000) at a concentration of 5.0 mg/ml was prepared. Also, 5 ml of an aqueous solution containing the coupling product of chitosan (MW: less than 1 ,600) and Pluronic (MW: 12,600), produced at a concentration of 5 mg/ml, 10 mg/ml, 15 mg/ml and 20 mg/ml in EXAMPLE 18, was prepared at a concentration of 1.0 mg/ml. While the hyaluronic acid solution was stirred, the chitosan-Pluronic coupling product solution was added thereto. While the mixture of both solutions was stirred, 1 ml of EDC was added to a concentration of 0.1471 mg/ml and 1 ml of NHS was also added to a concentration of 0.1765 mg/ml to perform an amidation reaction at 25°C for about 2 hours. After the reaction was completed, it was added to acetone to precipitate a hyaluronic acid derivative in which a hyaluronic acid had been crosslinked to a chitosan- Pluronic coupling product. A precipitate was separated from the solution and then dried (depending upon of the concentration of Pluronic in chitosan-Pluronic coupling products, 32.6 mg, 26.2 mg, 28.9 mg and 30 mg; yield: 59.27%, 47.64%, 52.55% and 54.54%). When the dried hyaluronic acid derivative was added to water at a concentration of 10 mg/ml, a soluble gel was formed having an increased viscoelasticity.
EXAMPLE 26: Preparation of hyaluronic acid derivative gel with hvaluronic acid crosslinked to Pluronic via chitosan - 3
10 ml of an aqueous solution containing sodium hyaluronate (MW: more than
1,000,000) at a concentration of 5.0 mg/ml was prepared. Also, 5 ml of an aqueous solution containing the coupling product of chitosan (MW: less than 1 ,600) and Pluronic (MW: 12,600), produced at a concentration of 5 mg/ml, 10 mg/ml, 15 mg/ml and 20 mg/ml in EXAMPLE 18, was prepared at a concentration of 1.0 mg/ml. While the hyaluronic acid solution was stirred, the chitosan-Pluronic coupling product solution was added thereto. While the mixture of both solutions was stirred, 1 ml of EDC was added to a concentration of 0.2941 mg/ml and 1 ml of
NHS was also added to a concentration of 0.3529 mg/ml to perform an amidation reaction at 25°C for about 2 hours. After the reaction was completed, it was added to acetone to precipitate a hyaluronic acid derivative in which a hyaluronic acid had been crosslinked to a chitosan- Pluronic coupling product A precipitate was separated from a solution and then dried (depending upon of the concentration of Pluronic in chitosan-Pluronic coupling products, 39.4 mg, 30 mg, 32.5 mg and 33.9 mg; yield: 71.64%, 54.55%, 59.10% and 61.64%). When the dried hyaluronic acid derivative was added to water at a concentration of 10 mg/ml, a soluble gel was formed having an increased viscoelasticity.
EXPERIMENTAL EXAMPLE 1: Measurement of viscoelasticity of hvaluronic acid derivative
5 ml of a solution for measurement was prepared containing the hyaluronic acid derivatives produced in EXAMPLES 11, 14, 21 and 26 and a hyaluronic acid at a concentration of 10 mg/ml, respectively. The complex viscosities of these solutions measured in a frequency range of 0.02 ~ 1 Hz are depicted in FIG. 1. Compared to the viscoelasticity of hyaluronic acid, the viscoelasticities of hyaluronic acid derivatives according to the present invention are higher, as shown in FIG. 1.
INDUSTRIAL APPLICABILITY
As described above, the hyaluronic acid derivatives according to the present invention are biocompatible and have a very high viscoelasticity, and thus can be used, in the forms of gel, film, thread, etc., for various applications such as post-operative adhesion-preventing gels, materials for wrinkle treatment, materials for plastic surgery, materials for arthritis treatment and drug delivery vehicles. Especially, where the hyaluronic acid derivatives according to the present invention are used as materials for wrinkle treatment, they can maintain a high viscoelasticity for a long time, thereby exhibiting excellent effects such as remarkably lengthening their shelf life.
As the present invention may be embodied in several forms without departing from the spirit or essential characteristics thereof, it should also be understood that the above-described examples are not limited by any of the details of the foregoing description, unless otherwise specified, but rather should be construed broadly within its spirit and scope as defined in the appended claims, and therefore all changes and modifications that fall within the meets and bounds of the claims, or equivalences of such meets and bounds are therefore intended to be embraced by the appended claims.