WO2004069979A2 - Laundry cleansing and conditioning compositions - Google Patents
Laundry cleansing and conditioning compositions Download PDFInfo
- Publication number
- WO2004069979A2 WO2004069979A2 PCT/EP2004/000668 EP2004000668W WO2004069979A2 WO 2004069979 A2 WO2004069979 A2 WO 2004069979A2 EP 2004000668 W EP2004000668 W EP 2004000668W WO 2004069979 A2 WO2004069979 A2 WO 2004069979A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- composition
- chloride
- cationic
- ammonium chloride
- polymer
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/37—Polymers
- C11D3/3703—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
- C11D3/373—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds containing silicones
- C11D3/3742—Nitrogen containing silicones
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/02—Anionic compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/02—Anionic compounds
- C11D1/04—Carboxylic acids or salts thereof
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/02—Anionic compounds
- C11D1/12—Sulfonic acids or sulfuric acid esters; Salts thereof
- C11D1/22—Sulfonic acids or sulfuric acid esters; Salts thereof derived from aromatic compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/0005—Other compounding ingredients characterised by their effect
- C11D3/001—Softening compositions
- C11D3/0015—Softening compositions liquid
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/37—Polymers
- C11D3/3746—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- C11D3/3769—(Co)polymerised monomers containing nitrogen, e.g. carbonamides, nitriles or amines
- C11D3/3773—(Co)polymerised monomers containing nitrogen, e.g. carbonamides, nitriles or amines in liquid compositions
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/37—Polymers
- C11D3/3746—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- C11D3/3769—(Co)polymerised monomers containing nitrogen, e.g. carbonamides, nitriles or amines
- C11D3/3776—Heterocyclic compounds, e.g. lactam
Definitions
- This invention relates to laundry conditioning compositions. More particularly, the invention is directed to laundry compositions containing at least one cationic polymer and at least one anionic surfactant that deliver an unexpected level of fabric softening.
- Hsu discloses the use of polymer JR in an anionic -surfactant containing liquid detergent and further requires a polysaccharide polymer such as xanthan gum, which leads to an unstable product .
- Fabric softener compositions have been disclosed in WO 00/70005 and U.S. Pat. No. 6,492,322.
- Liquid detergent compositions comprising polymeric suds enhancers have been disclosed in U.S. Pat. Appl. No. 2002/0169097.
- this invention is directed to a liquid laundry composition consisting essentially of one or more cationic polymers and one or more anionic surfactants wherein the composition has a percent transmittance of greater than about 50 at 570 nanometers measured in the absence of dyes and contains less than about 2% anionic polysaccharide .
- this invention is directed to a laundry composition
- a laundry composition comprising one or more cationic polymers and more than about 5% of one or more anionic surfactants having an HLB of greater than about 4 wherein the softening parameter is greater than 40 and one or more of the cationic polymers has a molecular weight of less than about 850,000 daltons.
- the composition can take many forms including liquid, powder, paste, granule, moulded solid or water soluble sheet .
- this invention is directed -to a laundry composition
- a laundry composition comprising one or more cationic polymers and more than about 5% of one or more anionic surfactants having an HLB of greater than about 4 wherein the softening parameter is greater than about 40.
- this invention is directed to a powdered laundry composition
- a powdered laundry composition comprising of one or more cationic polymers and one or more anionic surfactants wherein one or more of the cationic polymers has a dissolution parameter of 55 or greater, and more than about 5% of one or more anionic surfactants having an HLB of greater than about 4 wherein the softening parameter is greater than about 40.
- this invention is directed to a method for conditioning textiles comprising, in no particular order, the steps of: a. providing a laundry detergent or fabric softener composition comprising at least one anionic surfactant and at least one cationic polymer, in a ratio and concentration to effectively soften and condition fabrics under predetermined laundering conditions; b. contacting one or more articles with the composition at one or more points during a laundering process; and c. allowing the articles to dry or mechanically tumble-drying them.
- the softening parameter is greater than 40 and the composition comprises more than about 5% by weight of one or more anionic surfactants having an HLB of greater than about 4, and one or more of said cationic polymers have a molecular weight of less than about 850,000 daltons.
- the term “comprising” means including, made up of, composed of, consisting and/or consisting essentially of.
- a formula shall be considered physically "stable" when after 1 week at 21 degrees Celsius it exhibits no signs of phase separation.
- the present invention is directed to laundry compositions containing mixtures of one or more anionic surfactant and one or more cationic polymer that deliver an unexpectedly high level of conditioning to fabrics.
- the main objective of this invention is to render garments more pleasant to the touch, and provide other conditioning benefits.
- the compositions of the present invention yield softening parameters of greater than 40.
- the inventive compositions have a percent transmittance of greater than about 50 at 570 nanometers measured in the absence of dyes and contain less than about 2% anionic polysaccharide .
- compositions of this invention are intended to confer conditioning benefits to garments, home textiles, carpets and other fibrous or fibre-derived articles. These formulations are not to be limited to conditioning benefits, however, and will often be multi-functional. As such, in addition to conditioning fibre-derived articles, they may also clean, fragrance or otherwise treat them.
- Softening includes, but is not limited to, an improvement in the handling of a garment treated with the compositions of this invention relative to that of an article laundered under identical conditions but without the use of this invention. Consumers will often describe an article that is softened as “silky” or "fluffy", and generally prefer the feel of treated garments to those that are unsoftened. It is desirable that the formulae of this invention, when used as instructed, yield a softness parameter of more than 40. The preferred products give a softness parameter in excess of 55, however, while even more preferred products give a softness parameter of more than 70.
- the composition contain greater than about 5% anionic surfactant.
- compositions of these compositions are not limited to softening, however. They may, depending on the particular embodiment of the invention selected, also provide an antistatic benefit.
- the cationic polymer / anionic surfactant compositions of this invention are further believed to lubricate the fibres of textile articles, which can reduce wear, pilling and colour fading, and provide a shape-retention benefit.
- This lubricating layer may also, without wishing to be bound by theory, provide a substrate on the fabric for retaining fragrances and other benefit agents.
- the cationic polymers of this invention are also believed to inhibit the transfer, bleeding and loss of vagrant dyes from fabrics during the wash, further improving colour brightness over time.
- the present invention can take any of a number of forms. It can take the form of a dilutable fabric conditioner, that may be an isotropic liquid, a surfactant-structured liquid, a granular, spray-dried or dry-blended powder, a tablet, a paste, a molded solid or any other laundry detergent form known to those skilled in the art.
- a "dilutable fabric conditioning" composition is defined, for the purposes of this disclosure, as a product intended to be used by being diluted with water or a non-aqueous solvent by a ratio of more than 100:1, to produce a liquor suitable for treating textiles and conferring to them one or more conditioning benefits. Water soluble sheets or sachets, such as those described in U.S. Pat. Appl.
- compositions intended to be used as combination detergent / softeners, along with fabric softeners sold for application in the final rinse of a wash cycle and fabric softeners sold for application at the beginning of a wash cycle are all considered within the scope of this invention.
- these compositions are intended to be used by being diluted by a ratio of more than 100:1 with water or a non-aqueous solvent, to form a liquor suitable for treating fabrics.
- Particularly preferred forms of this invention include combination detergent / softener products, especially as a liquid or powder, and isotropic or surfactant-structured liquid products intended for application as a fabric softener during the wash cycle or the final rinse.
- fabric softener shall be understood to mean a consumer or industrial product added to the wash, rinse or dry cycle of a laundry process for the express or primary purpose of conferring one or more conditioning benefits.
- the preferred pH range of the composition is 2-12. Because many cationic polymers can decompose at high pH, especially when they contain amine or phosphine moieties, it is desirable to keep the pH of the composition below the pK a of the amine or phosphine group that is used to quaternise the selected polymer, below which the propensity for this to occur is greatly decreased. This reaction can cause the product to lose effectiveness over time and create an undesirable product odour. As such, a reasonable margin of safety, of 1-2 units of pH below the pK a should ideally be used in order to drive the equilibrium of this reaction to strongly favour polymer stability.
- wash liquor pH especially in the case of powdered softener and combination detergent / softener products, can often be less important, as the kinetics of polymer decomposition are often slow, and the time of one wash cycle is typically not sufficient to allow for this reaction to have a significant impact on the performance or odour of the product .
- a lower pH can also aid in the formulation of higher-viscosity products.
- the product depends on the presence of soluble anionic surfactants to provide softening, its pH should preferably be above the pK a of the surfactant acids used to formulate it.
- aqueous detergent products which are a highly preferred embodiment of this invention, are nearly impossible to formulate below the pK a of the surfactant acids used, as these molecules are rather insoluble in water when in acid form.
- the product pH should be above about 4, although in certain cases, such as when carboxylic acid salts, which often have a pK a around 4 or 5, are used, the pH of the product can need to be above about 7 or 8 to ensure effective softening. It is desirable to buffer the formulation at whatever the target pH of the composition is.
- a method for conditioning textiles comprising the steps, in no particular order of: a. providing a laundry detergent or fabric softener composition comprising at least one anionic surfactant and at least one cationic polymer, in a ratio and concentration to effectively soften and condition fabrics under predetermined laundering conditions; b. contacting one or more articles with the composition at one or more points during a laundering process; and c. allowing the articles to dry or mechanically tumble-drying them,
- the softening parameter is greater than 40 and the composition comprises more than about 5% by weight of one or more anionic surfactants having an HLB of greater than about 4.
- Amounts of composition used will generally range between about lOg and about 300g total product per 3 kg of conditioned fibrous articles, depending on the particular embodiment chosen and other factors, such as consumer preferences, that influence product use behaviour.
- a consumer that would use the present invention could also be specifically instructed to contact the fabrics with the inventive composition with the purpose of simultaneously cleaning and softening the said fabrics. This approach would be recommended when the composition takes the form of a softening detergent to be dosed at the beginning of the wash cycle.
- compositions of this disclosure be formulated with low levels, if any at all, of any matter that is substantially insoluble in the solvent intended to be used to dilute the product.
- substantially insoluble shall mean that the material in question can individually be dissolved at a level of less than 0.001% in the specified solvent.
- substantially insoluble matter in aqueous systems include, but are not limited to aluminosilicates, pigments, clays and the like.
- solvent-insoluble inorganic matter can be attracted and coordinated to the cationic polymers of this invention, which are believed to attach themselves to the articles being washed. When this occurs, it is thought that these particles can create a rough effect on the fabric surface, which in turn reduces the perception of softness.
- liquid compositions are a preferred embodiment of this invention, and insoluble matter is often difficult to formulate into a liquid, it is further desirable to minimise its level in the product.
- the liquid compositions be substantially transparent for aesthetic reasons.
- a percent transmittance of light of greater than about 50 using a 1 centimetre cuvette at a wavelength of 570 nanometers wherein the composition is measured in the absence of dyes.
- transparency of the composition may be measured as having an absorbence (A) at 570 nanometers of less than about 0.3 which is in turn equivalent to percent transmittance of greater than about 50 using the same ' cuvette as above.
- absorbance and percent transmittance is:
- insoluble and substantially insoluble matter will be limited to less than 10% of the composition, more preferably 5% .
- the composition will be essentially free of substantially insoluble matter.
- the anionic surfactants used in this invention can be any anionic surfactant that is substantially water soluble.
- Water soluble surfactants are, unless otherwise noted, here defined to include surfactants which are soluble or dispersible to at least the extent of 0.01% by weight in distilled water at 25 C.
- “Anionic surfactants” are defined herein as amphiphilic molecules with an average molecular weight of less than about 10,000, comprising one or more functional groups that exhibit a net anionic charge when in aqueous solution at the normal wash pH of between 6 and 11. It is preferred that at least one of the anionic surfactants used in this invention be an alkali or alkaline earth metal salt of a natural or synthetic fatty acid containing between 4 and 30 carbon atoms.
- anionic compounds are the water soluble salts, particularly the alkali metal salts, of organic sulphur reaction products having in their molecular structure an alkyl radical containing from about 6 to 24 carbon atoms and a radical selected from the group consisting of sulphonic and sulphuric acid ester radicals.
- R ⁇ -COOM i where R is a primary or secondary alkyl group of 4 to 30 carbon atoms and M is a solubilising cation.
- the alkyl group represented by j 1 may represent a mixture of chain lengths and may be saturated or unsaturated, although it is
- R groups have a chain length of between 8 and 18 carbon atoms.
- suitable alkyl group sources include the fatty acids derived from coconut oil, tallow, tall oil and palm kernel oil. For the purposes of minimising odour, however, it is often desirable to use primarily saturated carboxylic acids. Such materials are well known to those skilled in the art, and are available from many commercial sources, such as Uniqema (Wilmington, Del.) and Twin Rivers Technologies (Quincy, Mass.).
- the solubilising cation, M may be any cation that confers water solubility to the product, although monovalent such moieties are generally preferred.
- solubilising cations for use with this invention include alkali metals such as sodium and potassium, which are particularly preferred, and amines such as triethanolammonium, ammonium and morpholinium.
- alkali metals such as sodium and potassium, which are particularly preferred
- amines such as triethanolammonium, ammonium and morpholinium.
- R 2 is a primary alkyl group of 8 to 18 carbon atoms
- the alkyl group R may have a mixture of chain lengths . It is preferred that at least
- the solubilising cation may be a range of cations which are in general monovalent and confer water solubility.
- An alkali metal notably sodium, is especially envisaged.
- Other possibilities are ammonium and substituted ammonium ions, such as trialkanolammonium or trialkylammonium.
- R is a primary alkyl group of 8 to 18 carbon atoms
- n has an average value in the range from 1 to 6 and M is a
- the alkyl group R may have a mixture of chain lengths. It is preferred that at least two-thirds
- R alkyl groups have a chain length of 8 to 14 carbon 3 atoms. This will be the case if R is coconut alkyl, for example.
- n has an average value of 2 to 5.
- Ether sulphates have been found to provide viscosity build in certain of the formulations of this invention, and thus are considered a preferred ingredient.
- R is an alkyl group of 6 to 16 atoms
- R is an alkyl group of 1 to 4 carbon atoms
- M is a solubilising cation.
- the group R may have a mixture of chain lengths .
- At least two-thirds of these groups have 6 to 12
- R is a straight chain alkyl, notably methyl or ethyl .
- R is an alkyl group of 8 to 18 carbon atoms
- Ar is a benzene ring C ⁇ H
- M is a solubilising cation.
- the group R may be a mixture of chain lengths .
- a mixture of isomers is typically used, and a number of different grades, such as "high 2-phenyl” and “low 2-phenyl” are commercially available for use depending on formulation needs.
- alkylbenzenes typically they are produced by the sulphonation of alkylbenzenes, which can be produced by either the HF-catalyzed alkylation of benzene with olefins or an AICI 3 -catalyzed process that alkylates benzene with chlor-paraffins, and are sold by, for example, Petresa (Chicago, 111.) and Sasol (Austin, Tex.). Straight chains of 11 to 14 carbon atoms are usually preferred.
- Paraffin sulphonates having 8 to 22 carbon atoms, preferably 12 to 16 carbon atoms, in the alkyl moiety. They are usually produced by the sulphoxidation of petrochemically- derived normal paraffins . These surfactants are commercially available as, for example, Hostapur SAS from Clariant (Charlotte, N.C.).
- Olefin sulphonates having 8 to 22 carbon atoms, preferably 12 to 16 carbon atoms.
- U.S. Patent No. 3,332,880 contains a description of suitable olefin sulphonates, and is incorporated herein by reference. Such materials are sold as, for example, Bio-Terge AS-40, which can be purchased from Stepan (Northfield, 111.)
- R and R are alkyl groups with chain lengths of between 2 and 16 carbons, and may be linear or branched, saturated or unsaturated.
- a preferred sulphosuccinate is sodium bis (2- ethylhexyl) sulphosuccinate, which is commercially available under the tradename Aerosol OT from Cytec Industries (West Paterson, N.J. ) .
- Organic phosphate based anionic surfactants include organic phosphate esters such as complex mono- or diester phosphates of hydroxyl- terminated alkoxide condensates, or salts thereof. Included in the organic phosphate esters are phosphate ester derivatives of polyoxyalkylated alkylaryl phosphate esters, of ethoxylated linear alcohols and ethoxylates of phenol . Also included are nonionic alkoxylates having a sodium alkylenecarboxylate moiety linked to a terminal hydroxyl group of the nonionic through an ether bond. Counterions to the salts of all the foregoing may be those of alkali metal, alkaline earth metal, ammonium, alkanolammonium and alkylammonium types.
- fatty acid ester sulphonates with formula:
- R is either methyl or ethyl; primary alkyl sulphates with the formula:
- R 1 OS03M wherein R 11 i .s a primary alkyl group of 10 to 18 carbon atoms and M is a sodium cation; and paraffin sulphonates, preferably with 12 to 16 carbon atoms to the alkyl moiety.
- anionic surfactants preferred for use with this formulation include isothionates, sulphated triglycerides, alcohol sulphates, ligninsulphonates, naphthelene sulphonates and alkyl naphthelene sulphonates and the like. Additional anionic surfactants, falling into the general definition but not specifically mentioned above, should also be considered within the scope of this invention.
- a water soluble cationic polymer is here defined to include polymers which, because of their molecular weight or monomer composition, are soluble or dispersible to at least the extent of 0.01% by weight in distilled water at 25 C.
- Water soluble cationic polymers include polymers in which one or more of the constituent monomers are selected from the list of copolymerisable cationic or amphoteric monomers. These monomer units contain a positive charge over at least a portion of the pH range 6-11.
- a partial listing of monomers can be found in the "International Cosmetic Ingredient Dictionary," 5th Edition, edited by J.A. Wenninger and G.N. McEwe , The Cosmetic, Toiletry, and Fragrance Association, Washington DC, 1993, incorporated herein by reference. Another source of such monomers can be found in "Encyclopedia of Polymers and Thickeners for Cosmetics", by R.Y. Lochhead and W.R. Fron, Cosmetics & Toiletries, vol. 108, May 1993, pp 95-135.
- the cationic polymers of this invention are effective at surprisingly low levels .
- the ratio of cationic polymer to total surfactant in the composition should preferably be no greater than about 1:5, and more preferably less than about 1:10.
- the ratio of cationic polymer to anionic surfactant in the composition, on a mass basis, should be less than about 1:4, and ideally less than about 1:10, as well.
- the preferred compositions of this invention contain low levels, if any at all, of builder. Generally, these will comprise less than 10%, preferably less than 7% and most preferably less than 5% by weight of total phosphate and zeolite.
- compositions of this disclosure comprise less than 2%, more preferably less than 1% and most preferably less than 0.5% anionic polymer.
- anionic polymer is defined as a molecule with a molecular weight in excess of about 10,000 daltons comprised of monomer units where at least one of the monomer units making up the polymer contains a negative charge over a portion of the wash pH range of pH 6 to pH 11, those monomer units not containing anionic charges being nonionic in nature.
- monomers useful in this invention may be represented structurally as etiologically unsaturated compounds as in formula I .
- R is hydrogen, hydroxyl, methoxy, or a Ci to C3 0
- R is hydrogen, or a
- Ci- 3 0 straight or branched alkyl, a C1-.3 0 straight or branched alkyl substituted aryl, aryl substituted C 1 --3 0 straight or branched alkyl radical, or a polyoxyalkene
- R is a heteroatomic alkyl or aromatic radical containing either one or more quaternised nitrogen atoms or one or more amine groups which possess a positive charge over a portion of the pH interval pH 6 to 11.
- amine groups can be further delineated as having a pK a of about 6 or greater.
- Examples of cationic monomers of formula I include, but are not limited to, co-poly 2-vinyl pyridine and its co-poly 2- vinyl N-alkyl quaternary pyridinium salt derivatives; co- poly 4-vinyl pyridine and its co-poly 4-vinyl N-alkyl quaternary pyridinium salt derivatives; co-poly 4- vinylbenzyltrialkylammonium salts such as co-poly 4- vinylbenzyltrimethylammonium salt; co-poly 2-vinyl piperidine and co-poly 2-vinyl piperidinium salt; co-poly 4- vinylpiperidine and co-poly 4-vinyl piperidinium salt; co- poly 3-alkyl 1-vinyl imidazolium salts such as co-poly 3- methyl 1-vinyl imidazolium salt; acrylamido and methacrylamido derivatives such as co-poly dimethyl aminopropylmethacrylamide, co-poly acryl
- cationic monomers suitable for this invention are co-poly vinyl amine and co-polyvinylammonium salt; co-poly diallylamine, co-poly methyldiallylamine, and co-poly diallydimethylammonium salt; and the ionene class of internal cationic monomers.
- This class includes co-poly ethylene imine , co-poly ethoxylated ethylene imine and co- poly quaternised ethoxylated ethylene imine; co-poly [ (dimethylimino) trimethylene (dimethylimino) hexamethylene disalt] , co-poly [ ⁇ diethy1imino) trimethylene (dimethylimino) trimethylene disalt] ; co-poly [ (dimethylimino) 2-hydroxypropyl salt] ; co-polyquarternium- 2, co-polyquarternium-17, and co-polyquarternium 18, as defined in the "International Cosmetic Ingredient Dictionary" edited by Wenninger and McEwen.
- useful polymers are the cationic co-poly amido-a ine having the chemical structure of formula II.
- molecular weight of structures II and III can vary between about 10,000 and 10,000,000 Daltons and each is terminated with an appropriate terminating group such as, for example, a methyl group.
- An additional, and highly preferred class of cationic monomers suitable for this invention are those arising from natural sources and include, but are not limited to, cocodimethylammonium hydroxypropyl oxyethyl cellulose, lauryldimethylammonium hydroxypropyl oxyethyl cellulose, stearyldimethylammonium hydroxypropyl oxyethyl cellulose, and stearyldimethylammonium hydroxyethyl cellulose; guar 2- hydroxy-3- (trimethylammonium) propyl ether salt; cellulose 2-hydroxyethyl 2-hydroxy 3- (trimethyl ammonio) propyl ether salt.
- the counterion of the comprising cationic co-monomer is freely chosen from the halides: chloride, bromide, and iodide; or from hydroxide, phosphate, sulphate, hydrosulphate, ethyl sulphate, methyl sulphate, formate, and acetate.
- cationic polymer useful for the present invention are the cationic silicones . These materials are characterised by repeating dialkylsiloxane interspersed or end terminated, or both, with cationic substituted siloxane units. Commercially available materials of this class are the Abil Quat polymers from Degussa Goldschmidt (Virginia) .
- the weight fraction of the cationic polymer which is composed of the above-described cationic monomer units can range from 1 to 100%, preferably from 10 to 100%, and most preferably from 15 to 80% of the entire polymer.
- the remaining monomer units comprising the cationic polymer are chosen from the class of anionic monomers and the class of nonionic monomers or solely from the class of nonionic monomers.
- the polymer is an amphoteric polymer while in the latter case it can be a cationic polymer, provided that no amphoteric co-monomers are present .
- Amphoteric polymers should also be considered within the scope of this disclosure, provided that the polymer unit possesses a net positive charge at one or more points over the wash pH range of pH 6 to 11.
- the anionic monomers comprise a class of monounsaturated compounds which possess a negative charge over the portion of the pH range from pH 6 to 11 in which the cationic monomers possess a positive charge.
- the nonionic monomers comprise a class of monounsaturated compounds which are uncharged over the pH range from pH 6 to 11 in which the cationic monomers possess a positive charge. It is expected that the wash pH at which this invention would be employed would either naturally fall within the above mentioned portion of the pH range 6-11 or, optionally, would be buffered in that range.
- a preferred class of both the anionic and the nonionic monomers are the vinyl (ethylenically unsaturated) substituted compounds corresponding to formula IV.
- R , R , and R are independently hydrogen, a C to C 3 alkyl, a carboxylate group or a carboxylate group substituted with a Ci to C 30 linear or branched heteroatomic alkyl or aromatic radical, a heteroatomic radical or a poly oxyalkene condensate of an aliphatic radical .
- the class of anionic monomers are represented by the compound described by formula IV in which at least one of the R , R , or R comprises a carboxylate, substituted carboxylate, phosphonate, substituted phosphonate, sulphate, substituted sulphate, sulphonate, or substituted sulphonate group.
- Preferred monomers in this class include but are not limited to ⁇ -ethacrylic acid, ⁇ -cyano acrylic acid, ⁇ , ⁇ -dimethacrylic acid, methylenemalonic acid, vinylacetic acid, allylacetic acid, acrylic acid, ethylidineacetic acid, propylidineacetic acid, crotonic acid, methacrylic acid, maleic acid, fumaric acid, itaconic acid, sorbic acid, angelic acid, cinnamic acid, ⁇ -styryl acrylic acid (l-carboxy-4-phenyl butadiene-1, 3) , citraconic acid, glutaconic acid, aconitic acid, ⁇ -phenylacrylic acid, ⁇ -acryloxy propionic acid, citraconic acid, vinyl benzoic acid, N-vinyl succinamidic acid, and mesaconic acid.
- co-poly styrene sulphonic acid 2-methacryloyloxymethane-1-sulphonic acid, 3-methacryloyloxypropane-l-sulphonic acid, 3- (vinyloxy)propane-l-sulphonic acid, ethylenesulphonic acid, vinyl sulphuric acid, 4-vinylphenyl sulphuric acid, ethylene phosphonic acid and vinyl phosphoric acid.
- Most preferred monomers include acrylic acid, methacrylic acid and maleic acid.
- the polymers useful in this invention may contain the above monomers and the alkali metal, alkaline earth metal, and ammonium salts thereof.
- the class of nonionic monomers are represented by the compounds of formula IV in which none of the R 15 , R 16 , or R 17 contain the above mentioned negative charge containing radicals.
- Preferred monomers in this class include, but are not limited to, vinyl alcohol; vinyl acetate; vinyl methyl ether; vinyl ethyl ether; acrylamide, methacrylamide and other modified acrylamides ; vinyl propionate; alkyl acrylates (esters of acrylic or methacrylic acid) ; and hydroxyalkyl acrylate esters.
- a second class of nonionic monomers include co-poly ethylene oxide, co-poly propylene oxide, and co-poly oxymethylene.
- a third, and highly preferred, class of nonionic monomers includes naturally derived materials such as hydroxyethylcellulose and guar gum.
- the preferred ratio of cationic polymer: total surfactant will be less than about 1:.4, whereas the preferred ratio of cationic polymer: anionic surfactant will be less than about 1:5, and the preferred ratio of cationic polymer: nonionic surfactant will be less than about 1:5. More preferably, the ratios of cationic polymer: total surfactant, cationic polymer : anionic surfactant and cationic polymer: total surfactant will be less than about 1:10. In terms of absolute fraction, this often means that the concentration of cationic polymer will generally be less than about 5%, preferably less than about 2% and most preferably less than about 1% of the total product mass.
- compositions of this invention will preferably comprise at least about 2%, more preferably at least about 5%, and most preferably at least about 10% of one or more surfactants with a hydrophilic/lipophilic balance (HLB) of more than about 4.
- HLB hydrophilic/lipophilic balance
- cationic polymers can be synthesised in, and are commercially available in, a number of different molecular weights.
- the water-soluble cationic or amphoteric polymer used in this invention be of an appropriate molecular weight.
- polymers that are too high in mass can entrap soils and prevent them from being removed.
- the use of cationic polymers with an average molecular weight of less than about 850,000 daltons, and especially those with an average molecular weight of less than 500,000 daltons can help to minimise this effect without significantly reducing the softening performance of properly formulated products .
- polymers with a molecular weight of about 10,000 daltons or less are believed to be too small to give an effective softening benefit.
- lower molecular weight polymers can even improve the softening performance of the product. This is believed to be due to dissolution kinetics; materials of too high a molecular weight can fail to dissolve fully during the wash cycle, rendering them unavailable for softening fabrics.
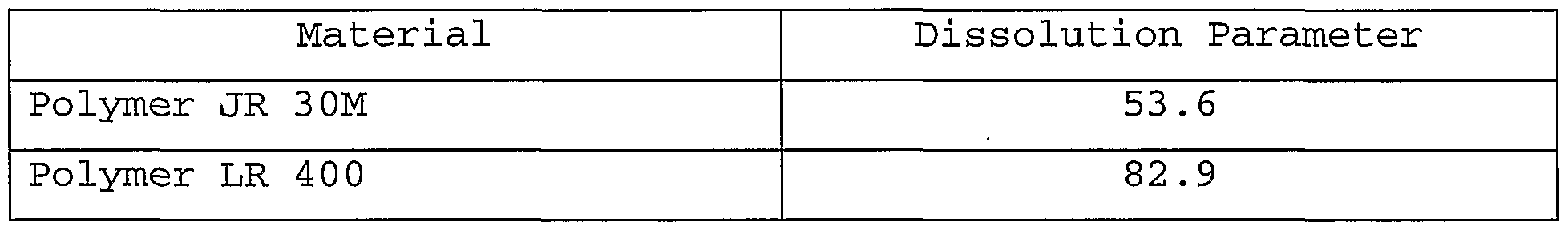
- the preferred powdered compositions of this invention include materials that have a dissolution parameter of more than about 55.
- Cleaning performance can further be improved by selecting a polymer with an appropriate level of cationic moiety.
- polymers with excessive levels of cationic charge can contribute to soil deposition, hindering the cleaning performance of either the fully formulated 2- in-1 detergent / softener or any laundry detergent that is used in conjunction with the compositions of this invention if they are to be standalone fabric softeners.
- Particularly appropriate materials are those that comprise less than about 2 % by weight, preferably less than about 1.8 % by weight of cationic nitrogen or phosphorus .
- the formulation may include one or more optional ingredients . While it is not necessary for these elements to be present in order to practice this invention, the use of such materials is often very helpful in rendering the formulation acceptable for consumer use.
- optional components include, but are not limited to: nonionic surfactants, amphoteric and zwitterionic surfactants, cationic surfactants, hydrotropes, fluorescent whitening agents, photobleaches, fibre lubricants, reducing agents, enzymes, enzyme stabilising agents, powder finishing agents, defoamers, builders, bleaches, bleach catalysts, soil release agents, antiredeposition agents, dye transfer inhibitors, buffers, colorants, fragrances, pro-fragrances, rheology modifiers, anti-ashing polymers, preservatives, insect repellents, soil repellents, water-resistance agents, suspending agents, aesthetic agents, structuring agents, sanitisers, solvents, fabric finishing agents, dye fixatives, wrinkle-reducing agents, fabric conditioning agents and deodorizers.
- nonionic surfactants amphoteric and zwitterionic surfactants, cationic surfactants, hydrotropes, fluorescent whitening agents, photobleaches, fibre lubricants, reducing agents,
- a soluble preservative may be added to this invention.
- Contamination of the product by microorganisms which can occur through both raw materials and consumer use, can have a number of undesirable effects. These include phase separation, the formation of bacterial and fungal colonies, the emission of objectionable odours and the like.
- the use of a preservative is especially preferred when the composition of this invention is a liquid, as these products tend to be especially susceptible to microbial growth.
- a broad-spectrum preservative which controls the growth of bacteria and fungi is preferred.
- Limited-spectrum preservatives which are only effective on a single group of microorganisms may also be used, either in combination with a broad-spectrum material or in a "package" of limited- spectrum preservatives with additive activities.
- biocidal materials i.e. substances that kill or destroy bacteria and fungi
- biostatic preservatives i.e. substances that regulate or retard the growth of microorganisms
- preservatives that are effective at low levels be used. Typically, they will be used only at an effective amount.
- the term "effective amount" means a level sufficient to control microbial growth in the product for a specified period of time, i.e., two weeks, such that the stability and physical properties of it are not negatively affected.
- an effective amount will be between about 0.00001% and about 0.5% of the total formula, based on weight. Obviously, however, the effective level will vary based on the material used, and one skilled in the art should be able to select an appropriate preservative and use level.
- Preferred preservatives for the compositions of this invention include organic sulphur compounds, halogenated materials, cyclic organic nitrogen compounds, low molecular weight aldehydes, quaternary ammonium materials, dehydroacetic acid, phenyl and phenoxy compounds and mixtures thereof .
- Examples of preferred preservatives for use in the compositions of the present invention include: a mixture of • about 77% 5-chloro-2-methyl-4-isothiazolin-3-one and about 23% 2-methyl-4-isothiazolin-3-one, which is sold commercially as a 1.5% aqueous solution by Rohm & Haas (Philadelphia, Pa.) under the trade name Kathon; 1,2- benzisothiazolin-3-one, which is sold commercially by Avecia (Wilmington, Del.) as, for example, a 20% solution in dipropylene glycol sold under the trade name Proxel GXL; and a 95:5 mixture of 1,3 bis (hydroxymethy1) -5, 5-dimethyl-2 , 4 imidazolidinedione and 3-butyl-2-iodopropynyl carbamate, which can be obtained, for example, as Glydant Plus from Lonza (Fair Lawn, N.J.).
- preservatives described above are generally only used at an effective amount to give product stability. It is conceivable, however, that they could also be used at higher levels in the compositions on this invention to provide a biostatic or antibacterial effect on the treated articles.
- Nonionic surfactants are useful in the context of this invention to both improve the cleaning properties of the compositions, when used as a detergent, and to contribute to product stability.
- nonionic surfactant shall be defined as amphiphilic molecules with a molecular weight of less than about 10,000, unless otherwise noted, which are substantially free of any functional groups that exhibit a net charge at the normal wash pH of 6-11. Any type of nonionic surfactant may be used, although preferred materials are further discussed below.
- R represents an alkyl chain of between 4 and 30 carbon atoms
- (EO) represents one unit of ethylene oxide monomer
- n has an average value between 0.5 and 20.
- R may be linear or branched.
- Such chemicals are generally produced by oligomerizing fatty alcohols with ethylene oxide in the presence of an effective amount catalyst, and are sold in the market as, for example, Neodols from Shell (Houston, Tex.) and Alfonics from Sasol (Austin, Tex.).
- fatty alcohol starting materials which are marketed under trademarks such as Alfol, Lial and Isofol from Sasol (Austin, Tex.) and Neodol, from Shell, may be manufactured by any of a number of processes known to those skilled in the art, and can be derived from natural or synthetic sources or a combination thereof.
- Commercial alcohol ethoxylates are typically mixtures, comprising varying chain
- fatty alcohol ethoxylates wherein R 18 represents an alkyl chain from 10-18 carbons and n is an average number between 5 and 12 are highly preferred.
- R 19 represents a linear or branched alkyl chain ranging from 4 to 30 carbons
- Ar is a phenyl (C 6 H 4 ) ring
- n is an oligomer chain comprised of an average of n moles of ethylene oxide.
- R 19 i .s comprised of between 8 and 12 carbons, and n is between 4 and 12.
- alkylphenol ethoxylate suitable for use in this invention is Triton X-100, available from Dow Chemical (Midland, Mich.) Ethylene Oxide / Propylene Oxide Block Polymers
- EO represents an ethylene oxide unit
- PO represents a propylene oxide unit
- x and y are numbers detailing the average number of moles ethylene oxide and propylene oxide in each mole of product .
- Such materials tend to have higher molecular weights than most nonionic surfactants, and as such can range between 1,000 and 30,000 daltons.
- BASF Mount Olive, N.J. manufactures a suitable set of derivatives and markets them under the Pluronic and Pluronic-R trademarks .
- nonionic surfactants should also be considered within the scope of this invention. These include condensates of alkanolamines with fatty acids, such as cocamide DEA, polyol-fatty acid esters, such as the Span series available from Uniqema (Wlimington, Del.), ethoxylated polyol-fatty acid esters, such as the Tween series available from Uniqema (Wilmington, Del.), Alkylpolyglucosides, such as the APG line available from Cognis (Gulph Mills, Pa.) and n- alkylpyrrolidones, such as the Surfadone series of products marketed by ISP (Wayne, N. ) . Furthermore, nonionic surfactants not specifically mentioned above, but within the definition, may also be used. Fluorescent Whi tening Agents
- Suitable fluorescent whitening agents include derivatives of diaminostilbenedisulphonic acid and their alkali metal salts. Particularly, the salts of 4, 4 ' -bis (2-anilino4- morpholino-1, 3, 5-triazinyl-6-amino) stilbene-2, 2 ' -disulphonic acid, and related compounds where the morpholino group is replaced by another nitrogen-comprising moiety, are preferred. Also preferred are brighteners of the 4,4'- bis (2-sulphostyryl) biphenyl type, which may optionally be blended with other fluorescent whitening agents at the option of the formulator.
- Typical fluorescent whitening agent levels in the preparations of this invention range between 0.001% and 1%, although a level between 0.1% and 0,3%, by mass, is normally used.
- Commercial supplies of acceptable fluorescent whitening agents can be sourced from, for example, Ciba Specialty Chemicals (High Point, N.C.) and Bayer (Pittsburgh, Pa.).
- Builders are often added to fabric cleaning compositions to complex and remove alkaline earth metal ions, which can interfere with the cleaning performance of a detergent by combining with anionic surfactants and removing them from the wash liquor.
- the preferred compositions of this invention especially when used as a combination detergent / softener, contain builders.
- Soluble builders such as alkali metal carbonates and alkali metal citrates, are particularly preferred, especially for the liquid embodiment of this invention.
- Other builders as further detailed below, may also be used, however. Often a mixture of builders, chosen from those described below and others known to those skilled in the art, will be used.
- Alkali and alkaline earth metal carbonates are suitable for use as builders in the compositions of this invention. They may be supplied and used either in anhydrous form, or including bound water. Particularly useful is sodium carbonate, or soda ash, which both is readily available on the commercial market and has an excellent environmental profile.
- the sodium carbonate used in this invention may either be natural or synthetic, and, depending on the needs of the formula, may be used in either dense or light form.
- Natural soda ash is generally mined as trona and further refined to a degree specified by the needs of the product it is used in.
- Synthetic ash is usually produced via the Solvay process or as a coproduct of other manufacturing operations, such as the synthesis of caprolactam. It is sometimes further useful to include a small amount of calcium carbonate in the builder formulation, to seed crystal formation and increase building efficacy.
- Organic detergent builders can also be used as nonphosphate builders in the present invention.
- organic builders include alkali metal citrates, succinates, malonates, fatty acid sulphonates, fatty acid carboxylates, nitrilotriacetates, oxydisuccinates, alkyl and alkenyl disuccinates, oxydiacetates, carboxymethyloxy succinates, ethylenediamine tetraacetates, tartrate monosuccinates, tartrate disuccinates, tartrate monoacetates, tartrate diacetates, oxidized starches, oxidized heteropolymeric polysaccharides, polyhydroxysulphonates, polycarboxylates such as polyacrylates, polymaleates, polyacetates, polyhydroxyacrylates, polyacrylate/polymaleate and polyacrylate/ polymethacrylate copolymers, acrylate/maleate/vinyl alcohol terpolymers, aminopolycarboxylates and polyacetal carboxylates,
- Such carboxylates are described in U.S. Patent Nos. 4,144,226, 4,146,495 and 4,686,062.
- Alkali metal citrates, nitrilotriacetates, oxydisuccinates, acrylate/maleate copolymers and acrylate/maleate/vinyl alcohol terpolymers are especially preferred nonphosphate builders. Phosphates
- compositions of the present invention which utilise a water-soluble phosphate builder typically contain this builder at a level of from 1 to 90% by weight of the composition.
- water-soluble phosphate builders are the alkali metal tripolyphosphates, sodium, potassium and ammonium pyrophosphate, sodium and potassium orthophosphate, sodium polymeta/phosphate in which the degree of polymerisation ranges from about 6 to 21, and salts of phytic acid. Sodium or potassium tripolyphosphate is most preferred.
- the preferred compositions of this invention comprise phosphates at a level of less than about 10% by weight, more preferably less than about 5% by weight.
- the most preferred compositions of this invention are formulated to be substantially free of phosphate builders .
- Zeolites may also be used as builders in the present invention.
- a number of zeolites suitable for incorporation into the products of this disclosure are available to the formulator, including the common zeolite 4A.
- zeolites of the MAP variety such as those taught in European Patent Application EP-B-384, 070 , which are sold commercially by, for example, Ineos Silicas (UK) , as Doucil A24, are also acceptable for incorporation.
- MAP is defined as an alkali metal aluminosilicate of zeolite P type having a silicon to aluminium ratio not exceeding 1.33, preferably within the range of from 0.90 to 1.33, more preferably within the range of from 0.90 to 1.20.
- zeolite MAP having a silicon to aluminium ratio not exceeding 1.07, more preferably about 1.00.
- the particle size of the zeolite is not critical. Zeolite A or zeolite MAP of any suitable particle size may be used. In any event, as zeolites are insoluble matter, it is advantageous to minimise their level in the compositions of this invention. As such, the preferred formulations contain less than about 10% of zeolite builder, while especially preferred compositions compress less than about 5% zeolite.
- enzyme stabiliser When enzymes, and especially proteases are used in liquid detergent formulations, it is often necessary to include a suitable quantity of enzyme stabiliser to temporarily deactivate it until it is used in the wash.
- suitable enzyme stabilisers are well-known to those skilled in the art, and include, for example, borates and polyols such as propylene glycol. Borates are especially suitable for use as enzyme stabilisers because in addition to this benefit, they can further buffer the pH of the detergent product over a wide range, thus providing excellent flexibility.
- a borate-based enzyme stabilisation system along with one or more cationic polymers that are at least partially comprised of carbohydrate moeities, stability problems can result if suitable co-stabilisers are not used. It is believed that this is the result of borates' natural affinity for hydroxyl groups, which can create an insoluble borate-polymer complex that precipitates from solution either over time or at cold temperatures . Incorporating into the formulation a co-stabiliser, which is normally a diol or polyol, sugar or other molecule with a large number of hydroxyl groups, can ordinarily prevent this.
- sorbitol used at a level that is at least about 0.8 times the level of borate in the system, more preferably 1.0 times the level of borate in the system and most preferably more than 1.43 times the level of borate in the system, is sorbitol, which is effective, inexpensive, biodegradable and readily available on the market.
- Similar materials including sugars such as glucose and sucrose, and other polyols such as propylene glycol, glycerol, mannitol, maltitol and xylitol, should also be considered within the scope of this invention.
- fibre lubricants in the formulation.
- Such ingredients are well known to those skilled in the art, and are intended to reduce the coefficient of friction between the fibres and yarns in articles being treated, both during and after the wash process . This effect can in turn improve the consumer's perception of softness, minimise the formation of wrinkles and prevent damage to textiles during the wash.
- "fibre lubricants” shall be considered non-cationic materials intended to lubricate fibres for the purpose of reducing the friction between fibres or yarns in an article comprising textiles which provide one or more wrinkle-reduction, fabric conditioning or protective benefit.
- suitable fibre lubricants include oily sugar derivatives, functionalised plant and animal-derived oils, silicones, mineral oils, natural and synthetic waxes and the like. Such ingredients often have low HLB values, less than about 10, although exceeding this level is not outside of the scope of this invention.
- Oily sugar derivatives suitable for use in this invention are taught in WO 98/16538, which is incorporated herein by reference. These are especially preferred as fibre lubricants, due to their ready availability and favorable environmental profile. When used in the compositions of this invention, such materials are typically present at a level between about 1% and about 10% of the finished composition.
- Another class of acceptable ingredients includes hydrophilically-modified plant and animal oils and synthetic triglycerides . Suitable and preferred hydrophilically modified plant, animal, and synthetic triglyceride oils and waxes have been identified as effective fibre lubricants.
- Such suitable plant derived triglyceride materials include hydrophilically modified triglyceride oils, e.g.
- Suitable animal derived triglyceride materials include hydrophilically modified fish oil, tallow, lard, and lanolin wax, and the like.
- An especially preferred functionalised oil is sulphated castor oil, which is sold commercially as, for example, Freedom SCO-75, available from Noveon (Cleveland, Ohio) .
- Various levels of derivatisation may be used provided that the derivatisation level is sufficient for the oil or wax derivatives to become soluble or dispersible in the solvent it is used in so as to exert a fibre lubrication effect during laundering of fabrics with a detergent containing the oil or wax derivative.
- this invention includes a functionalised oil of synthetic origin, preferably this oil is a silicone oil. More preferably, it is either a silicone poly ether or amino- functional silicone. If this invention incorporates a silicone polyether, it is preferably of one of the two general structures shown below: Structure A
- Me represents methyl
- EO represents ethylene oxide
- PO represents 1,2 propylene oxide
- Z represents either a hydrogen or a lower alkyl radical
- x, y, m, n are constants and can be varied to alter the properties of the functionalised silicone.
- a molecule of either structure can be used for the purposes of this invention.
- this molecule contains more than 30% silicone, more than 20% ethylene oxide and less than 30% propylene oxide by weight, and has a molecular weight of more than 5,000.
- An example of a suitable, commercially available such material is L-7622, available from Crompton Corporation, (Greenwich, Ct.)
- Amino-functional silicones come in a wide variety of structures, which are well-known to those skilled in the art. These are also useful in the context of this invention, although over time many of these materials can oxidize on fabrics, leading to yellowing. As this is not a desirable property of a fabric care composition, if an amino-functional silicone is used, preferably it is a hindered amine light stabilised product, which exhibits a greatly reduced tendency to show this behavior.
- a commercially available example of such a silicone is Hydrosoft, available from Rhodia - US (Cranbury, N.J.)
- fibre lubricant When the use of a fibre lubricant is elected, it will generally be present as between 0.1% and 15% of the total composition weight .
- An effective amount of a. bleach catalyst can also be present in the invention.
- a number of organic catalysts are available such as the sulphonimines as described in U.S. Patents 5,041,232; 5,047,163 and 5,463,115.
- Transition metal bleach catalysts are also useful, especially those based on manganese, iron, cobalt, titanium, molybdenum, nickel, chromium, copper, ruthenium, tungsten and mixtures thereof. These include simple water-soluble salts such as those of iron, manganese and cobalt as well as catalysts containing complex ligands .
- Suitable examples of manganese catalysts containing organic ligands are described in U.S. Pat. 4,728,455, U.S. Pat. 5,114,606, U.S. Pat 5,153,161, U.S. Pat. 5,194,416, U.S. Pat. 5,227,084, U.S. Pat. 5,244,594, U.S. Pat .5, 246, 612 , U.S. Pat. 5,246,621, U.S. Pat. 5,256,779, U.S. Pat. 5,274,147, U.S. Pat. 5,280,117 and European Pat. App. Pub. Nos. 544,440, 544,490, 549,271 and 549,272.
- Preferred examples of these catalysts include Mn 2 (u-O) 2 (1/ , 7- trimethyl-1, 4, 7-triazacyclononane) 2 (PF ⁇ ) 2 r Mn 2( u ⁇ °)l( u_ OAc) 2(1, ,7- trimethyl-1, 4, 7-triazacyclononane) 2 (CIO4) 2, Mn 4 (u-O) 6 (1 / 4, 7-triazacyclononane) 4 (0104) 4 , M Mn 4 (u- O) 1 (u-OAc) 2 (1, 4, 7-trimethyl-l, 4, 7-triazacyclononane) 2 (CIO 4 ) 3, Mn (1,4, 7-trimethyl-l, 4, 7-triazacyclononane) - (OCH 3 ) 3 (PF ⁇ ) , and mixtures thereof.
- metal-based bleach catalysts include those disclosed in U.S. Pat. 4,430,243 and U.S. Pat. 5,114,611.
- complexes of transition metals include Mn gluconate, Mn(CF 3 S0 3 )2 and binuclear Mn complexed with tetra-N-dentate and bi-N-dentate ligands, including
- Iron and manganese salts of aminocarboxylic acids in general are useful herein including iron and manganese aminocarboxylate salts disclosed for bleaching in the photographic colour processing arts.
- a particularly useful transition metal salt is derived from ethylenediaminedisuccinate and any complex of this ligand with iron or manganese.
- Another type of bleach catalyst is a water soluble complex of manganese (II), (III) , and/or (IV) with a ligand which is a non-carboxylate polyhydroxy compound having at least three consecutive C-OH groups.
- Preferred ligands include sorbitol, iditol, dulsitol, mannitol, xylitol, arabitol, adonitol, eso- erythritol, meso-inositol, lactose and mixtures thereof. Especially preferred is sorbitol.
- bleach catalysts are described, for example, in European ' Pat . App. Pub. Nos. 408,131 (cobalt complexes), 384,503 and 306,089 (metallo-porphyrins) , U.S. Pat. 4,728,455 (manganese/multidenate ligand), U.S. Pat. 4,711,748 (absorbed manganese on aluminosilicate) , U.S. Pat. 4,601,845 (aluminosilicate support with manganese, zinc or magnesium salt), U.S. Pat. 4,626,373 (manganese/1igand) , U.S. Pat. 4,119,557 (ferric complex), U.S. Pat. 4,430.243 (Chelants with manganese cations and non-catalytic metal cations), and U.S. Pat. 4,728,455 (manganese gluconates) .
- WO 96/23860 describe cobalt catalysts of the z . type [Co n L m X p ] Y z , where L is an organic ligand molecule containing more than one heteroatom selected from N, P, O and S; X is a co-ordinating species; n is preferably 1 or 2; m is preferably 1 to 5; p is preferably 0 to 4 and Y is a counterion.
- L is an organic ligand molecule containing more than one heteroatom selected from N, P, O and S; X is a co-ordinating species; n is preferably 1 or 2; m is preferably 1 to 5; p is preferably 0 to 4 and Y is a counterion.
- a catalyst is N,N'-
- Co (III) complexes with ammonia and mono-, bi ⁇ , tri- and tetradentate ligands such as [Co (NH 3 ) 5 OAC] + with Cl “ , OAc " , PF ⁇ , S ⁇ 4 ⁇ , and BF 4 anions.
- Certain transition-metal containing bleach catalysts can be prepared in the situ by the reaction of a transition-metal salt with a suitable chelating agent, for example, a mixture of manganese sulphate and ethylenediaminedisuccinate. Highly coloured transition metal-containing bleach catalysts may be co-processed with zeolites to reduce the colour impact .
- the bleach catalyst is typically incorporated at a level of about 0.0001 to about 10% by wt., preferably about 0.001 to about 5% by weight.
- hydrotropes Two types are typically used in detergent formulations and are applicable to this invention.
- the first of these are short-chain functionalised amphiphiles.
- short-chain amphiphiles include the alkali metal salts of xylenesulphonic acid, cumenesulphonic acid and octyl sulphonic acid, and the like.
- organic solvents and monohydric and polyhydric alcohols with a molecular weight of less than about 500 such as, for example, ethanol, isoporopanol, acetone, propylene glycol and glycerol, may also be used as hydrotropes.
- soil release Agents In order to prevent the resoiling of fabrics during and after the wash, one or more soil release agents may also be added to the products of this invention. Many different types of soil release agents are known to those skilled in the art, depending on the formulation in use and the desired benefit.
- the soil release agents useful in the context of this invention are typically either antiredeposition aids or stain-repelling finishes. Examples of anti-redeposition agents include soil release polymers, such as those described in WO 99/03963, which is incorporated herein by reference .
- the cationic polymers of this invention are particularly advantageous when used in conjunction with a stain-repelling finish.
- Such materials are typically either fluoropolymers or fluorosurfactants, although the use of other amphiphilic materials with extremely hydrophobic lyophobes, such as silicone surfactants, is also conceivable.
- suitable anionic fluorosurfactants are taught in U.S. Patent No. 6,040,053, which is incorporated herein by reference. Without wishing to be bound by theory, it is believed that the cationic polymers of this invention coordinate to the fabric surface and act as a substrate and deposition aid for the stain- repelling finish.
- Fabric was washed with a variety of products, the formulations for which are set forth hereinbelow.
- the washed fabric was then tested by consumer panels for perceived softening.
- product was added to a top loading Whirlpool washing machine that contained 17 gallons of water and 6 pounds of fabric. There were several 86% cotton/14% polyester hand towels in each machine along with 100% cotton sheets to bring the total weight of the fabric to 6 pounds .
- the temperature of the water for the washes was 32°C and the fabrics were washed for 12 minutes. After the rinse cycle, the fabrics were tumble dried. Two washes were done with each product.
- model liquid fabric softener TABLE 3. Model Liquid Fabric Softener
- St is the softening score for the formula being tested
- S d is the softening score for model detergent
- S c is the softening score for the model detergent + model liquid fabric softener.
- Detergency experiments were carried out via a modification of ATSM Method D 3050-87 using a Terg-O-Tometer (available from SCS, Fairfield, N.J.) set to 100 RPM in 1000 ml of 90F water standardised to 120ppm hardness with a Ca/Mg ratio of 2:1. Cloths were washed for 10 minutes with 2.21g of detergent, followed by a 2 minute rinse and then tumble dried. Two types of standard soil cloth were used for each experiment: pigment / synthetic sebum on cotton (WFK-lOd, available from WFK Testgewebe Gmbh, Bruggen-Bracht Germany ) and pigment / oil on poly-cotton (PC-9, Available from C.F.T, Vlaardingen, Holland).
- WFK-lOd available from WFK Testgewebe Gmbh, Bruggen-Bracht Germany
- PC-9 Available from C.F.T, Vlaardingen, Holland.
- Rp average reflectance of the monitor cloths after washing
- the softening results show that many of the cationic polymers tested yielded superior softening through the wash when used in combination with anionic surfactants . Specifically, the cationic polymers used in experimental formulations 8-19 were deemed to be superior .
- linear alkyl benzene sulphonic acid e . g . linear alkyl benzene sulphonic acid; neutralised fatty acids (including oleic; coconut; stearic) ; secondary alkane sulphonate; alcohol ethoxy sulphate
- one wash with a detergent prepared with and without the inventive cationic polymer/ anionic surfactant mixture is performed using approximately 90-150g of liquid detergent in 17 gallons (77 .3 litres) of water at 35°C .
- linear alkyl benzene sulphonic acid e . g. linear alkyl benzene sulphonic acid; neutralised fatty acids (including oleic ; coconut; stearic) ; secondary alkane sulphonate ; alcohol ethoxy sulphate
- one wash (either added at the beginning of the wash or beginning of the rinse cycle) with a softener prepared with and without the inventive cationic polymer/anionic surfactant mixture is performed using approximately 25-150g of liquid softener in 17 gallons (77 .3 litres) of water at 35°C .
- linear alkyl benzene sulphonic acid e.g. linear alkyl benzene sulphonic acid; neutralised fatty acids (including oleic ; coconut; stearic) ; secondary alkane sulphonate; alcohol ethoxy sulphate
- linear alkyl benzene sulphonic acid e . g . linear alkyl benzene sulphonic acid; neutralised fatty acids (including oleic ; coconut ; stearic) ; secondary alkane sulphonate; alcohol ethoxy sulphate
- linear alkyl benzene sulphonic acid e . g . linear alkyl benzene sulphonic acid; neutralised fatty acids (including oleic ; coconut; stearic) ; secondary alkane sulphonate; alcohol ethoxy sulphate
- one wash with a conditioner prepared with and without the inventive cationic polymer/anionic surfactant mixture is performed using approximately 40-150g of powdered fabric conditioner in 17 gallons (77 .3 litres) of water at 35°C .
- linear alkyl benzene sulphonic acid e.g. linear alkyl benzene sulphonic acid; neutralised fatty acids (including oleic; coconut; stearic) ; secondary alkane sulphonate; alcohol ethoxy sulphate
- one wash with a softener prepared with and without the inventive cationic polymer/anionic surfactant mixture is performed using 1 or 2 approximately 15-35g sheets in 17 gallons (77.3 litres) of water at 35°C.
- linear alkyl benzene sulphonic acid e.g. linear alkyl benzene sulphonic acid; neutralised fatty acids (including oleic; coconut; stearic); secondary alkane sulphonate; alcohol ethoxy sulphate
- one wash with a softener prepared with and without the inventive cationic polymer/anionic surfactant mixture is performed using 1 or 2 approximately 20-50g sachets in 17 gallons (77 .3 litres) of water at 35°C .
- one wash with prepared with and without the inventive cationic polymer/anionic fluorocarbon surfactant mixture added at the beginning of the rinse cycle is performed using approximately 50-200g of stain repellency liquid in 17 gallons (77.3 litres) of water.
- inventive cationic polymer/anionic surfactant mixtures may be incorporated in liquid, powdered/granular, semi-solid or paste, moulded solid or tablet, and water soluble sheet compositions.
- the Softening parameter of the Comparative Formulation 1 was determined to be 35.
- EXAMPLE 6 This example shows that the use of polymer JR in an anionic surfactant-containing liquid detergent in combination with a polysaccharide polymer such as xanthan gum leads to an unacceptable product .
- Comparative formulation 3 employs ratios taught in U.S. Pat. Appl. Nos, 2002/0151454, 2002/0155981, 2002/0055451 and 2002/0058604.
- the cationic polymer : surfactant ratio of comparative formulation 3 is 1:5; the cationic polymer : anionic surfactant ratio is 2:7; the cationic polymer : nonionic surfactant ratio is 1:3.
- the cationic polymer : surfactant ratio of formulation 29 is 1:66.7; the cationic polymer: anionic surfactant ratio is 1: 46.7; the cationic polymer : nonionic surfactant ratio is 1: 20.
- the viscosity of each formula was measured with a Brookfield LV Viscometer (available from Brookfield Engineering, Stoughton, MA ) .
- the viscosity of comparative formulation 3 could not be measured, as the product was sufficiently thick to be out of the range (1,000,000 cP) of the viscometer.
- the viscosity of formulation 28 was measured as 430 cP with a #1 spindle at 12 rpm, which is well within the accepted range for consumer liquid laundry detergents (50-1000 cP) .
- liquid laundry detergent formulations comprising zeolites, layered silicates and phosphates, along with cationic polymers tend to be unstable and aesthetically unacceptable for commercial sale.
- U.S. Pat. Appl. Nos. 2002/0151454, 2002/0155981, 2002/0055451 and 2002/0058604 teach the use of one or more of zeolite, layered silicate and phosphate.
- Comparative Formulation 4 - Comprises zeolite
- va a e rom cas, o e Available from Amerchol division of Dow Chemical, Edison N.J.
- Comparative Formulation 6 - comprises layered silicate
- Phase A in each was made and kept at 140F (60°C) until it was added at the point designated in the formula. Between additions, 5 minutes of constant mixing using an IKA RW 20 DZM.n mechanical stirrer equipped with a double-blade impeller took place to allow uniform blending to take place.
- the following example illustrates how the cleaning performance of fabric softening compositions comprising cationic polymers can be improved without negatively impacting their conditioning properties by selecting a polymer of appropriate molecular weight and charge density.
- Formulation 30 Comprises high molecular- weight, highly substituted cationic polymer.
- Formulation 31 Comprises lower molecular-weight, highly substituted cationic polymer.
- Formulation 32 Comprises lower molecular-weight, less substituted cationic polymer.
- Example 10 The following example demonstrates how the selection of a lower molecular-weight polymer can also improve softening performance in applications such as powdered detergent compositions .
- Formulation 33 Powdered Detergent comprising high molecular-weight cationic polymer
- Formulation 34 Powdered Detergent comprising low molecular-weight cationic polymer
- the ingredients were first combined and spray- dried into a base powder. Following this, the sodium cocoate and polymer were post-dosed, and all components were agitated for 60 seconds in a Waring Laboratory Blender on the low speed. For each formulation, the powder was dosed at 66.79g/wash.
- the following example illustrates how the odour profile of fabric softening compositions comprising cationic polymers can be improved without negatively impacting their conditioning properties by selecting a pH value between the pK a of coconut oil fatty acid, one of the anionic surfactant acids and the pK a of the amino or phosphino group that is used to quaternise the selected polymer.
- the pK a of trimethylamine, the amino group used to quaternise Polymer LR 400 is 9.8. Prior to pH adjustment, when the pH of the formulations was approximately 5, they were physically unstable, as the pK a of the fatty acid had not been reached.
Abstract
Description
Claims
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CA2514766A CA2514766C (en) | 2003-02-03 | 2004-01-23 | Laundry cleansing and conditioning compositions |
| EP04704608.1A EP1590426B1 (en) | 2003-02-03 | 2004-01-23 | Laundry cleansing and conditioning compositions |
| BRPI0407114A BRPI0407114B1 (en) | 2003-02-03 | 2004-01-23 | laundry composition |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/357,248 | 2003-02-03 | ||
| US10/357,248 US20040152616A1 (en) | 2003-02-03 | 2003-02-03 | Laundry cleansing and conditioning compositions |
| US10/446,202 US6949498B2 (en) | 2003-02-03 | 2003-05-27 | Laundry cleansing and conditioning compositions |
| US10/446,202 | 2003-05-27 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2004069979A2 true WO2004069979A2 (en) | 2004-08-19 |
| WO2004069979A3 WO2004069979A3 (en) | 2005-11-24 |
Family
ID=32853092
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2004/000668 WO2004069979A2 (en) | 2003-02-03 | 2004-01-23 | Laundry cleansing and conditioning compositions |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP1590426B1 (en) |
| BR (1) | BRPI0407114B1 (en) |
| CA (1) | CA2514766C (en) |
| WO (1) | WO2004069979A2 (en) |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005103219A1 (en) * | 2004-04-21 | 2005-11-03 | Henkel Kommanditgesellschaft Auf Aktien | Textile care product |
| WO2006012984A1 (en) * | 2004-08-03 | 2006-02-09 | Unilever Plc | Softening laundry detergent |
| WO2006105991A1 (en) * | 2005-04-05 | 2006-10-12 | Unilever Plc | Fabric softening composition with cationic polymer, soap, and amphoteric surfactant |
| WO2007107215A1 (en) * | 2006-03-18 | 2007-09-27 | Unilever Plc | Fabric treatment composition and process for preparation thereof |
| WO2010025116A1 (en) * | 2008-08-28 | 2010-03-04 | The Procter & Gamble Company | Fabric care compositions, process of making, and method of use |
| WO2010072627A1 (en) * | 2008-12-22 | 2010-07-01 | Unilever Plc | Laundry compositions |
| WO2010072628A1 (en) * | 2008-12-22 | 2010-07-01 | Unilever Plc | Laundry compositions |
| WO2011002475A1 (en) * | 2009-06-30 | 2011-01-06 | The Procter & Gamble Company | Fabric care compositions, process of making, and method of use |
| EP2399980A1 (en) | 2010-06-24 | 2011-12-28 | The Procter & Gamble Company | Stable compositions comprising cationic cellulose polymer and cellulase |
| US8529927B2 (en) | 2004-04-30 | 2013-09-10 | Allergan, Inc. | Alpha-2 agonist polymeric drug delivery systems |
| EP2318500B1 (en) | 2008-08-28 | 2018-02-28 | The Procter and Gamble Company | Methods for providing a benefit |
| US10195134B2 (en) | 2014-11-25 | 2019-02-05 | Dow Global Technologies Llc | Personal care compositions containing cationic polymers |
| EP1975226B2 (en) † | 2007-03-20 | 2019-03-13 | The Procter and Gamble Company | Liquid treatment composition |
| US10507176B2 (en) | 2014-11-25 | 2019-12-17 | Dow Global Technologies Llc | Hair care compositions containing cationic polymers |
| EP3926030A1 (en) | 2020-06-18 | 2021-12-22 | The Procter & Gamble Company | Water-soluble unit dose article comprising a polyvinylalcohol film and a cationic poly alpha-1,6-glucan ether compound |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2553077B1 (en) | 2010-03-31 | 2015-10-14 | Henkel AG & Co. KGaA | Washing composition for sensitive textiles |
Citations (55)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3332880A (en) | 1965-01-04 | 1967-07-25 | Procter & Gamble | Detergent composition |
| US3472840A (en) | 1965-09-14 | 1969-10-14 | Union Carbide Corp | Quaternary nitrogen-containing cellulose ethers |
| DE2321001A1 (en) | 1972-04-28 | 1973-11-15 | Procter & Gamble | COMPOSITION WITH CRYSTALLIZATION INOCULATION |
| US4119557A (en) | 1975-12-18 | 1978-10-10 | Lever Brothers Company | Bleaching compositions and process for cleaning fabrics |
| US4144226A (en) | 1977-08-22 | 1979-03-13 | Monsanto Company | Polymeric acetal carboxylates |
| US4146495A (en) | 1977-08-22 | 1979-03-27 | Monsanto Company | Detergent compositions comprising polyacetal carboxylates |
| US4299817A (en) | 1977-08-24 | 1981-11-10 | Union Carbide Corporation | Hair care compositions |
| US4430243A (en) | 1981-08-08 | 1984-02-07 | The Procter & Gamble Company | Bleach catalyst compositions and use thereof in laundry bleaching and detergent compositions |
| US4601845A (en) | 1985-04-02 | 1986-07-22 | Lever Brothers Company | Bleaching compositions containing mixed metal cations adsorbed onto aluminosilicate support materials |
| US4626373A (en) | 1983-11-08 | 1986-12-02 | Lever Brothers Company | Manganese adjuncts, their preparation and use |
| US4686062A (en) | 1985-02-23 | 1987-08-11 | The Procter & Gamble Company | Detergent composition |
| US4711748A (en) | 1985-12-06 | 1987-12-08 | Lever Brothers Company | Preparation of bleach catalyst aggregates of manganese cation impregnated aluminosilicates by high velocity granulation |
| US4728455A (en) | 1986-03-07 | 1988-03-01 | Lever Brothers Company | Detergent bleach compositions, bleaching agents and bleach activators |
| EP0306089A2 (en) | 1987-09-04 | 1989-03-08 | Unilever N.V. | Metallo-porphirins as bleach catalyst and process for cleaning fabrics |
| US4844821A (en) | 1988-02-10 | 1989-07-04 | The Procter & Gamble Company | Stable liquid laundry detergent/fabric conditioning composition |
| US4913828A (en) | 1987-06-10 | 1990-04-03 | The Procter & Gamble Company | Conditioning agents and compositions containing same |
| EP0384503A1 (en) | 1989-02-22 | 1990-08-29 | Unilever N.V. | Metallo-porphyrins for use as bleach catalyst |
| EP0408131A2 (en) | 1989-07-10 | 1991-01-16 | Unilever N.V. | Bleach activation |
| US5041232A (en) | 1990-03-16 | 1991-08-20 | Lever Brothers Company, Division Of Conopco, Inc. | Sulfonimines as bleach catalysts |
| US5047163A (en) | 1990-03-16 | 1991-09-10 | Lever Brothers Company, Division Of Conopco, Inc. | Activation of bleach precursors with sulfonimines |
| US5073274A (en) | 1988-02-08 | 1991-12-17 | The Procter & Gamble Co. | Liquid detergent containing conditioning agent and high levels of alkyl sulfate/alkyl ethoxylated sulfate |
| US5114606A (en) | 1990-02-19 | 1992-05-19 | Lever Brothers Company, Division Of Conopco, Inc. | Bleaching composition comprising as a bleaching catalyst a complex of manganese with a non-carboxylate polyhydroxy ligand |
| US5114611A (en) | 1989-04-13 | 1992-05-19 | Lever Brothers Company, Divison Of Conopco, Inc. | Bleach activation |
| US5153161A (en) | 1991-11-26 | 1992-10-06 | Lever Brothers Company, Division Of Conopco, Inc. | Synthesis of manganese oxidation catalyst |
| US5194416A (en) | 1991-11-26 | 1993-03-16 | Lever Brothers Company, Division Of Conopco, Inc. | Manganese catalyst for activating hydrogen peroxide bleaching |
| EP0544490A1 (en) | 1991-11-26 | 1993-06-02 | Unilever Plc | Detergent bleach compositions |
| EP0544440A2 (en) | 1991-11-20 | 1993-06-02 | Unilever Plc | Bleach catalyst composition, manufacture and use thereof in detergent and/or bleach compositions |
| EP0549271A1 (en) | 1991-12-20 | 1993-06-30 | Unilever Plc | Bleach activation |
| EP0549272A1 (en) | 1991-12-20 | 1993-06-30 | Unilever Plc | Bleach activation |
| US5227084A (en) | 1991-04-17 | 1993-07-13 | Lever Brothers Company, Division Of Conopco, Inc. | Concentrated detergent powder compositions |
| US5244594A (en) | 1990-05-21 | 1993-09-14 | Lever Brothers Company, Division Of Conopco, Inc. | Bleach activation multinuclear manganese-based coordination complexes |
| US5246612A (en) | 1991-08-23 | 1993-09-21 | Lever Brothers Company, Division Of Conopco, Inc. | Machine dishwashing composition containing peroxygen bleach, manganese complex and enzymes |
| US5256779A (en) | 1992-06-18 | 1993-10-26 | Lever Brothers Company, Division Of Conopco, Inc. | Synthesis of manganese oxidation catalyst |
| US5274147A (en) | 1991-07-11 | 1993-12-28 | Lever Brothers Company, Division Of Conopco, Inc. | Process for preparing manganese complexes |
| US5280117A (en) | 1992-09-09 | 1994-01-18 | Lever Brothers Company, A Division Of Conopco, Inc. | Process for the preparation of manganese bleach catalyst |
| EP0384070B1 (en) | 1988-11-03 | 1995-09-06 | Unilever Plc | Zeolite P, process for its preparation and its use in detergent compositions |
| WO1996023859A1 (en) | 1995-02-02 | 1996-08-08 | The Procter & Gamble Company | Automatic dishwashing compositions comprising cobalt catalysts |
| WO1996023861A1 (en) | 1995-02-02 | 1996-08-08 | The Procter & Gamble Company | Automatic dishwashing compositions comprising cobalt (iii) catalysts |
| WO1996023860A1 (en) | 1995-02-02 | 1996-08-08 | The Procter & Gamble Company | Automatic dishwashing compositions comprising cobalt chelated catalysts |
| US5559261A (en) | 1995-07-27 | 1996-09-24 | The Procter & Gamble Company | Method for manufacturing cobalt catalysts |
| WO1998004239A2 (en) | 1996-07-25 | 1998-02-05 | The Procter & Gamble Company | Shampoo compositions |
| WO1998004241A2 (en) | 1996-07-25 | 1998-02-05 | The Procter & Gamble Company | Shampoo compositions |
| WO1998016538A1 (en) | 1996-10-16 | 1998-04-23 | Unilever Plc | Fabric softening composition |
| WO1999003963A1 (en) | 1997-07-15 | 1999-01-28 | Unilever Plc | Liquid detergent compositions and process for their preparation |
| US6040053A (en) | 1996-07-19 | 2000-03-21 | Minnesota Mining And Manufacturing Company | Coating composition having anti-reflective and anti-fogging properties |
| WO2000070005A1 (en) | 1999-05-17 | 2000-11-23 | Unilever Plc | Fabric softening compositions |
| US6369018B1 (en) | 1998-12-16 | 2002-04-09 | Unilever Home & Personal Care, Usa, Division Of Conopco, Inc. | Process for preparing pourable, transparent/translucent liquid detergent with non-continuous suspending system |
| US20020055451A1 (en) | 2000-09-08 | 2002-05-09 | Ditmar Kischkel | Detergent tablets |
| US20020058604A1 (en) | 2000-09-08 | 2002-05-16 | Ditmar Kischkel | Laundry detergent tablets |
| US6461387B1 (en) | 1995-03-06 | 2002-10-08 | Lever Brothers Company, Division Of Conopco, Inc. | Dry cleaning system with low HLB surfactant |
| US20020151454A1 (en) | 2000-09-08 | 2002-10-17 | Ditmar Kischkel | Laundry detergent compositions |
| US20020155981A1 (en) | 2000-09-08 | 2002-10-24 | Ditmar Kischkel | Laundry detergent compositions |
| US20020169097A1 (en) | 1997-11-21 | 2002-11-14 | Chandrika Kasturi | Liquid detergent compositions comprising polymeric suds enhancers |
| US6492322B1 (en) | 1996-09-19 | 2002-12-10 | The Procter & Gamble Company | Concentrated quaternary ammonium fabric softener compositions containing cationic polymers |
| US20020187909A1 (en) | 2001-02-28 | 2002-12-12 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Unit dose cleaning product |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5733854A (en) * | 1996-10-25 | 1998-03-31 | Rhone-Poulenc Inc. | Cleaning compositions including derivatized guar gum composition including nonionic and cationic groups which demonstrate excellent solution clarity properties |
| US6533873B1 (en) * | 1999-09-10 | 2003-03-18 | Unilever Home & Personal Care Usa Division Of Conopco, Inc. | Suspending clear cleansing formulation |
| GB0009029D0 (en) * | 2000-04-12 | 2000-05-31 | Unilever Plc | Laundry wash compositions |
| US6903061B2 (en) * | 2000-08-28 | 2005-06-07 | The Procter & Gamble Company | Fabric care and perfume compositions and systems comprising cationic silicones and methods employing same |
| US7067499B2 (en) * | 2002-05-06 | 2006-06-27 | Hercules Incorporated | Cationic polymer composition and its use in conditioning applications |
-
2004
- 2004-01-23 EP EP04704608.1A patent/EP1590426B1/en not_active Revoked
- 2004-01-23 CA CA2514766A patent/CA2514766C/en not_active Expired - Fee Related
- 2004-01-23 BR BRPI0407114A patent/BRPI0407114B1/en not_active IP Right Cessation
- 2004-01-23 WO PCT/EP2004/000668 patent/WO2004069979A2/en active Application Filing
Patent Citations (57)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3332880A (en) | 1965-01-04 | 1967-07-25 | Procter & Gamble | Detergent composition |
| US3472840A (en) | 1965-09-14 | 1969-10-14 | Union Carbide Corp | Quaternary nitrogen-containing cellulose ethers |
| DE2321001A1 (en) | 1972-04-28 | 1973-11-15 | Procter & Gamble | COMPOSITION WITH CRYSTALLIZATION INOCULATION |
| US4119557A (en) | 1975-12-18 | 1978-10-10 | Lever Brothers Company | Bleaching compositions and process for cleaning fabrics |
| US4144226A (en) | 1977-08-22 | 1979-03-13 | Monsanto Company | Polymeric acetal carboxylates |
| US4146495A (en) | 1977-08-22 | 1979-03-27 | Monsanto Company | Detergent compositions comprising polyacetal carboxylates |
| US4299817A (en) | 1977-08-24 | 1981-11-10 | Union Carbide Corporation | Hair care compositions |
| US4430243A (en) | 1981-08-08 | 1984-02-07 | The Procter & Gamble Company | Bleach catalyst compositions and use thereof in laundry bleaching and detergent compositions |
| US4626373A (en) | 1983-11-08 | 1986-12-02 | Lever Brothers Company | Manganese adjuncts, their preparation and use |
| US4686062A (en) | 1985-02-23 | 1987-08-11 | The Procter & Gamble Company | Detergent composition |
| US4601845A (en) | 1985-04-02 | 1986-07-22 | Lever Brothers Company | Bleaching compositions containing mixed metal cations adsorbed onto aluminosilicate support materials |
| US4711748A (en) | 1985-12-06 | 1987-12-08 | Lever Brothers Company | Preparation of bleach catalyst aggregates of manganese cation impregnated aluminosilicates by high velocity granulation |
| US4728455A (en) | 1986-03-07 | 1988-03-01 | Lever Brothers Company | Detergent bleach compositions, bleaching agents and bleach activators |
| US4913828A (en) | 1987-06-10 | 1990-04-03 | The Procter & Gamble Company | Conditioning agents and compositions containing same |
| EP0306089A2 (en) | 1987-09-04 | 1989-03-08 | Unilever N.V. | Metallo-porphirins as bleach catalyst and process for cleaning fabrics |
| US5073274A (en) | 1988-02-08 | 1991-12-17 | The Procter & Gamble Co. | Liquid detergent containing conditioning agent and high levels of alkyl sulfate/alkyl ethoxylated sulfate |
| US4844821A (en) | 1988-02-10 | 1989-07-04 | The Procter & Gamble Company | Stable liquid laundry detergent/fabric conditioning composition |
| EP0384070B1 (en) | 1988-11-03 | 1995-09-06 | Unilever Plc | Zeolite P, process for its preparation and its use in detergent compositions |
| EP0384503A1 (en) | 1989-02-22 | 1990-08-29 | Unilever N.V. | Metallo-porphyrins for use as bleach catalyst |
| US5114611A (en) | 1989-04-13 | 1992-05-19 | Lever Brothers Company, Divison Of Conopco, Inc. | Bleach activation |
| EP0408131A2 (en) | 1989-07-10 | 1991-01-16 | Unilever N.V. | Bleach activation |
| US5114606A (en) | 1990-02-19 | 1992-05-19 | Lever Brothers Company, Division Of Conopco, Inc. | Bleaching composition comprising as a bleaching catalyst a complex of manganese with a non-carboxylate polyhydroxy ligand |
| US5463115A (en) | 1990-03-16 | 1995-10-31 | Lever Brothers Company, Division Of Conopco, Inc. | Sulfonimines as bleach catalysts |
| US5041232A (en) | 1990-03-16 | 1991-08-20 | Lever Brothers Company, Division Of Conopco, Inc. | Sulfonimines as bleach catalysts |
| US5047163A (en) | 1990-03-16 | 1991-09-10 | Lever Brothers Company, Division Of Conopco, Inc. | Activation of bleach precursors with sulfonimines |
| US5244594A (en) | 1990-05-21 | 1993-09-14 | Lever Brothers Company, Division Of Conopco, Inc. | Bleach activation multinuclear manganese-based coordination complexes |
| US5246621A (en) | 1990-05-21 | 1993-09-21 | Lever Brothers Company, Division Of Conopco, Inc. | Bleach activation by manganese-based coordination complexes |
| US5227084A (en) | 1991-04-17 | 1993-07-13 | Lever Brothers Company, Division Of Conopco, Inc. | Concentrated detergent powder compositions |
| US5274147A (en) | 1991-07-11 | 1993-12-28 | Lever Brothers Company, Division Of Conopco, Inc. | Process for preparing manganese complexes |
| US5246612A (en) | 1991-08-23 | 1993-09-21 | Lever Brothers Company, Division Of Conopco, Inc. | Machine dishwashing composition containing peroxygen bleach, manganese complex and enzymes |
| EP0544440A2 (en) | 1991-11-20 | 1993-06-02 | Unilever Plc | Bleach catalyst composition, manufacture and use thereof in detergent and/or bleach compositions |
| US5153161A (en) | 1991-11-26 | 1992-10-06 | Lever Brothers Company, Division Of Conopco, Inc. | Synthesis of manganese oxidation catalyst |
| US5194416A (en) | 1991-11-26 | 1993-03-16 | Lever Brothers Company, Division Of Conopco, Inc. | Manganese catalyst for activating hydrogen peroxide bleaching |
| EP0544490A1 (en) | 1991-11-26 | 1993-06-02 | Unilever Plc | Detergent bleach compositions |
| EP0549271A1 (en) | 1991-12-20 | 1993-06-30 | Unilever Plc | Bleach activation |
| EP0549272A1 (en) | 1991-12-20 | 1993-06-30 | Unilever Plc | Bleach activation |
| US5256779A (en) | 1992-06-18 | 1993-10-26 | Lever Brothers Company, Division Of Conopco, Inc. | Synthesis of manganese oxidation catalyst |
| US5280117A (en) | 1992-09-09 | 1994-01-18 | Lever Brothers Company, A Division Of Conopco, Inc. | Process for the preparation of manganese bleach catalyst |
| WO1996023859A1 (en) | 1995-02-02 | 1996-08-08 | The Procter & Gamble Company | Automatic dishwashing compositions comprising cobalt catalysts |
| WO1996023861A1 (en) | 1995-02-02 | 1996-08-08 | The Procter & Gamble Company | Automatic dishwashing compositions comprising cobalt (iii) catalysts |
| WO1996023860A1 (en) | 1995-02-02 | 1996-08-08 | The Procter & Gamble Company | Automatic dishwashing compositions comprising cobalt chelated catalysts |
| US6461387B1 (en) | 1995-03-06 | 2002-10-08 | Lever Brothers Company, Division Of Conopco, Inc. | Dry cleaning system with low HLB surfactant |
| US5559261A (en) | 1995-07-27 | 1996-09-24 | The Procter & Gamble Company | Method for manufacturing cobalt catalysts |
| US6040053A (en) | 1996-07-19 | 2000-03-21 | Minnesota Mining And Manufacturing Company | Coating composition having anti-reflective and anti-fogging properties |
| WO1998004241A2 (en) | 1996-07-25 | 1998-02-05 | The Procter & Gamble Company | Shampoo compositions |
| WO1998004239A2 (en) | 1996-07-25 | 1998-02-05 | The Procter & Gamble Company | Shampoo compositions |
| US6492322B1 (en) | 1996-09-19 | 2002-12-10 | The Procter & Gamble Company | Concentrated quaternary ammonium fabric softener compositions containing cationic polymers |
| WO1998016538A1 (en) | 1996-10-16 | 1998-04-23 | Unilever Plc | Fabric softening composition |
| WO1999003963A1 (en) | 1997-07-15 | 1999-01-28 | Unilever Plc | Liquid detergent compositions and process for their preparation |
| US20020169097A1 (en) | 1997-11-21 | 2002-11-14 | Chandrika Kasturi | Liquid detergent compositions comprising polymeric suds enhancers |
| US6369018B1 (en) | 1998-12-16 | 2002-04-09 | Unilever Home & Personal Care, Usa, Division Of Conopco, Inc. | Process for preparing pourable, transparent/translucent liquid detergent with non-continuous suspending system |
| WO2000070005A1 (en) | 1999-05-17 | 2000-11-23 | Unilever Plc | Fabric softening compositions |
| US20020055451A1 (en) | 2000-09-08 | 2002-05-09 | Ditmar Kischkel | Detergent tablets |
| US20020058604A1 (en) | 2000-09-08 | 2002-05-16 | Ditmar Kischkel | Laundry detergent tablets |
| US20020151454A1 (en) | 2000-09-08 | 2002-10-17 | Ditmar Kischkel | Laundry detergent compositions |
| US20020155981A1 (en) | 2000-09-08 | 2002-10-24 | Ditmar Kischkel | Laundry detergent compositions |
| US20020187909A1 (en) | 2001-02-28 | 2002-12-12 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Unit dose cleaning product |
Cited By (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005103219A1 (en) * | 2004-04-21 | 2005-11-03 | Henkel Kommanditgesellschaft Auf Aktien | Textile care product |
| US8529927B2 (en) | 2004-04-30 | 2013-09-10 | Allergan, Inc. | Alpha-2 agonist polymeric drug delivery systems |
| WO2006012984A1 (en) * | 2004-08-03 | 2006-02-09 | Unilever Plc | Softening laundry detergent |
| WO2006105991A1 (en) * | 2005-04-05 | 2006-10-12 | Unilever Plc | Fabric softening composition with cationic polymer, soap, and amphoteric surfactant |
| WO2007107215A1 (en) * | 2006-03-18 | 2007-09-27 | Unilever Plc | Fabric treatment composition and process for preparation thereof |
| EP1975226B2 (en) † | 2007-03-20 | 2019-03-13 | The Procter and Gamble Company | Liquid treatment composition |
| EP2318500B1 (en) | 2008-08-28 | 2018-02-28 | The Procter and Gamble Company | Methods for providing a benefit |
| EP2857489A2 (en) | 2008-08-28 | 2015-04-08 | The Procter and Gamble Company | Process for preparing a fabric care composition |
| WO2010025116A1 (en) * | 2008-08-28 | 2010-03-04 | The Procter & Gamble Company | Fabric care compositions, process of making, and method of use |
| WO2010072628A1 (en) * | 2008-12-22 | 2010-07-01 | Unilever Plc | Laundry compositions |
| WO2010072627A1 (en) * | 2008-12-22 | 2010-07-01 | Unilever Plc | Laundry compositions |
| WO2011002475A1 (en) * | 2009-06-30 | 2011-01-06 | The Procter & Gamble Company | Fabric care compositions, process of making, and method of use |
| EP2399980A1 (en) | 2010-06-24 | 2011-12-28 | The Procter & Gamble Company | Stable compositions comprising cationic cellulose polymer and cellulase |
| WO2011163112A1 (en) | 2010-06-24 | 2011-12-29 | The Procter & Gamble Company | Stable compositions comprising cationic cellulose polymers and cellulase |
| US10195134B2 (en) | 2014-11-25 | 2019-02-05 | Dow Global Technologies Llc | Personal care compositions containing cationic polymers |
| US10507176B2 (en) | 2014-11-25 | 2019-12-17 | Dow Global Technologies Llc | Hair care compositions containing cationic polymers |
| EP3926030A1 (en) | 2020-06-18 | 2021-12-22 | The Procter & Gamble Company | Water-soluble unit dose article comprising a polyvinylalcohol film and a cationic poly alpha-1,6-glucan ether compound |
| WO2021257932A1 (en) | 2020-06-18 | 2021-12-23 | The Procter & Gamble Company | Water-soluble unit dose article comprising a polyvinylalcohol film and a cationic poly alpha-1,6-glucan ether compound |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1590426A2 (en) | 2005-11-02 |
| BRPI0407114B1 (en) | 2018-09-11 |
| CA2514766C (en) | 2012-09-25 |
| EP1590426B1 (en) | 2014-01-08 |
| WO2004069979A3 (en) | 2005-11-24 |
| BRPI0407114A (en) | 2006-01-24 |
| CA2514766A1 (en) | 2004-08-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CA2742133C (en) | Laundry cleansing and conditioning compositions | |
| EP2064306B2 (en) | Laundry compositions | |
| EP1773974B2 (en) | Softening laundry detergent | |
| CA2547663C (en) | Softening laundry detergent | |
| CA2514766C (en) | Laundry cleansing and conditioning compositions | |
| CA2658452C (en) | Softening laundry detergent | |
| CA3041104C (en) | Fabric treatment compositions having low calculated cationic charge density polymers and fabric softening actives and methods for providing a benefit | |
| US11560534B2 (en) | Surfactant compositions for improved transparency of DADMAC-acrylamide co-polymers | |
| US11505766B2 (en) | Surfactant compositions for improved transparency of DADMAC-acrylic acid co-polymers | |
| CA2968071A1 (en) | Compositions to boost fabric softener performance |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A2 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A2 Designated state(s): BW GH GM KE LS MW MZ SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LU MC NL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| DPEN | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed from 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2004704608 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2005/05997 Country of ref document: ZA Ref document number: 200505997 Country of ref document: ZA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2514766 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 00851/MUMNP/2005 Country of ref document: IN Ref document number: 851/MUMNP/2005 Country of ref document: IN |
|
| WWP | Wipo information: published in national office |
Ref document number: 2004704608 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: PI0407114 Country of ref document: BR |