PHARMACEUTICAL COMPOSITION HAVING CASING WITH MULTIPLE MICRO TABLETS
FIELD OF INVENTION The present invention relates to a modified extended or controlled release pharmaceutical composition, containing at least one pharmaceutical active substance, having a casing containing two or more micro tablets. The invention further relates to a process for the preparation of said modified release dosage form comprising steps of making core particles by granulating active ingredient or substance with release controlling agent(s), optionally coating of core particles with release controlling agent(s), compression of coated core particles into micro tablets, optionally coating of micro tablets with release controlling agent(s) and then filling of two or more coated and/or uncoated micro tablets in a casing. At least one pharmaceutical active ingredient or substance is selected from the group consisting of venlafaxine HC1, sodium valproate, domperidone maleate, diltiazem HC1 and mixtures thereof. The present invention also relates to solid oral dosage forms comprising a pharmaceutical composition according to the invention. The present invention also provides a novel process for preparing the novel formulations of the invention. The present invention further provides a method of treating a mammal, particularly a human in need of treatment utilizing at least one active pharmaceutical substance, comprising administering a therapeutically effective amount of composition or solid oral dosage form, preferably once or twice daily. BACKGROUND OF THE INVENTION During the past three decades significant advances have been made in the area of controlled drug delivery. This was, in part, due to the evolving disciplines of biopharmaceutics, pharmacokinetics and pharmacodynamics. In a typical therapeutic regimen, the drug dose and the dosing interval are optimized to maintain drug concentration within the therapeutic window, thus ensuring efficacy while minimizing toxic side effects. Due to different physico-chemical and pharmacokinetic properties of different active ingredients or substances, different types of release profiles like zero order, first order, pulsating profiles, etc. are required to develop an effective modified
release dosage form. This warrants a flexible technology that can offer different kinds of release profiles. Modified release pharmaceutical compositions and dosage forms are designed to improve the delivery profile drugs to be internally administered to an animal, including humans. A modified release composition is typically used to improve the effects of administered substances by optimizing the kinetics of delivery, thereby increasing bio-availability, convenience, and patient compliance, as well as minimizing side effects associated with inappropriate immediate release rates such as a high initial release rate and, if undesired, uneven blood or tissue levels. There are a number of different modified release dosage forms available commercially and their methods of manufacturing are disclosed in books like Encyclopedia of Control Drug Delivery, Pharmaceutical Pelletization Technology, Multiparticulate Oral Drug delivery and Sustained and Controlled Release Drug Delivery Systems. The most popular way to make modified release formulation is tablet. The tablet can be either monolithic matrix tablet or heterogeneous matrix tablet or tablets coated with release controlling membrane. These type of modified release formulations offer various advantages like simple manufacturing process, compact dosage form, use of conventional manufacturing equipments, high throughput, easy scale-up, economic, etc. However, such matrices do not provide adequate control over the release rate, instead resulting in a release that approximates first-order kinetics and may have a problem of dose dumping or burst release. However, since many modified release dosage forms contain comparatively large amounts of active ingredient or substance it is often necessary to include large amounts of suitable excipients to achieve appropriate controlled release profiles. Clearly, this will tend to increase the size of the dosage form. Tablet based matrix formulations have variations in gastric emptying rates and overall transit times leading to intra- and inter-subject variability of plasma profiles. Moreover these systems are also not suitable for modified release of active ingredients or substances with poor water solubility and active ingredients or substances with high water solubility and often difficult to combine two chemically incompatible active ingredients or substances in a singe unit. Another popular way to make modified release formulation is multiparticulate formulations based on pellets or spheroids. Pelletized products not only offer flexibility in dosage form design and development, but are also utilized to improve the safety and
efficacy of active ingredients or substances. When pellets containing the active ingredient or substance are administered in vivo in the form of suspensions, capsules, or disintegrating tablets, they offer significant therapeutic advantages over single unit dosage forms like matrix-based tablets. Because pellets disperse freely in the gastrointestinal tract, they invariably maximize drug absorption, reduce peak plasma fluctuations, and minimize potential side effects without appreciably lowering drug bioavailability. Pellets also reduce variations in gastric emptying rates and overall transit times. Thus, intra- and inter-subject variability of plasma profiles, which are common with single unit regimens, is minimized. Another advantage of pellets over single-unit dosage forms is that high local concentrations of bioactive agents, which may inherently be irritative or anesthetic, can be avoided. When formulated as modified-release dosage forms, pellets are less susceptible to dose dumping than the reservoir-type, single unit formulations. Pellets also provide tremendous flexibility; for instance, pellets composed of different active ingredients or substances can be blended and formulated in a single dosage form. It allows the combined delivery of two or more active ingredients or substances that may or may not be chemically compatible, at the same site or at different sites with in the gastrointestinal tract. It also permits the combination of dosage form. But production of pellets generally involves expensive processes or highly specialized equipment. Equipment, which is readily available in a given setting due to its suitability for other applications such as coating, tends to obviate the need for the purchase of a new and specialized machine. Unfortunately, except in special cases, the pelletization processes are usually lengthy and expensive. Processing of a single batch may sometimes require hours or even days to be completed. Conversely, if a short processing time is desired, it becomes mandatory to utilize highly efficient and, at times, unique pieces of equipment that require the allocation of substantial capital investment. Extruders, spheronizers, and rotor granulators fall under this category. Although pellets could conceivably be coated in any tablet coating equipment, they generally require specialized coating machinery for optimum processability. Moreover, irrespective of the pelletization process, pellets are not uniform in size and generally represent a narrow mesh fraction, which could lead to fewer yields and increases the cost of the product. Apart from this pellet based products may also be
difficult to fill in the capsule if they differ in size or density, as it leads to segregation. Other factors that lead to segregation are static electricity and surface morphology. PRIOR ART US patent application 2003/0050620 Al discloses a controlled release delivery device, which comprises more than one vehicle for the controlled release of the drugs. The vehicle can be in the form of granules; pellets, beads or tablets filled in a housing. The vehicle essentially contains an amino acid, a polymer and a buffer. PCT application WO 98/29095 discloses a sustained release cisapride mini tablet formulation for oral administration. Cisapride is a poorly soluble ingredient or substance. The comprises of plurality of mini tablets containing cisapride with an organic acid. The tablets are capable of releasing cisapride at different sites of gastrointestinal tract in response to the pH environment. The tablets are coated with a pH dependent polymer, namely a copolymer of methacrylic acid and methacrylic acid esters. PCT application WO 00/71099 Al refers to a tablet or a multiparticulate controlled release selective serotinin reuptake inhibitor for oral administration. The formulation contains fluvoxamine, is suitable for once or twice daily administration and can comprise a blend of two or more population of particles, pellets or mini tablets. The composition or method to manufacture of said mini tablets is not described. PCT applications WO 03/041692, WO 02/102129 A2 and WO 99/22724 disclose information about sustained release formulations of venlafaxine hydrochloride. These formulations are based on pellets, beads or spheres. The pellets or core is prepared by either extrusion spheronization or layering techniques and a polymer layer is provided over the core for the sustained release of venlafaxine hydrochloride. PCT application WO 03/055475 and US patent application 2003/0059466 Al disclose about modified release formulations of venlafaxine hydrochloride. The formulations are tablet based and in the form of single unit. Venlafaxine, chemically named (±) l-[2-(dimethylamino)-l-(4-methoxyphenyl) ethyl] -cyclohexanol, is an antidepressant disclosed in EP-A-0 112 669. Presently venlafaxine hydrochloride is administered to adults as conventional immediate release tablets or as 24 hour extended-release multiparticulate capsules. Venlafaxine hydrochloride is highly soluble in water. It is known that it is very difficult to develop a pharmaceutical form with a very slow dissolution rate of freely soluble drug. Besides that, venlafaxine hydrochloride is polymorphic, so dissolution is
dependent also on polymorphic form and particle size of particular polymorphic form. Therefore, it is a special task to develop such a pharmaceutical formulation that would sustain and control the dissolution of freely soluble drug over 24 hour period. EP-A-0 797 991 and WO 99/22724 disclose encapsulated venlafaxine extended release dosage formulation of venlafaxine hydrochloride, which provides in a single dose, a therapeutic blood serum level over a twenty four hour period. Gelatine capsules are filled with film coated spheroids containing venlafaxine hydrochloride. EP-A-0 797 991 states that numerous attempts to produce extended release tablets by hydrogel technology proved to be fruitless because the compressed tablets were either physically unstable (poor compressibility or capping problems) or dissolved too rapidly in dissolution studies. Typically, the tablets prepared as hydrogel sustained release formulations gave 40-50% dissolution at 2 hours, 60-70% dissolution at 4 hours and 85-100% dissolution at 8 hours (EP-A-0 797 991). WO 94/27589 and WO 01/37815 describe osmotic dosage forms containing venlafaxine hydrochloride. EP 1 028 718 Bl discloses an improved core of extended release spheroids comprised of venlafaxine hydrochloride and microcrystalline cellulose, which are free of hydroxypropylmethylcellulose. EP 1 028 718 Bl provides extended release, encapsulated formulations containing the referred spheroid cores comprising venlafaxine hydrochloride as an active drug component, which provides in a single dose a therapeutic blood serum level over a 25 hour period. US Patent Application 2002/0044962 Al discloses an extended or controlled release encapsulated product which includes at least one active ingredient or substance, at least one erodible polymer and at least one lubricating material, wherein the encapsulated product is in the form of a caplet having a diameter from about 1 millimeter to about 7 millimeter and a length from about 1 millimeter to about 7 millimeter. As active ingredient or substance among other things also antidepressants are mentioned. US Patent 6,270,797 Bl discloses a sustained release glipizide composition that releases glipizide in conformity to a zero-order kinetic. Also a process for the preparation of this sustained release pharmaceutical composition is described, including the steps of granulating glipizide, a hydrophilic material and a diluent, drying the granulated product and lubricating the product with a flow regulating agent and lubricant.
US Patent Application 2002/0015730 Al discloses an oral pharmaceutical formulation with variably adjustable release rate, which comprises one or more active ingredients or substances and one or more sucrose ester of a fatty acid as the sole release-controlling agent for said active ingredient or substance wherein when the dosage form is a granule or a pellet, the formulation is made by melting the oral formulation, and granulating or pelletizing the melt. In German Patent No. 198 40 152 CI a retard formulation is disclosed, which contains calcium valproate, at least one acrylic polymer and at least one sugar ester, wherein the desired retarding effect being achieved by the acrylic polymer that is used. In International Patent Application WO 93/00093 a new controlled release formulation for Diltiazem in the form of spheroids is disclosed, which is composed of the active ingredient or substance, a wetting agent and a polymer coating for controlling the release. Sucrose esters of fatty acids are used as a wetting agent. International Patent Application WO 03/022267 Al discloses pharmaceutical compositions for oral administration comprising terbinafine and a method for administrating high dosages while minimizing effects associated with e.g. a high dosage load, e.g. coated tablets or multiparticulate formulations such as mini tablets or pellets, e.g. in capsules. US Patent No. 4,578,264 describes a pharmaceutical dosage unit composition consisting essentially of a gelatin capsule containing a disintegrating core comprising clonidine. Said disintegrating core having a readily water-soluble coating and a plurality of non-disintegrating cores comprising clonidine. International Application WO 94/27589 discloses an antidepressant dosage form and to administering this medicament for an extended period of time in a rate- known dose. As antidepressant Venlafaxine hydrochloride is used. International Application WO 97/02017 discloses controlled release formulations for poorly soluble drugs. The formulation can been in a multi-particulate form such as pellets or mini-tablets or in the form of tablets. Examples of active ingredients or substances are cisapride, cyclosporin, diclofenac etc. There still exists a need for compositions and process for making orally deliverable dosage form containing active ingredient or substance as modified release that overcomes the problems discussed above for the matrix based tablets and multiparticulate systems based on pellets and spheroids. This invention addresses the need.
Objects of the invention Therefore, it would be of considerable clinical and commercial benefit to design a modified release dosage form, which has the advantages of matrix based tablets and multiparticulate systems based on pellets and spheroids but devoid of the disadvantages described above for matrix based tablets and multiparticulate systems based on pellets and spheroids. Therefore, an object of the present invention is to prepare a modified release dosage form, which has advantages of matrix based tablets but multiparticulate systems based on pellets or spheroids and devoid of disadvantages described above for matrix based tablets and multiparticulate systems based on pellets or spheroids. The second object of the present invention is to prepare a modified release dosage form, which allows the combined delivery of two or more active ingredients or substances that may or may not be chemically compatible, at the same site or at different sites within the gastrointestinal tract. It also permits the combination of dosage form. Accordingly, an object of the present invention is to have a dosage form, which is flexible enough to provide different release profiles like zero order, first order, pulsating type profiles. A further object of the present invention is to have a dosage form, which offers advantages like simple manufacturing process, compact dosage form, use of conventional manufacturing equipment, high throughput, easy scale-up, economic, etc. A further object of the present invention is to have a modified release dosage form, which can be given twice a day or more preferably can be given once a day. Yet another object of the present invention is to have a modified release dosage form of poorly soluble active ingredients or substances, soluble active ingredients or substances and highly soluble active ingredients or substances.
Additional object of the invention, advantage and novel features of the invention will be set forth in the part of the description.
SUMMARY DESCRIPTION OF THE INVENTION According to the present invention the following- modified release dosage forms according to embodiments A., B. and C. are prepared:
A. A modified extended or controlled release pharmaceutical composition, comprising at least one pharmaceutical active substance, having a casing containing two or more micro tablets, the said micro tablets are manufactured by following steps-
(i) core particles, wherein the pharmaceutically active substance is granulated along with release controlling agent(s)and optionally one or more auxiliary pharmaceutical excipient(s);
(ii) coating of core' particles, wherein the core particles prepared in step (i) are coated with release controlling agent(s)and optionally one or more auxiliary pharmaceutical excipient(s), wherein the coated core particles may optionally be mixed with lubricants;
(iii) compression, wherein the coated core particles prepared in step (ii) are compressed to micro tablets;
(iv) coating of micro tablets, wherein the micro tablets prepared in step (iii) are coated with release controlling agent(s)and optionally one or more auxiliary pharmaceutical excipient(s); and
(v) filling, wherein the coated micro tablets equivalent to desired weight of pharmaceutically active agent are filled in casing.
B. A modified release pharmaceutical composition comprising a casing containing two or more micro tablets. The said micro tablets are manufactured by following steps-
(i) core particles, wherein a pharmaceutically active substance is granulated along with release controlling agent(s)and optionally one or more auxiliary pharmaceutical excipient(s).
(ii) coating of core particles, wherein the core particles prepared in step (i) are coated with release controlling agent(s)and optionally one or more auxiliary pharmaceutical excipient(s). The coated core particles may optionally be mixed with lubricants. •
(iii) compression, wherein the coated "core particles prepared in step (ii) are compressed to micro tablets. (iv) filling, wherein the micro tablets equivalent to desired weight of pharmaceutically active agent are filled in casing.
C. A modified release pharmaceutical composition comprising a casing containing two or more micro tablets. The said micro tablets are manufactured by following steps-
(i) making core particles, wherein a pharmaceutically active substance is granulated along with release controlling agent(s)and optionally one or more auxiliary pharmaceutical excipient(s).
(ii) compression, wherein the core particles prepared in step (i) are compressed to micro tablets.
(iii) coating of micro tablets, wherein the micro tablets prepared in step (ii) are coated with release controlling agent(s)and optionally one or more auxiliary pharmaceutical excipient(s).
(iv) filling, wherein the coated micro tablets equivalent to desired weight of pharmaceutically active agent are filled in casing. In one of the embodiment, the present invention described above (scheme 1, 2 and 3) comprises casing containing two or more identical micro tablets of a single active ingredient or substance. In another embodiment, the present invention described above (scheme 1, 2 and 3) comprises casing containing two or more micro tablets of a single active ingredient or substance having different release profiles. In another embodiment, the present invention described above (scheme 1, 2 and 3) comprises casing containing two or more micro tablets of two or more active ingredients or substances. In another embodiment, the present invention comprises casing containing two or more micro tablets of active ingredients or substances prepared by scheme 1, 2 and 3 described above or combination thereof. The present invention also relates to solid oral dosage forms comprising a pharmaceutical composition according to the invention. The present invention also provides a novel process for preparing the novel formulations of the invention. The present invention further provides a method of treating a mammal, particularly a human in need of treatment utilizing the active agent(s), comprising administering a therapeutically effective amount of composition or solid oral dosage form, preferably once daily DETAILED DESCRIPTION OF THE INVENTION This invention relates to a modified extended or controlled release pharmaceutical composition, comprising at least one active ingredient or substance, having a casing containing two or more micro tablets. The invention further relates to a process for the preparation of said modified release dosage form comprising steps of making core particles by granulating active ingredient or substance with release controlling agent(s), optionally coating of core particles with release controlling agent(s), compression of coated core particles into micro tablets, optionally coating of
micro tablets with release controlling agent(s) and then filling of two or more coated and/or uncoated micro tablets in a casing.
The term "modified extended or controlled release" as used herein in relation to the composition according to the invention or a rate controlling polymer or used in any other context means release, which is not immediate release and is taken to encompass controlled release, sustained release, prolonged release timed release, retarded release, extended release and delayed release from the encapsulated product into the mammal.
The terms refer to the release of the drug over a period of time, for example from one hour to twenty-four hours. The term "modified release dosage form" as used herein can be described as dosage forms, whose drug-release characteristics of time course and/or location are chosen to accomplish therapeutic or convenience objectives not offered by conventional dosage forms, such as a solution or an immediate release dosage form.
Modified release solid oral dosage forms include both delayed and extended release drug products (as per US FDA guideline for 'SUP AC-MR: Modified Release Solid Oral
Dosage Forms'). The term "dosage form" denotes any form of the formulation that contains an amount sufficient to achieve a therapeutic effect with a single administration. The term "active ingredient or substance" refers to an agent, active ingredient or substance compound or other substance, or compositions and mixture thereof that provide some pharmacological, often beneficial, effect. The term "active ingredient or substance" therefore includes without limitation flavors, sweeteners, herbal ingredients or substances, pharmaceuticals, vitamins, minerals and mixture thereof. Reference to a specific active ingredient shall include where appropriate the active ingredient or substance and it's pharmaceutically acceptable salts. The term "highly soluble active ingredient or substance" as used herein means that from less 30 parts of the water will require dissolving 1 part of active ingredient or substance. The term "soluble active ingredient or substance" as used herein means that from 30 to 1000 parts of the water will require dissolving 1 part of active ingredient or substance. The term "poor soluble active ingredient or substance" as used herein means that more than 1000 parts of the water will require dissolving 1 part of active ingredient or substance.
The term "casing" as used herein refers to a hard gelatin capsule shell or casing prepared by pharmaceutically acceptable polymers. The term "micro tablets" herein refers to compressed tablets of any shape and size, which can be filled in casings available for human swallowing. Preferably the micro tablets according to the present invention have a diameter from about 1.5 millimeter to about 9.5 millimeter. The term "mammal" includes without limitation a mammalian subject, such as human, mice, rates, guinea pigs, cats, dogs, beings, cows, horses, sheep or other livestock. The term "release controlling agent(s)" used in steps (i), (ii), (iii) and in step (iv) are pharmaceutical excipients and can be hydrophilic (water soluble/swellable/soluble at particular pH) or hydrophobic (water in-soluble, non-swellable, in-soluble in particular pH) or mixtures there of. The amount of "release controlling agent (s)" used in steps (i), (ii), (iii) and (iv) will depend on the desired release profile. The "release controlling agent (s)" will be explained in more detail hereinafter. The invention relates to following modified controlled release dosage forms, especially for oral administration. The compositions according to the invention are preferably suitable for once-daily-administration.
A. A modified release pharmaceutical composition comprising a casing containing two or more micro tablets. The said micro tablets are manufactured by following steps (ref: scheme -1)-
(i) core particles, wherein a pharmaceutically active substance is granulated along with release controlling agent(s)and optionally one or more auxiliary pharmaceutical excipient(s).
(ii) coating of core particles, wherein the core particles prepared in step (i) are coated with release controlling agent(s)and optionally one or more auxiliary pharmaceutical excipient(s). The coated core particles may optionally be mixed with lubricants.
(iii) compression, wherein the coated core particles prepared in step (ii) are compressed to micro tablets. (iv) coating of micro tablets, wherein the micro tablets prepared in step (iii) are coated with release controlling agent(s)and optionally one or more auxiliary pharmaceutical excipient(s).
(v) filling, wherein the coated micro tablets equivalent to desired weight of pharmaceutically active agent are filled in casing.
B. A modified release pharmaceutical composition comprising a casing containing two or more micro tablets. The said micro tablets are manufactured by following steps (ref: scheme -2)- (i) core particles, wherein a pharmaceutically active substance is granulated along with release controlling agent(s)and optionally one or more auxiliary pharmaceutical excipient(s).
(ii) coating of core particles, wherein the core particles prepared in step (i) are coated with release controlling agent(s)and optionally one or more auxiliary pharmaceutical excipient(s). The coated core particles may optionally be mixed with lubricants.
(iii) compression, wherein the coated core particles prepared in step (ii) are compressed to micro tablets.
(iv) filling, wherein the micro tablets equivalent to desired weight of pharmaceutically active agent are filled in casing.
C. A modified release pharmaceutical composition comprising a casing containing two or more micro tablets. The said micro tablets are manufactured by following steps(ref: scheme -3)-
(i) making core particles, wherein a pharmaceutically active substance is granulated along with release controlling agent(s)and optionally one or more auxiliary pharmaceutical excipient(s).
(ii) compression, wherein the core particles prepared in step (i) are compressed to micro tablets.
(iii) coating of micro tablets, wherein the micro tablets prepared in step (ii) are coated with release controlling agent(s)and optionally one or more auxiliary pharmaceutical excipient(s).
(iv) filling, wherein the coated micro tablets equivalent to desired weight of pharmaceutically active agent are filled in casing. In one of the embodiment, the present invention described above (Scheme 1, 2 and 3) comprises casing containing two or more identical micro tablets of a single active ingredient or substance.
In another embodiment, the present invention described above (Scheme 1, 2 and
3) comprises casing containing two or more micro tablets of a single active ingredient or substance having different release profiles. In another embodiment, the present invention described above (Scheme 1, 2 and 3) comprises casing containing micro tablets of two or more active ingredients or substances. The present invention also provides solid oral dosage form comprising a composition according to the invention. The present invention also provides a novel process for preparing the novel formulations of the invention. The present invention further provides a method of treating a mammal, particularly a human in need of treatment utilizing the active agents, comprising administering a therapeutically effective amount of composition or solid oral dosage form to provide administration of active ingredients or substances. The active ingredients or substances can be present in the form of a free base or in the form of pharmaceutically acceptable salts. Pharmaceutically acceptable salts forming part of this invention are intended to define but not limited to salts of the carboxylic acid moiety such as alkali metal salts like Li, Na and K salts; alkaline earth metal salts like Ca and Mg salts; salts of organic bases such as lysine, arginine, guanidine, diethanolamine," choline, and the like; ammonium or substituted ammonium salts and aluminum salts. Salts may be acid addition salts which defines but not limited to sulfates, nitrates, phosphates, perchlorates, borates, hydrohalides, acetates, tartrates, maleates, citrates, succinates, palmoates, methanesulfonates, benzoates, salicylates, hydroxynaphthoates, benzensulfonates, ascorbates, glycerophosphates, ketoglutarates and the like. Further, active ingredients or substances, where applicable, may be present either in the form of one substantially optically pure enantiomer or as a mixture of enantiomers or polymorphs thereof. The active ingredient or substance can be poorly soluble, soluble or highly soluble. The active ingredients or substances are comprising of the following therapeutic classes but not limited to general anesthetics, angiotensin converting enzyme inhibitors, angiotensin receptor antagonist, antacids, anti- rheumatoid, anti- asthmatics, antiallergics, antiarrhythmic, antibiotics, anti-convulsants, anti-depressants, anti-diarrheals antiemetics, anti-histamines, anti-infective, anti-bacterials, antiviral,
antifungal, antiprotozoals, anti-inflammatory agents, anti-nauseants, anti- hyperlipidemic drugs, anti-parkinson, antihelmintics, anti-psychotics, antipyretics, anti- spasmodic, antithrombotic drugs, anticoagulants, antiplatelets, anti-tumor drugs, anti- uricaemic drugs, anxiolytic agents, appetite stimulants, appetite suppressants, bronchodialators, antihypertensives(diuretics, beta-blockers, ACE inhibitors, calcium channel blockers, vasodialators),chelating agents, cholecystokinin antagonists, cognition activators, cough suppressants, erythropoietic drugs, fertility agents, antidiarrhoeals, laxatives, antiulcer agents, growth regulators, immunomodulating agents, neuroleptics, neuromuscular agents, potassium channel blocker, potassium channel opener, prostaglandins, respiratory stimulants, selective aldosterone receptor blocker, sedatives & hypnotics, synthetic hormones, tachykinin nk 1 antagonist, vasoconstrictors and vertigo agents and vitamins. Examples of active ingredients or substances comprise of, but not limited to AAE581, AAG561, abacavit sulfate, abciximab, ABT-510, ABT-751, acarbose, acetaminophen, acyclovir, aesloratadine, AG-1749, aldosterone antagonist, alendronate sodium, almotriptan, alprazolam, aminocaproic acid, amlodipine besylate, AMP397, amphetamine, anagrelide hydrochloride, anastrazole, aprepitant, aripiprazole, atorvastatin calcium, atrasentan, AZD0328, AZD0865, AZD0902,
AZD2171, AZD3409, AZD4282, AZD4750, AZD5106, AZD7140, azelnidipine, azithromycine, balsalazide disodium, bazedoxifene, betaxolol hydrochloride, bicalntamid, BILN-2061, biperiden HCl, bisoprolol fumarate, bromfenac sodium, buformin, candesartan cilexetil, captopril, carboplatin,. carvedilol, CCI-779, cefadroxil, ceftriaxone sodium, celecoxib, CEP 7055, cerivastatin, cetrizine HCl, CHC12103, chlorthalidone, CHS 13340, ciglitazone, cilastazole, ciprofloxacin, clindamycin, clofapine, clonazepam, clopidogrel bisulfate, cloraze ate dipotassium, clorpropamide, clozapine, CP-122,721, CP-526,555, CP-529,414, CRF-1 antagonists, CS-003, CS-011, CS-023, CS-502, CS-505, CS-706, CS-747, CS-866, cyclophosphamide, cyclosporine, deloratadine, desloratadine, dexmethylphenidate HCl, dextroamphetamine sulfate, diclofenac sodium, dicyclomine hydrochloride, digoxin, diltiazem, divalproex sodium, docetaxel, dofetilide, domperidone, doxazosin mesylate, doxercaϊciferol, doxorubicin HCl, dronedarone, dulaxetine, emiglitate, enalapril maleate, enbrel, endesartan, englitazone, enoxaparin sodium, enzothiadiazine, eplernone, eplivanserin, epoetin alpha, eprosartan, eptifibatide, erythromycin, escitalopram, esomeprazole magnesium, esters of ampicillin, estradiol,
estropipate, ethacrynate sodium, etoricoxib, exemestane, famotidine, fexofenadine HCl, fidalestat, fluoxetine, fluoxetine HCl, fluticazone peopianate, fluvastatin sodium, fluvoxamine maleate, fondaparinux sodium, frovatriptan, FTY720, fudosteine, fulvestrant, gabapentin, galantamine HCl, galopentin, ganirelix acetate, gemitatrine HCl, glatiramer acetate, glibenclamide (glyburide), glibornuride, gliclazide, glimepiride, glipizide, gliquidone, glisoxepid, g'osereline acetate implant, granisetron, guanabenz acetate, guanylate cyclase inhibitors, haloperidol, hydralazine, hydrochlorothiazide, hydromorphone HCl, hydroxyurea, ICL670, idraparinux, imatinib mesylate, indapamide, infliximab, ipratropium bromide, irbesartan, isosorbide 5-imononitrate, isosorbide d nitrate, isotretinoin, itrozole tablets, J695, ketorolac trimethamine, KUC 7483, lactobionate, 1AF237, lafutidine, lamotrigine, landiolol, latanoprost, leflunomide, letrozole, levetiracetam, levofloxaine, levonorgestrel, levothroxine sodium, levothyroxine sodium, linezolid, liothyronine sodium, lisinopril, loperamide, loratidine, lorazepam, losartan, lovastatin, ludrocortisone acetate, mabthera, MCC-135, meloxicam, mephalan, metformin hydrochloride, methylphenidate HCl, MH-15E, miglitol, minoxidil, misoprostol, mometasone furote-monohydrate, montelkast sodium, moxifloxacin HCl, MS 209, MS 275, naratriptan hydrochloride, nateglinide, nebirapine, nebivolol, neorecormon, neteglinide, niacin, nicorandil, nifedipine, nitricoxide, nitroprusside, NK-104, NKS 104, NM 283, norethindrone acetate, norgestrel, NS 2330, octreotide acetate, olanzepine, olmesartan, omeprazole, ondensetron HCl, orlistat, osanetant, oseltamivir phosphate, oxaliplatin, oxcarbazepine, oxistat, oxprenolol, oxybutynin chloride, paloxetine HCl, pantoprazole sodium, paroxetine, paroxetine HCl, pazufloxacin, pemoline, pentoxyphylline, perindopril, phenbutamide, picotamide, pimecrolimus, pioglitazone, potassium chloride, pralnacasan, pravastatin sodium, propranolol, prucolapride, PTK787, quetiapine, quetiapine fumarate, quinapril, R1068, R1204, R- 142440, R1439, R1440, R1453, R1487, R1518, R1549, R1559, R411, R450, R673, R701, R744, rabeprazole sodium, radOOl, raloxifene HCl, ramipril, ranitidine hydrochloride, renzapride, RHIL-11, ribavarin, rimonabant, risedronate sodium, risperidone, rituximab, rivastigmine tartrate, rofecoxib, roloxifene HCl, ropinirol, rosiglitazone maleate, rosuvastatin, SAB378, salmeteyol xinafoate, saredutant, serm 6471, sertraline HCl, sildenafil citrate, simvastatin, sirolimus, sitafloxacin, sodium valproate, somatropin, sotalol, SR 123781, SSR 126517, SSR 180575, SSR 250411, SSR 591813, sulodexide, sumatriptan succinate, T-1249, TAK-013, TAK-370, TAK-
559, TAK-637, tamsuloin HCl, TAP-144SR, TCH346, TCV - 116, telbivudine, telmisaltan, telmisartan, temozolomide, terbinafine HCl, timolol, tipranavir, tirapazamine, tolazamide, tolbutamide, tolcyclamide, tolterodine taltacate, tolterodine tartrate, tomoxetine HCl, topiramate, topotecan HCl, tramadol, trandolapril, trastuzumab, troglitazone, UK-338,003, UK-369,003, valacyclovir HCl, valdecoxib, valsartan, valtorcitabine, vancomycin hydrochloride, vegf tki, venlafaxine, voglibose, voriconazole, warfarin sodium, xaliproden, zafirlukast, zalcitabine, ziprasidone, ziprasidone mesylate, zofenopril calcium, ZO1446, zoledronic acid, zolpidem tartarate, ZP 10, etc. In a preferred embodiment of the present invention the active ingredient or substance is selected from the group consisting of venlafaxine HCl, sodium valproate, domperidone maleate, diltiazem HCl and mixtures thereof. In a most preferred aspect the invention venlafaxine is used in the form of venlafaxine hydrochloride. The amount of venlafaxine in the pharmaceutical formulation for once-daily administration is from 10 to 400 mg. Especially preferred is an amount of venlafaxine of 10 to 150 mg. Venlafaxine hydrochloride is polymorphic. Of the forms isolated and characterized to date, From I is considered to be the kinetic product of crystallization, which can be converted to Form II upon heating in the crystallization solvent. Forms I and II cannot be distinguished by their melting points but do exhibit some differences in their infrared spectra and X-ray diffraction patterns. Any of the polymorphic forms such as Form I or Form II may be used in the formulation of the present invention. Also, other polymorphs of venlafaxine hydrochloride and also solvate forms have been characterized. However, in the pharmaceutical formulation of the present invention the racemic form of venlafaxine hydrochloride is used. But its stereo isomer(s) can also be used. As indicated above the present invention may comprise auxiliary excipients such as for example lubricants, plasticisers, anti-tack agents, opacifying agents, pigments, and such like. As will be appreciated by those skilled in the art, the exact choice of excipient and their relative amounts will depend to some extent on the final oral dosage form.
Suitable lubricants, including agents that act on the flowability of the powder to be compressed are, for example, colloidal silicon dioxide; talc; stearic acid, magnesium stearate, calcium stearate and sodium stearyl fumarate. Preferred lubricants can be selected from magnesium stearate, stearic acid, sodium stearyl fumarate, etc. The auxiliary pharmaceutical excipients will also be explained hereinafter in more detail. In core particles, the active ingredient or substance and one or more release controlling agents are preferably present in a ratio of from 100: 1 to 1 : 100. Core particles and coating of one or more release controlling agents are preferably present in a ratio of from 100:0.5 to 0.5:100. Micro tablets and coating of one or more release controlling agents are present in a ratio of from 100:0.5 to 0.5:100. According to one embodiment the release controlling agents are pharmaceutical excipients, which are hydrophobic in nature. According to another embodiment the release controlling agents are pharmaceutical excipients, which are hydrophilic in nature. According to yet another embodiment the release controlling agents are pharmaceutical excipients, which is combination of hydrophilic release controlling agents and hydrophobic release controlling agents. The release controlling agents that can be used in core particles or to coat core particles and micro tablets micromatrix are described in greater detail herein below. The hydrophobic release controlling agents are selected from but are not limited to Ammonio methacrylate copolymers type A and B as described in USP, methacrylic acid copolymer type A, B and C as described in USP, Polyacrylate dispersion 30% as described in Ph. Eur., Polyvinyl acetate dispersion, ethylcellulose, cellulose acetate, cellulose propionate (lower, medium or higher molecular weight), cellulose acetate propionate, cellulose acetate butyrate, cellulose acetate phthalate, cellulose triacetate, ploy(methyl methacrylate), ploy(ethyl methacrylate), poly(butyl methacrylate), poly(isobutyl methacrylate), and poly (hexyl methacrylate). poly(isodecyl methacrylate), poly (lauryl methacrylate), poly(phenyl methacrylate), poly (methyl acrylate), poly (isopropyl acrylate), poly (isobutyl acrylate), poly (octadecyl acrylate), waxes such as beeswax, carnauba wax, microcrystalline wax, and ozokerite; fatty alcohols such as ceto stearyl alcohol, stearyl alcohol; cetyl alcohol and myristyl alcohol;
and fatty acid esters such as glyceryl monostearate; glycerol monooleate, acetylated monoglycerides, tristearin, tripalmitin, cetyl esters wax, glyceryl palmitostearate, glyceryl behenate, and hydrogenated castor oil.
Preferred examples of hydrophobic release controlling agent are Ethyl cellulose, Cellulose acetate, Eudragit RS, Hydrogenated castor oil, Eudragit S, etc. According to an especially preferred embodiment the release controlling agents contain ammonio methacrylate co-polymers and fatty acid esters as hereinafter described. The suitable hydrophobic agents are polymers sold under the Trade Mark Eudragit RS (Ammonio Methacrylate Copolymer type B USP),Eudragit NE 30D (Poly acrylate dispersion 30% Ph., Eur.), Eudragit RL (Ammonio Methacrylate Copolymer type A USP) and Kollicoat SR 30 D and fatty acid esters such as glyceryl behenate, and hydrogenated castor oil. Eudragit polymers are polymeric lacquer substances based on acrylate and/or methacrylates. The hydrophilic release controlling agents are selected from but are not limited to hydroxypropyl methylcellulose (HPMC), hydroxypropyl cellulose (HPC), poly (ethylene oxide), poly(vinyl alcohol), xanthan gum, carbomer, carrageenan, carboxymethyl cellulose, sodium alginate or mixture thereof. A preferred release controlling agents is HPMC. Other similar hydrophilic release controlling agents may also be employed. In use, the hydrophilic release controlling agents is swollen by, and eventually dissolves, in water. The active ingredient or substance dissolution rate of from the present invention may be controlled by the amount and molecular weight of hydrophilic release controlling agent employed. In general, using a greater amount of the hydrophilic release controlling agent decreases the dissolution rate, as does using a higher molecular weight polymer. Preferred examples of hydrophilic release controlling agent are HPMC, HPC, Eudragit RL, povidone, Eudragit L, etc. The dosage form can also include one or more commonly used excipients in oral pharmaceutical formulations. Auxiliary pharmaceutical excipients are known in the art and can be selected from plasticisers, fillers, coating adds, anti-foaming, anti-tacking agents, diluents, etc. Preferred commonly used excipients in oral pharmaceutical formulations include talc, fumed silica, glyceryl monostearate, magnesium stearate, calcium stearate, kaolin, colloidal silica, gypsum, Tween 80, Geleol pastiles (trade mark), micronised silica and magnesium trisilicate.
The quantity of commonly used excipients in oral pharmaceutical formulations used is from about 0.5% to about 99.5 % by weight. The dosage form can also include a material that improves the processing of the release controlling agents. Such materials are generally referred to as "plasticisers" and include, for example, adipates, azelates, benzoates, citrates, isoebucaes, phthalates, sebacates, stearates, tartrates, polyhydric alcohols and glycols. Preferred plasticisers include acetylated monoglycerides, butyl phthalyl butyl gylcolate, dibutyl tartrate, diethyl phthalate, dimethyl phthalate, ethyl phthalyl ethyl glycolate, glycerin, ethylene glycol, propylene glycol, Triethyl citrate, triacetin, tripropinoin, diacetin, dibutyl phthalate, acetyl monoglyceride, polyethylene glycols, castor oil, triethyl citrate, polyhydric alcohols, acetate esters, glycerol triacetate, acetyl triethyl citrate, dibenzyl phthalate, dihexyl phthalate, butyl octyl phthalate, diisononyl phthalate, butyl octyl phthalate, dioctyl azelate, epoxidised tallate, triisoctyl trimellitate, diethylexyl phthalate, di-n-octyl phthalate, di-I-octyl phthalate, di-I-decyl phthalate, di- n-undecyl phthalate, di-n-tridecyl phthalate, tri-2-ethylexyl trimellitate, di-2-ethylexyl adipate, di-2-ethylhexyl sebacate, di-2-ethylhexyl azelate, dibutyl sebacate, glyceryl monocaprylate, glycerol distearate and glyceryl monocaprate. The amount of plasticiser to be used is from about 1% to 50% based on the ' weight of the dry release controlling agent(s). The amount of release controlling agent(s) to be used in forming the outer portion will be determined based on various parameters such as the desired delivery properties, including the amount of antidiabetic active ingredient or substance to be delivered, the antidiabetic active ingredient or substance release rate desired, and the size of the micro matrix particles. The modified release dosage form of the present invention can be manufactured by the following procedure: The core particles can be manufactured in accordance with usual techniques in which the active ingredient or substance and one or more release controlling agents are mixed and granulated by adding solvent in a low or high shear mixer or by fluidized bed granulator. The granulate is dried, preferably in a fluidized bed dryer. The dried granulate is sized. The sizing of the micromatrix particles can be done using oscillating granulator, comminuting mill or any other conventional method. The sieve used for the sizing can have openings from 0.25 mm to 5 mm. Alternatively the core particles can be made by extrusion, spheronization, melt granulation or by roller compaction. The
core particles can be coated by a solution of one or more release controlling agents by any known method, including spray application. Spraying can be carried out using a fluidized bed coater (preferably Wurster coating), or in a pan coating system. Alternatively the coating of the core particles with one or more rate controlling agents can be done by hot melt process using a granulator or fluidized bed coater (preferably Wurster coating), or in a pan coating system. The compression of micro tablets is carried out on usual compression machines (e.g. machines of the Manesty, Cadmach or Kilian). The micro tablets can be made of various sizes and shapes like round, oval, oblong, capsule shaped, triangular, square, etc. The preferred shape of the micro tablet is round, biconvex and the preferred diameter of the micro tablet is 1.5 mm to 9.5 mm. The micro tablets can be coated by a solution of one or more release controlling agents by any known method, including spray application. Spraying can be carried out using a fluidized bed coated (preferably Wurster coating), or in a pan coating system. Alternatively the coating of the micro tablets with one or more rate controlling agents can be done by hot melt process using a fluidized bed coated (preferably Wurster coating), or in a pan coating system. The micro tablets can be filled in the casing using manually operated, semiautomatic or automatic capsule filling machine. BRTEF DESCRIPTION OF THE DRAWINGS Scheme 1, 2 and 3 shows the flow diagram of manufacturing steps of different embodiments of modified release dosage form as per the present invention. These schemes are given for the purpose of illustration only. Scheme 1 is a flow diagram of different processing steps of manufacturing the modified release dosage form as per the one embodiment of present invention. The different steps are (i) making core particles, (ii) coating of core particles, (iii) compression of micro tablets, (iv) coating of micro tablets and (v) filling of coated micro tablets into a casing. Scheme 2 is a flow diagram of different processing steps of manufacturing the modified release dosage form as per the one embodiment of present invention. The different steps are (i) making core particles, (ii) coating of core particles, (iii) compression of micro tablets and (iv) filling of micro tablets into a casing. Scheme 3 is a flow diagram of different processing steps of manufacturing the modified release dosage form as per the one embodiment of present invention. The different steps are (i) making core particles, (ii) compression of micro tablets (iii) coating of micro tablets and (iv) filling of micro tablets into a casing.
DESCRIPTION OF PREFERRED EMBODIMENTS Following embodiments further illustrate but by no means limit the present invention.
The dissolution of novel dosage form of the present invention was determined by following method. For sodium valproate- Instrument - Apparatus I, USP (basket) Revolution - 75 / min. Temperature - 37±0.5°C
Dissolution medium - 1000 ml pH 6.8 buffer For domperidone maleate- Instrument - Apparatus I, USP (Basket) Revolution - 100 / min. Temperature - 37±0.5°C
Dissolution medium - 900 ml 0.01 N HCl in 1 % SLS For venlafaxine hydrochloride- Instrument - Apparatus I, USP (Basket) Revolution - 100 / min. Temperature - 37±0.5°C
Dissolution medium - 500 ml water For diltiazem HC1- Instrument - Apparatus II, USP (Paddle) Revolution - 100 / min. Temperature - 37±0.5°C
Dissolution medium - 900 ml 0. I N HCl Throughout this specification and the appended claims it is to be understood that the words "comprise" and "include" and variations such as "comprises", "comprising", "includes", "including" are to be interpreted inclusively, unless the context requires otherwise. That is, the use of these words may imply the inclusion of an element or elements not specifically recited. The present invention has been described by way of example only, and it is to be recognized that modifications thereto which fall within the scope and spirit of the appended claims, and which would be obvious to a skilled person based upon the disclosure herein, are also considered to be included within the invention.
EXAMPLES EXAMPLE 1
A) Core particles- Composition- Venlafaxine HCl 80.93 %w/w Eudragit RSPO 19.07 %w/w
Method- Venlafaxine HCl and Eudragit RSPO were blended in a low shear mixer (Planetary Mixer) and granulated with solvent mixture of acetone and methylene chloride (with ratio of 37.44:62.56 %w/w). The wet mass was dried and sized.
B) Coating of core particles- Composition- Core particles 52.43 %w/w Hydrogenated castor oil 46.57 %w/w Magnesium stearate 01.00 %w/w Method- Core particles and hydrogenated castor oil were blended in a low shear mixer (Planetary Mixer). In order to coat the core particles with hydrogenated castor oil, the blend was heated to 80-85°C with the help of water bath while continuing the mixing operation for one hour. The coated core particles were cooled and sifted through sieve and blended with magnesium stearate. C) Compression of micro tablets 50 mg coated core particles are pressed to tablet (equivalent to 18.75 mg venlafaxine) using 4.76 mm round flat punches. D) Filling of micro tablets into casing (a) Two micro tablets equivalent to 37.5 mg venlafaxine were filled in each hard gelatin capsule. (b) Four micro tablets equivalent to 75 mg venlafaxine were filled in each hard gelatin capsule.
The dissolution rate of the modified release dosage forms prepared above was determined (Table 1)
EXAMPLE 2
A) Core particles- Composition- Venlafaxine HCl 58.58 %w/w Eudragit RSPO 41.42 %w/w
Method- Venlafaxine HCl and Eudragit RSPO were blended in a low shear mixer (Planetary Mixer) and granulated with solvent- mixture of acetone and methylene chloride (with ratio of 58.27:41.73 %w/w). The wet mass was dried and sized. B) Coating of core particles- Composition- Core particles 60.36 %w/w Hydrogenated castor oil 38.64 %w/w Magnesium stearate 01.00 %w/w Method- Core particles and hydrogenated castor oil were blended in a low shear mixer (Planetary Mixer). In order to coat the core particles with hydrogenated castor oil, the blend was heated to 80-85°C with the help of water bath while continuing the mixing operation for one hour. The coated core particles were cooled and sifted through sieve and blended with magnesium stearate.
C) Compression of micro tablets 60 mg coated core particles are pressed to tablet (equivalent to 18.75 mg venlafaxine) using 4.76 mm round flat punches.
D) Filling of micro tablets into casing (a) Two micro tablets equivalent to 37.5 mg venlafaxine were filled in each hard gelatin capsule.
(b) Four micro tablets equivalent to 75 mg venlafaxine were filled in each hard gelatin capsule.
The dissolution rate of the modified release dosage forms prepared above was determined (Table 2) Table 2: Dissolution profile
EXAMPLE 3
A) Core particles- Composition- Venlafaxine HCl 80.93 %w/w Eudragit RSPO 19.07 %w/w
Method- Venlafaxine HCl and Eudragit RSPO were blended in a high shear mixer (Rapid Mixer Granulator) and granulated with solvent mixture of acetone and methylene chloride (with ratio of 84.34:15.66 %w/w). The wet mass was dried and sized.
B) Coating of core particles- Composition- Core particles 82.34 %w/w Hydrogenated castor oil 16.66 %w/w Magnesium stearate 01.00 %w/w Method- Core particles were loaded in fluidized bed coater and coated with hydrogenated castor oil solution in methylene chloride. The coated core particles were sifted through sieve and blended with magnesium stearate.
C) Compression of micro tablets 31.84 mg coated core particles are pressed to tablet (equivalent to 18.75 mg venlafaxine) using 3.97 mm round standard concave punches.
D) Coating of micro tablets Coating composition Hydrogenated castor oil 07.04 %w/w Methylene chloride 92.96 %w/w Micro tablets were coated in perforated coating pan coating machine using the coating solution prepared above. The percent of coating on micro tablets was 6.29 %w/w. E) Filling of micro tablets into casing Four micro tablets equivalent to 75 mg venlafaxine were filled in each hard gelatin capsule. The dissolution rate of the modified release dosage forms prepared above was determined (Table 3) Table 3: Dissolution profile
EXAMPLE 4
A) Core particles- Composition- Same as of example 3.
B) Coating of core particles- Composition- Core particles 70.49 %w/w Hydrogenated castor oil 28.52 %w/w Magnesium stearate 00.99 %w/w Method- Core particles were loaded in fluidized bed coater and coated with hydrogenated castor oil solution in methylene chloride. The coated core particles were sifted through sieve and blended with magnesium stearate.
C) Compression of micro tablets
37.20 mg coated core particles are pressed to tablet (equivalent to 18.75 mg venlafaxine) using 3.97 mm round standard concave punches. D) Filling of micro tablets into casing Four micro tablets equivalent to 75 mg venlafaxine were filled in each hard gelatin capsule.
The dissolution rate of the modified release dosage forms prepared above was determined (Table 4) Table 4: Dissolution profile
EXAMPLE 5
A) Core particles- Composition- same as of example 3.
B) Coating of core particles- Composition- same as of example 4.
C) Compression of micro tablets Same as of example 4.
D) Coating of micro tablets Coating composition Hydrogenated castor oil 07.04 %w/w Methylene chloride 92.96 %w/w Micro tablets were coated in perforated coating pan coating machine using the coating solution prepared above. The weight gain of coating was 6.62 %w/w. E) Filling of micro tablets into casing Four micro tablets equivalent to 75 mg venlafaxine were filled in each hard gelatin capsule.
The dissolution rate of the modified release dosage forms prepared above was determined (Table 5)
Table 5: Dissolution profile
EXAMPLE 6
A) Core particles- Composition- same as of example 3.
B) Coating of core particles- Composition- same as of example 3.
C) Compression of micro tablets Same as of example 3.
D) Coating of micro tablets Coating composition Eudragit RSPO 09.54 %w/w Eudragit L 100 00.95 %w/w Diethyl phthalate 01.04 %w/w Talc 05.95 %w/w Isopropyl alcohol 30.75 %w/w Methylene chloride 51.77 %w/w Micro tablets were coated in perforated coating pan coating machine using the coating solution prepared above. Micro tablets were coated for two different levels of percent coating. The percent coating on micro tablets was (a) 7.46 %w/w and (b) 9.17 %w/w. E) Filling of micro tablets into casing Four micro tablets equivalent to 75 mg venlafaxine were filled in each hard gelatin capsule.
The dissolution rate of the modified release dosage forms prepared above was determined (Table 6).
Table 6: Dissolution profile
EXAMPLE 7
A) Core particles- Composition- same as of example 3.
B) Coating of core particles- Composition- Core particles 79.68 %w/w Eudragit RSPO 12.89 %w/w Eudragit LI 00 03.23 %w/w Triethyl citrate 03.23 %w/w Magnesium stearate 00.97 %w/w Method- Core particles were loaded in fluidized bed coater and coated with coating solution prepared by dissolving Eudragit RSPO, Eudragit LI 00 and Triethyl citrate in solvent mixture of isopropyl alcohol and methylene chloride
(ratio of 37.26:62.74 %w/w). The coated bore particles were sifted through sieve and blended with magnesium stearate.
C) Compression of micro tablets 32.90 mg coated core particles are pressed to tablet (equivalent to 18.75 mg venlafaxine) using 3.97 mm round standard concave punches.
D) Coating of micro tablets Coating composition Eudragit RSPO 05.39 %w/w Eudragit L 100 01.35 %w/w Triethyl citrate 01.35 %w/w Isopropyl alcohol 33.00 %w/w Methylene chloride 55.56 %w/w Purified water 03.35 %w/w Micro tablets were coated in perforated coating pan coating machine using the coating solution prepared above. The percent coating on micro tablets was 7.1 %w/w. E) Filling of micro tablets into casing Four micro tablets equivalent to 75 mg venlafaxine were filled in each hard gelatin capsule.
The dissolution rate of the modified release dosage forms prepared above was determined (Table 7). Table 7: Dissolution profile
EXAMPLE 8 A) Core particles- Composition- Venlafaxine HCl 66.62 %w/w Hydrogenated castor oil 33.38 %w/w
Method- Venlafaxine and hydrogenated castor oil were blended and heated to approximate 120°C in a steam jacketed vessel. The molten semisolid mass was spread on trays. The dried mass was then sized through oscillating granulator. B) Coating of core particles-
Composition- Core particles 57.99 %w/w Eudragit RSPO 36.42 %w/w Triethyl citrate 03.64 %w/w Colloidal silicon dioxide 00.49 %w/w Magnesium stearate 01.46 %w/w Method- Core particles were loaded in fluidized bed coater and coated with coating solution prepared by dissolving Eudragit RSPO and Triethyl citrate in solvent mixture of in methylene chloride and isopropyl alcohol (in the ratio of 62.74:37.26 %w/w). The coated core particles were sifted through sieve and blended with magnesium stearate.
C) Compression of micro tablets 21.97 mg coated core particles are pressed to tablet (equivalent to 18.75 mg venlafaxine) using 3.2 mm round standard concave punches.
D) Coating of micro tablets Coating composition Eudragit RSPO 05.23 %w/w Diethyl phthalate 00.52 %w/w Talc 02.61 %w/w Isopropyl alcohol 34.15 %w/w Methylene chloride 57.49 %w/w Micro tablets were coated in perforated coating pan coating machine using the coating solution prepared above. Micro tablets were coated for two different levels of percent coating. The percent coating on micro tablets was (a) 7.09 %w/w and (b) 10.2 %w/w. E) Filling of micro tablets into casing Ten micro tablets equivalent to 75 mg venlafaxine were filled in each hard gelatin capsule.
The dissolution rate of the modified release dosage forms prepared above was determined (Table 8).
Table 8: Dissolution profile
EXAMPLE 9
A) Core particles- Composition- Sodium valproate 80.00 %w/w Eudragit RSPO 20.00 %w/w
Method- Sodium valproate and Eudragit RSPO were blended in a low shear mixer (Planetary Mixer) and granulated with solvent mixture of acetone and methylene chloride (with ratio of 37.44:62.56 %w/w). The wet mass was dried and sized.
B) Coating of core particles- Composition- Core particles 54.64 %w/w Hydrogenated castor oil 43.72 %w/w Colloidal silicon dioxide 00.55 %w/w Magnesium stearate 01.09 %w/w Method- Core particles and hydrogenated castor oil were blended in a low shear mixer (Planetary Mixer). In order to coat the core particles with hydrogenated castor oil, the blend was heated to 80-85°C with the help of water bath while continuing the mixing operation for one hour. The coated core particles were cooled and sifted through sieve and blended with magnesium stearate and colloidal silicon dioxide. C) Compression of micro tablets 45.75 mg coated core particles are pressed to tablet (equivalent to 20 mg sodium valproate) using 4.76 mm flat round punches.
D) Filling of micro tablets into casing Ten micro tablets equivalent to 200 mg sodium valproate were filled in each hard gelatin capsule.
The dissolution rate of the modified release dosage forms prepared above was determined (Table 9). Table 9: Dissolution profile
EXAMPLE 10
A) Core particles- Composition- Same as of example 9.
B) Coating of core particles- Composition- Core particles 54.17 %w/w Hydrogenated castor oil 43.33 %w/w Colloidal silicon dioxide 00.50 %w/w Magnesium stearate 02.00 %w/w Method- Core particles and hydrogenated castor oil were blended in a low shear mixer (Planetary Mixer). In order to coat the core particles with hydrogenated castor oil, the blend was heated to 80-85°C with the help of water bath while continuing the mixing operation for one hour. The coated core particles were cooled and sifted through sieve and blended with magnesium stearate and colloidal silicon dioxide.
C) Compression of micro tablets 30.77 mg coated core particles are pressed to tablet (equivalent to 13.333 mg sodium valproate) using 3.97 mm flat round punches.
D) Coating of micro tablets Coating composition Eudragit RSPO 05.23 %w/w
Diethyl phthalate 00.52 %w/w Talc 02.61 %w/w Isopropyl alcohol 34.15 %w/w Methylene chloride 57.49 %w/w Micro tablets were coated in perforated coating pan coating machine using the coating solution prepared above. Micro tablets were coated for three different levels of percent coating. The percent coating on micro tablets was (a) 4.59
%w/w, (b) 6.71 %w/w and (c) 8.41 %w/w. E) Filling of micro tablets into casing Fifteen micro tablets equivalent to 200 mg sodium valproate were filled in each hard gelatin capsule.
The dissolution rate of the modified release dosage forms prepared above was determined (Table 10).
Table 10: Dissolution profile
EXAMPLE 11
A) Core particles- Composition- Domperidone maleate 41.93 %w/w Eudragit RLPO 08.79 %w/w Lactose 49.28 %w/w
Method- Domperidone maleate, lactose and Eudragit RLPO were blended in a low shear mixer (Planetary Mixer) and granulated with solvent mixture of acetone and methylene chloride (with ratio of 70.54:29.46 %w/w). The wet mass was dried and sized.
B) Coating of core particles- Composition- Core particles 94.28 %w/w Ethyl cellulose 45 cps 02.76 %w/w HPMC 6 cps 01.38 %w/w Colloidal silicon dioxide 00.54 %w/w Magnesium stearate 01.04 %w/w Method- Core particles were coated in fluid bed coater by coating solution by dissolving ethyl cellulose 45 cps and HPMC 6 cps solvent mixture of methylene
chloride and methanol (in ratio of 62.50:37.50 %w/w). The coated core particles were sifted through sieve and blended with magnesium stearate and colloidal silicon dioxide.
C) Compression of micro tablets 24.13 mg coated core particles are pressed to tablet (equivalent to 7.5 mg domperidone) using 3.97 mm round flat punches.
D) Filling of micro tablets into casing Four micro tablets equivalent to 30 mg domperidone were filled in each hard gelatin capsule.
The dissolution rate of the modified release dosage forms prepared above was determined (Table 11) Table 11 : Dissolution profile
EXAMPLE 12 A) Core particles- Composition- Diltiazem HCl 81.63 %w/w Eudragit RSPO 18.37 %w/w
Method- Diltiazem HCl and Eudragit RSPO were blended in a high shear mixer (Rapid Mixer Granulator) . and granulated with solvent mixture of isopropyl alcohol and methylene chloride (with ratio of 37.26:62.74 %w/w). The wet mass was dried and sized. B) Coating of core particles- Composition-
Core particles 81.67 %w/w Eudragit RSPO 15.00 %w/w Diethyl phthalate 01.83 %w/w Colloidal silicon dioxide 00.50 %w/w Magnesium stearate 01.00 %w/w Method- Core particles were loaded in fluidized bed coater and coated with coating solution prepared by dissolving Eudragit RSPO, and diethyl phthalate in solvent mixture of isopropyl alcohol and methylene chloride (ratio of 37.26:62.74 %w/w). The coated core particles were sifted through sieve and blended with magnesium stearate and colloidal silicon dioxide. C) Compression of micro tablets 30 mg coated core particles are pressed to tablet ' (equivalent to 20 mg diltiazem HCl) using 3.97 mm round flat punches.
D) Filling of micro tablets into casing Twelve micro tablets equivalent to 240 mg diltiazem HCl were filled in each hard gelatin capsule.
The dissolution rate of the modified release dosage forms prepared above was determined (Table 12) •Table 12: Dissolution profile
EXAMPLE 13
A) Core particles- Composition- Same as of example 12
B) Coating of core particles- Composition- Core particles 90.74 %w/w Eudragit RSPO 05.61 %w/w Eudragit RLPO 01.12 %w/w Diethyl phthalate 00.68 %w/w Colloidal silicon dioxide 00.50 %w/w Magnesium stearate 01.35 %w/w Method- Core particles were loaded in fluidized bed coater and coated with coating solution prepared by dissolving Eudragit RSPO, Eudragit RLPO and diethyl phthalate in solvent mixture of isopropyl alcohol and methylene chloride (ratio of 37.26:62.74 %w/w). The coated core particles were sifted through sieve and blended with magnesium stearate and colloidal silicon dioxide.
C) Compression of micro tablets 27 mg coated core particles are pressed to tablet (equivalent to 20 mg diltiazem HCl) using 3.97 mm round standard concave punches.
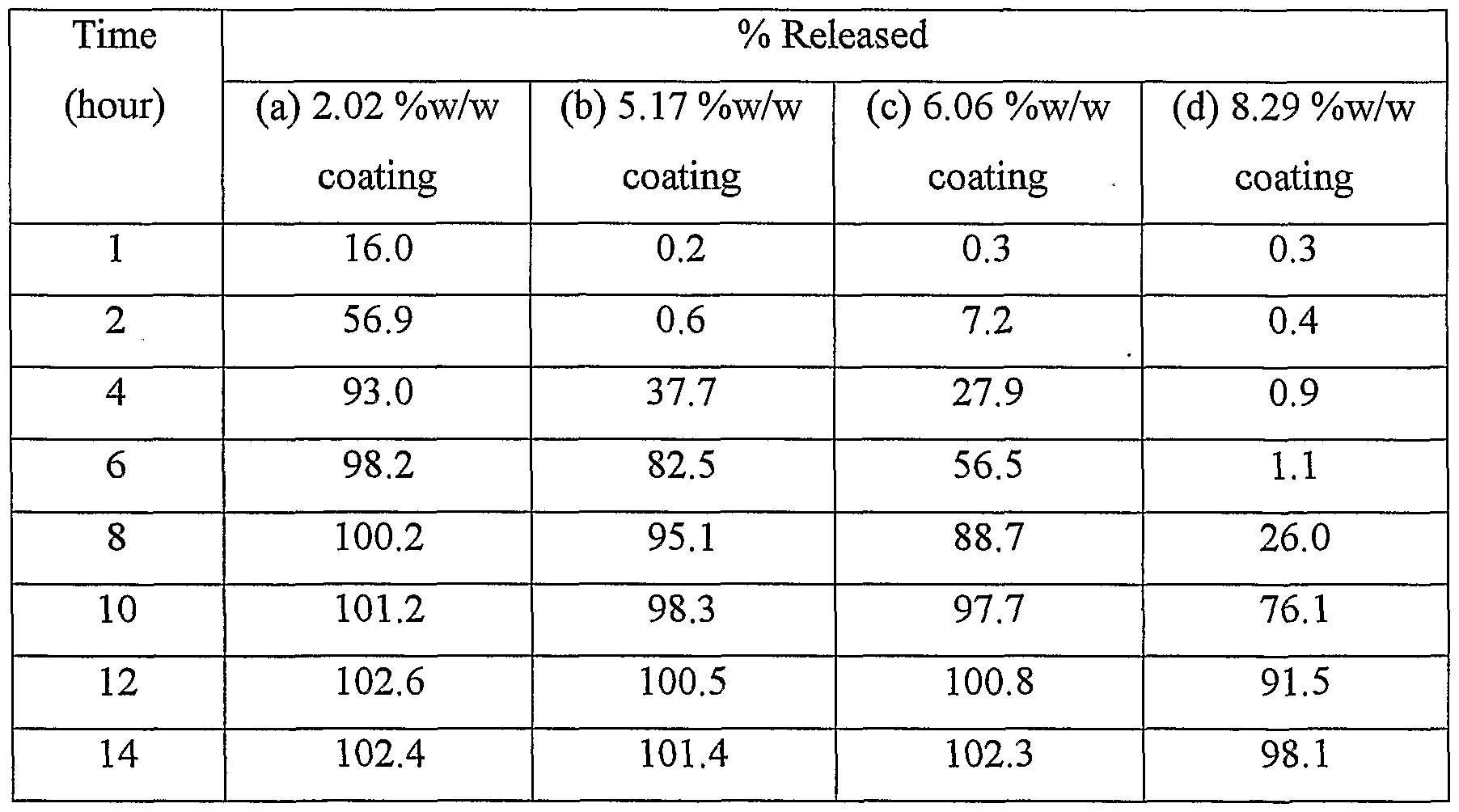
D) Coating of micro tablets Coating composition Eudragit RSPO 05.23 %w/w Eudragit RLPO 00.52 %w/w Diethyl phthalate 00.57 %w/w Talc 03.26 %w/w Isopropyl alcohol 33.69 %w/w Methylene chloride 56.73 %w/w Micro tablets were coated in perforated coating pan coating machine using the coating solution prepared above. Micro tablets were coated for three different levels of percent coating. The percent coating on micro tablets was (a) 2.02
%w/w, (b) 5.17 %w/w, (c)6.06 %w/w and .(d) 8.29 %w/w. E) Filling of micro tablets into casing Twelve micro tablets equivalent to 240 mg Diltiazem HCl were filled in each hard gelatin capsule.
The dissolution rate of the modified release dosage forms prepared above was determined (Table 13). Table 13: Dissolution profile
Examples 14 to 19 These examples further illustrate the granulation, coating and dissolution profile of the micro tablets of the present invention.