FRESHENING COMPOSITIONS COMPRISING MALODOR BINDING POLYMERS AND
MALODOR CONTROL COMPONENTS
FIELD OF THE INVENTION
The present invention relates to freshening compositions comprising a malodor binding polymer, malodor control components, and an aqueous carrier; and methods thereof.
BACKGROUND OF THE INVENTION
Freshening products for reducing or masking malodors on fabrics and in air are currently available and are described in the patent literature. The Procter & Gamble Company sells fabric and air freshening products under the FEBREZE® brand name. These products typically contain perfume, solubilizer, cyclodextrin, and an aqueous carrier. S. C. Johnson sells products such as Glade® Fabric and Air Odor Eliminator and Oust® Surface Disinfectant and Air Sanitizer. Reckitt-Benckiser sells products such as Lysol® Disinfectant Spray.
Certain freshening compositions do not effectively neutralize a broad range of malodors on fabrics and in the air. Further, the time required for a composition to noticeably combat malodors may create consumer doubt as to a product's efficacy on malodors. For example, the consumer may leave the treated space before the product begins to noticeably reduce the malodor.
There remains a need for improved freshening compositions that neutralize a broad range of malodors, including amine-base and sulfur-based malodors and malodors caused by microbes, while not overpowering malodors with overwhelming perfume.
SUMMARY OF THE INVENTION
The present invention relates to a freshening composition for reducing malodor. According to one embodiment, there is provided a freshening composition for reducing malodor comprising: an effective amount of a malodor binding polymer; a malodor control component comprising an effective amount of a mixture of two or more volatile aldehydes for neutralizing a malodor, wherein said two or more volatile aldehydes are selected from the group consisting of 2-ethoxy benzylaldehyde, 2-isopropyl-5-methyl-2-hexenal, 5-methyl furfural, 5-methyl- thiophene-carboxaldehyde, adoxal, p-anisaldehyde, benzylaldehyde, bourgenal, cinnamic
aldehyde, cymal, decyl aldehyde, floral super, florhydral, helional, lauric aldehyde, ligustral, lyral, melonal, o-anisaldehyde, pino acetaldehyde, P.T. bucinal, thiophene carboxaldehyde, trans- 4-decenal, trans trans 2,4-nonadienal, undecyl aldehyde, and mixtures thereof; and an aqueous carrier.
In another embodiment, there is provided a freshening composition for reducing malodor comprising: an effective amount of a malodor binding polymer; and a malodor control component comprising at least one volatile aldehyde and an acid catalyst having a vapor pressure of about 0.01 to about 13 at 25°C.
The present invention also relates to methods of reducing malodor comprising the steps of: providing a freshening composition comprising an effective amount of a malodor binding polymer and a malodor control component; and dispersing an effective amount of said freshening composition on an inanimate surface or in the air.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a bar graph showing the reduction of aldehydic malodors evaporating off fabrics treated with freshening compositions according to the present invention containing a malodor binding polymer in comparison to a freshening composition lacking such malodor binding polymer.
Fig. 2 is a bar graph showing microbe reduction with a freshening composition according to the present invention containing a malodor binding polymer in comparison to a freshening composition lacking such malodor binding polymer.
Fig. 3 is a graph showing the performance of one embodiment of a malodor control component, in accordance with the present invention, on a sulfur-based malodor.
Fig. 4 is a graph showing the performance of one embodiment of a malodor control component, in accordance with the present invention, on an amine-based malodor.
Fig. 5 is a graph showing butanethiol reduction by thiophene carboxaldehyde in combination with various acid catalysts.
DETAILED DESCRIPTION OF THE INVENTION
The freshening composition of the present invention is designed to deliver genuine malodor reduction and not function merely by using perfume to cover up or mask odors. The freshening composition reduces malodor in the air or on inanimate surfaces, for example, fabrics
that are contaminated with environmental odors such as food and tobacco odors, or wetted with perspiration. The freshening composition may also reduce microbes on inanimate surfaces or in air. The freshening composition may also act as a barrier to prevent malodors from adhering to or penetrating an inanimate surface.
A genuine malodor reduction provides a sensory and analytically measurable (e.g. gas chromatograph) malodor reduction. Thus, if the freshening composition delivers a genuine malodor reduction, the freshening composition will neutralize malodors in the air and/or on fabrics. "Neutralize" or "neutralization" as used herein means chemically reacting with malodor components (e.g. the reaction of primary amines with aldehydes to form imines, reductive alkylation of amines, protonation and deprotonation of amines, polymerization or de- polymerization); or suppressing the volatility of malodorous components such that other parts of the composition may react (e.g. acid - base neutralization); or physically entrapping odorous molecules such that they are not re-released into the air (e.g. cyclodextrin inclusion complexes as described herein). Odor neutralization may be distinguished from odor masking or odor blocking by a change in the malodorous compound, as opposed to a change in the ability to perceive the malodor without any corresponding change in the condition of the malodorous compound.
I. Freshening Composition
The freshening composition for reducing malodor comprises a malodor binding polymer, a malodor counteractant comprising at least one aliphatic aldehyde, and an aqueous carrier, wherein said composition is essentially free of materials that soil or stain fabric. The total amount of surfactants (e.g. solubilizer, wetting agent) in the freshening composition is from 0% to 3% or no more than 3%, alternatively from 0% to 1% or no more than 1%, alternatively from 0% to 0.9% or no more than 0.9%, alternatively from 0% to 0.7 or no more than 0.7%, alternatively from 0% to 0.5% or no more than 0.5%, alternatively from 0% to 0.3% or no more than 0.3%, by weight of the composition. Compositions with higher concentrations can make fabrics susceptible to soiling and/or leave unacceptable visible stains on fabrics as the solution evaporates. A. Malodor Binding Polymer
The freshening composition of the present invention includes a malodor binding polymer. A malodor binding polymer is polymer having an available functional group (e.g. at least one primary amine) that has the affinity to neutralize malodor components. Monomers having an
available function group with an affinity to neutralize malodor components are also contemplated.
A malodor binding polymer may include amine based compounds, such as monoamines, amino acids, polyethyleneimine polymers (PEIs), modified PEIs, substituted PEIs; acrylic acid polymers, such as polyacrylate co-polymer (e.g. Acumer™ 9000 from Rohm & Haas), polyacrylic acid polymers (e.g. Acusol™ from Rohm & Haas), and modified acrylate copolymers (e.g. Aculyn™ from Rohm & Haas); and modified methacrylate copolymers (e.g. HydroSal™ from Salvona Technologies); or mixtures thereof.
1. Amine based compounds
In some embodiments, the malodor binding polymer is an amine based compound with a molecular weight greater than 100 Daltons and at least 10% of its amine groups are primary amines. In one embodiment, the amine-based compound will be a polyamine with a molecular weight greater than 150 Daltons and 15% to 80% of its amine groups are primary amines. In another embodiment, the malodor binding polymer is an amine-based compound with a molecular weight greater than 1000 Daltons and from 0% to about 10% or less than 10% of its amine groups are primary amines.
A general structure for a primary amine compound useful in this invention is as follows:
B-(NH2)n;
wherein B is a carrier material, and n is an index of value of at least 1. Suitable B carriers include both inorganic and organic carrier moieties. By "inorganic carrier", it is meant a carrier which is comprised of non- or substantially non-carbon based backbones.
Compounds containing a secondary amine group have a structure similar to the above with the exception that the compound comprises one or more -NH- groups as well as -N¾ groups. The amine compounds of this general type may be relatively viscous materials.
Exemplary amine based compounds are those selected from monoamines, aminoaryl derivatives, poly amines and derivatives thereof, poly amino acids and copolymers thereof, glucamines, dendrimers, PEIs, substituted amines and amides monoamines, or mixtures thereof, a. Monoamines
Monoamines may be utilized in the present invention. Nonlimiting examples of suitable monoamines for use in the present invention include, but are not limited to, primary amines that also contain hydroxy and/or alkoxy functional groups, such as the 2-hydroxyamines and/or 3- hydroxy amines; primary or secondary amines that also contain a functional group that enhances deposition of the monoamine compared to monoamines that lack that functional group, especially
when the monoamine is interacting with the benefit agent. Primary monoamines may also be used herein in combination with secondary monoamines. However, sufficient levels of the primary monoamine must be used to provide at least 10% of the total amine groups within such combinations as primary amine groups.
b. Aminoaryl derivatives
Exemplary aminoaryl derivatives are the amino-benzene derivatives including the alkyl esters of 4-amino benzoate compounds, ethyl-4-amino benzoate, phenylethyl-4-aminobenzoate, phenyl-4-aminobenzoate, 4-amino-N'-(3-aminopropyl)-benzarnide, or mixtures thereof.
c. Polyamines
Examples of suitable amino functional polymers containing at least one primary amine group for the purposes of the present invention are:
- Polyvinylamine with a MW of 300-2.10E6 Daltons (e.g Lupamine series 1500, 4500, 5000, 9000 available from BASF);
- Polyvinylamine alkoxylated with a MW of > 600 Daltons and a degree of ethoxylation of at least 0.5;
- Polyvinylamine vinylalcohol - molar ratio 2:1, polyvinylaminevinylformamide - molar ratio 1:2 and polyvinylamine vinylformamide-molar ratio 2:1;
- Triethylenetetramine, diethylenetriamine, tetraethylenepentamine;
- Bis-aminopropylpiperazine;
- amino substituted polyvinylalcohol with a MW ranging from 400-300,000 Daltons;
- polyoxyethylene bis [amine] available from e.g. Sigma;
- polyoxyethylene bis [6-aminohexyl] available from e.g. Sigma;
- N,N'-bis-(3-aminopropyl)-l,3-propanediamine linear or branched (TPTA);
- N,N'-bis-(3-arninopropyl)ethylenediarnine;
- bis (amino alkyl) alkyl diamine, linear or branched; and
- l,4-bis-(3-aminopropyl) piperazine (BNPP).
d. Polyamino Acids
Suitable amine based compounds include polyamino acids. Polyamino acids are made up of amino acids or chemically modified amino acids. The amino acids may be selected from cysteine, histidine, isoleucine, tyrosine, tryptophane, leucine, lysine, glutamic acid, glutamine, glycine, alanine, aspartic acid, arginine, asparagine, phenylalanine, proline, serine, histidine, threonine, methionine, valine, and mixtures thereof. Amino acid derivatives may be tyrosine
ethylate, glycine methylate, tryptophane ethylate, or mixtures thereof; homopolymers of amino acids; hydroxy amines; polyamino acids; or mixtures thereof.
In chemically modified amino acids, the amine or acidic function of the amino acid has reacted with a chemical reagent. This is often done to protect these chemical amine and acid functions of the amino acid in a subsequent reaction or to give special properties to the amino acids, like improved solubility. Examples of such chemical modifications are benzyloxycarbonyl, aminobutyric acid, butyl ester, and pyroglutamic acid. More examples of common modifications of amino acids and small amino acid fragments can be found in the Bachem, 1996, Peptides and Biochemicals Catalog.
One polyamino acid is polylysine, alternatively poly lysines or polyamino acids where more than 50% of the amino acids are lysine, since the primary amine function in the side chain of the lysine is the most reactive amine of all amino acids. One polyamino acid has a molecular weight of 500 to 10,000,000, alternatively between 2000 and 25,000.
The polyamino acid can be cross linked. The cross linking can be obtained for example by condensation of the amine group in the side chain of the amino acid like lysine with the carboxyl function on the amino acid or with protein cross linkers like PEG derivatives. The cross linked polyamino acids still need to have free primary and/or secondary amino groups left for neutralization. Cross linked polyamino acid has a molecular weight of 20,000 to 10,000,000; alternatively between 200,000 and 2,000,000.
The polyamino acid or the amino acid can be co-polymerized with other reagents like for instance with acids, amides, acyl chlorides, aminocaproic acid, adipic acid, ethylhexanoic acid, caprolactam, or mixtures thereof. The molar ratio used in these copolymers ranges from 1:1 (reagent/ amino acid (lysine)) to 1:20, alternatively from 1:1 to 1:10. The polyamino acid like polylysine can be unethoxylated or partially ethoxylated so long as the requisite amount of primary amine remains in the polymer.
e. Dendrimers
Also useful amine based compounds are polypropylenimine dendrimers and the commercially available Starburst® polyamidoamines (PAMAM) dendrimers, generation G0-G10 from Dendritech and the dendrimers Astromols®, generation 1-5 from DSM being DiAminoButane Poly Amine DAB (PA)x dendrimers with x = 2nx4 and n being generally comprised between 0 and 4.
f. PEIs
In one embodiment, the malodor binding polymer is a PEL It has been surprisingly discovered that amine based polymers at a pH of about 4 to about 8, alternatively above 5 to about 8, alternatively 7 can neutralize amine based odors. PEIs have the following general formula:
- ( CH2 - CH2 - NH )n - ; n = 10 - 105
Homopolymeric PEIs are branched, spherical polyamines with a well defined ratio of primary, secondary and tertiary amine functions. They are best described in the following partial structural formula:
The chemical structure of homopolymeric PEIs follows a simple principle: one amine function - two carbons.
The freshening composition may comprise a homopolymeric polyethylenimine having a molecular weight of about 800 to about 2,000,000, alternatively about 1,000 to about 2,000,000, alternatively about 1,200 to about 25,000, alternatively about 1,300 to about 25,000, alternatively about 2,000 to about 25,000, alternatively about 10,000 to about 2,000,000, alternatively about 25,000 to about 2,000,000, alternatively about 25,000. Exemplary homopolymeric PEIs include those that are commercially available under the tradename Lupasol® from BASF. Lupasol products are usually obtained through polymerization of the ethylenimine monomer. The ethylenimine monomer has totally reacted in the polymer matrix. Suitable Lupasol products include Lupasol FG (MW 800), G20wfv (MW 1300), PR8515 (MW 2000), WF (MW 25,000), FC (MW 800), G20 (MW 1300), G35 (MW 1200), GlOO (MW 2000), HF (MW 25,000), P (MW 750,000), PS (MW 750,000), SK (MW 2,000,000), SNA (MW 1,000,000).
In some embodiments, the freshening composition comprises Lupasol HF or WF (MW
25,000), P (MW 750,000), PS (MW 750,000), SK (MW 2,000,000), 620wfv (MW 1300) or PR 1815 (MW 2000), or Epomin SP-103, Epomin SP-110, Epomin SP-003, Epomin SP-006, Epomin SP-012, Epomin SP-018, Epomin SP-200, or partially alkoxylated polyethyleneimine,
like polyethyleneirnine 80% ethoxylated from Aldrich. In one embodiment, the freshening composition contains Lupasol WF (MW 25,000).
Also suitable amine based compounds for use in the freshening composition are modified PEIs, partially alkylated polyethylene polymers, PEIs with hydroxyl groups, 1,5-pentanediamine, 1,6-hexanediamine, 1,3 pentanediamine, 3-dimethylpropanediamine, 1,2-cyclohexanediamine, l,3-bis(aminomethyl)cyclohexane, tripropylenetetraamine, bis (3-aminopropyl)piperazine, dipropylenetriamine, tris(2-arninoethylamine), tetraethylenepentamine, bishexamethylenetriamine, bis(3-aminopropyl) 1,6 - hexamethylenediamine, 3,3'-diamino-N- methyldipropylamine, 2-methyl- 1 ,5 -pentanediamine, Ν,Ν,Ν' ,Ν' -tetra(2- aminoethyl)ethylenediamine, N,N,N',N'-tetra(3-aminopropyl)-l,4-butanediamine, pentaethylhexamine, l,3-diamino-2-propyl-tert-butylether, isophorondiamine, 4,4',- diaminodicyclohylmethane, N-methyl-N-(3-aminopropyl)ethanolamine, spermine, spermidine, 1- piperazineethaneamine, 2-(bis(2-aminoethyl)amino)ethanol, ethoxylated N-
(tallowalkyl)trimethylene diamines,poly[oxy(methyl-l,2-ethanediyl)], a-(2-aminomethyl- ethoxy)- (= C.A.S No. 9046-10-0); poly[oxy(methyl-l,2-ethanediyl)], a-hydro-)-co-(2- aminomethylethoxy)-, ether with 2-ethyl-2-(hydroxymethyl)-l,3-propanediol (= C.A.S. No. 39423-51-3); commercially available under the tradename Jeffamines T-403, D-230, D-400, D- 2000; 2,2\2"-triarninotriethylarnine; 2,2'-diamino-diethylamine; 3,3'-diamino-dipropylamine, 1,3 bis aminoethyl-cyclohexane commercially available from Mitsubishi, and the C12 Sternamines commercially available from Clariant like the C12 Sternamin(propylenamine)n with n=3/4.
In one embodiment, the malodor binding polymer may be used in an effective amount to provide a reduction of microbes on fabric and/or in the air. When using a malodor binding polymer, an effective amount reduces microbes by at least 1 log difference as compared to a composition lacking the malodor binding polymer. This difference is then attributed to the use of the malodor binding polymer and not the inherent variability in the microbial species.
Suitable levels of malodor binding polymer are from about 0.01% to about 2%, alternatively from about 0.01% to about 1%, alternatively about 0.01% to about 0.8%, alternatively about 0.01% to about 0.6%, alternatively about 0.01% to about 0.1%, alternatively about 0.01% to about 0.07%, alternatively about 0.07%, by weight of the freshening composition. Compositions with higher amount of malodor binding polymer may make fabrics susceptible to soiling and/or leave unacceptable visible stains on fabrics as the solution evaporates off of the fabric.
B. Malodor Control Components
The freshening composition may include malodor control components. The malodor control components are designed to deliver genuine malodor neutralization and not function merely by covering up or masking odors. A genuine malodor neutralization provides a sensory and analytically measurable (e.g. gas chromatograph) malodor reduction. Thus, if the malodor control component delivers a genuine malodor neutralization, the composition will reduce malodors in the vapor and/or liquid phase. When used in combination with the malodor binding polymer, the composition may neutralize a broader range of malodor causing materials which, in turn, further reduces malodors in the air or on inanimate surfaces.
1. Perfume Materials
The malodor control component includes perfume materials which may include a mixture of volatile aldehydes. Volatile aldehyes neutralize malodors in vapor and/or liquid phase via chemical reactions. Aldehydes that are partially volatile may be considered a volatile aldehyde as used herein. Volatile aldehydes may react with amine-based odors, following the path of Schiff-base formation. Volatiles aldehydes may also react with sulfur-based odors, forming thiol acetals, hemi thiolacetals, and thiol esters in vapor and/or liquid phase. It may be desirable for these vapor and/or liquid phase volatile aldehydes to have virtually no negative impact on the desired perfume character of a product.
Suitable volatile aldehydes may have a vapor pressure (VP) in the range of about 0.0001 torr to 100 torr, alternatively about 0.0001 torr to about 10 torr, alternatively about 0.001 torr to about 50 torr, alternatively about 0.001 torr to about 20 torr, alternatively about 0.001 torr to about 0.100 torr, alternatively about 0.001 torr to 0.06 torr, alternatively about 0.001 torr to 0.03 torr, alternatively about 0.005 torr to about 20 torr, alternatively about 0.01 torr to about 20 torr, alternatively about 0.01 torr to about 15 torr, alternatively about 0.01 torr to about 10 torr, alternatively about 0.05 torr to about 10 torr, measured at 25 °C.
The volatile aldehydes may also have a certain boiling point (B.P.) and octanol/water partition coefficient (P). The boiling point referred to herein is measured under normal standard pressure of 760 mmHg. The boiling points of many volatile aldehydes, at standard 760 mm Hg are given in, for example, "Perfume and Flavor Chemicals (Aroma Chemicals)," written and published by Steffen Arctander, 1969.
The octanol/water partition coefficient of a volatile aldehyde is the ratio between its equilibrium concentrations in octanol and in water. The partition coefficients of the volatile aldehydes used in the malodor control component may be more conveniently given in the form of
their logarithm to the base 10, logP. The logP values of many volatile aldehydes have been reported. See, e.g., the Pomona92 database, available from Daylight Chemical Information Systems, Inc. (Daylight CIS), Irvine, California. However, the logP values are most conveniently calculated by the "CLOGP" program, also available from Daylight CIS. This program also lists experimental logP values when they are available in the Pomona92 database. The "calculated logP" (ClogP) is determined by the fragment approach of Hansch and Leo (cf., A. Leo, in Comprehensive Medicinal Chemistry, Vol. 4, C. Hansch, P. G. Sammens, J. B. Taylor and C. A. Ramsden, Eds., p. 295, Pergamon Press, 1990). The fragment approach is based on the chemical structure of each volatile aldehyde, and takes into account the numbers and types of atoms, the atom connectivity, and chemical bonding. The ClogP values, which are the most reliable and widely used estimates for this physicochemical property, are preferably used instead of the experimental logP values in the selection of volatile aldehydes for the malodor control component.
The ClogP values may be defined by four groups and the volatile aldehydes may be selected from one or more of these groups. The first group comprises volatile aldehydes that have a B.P. of about 250 °C or less and ClogP of about 3 or less. The second group comprises volatile aldehydes that have a B.P. of 250°C or less and ClogP of 3.0 or more. The third group comprises volatile aldehydes that have a B.P. of 250°C or more and ClogP of 3.0 or less. The fourth group comprises volatile aldehydes that have a B.P. of 250°C or more and ClogP of 3.0 or more. The malodor control component may comprise any combination of volatile aldehydes from one or more of the ClogP groups.
In some embodiments, the malodor control component of the present invention may comprise, by total weight of the malodor control component, from about 0% to about 30% of volatile aldehydes from group 1, alternatively about 25%; and/or about 0% to about 10% of volatile aldehydes from group 2, alternatively about 10%; and/or from about 10% to about 30% of volatile aldehydes from group 3, alternatively about 30%; and/or from about 35% to about 60% of volatile aldehydes from group 4, alternatively about 35%.
Exemplary volatile aldehydes which may be used in a malodor control component include, but are not limited to, Adoxal (2,6,10-Trimethyl-9-undecenal), Bourgeonal (4-t- butylbenzenepropionaldehyde), Lilestralis 33 (2-methyl-4-t-butylphenyl)propanal), Cinnamic aldehyde, cinnamaldehyde (phenyl propenal, 3-phenyl-2-propenal), Citral, Geranial, Neral (dimethyloctadienal, 3,7-dimethyl-2,6-octadien-l-al), Cyclal C (2,4-dimethyl-3-cyclohexen-l- carbaldehyde), Florhydral (3-(3-Isopropyl-phenyl)-butyraldehyde), Citronellal (3,7-dimethyl 6-
octenal), Cymal, cyclamen aldehyde, Cyclosal, Lime aldehyde (Alpha- methyl-p-isopropyl phenyl propyl aldehyde), Methyl Nonyl Acetaldehyde, aldehyde C12 MNA (2-methyl-l-undecanal), Hydroxycitronellal, citronellal hydrate (7-hydroxy-3,7-dimethyl octan-l-al), Helional (alpha- methyl-3,4-(methylenedioxy)-hydrocinnamaldehyde, hydrocinnamaldehyde (3-phenylpropanal, 3-phenylpropionaldehyde), Intreleven aldehyde (undec-10-en-l-al), Ligustral, Trivertal (2,4- dimethyl-3-cyclohexene-l-carboxaldehyde), Jasmorange, satinaldehyde (2-methyl-3- tolylproionaldehyde, 4-dimethylbenzenepropanal), Lyral (4-(4-hydroxy-4-methyl pentyl)-3- cyclohexene-l-carboxaldehyde), Melonal (2,6-Dimethyl-5-Heptenal), Methoxy Melonal (6- methoxy-2,6-dimethylheptanal), methoxycinnamaldehyde (trans-4-methoxycinnamaldehyde), Myrac aldehyde isohexenyl cyclohexenyl-carboxaldehyde, trifernal ((3-methyl-4-phenyl propanal, 3-phenyl butanal), lilial, P.T. Bucinal, lysmeral, benzenepropanal (4-tert-butyl-alpha- methyl-hydrocinnamaldehyde), Dupical, tricyclodecylidenebutanal (4-Tricyclo5210- 2,6decylidene-8butanal), Melafleur (1,2,3,4,5, 6,7, 8-octahydro-8,8-dimethyl-2-naphthaldehyde), Methyl Octyl Acetaldehyde, aldehyde C-l l MOA (2-mehtyl deca-l-al), Onicidal (2,6,10- trimethyl-5,9-undecadien-l-al), Citronellyl oxyacetaldehyde, Muguet aldehyde 50 (3,7-dimethyl- 6-octenyl) oxyacetaldehyde), phenylacetaldehyde, Mefranal (3 -methyl-5 -phenyl pentanal), Triplal, Vertocitral dimethyl tetrahydrobenzene aldehyde (2,4-dimethyl-3-cyclohexene-l- carboxaldehyde), 2-phenylproprionaldehyde, Hydrotropaldehyde, Canthoxal, anisylpropanal 4- methoxy-alpha-methyl benzenepropanal (2-anisylidene propanal), Cylcemone A (1,2,3,4,5,6,7,8- octahydro-8,8-dimethyl-2-naphthaldehyde), and Precylcemone B (1-cyclohexene-l- carboxaldehyde) .
Still other exemplary aldehydes include, but are not limited to, acetaldehyde (ethanal), pentanal, valeraldehyde, amylaldehyde, Scentenal (octahydro-5-methoxy-4,7-Methano-lH- indene-2-carboxaldehyde), propionaldehyde (propanal), Cyclocitral, beta-cyclocitral, (2,6,6- trimethyl-l-cyclohexene-1 -acetaldehyde), Iso Cyclocitral (2,4,6-trimethyl-3-cyclohexene-l- carboxaldehyde), isobutyraldehyde, butyraldehyde, isovaleraldehyde (3-methyl butyraldehyde), methylbutyraldehyde (2-methyl butyraldehyde, 2-methyl butanal), Dihydrocitronellal (3,7- dimethyl octan-l-al), 2-Ethylbutyraldehyde, 3-Methyl-2-butenal, 2-Methylpentanal, 2-Methyl Valeraldehyde, Hexenal (2-hexenal, trans-2-hexenal), Heptanal, Octanal, Nonanal, Decanal, Laurie aldehyde, Tridecanal, 2-Dodecanal, Methylthiobutanal, Glutaraldehyde, Pentanedial, Glutaric aldehyde, Heptenal, cis or trans-Heptenal, Undecenal (2-, 10-), 2,4-octadienal, Nonenal (2-, 6-), Decenal (2-, 4-), 2,4-hexadienal, 2,4-Decadienal, 2,6-Nonadienal, Octenal, 2,6-dimethyl 5-heptenal, 2-isopropyl-5-methyl-2-hexenal, Trifernal, beta methyl Benzenepropanal, 2,6,6-
Trimethyl-l-cyclohexene-l-acetaldehyde, phenyl Butenal (2 -phenyl 2-butenal), 2.Methyl-3(p- isopropylphenyl)-propionaldehyde, 3-(p-isopropylphenyl)-propionaldehyde, p-Tolylacetaldehyde (4-methylphenylacetaldehyde), Anisaldehyde (p-methoxybenzene aldehyde), Benzaldehyde, Vernaldehyde (l-Methyl-4-(4-methylpentyl)-3-cyclohexenecarbaldehyde), Heliotropin (piperonal) 3,4-Methylene dioxy benzaldehyde, alpha- Amylcinnamic aldehyde, 2-pentyl-3- phenylpropenoic aldehyde, Vanillin (4-methoxy 3 -hydroxy benzaldehyde), Ethyl vanillin (3- ethoxy 4-hydroxybenzaldehyde), Hexyl Cinnamic aldehyde, Jasmonal H (alpha-n-hexyl- cinnamaldehyde), Floralozone, (para-ethyl-alpha,alpha-dimethyl Hydrocinnamaldehyde), Acalea (p-methyl-alpha-pentylcinnamaldehyde), methylcinnamaldehyde, alpha-Methylcinnamaldehyde (2-methyl 3-pheny propenal), alpha-hexylcinnamaldehyde (2-hexyl 3-phenyl propenal), Salicylaldehyde (2 -hydroxy benzaldehyde), 4-ethyl benzaldehyde, Cuminaldehyde (4-isopropyl benzaldehyde), Ethoxybenzaldehyde, 2,4-dimethylbenzaldehyde, Veratraldehyde (3,4- dimethoxybenzaldehyde), Syringaldehyde (3,5-dimethoxy 4-hydroxybenzaldehyde), Catechaldehyde (3,4-dihydroxybenzaldehyde), Safranal (2,6,6-trimethyl-l,3-diene methanal), Myrtenal (pin-2-ene-l-carbaldehyde), Perillaldehyde L-4(l-methylethenyl)-l-cyclohexene-l- carboxaldehyde), 2,4-Dimethyl-3-cyclohexene carboxaldehyde, 2-Methyl-2-pentenal, 2- methylpentenal, pyruvaldehyde, formyl Tricyclodecan, Mandarin aldehyde, Cyclemax, Pino acetaldehyde, Corps Iris, Maceal, and Corps 4322.
In one embodiment, the malodor control component includes a mixture of two or more volatile aldehydes selected from the group consisting of 2-ethoxy Benzylaldehyde, 2-isopropyl- 5-methyl-2-hexenal, 5-methyl Furfural, 5-methyl-thiophene-carboxaldehyde, Adoxal, p- anisaldehyde, Benzylaldehyde, Bourgenal, Cinnamic aldehyde, Cymal, Decyl aldehyde, Floral super, Florhydral, Helional, Laurie aldehyde, Ligustral, Lyral, Melonal, o-anisaldehyde, Pino acetaldehyde, P.T. Bucinal, Thiophene carboxaldehyde, trans-4-Decenal, trans trans 2,4- Nonadienal, Undecyl aldehyde, and mixtures thereof.
In some embodiments, the malodor control component includes fast reacting volatile aldehydes. "Fast reacting volatile aldehydes" refers to volatile aldehydes that either (1) reduce amine odors by 20% or more in less than 40 seconds; or (2) reduce thiol odors by 20% or more in less than 30 minutes.
In one embodiment, the malodor control component includes a mixture of the volatile aldehydes listed in Table 1 and referred to herein as Accord A.
Table 1
In another embodiment, the malodor control component includes a mixture of the volatile aldehydes listed in Table 2 and referred to herein as Accord B.
Table 2
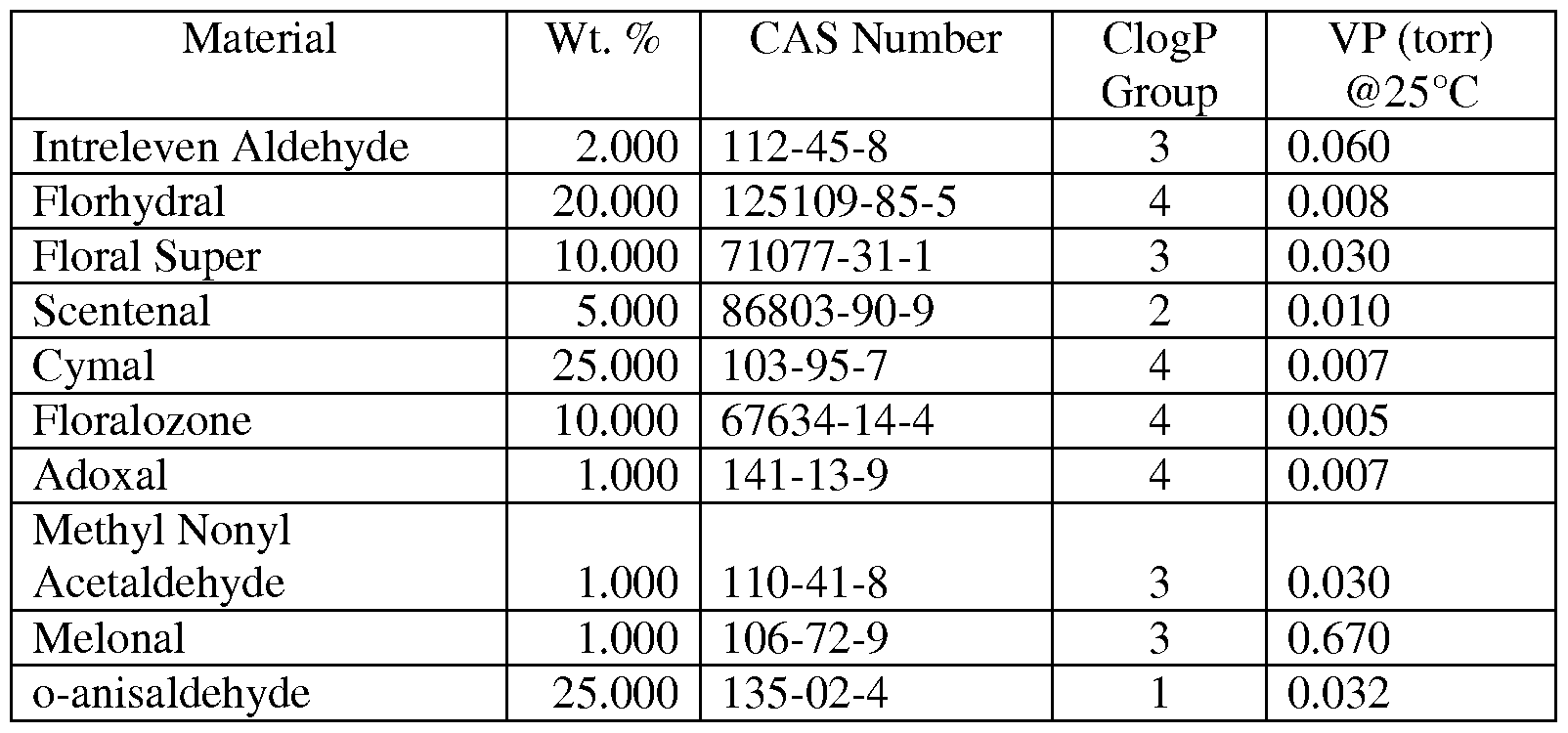
In another embodiment, the malodor control component includes a mixture of about 71.2% volatile aldehydes, the remainder being other an ester and an alcohol perfume raw material. This mixture is listed in Table 3 and referred to herein as Accord C.
Table 3
Material Wt. % CAS Number ClogP VP (torr)
Group @25°C
Intreleven Aldehyde 2.000 112-45-8 3 0.060
Florhydral 10.000 125109-85-5 4 0.008
Floral Super 5.000 71077-31-1 3 0.030
Scentenal 2.000 86803-90-9 2 0.010
Cymal 15.000 103-95-7 4 0.007
Floralozone 12.000 67634-14-4 4 0.005
Adoxal 1.000 141-13-9 4 0.007
Methyl Nonyl
Acetaldehyde 1.000 110-41-8 3 0.030
Melonal 1.000 106-72-9 3 0.670
Flor Acetate 11.800 5413-60-5 1 0.060
Frutene 7.000 17511-60-3 4 0.020
Helional 5.000 1205-17-0 2 0.0005
Bourgeonal 2.000 18127-01-0 4 0.004
Linalool 10.000 78-70-6 3 0.050
Benzaldehyde 0.200 100-52-7 1 1.110
o-anisaldehyde 15.000 135-02-4 1 0.320
Accords A, B, or C can be formulated in with other perfume raw materials in an amount, for example, of about 10% by weight of the malodor control component. Additionally, the individual volatile aldehydes or a various combination of the volatile aldehydes can be formulated into a malodor control component. In certain embodiments, the volatile aldehydes may be present in an amount up to 100%, by weight of the malodor control component, alternatively from 1% to about 100%, alternatively from about 2% to about 100%, alternatively from about 3% to about 100%, alternatively about 50% to about 100%, alternatively about 70% to about 100%, alternatively about 80% to about 100%, alternatively from about 1% to about 20%, alternatively from about 1% to about 10%, alternatively from about 2% to about 20%, alternatively from about 3% to about 20%, alternatively from about 4% to about 20%, alternatively from about 5% to about 20%.
In some embodiments where volatility is not important for neutralizing a malodor, the present invention may include poly- aldehydes, for example, di-, tri-, tetra-aldehydes. Such embodiments may include laundry detergents, additive, and the like for leave-on, through the wash, and rinse-off type of applications.
In one embodiment, the freshening composition comprises a perfume mixture having one or more fabric-safe, non-yellowing aliphatic aldehydes. Certain types of aldehydes that predominately comprise a straight chain aliphatic backbone will not discolor fabrics, unlike products that utilize types of aldehydes that contain multiple double bonds and benzene rings. The following table illustrates the selection of aldehydes to avoid fabric yellowing.
Aldehyde Solution Tested Fadometer Test on treated Fabric
(0.75 grams of product are pipetted onto a 4 inch X 4 inch (10 cm X 10 cm)
swatch which is then subjected to 5 hours
of exposure to simulated sunlight using a
SUNTEST CPS+ model Fadometer
supplied by Atlas, Chicago, Illinois,
USA.
Control- untreated fabric swatch No yellowing
1000 ppm amy lie cinnamic aldehyde Yellowish brown
(aromatic)
1000 ppm citronellal (aromatic) Yellowish brown
lOOOppm citral aldehyde (aliphatic) No yellowing
1000 ppm lauric aldehyde (aliphatic) No yellowing
Examples of suitable aliphatic aldehydes are R-COH where R is saturated C7 to C22 linear and/or branched with no more than two double bonds. Examples of suitable aliphatic aldehydes are bourgeonal, citral, citronellyl oxyacetaldehyde, cymal, decyl aldehyde, helional, hexyl cinnamic aldehyde, lauric aldehyde, ligustral, lyral, melonal, methyl dihydro jasmonate, methyl nonyl acetaldehyde, methyl phenyl carbinyl acetate, nonyl aldehyde, octyl aldehyde, oxane, P. T. bucinal, polysantol, rhubafuran, tripal, or mixtures thereof.
In one embodiment, the composition includes at least one aliphatic aldehyde selected from the group consisting of: bourgeonal, citral, citronellyl oxyacetaldehyde, cymal, decyl aldehyde, helional, hexyl cinnamic aldehyde, lauric aldehyde, ligustral, lyral, melonal, methyl dihydro jasmonate, methyl nonyl acetaldehyde, methyl phenyl carbinyl acetate, nonyl aldehyde, 2, 6 - nonadien-l-al, octyl aldehyde, oxane, P.T. bucinal, polysantol, rhubafuran, tripal, and mixtures thereof.
In another embodiment, the composition includes at least one aliphatic aldehyde selected from the group consisting of: burgeonal, cymal, hexyl cinnamic aldehyde, mmethyl dihydro jasmonate, methyl nonyl acetaldehyde, P.T. bucinal, and mixtures therof.
The aliphatic aldehydes may be present in an amount from about 0.001% to about 10%, alternatively from about 0.001% to about 5%, alternatively from about 0.01% to about 1%, alternatively from about 0.02% to about 1%, alternatively from about 0.02% to about 0.5%, alternatively from about 0.02% to about 0.06%, alternatively about 0.06%, by weight of the composition.
In addition to aliphatic aldehydes, the composition may also include perfume materials for their scent experience including enones, ketones, ionones including ionone alpha, ionone beta, ionone gamma methyl, or mixtures thereof. Suitable perfume materials are discussed in US
5,714,137. The composition may contain an effective amount of perfume to provide the freshening fragrance when first sprayed, some lingering fragrance, and some extra fragrance to be released upon fabric rewetting. It may be desirable for the aliphatic aldehydes to have virtually no negative impact on the desired perfume character.
Certain malodor counteractants may be odoriferous and negatively impact the overall character of the fragrance. In this case, a perfume/malodor counteractant premix is formed such that the perfume raw materials used are selected to neutralize any odor of the malodor counteractants. This odor neutralized premix can then be added to a parent perfume mixture without affecting the character of the parent fragrance. This permits the malodor counteractants to be used broadly with a large variety of fragrance types.
The following are non-limiting examples of perfume formulations that include fabric-safe malodor counteractants.
(1) Pine
Material Name Amount
Rosemary 10.00
Spike Lavender 10.00
Lavandin Grosso 5.00
Spruce (conf.-manh) 5.00
Camphor Gum 5.00
Melonal 0.30
Eucalyptol 15.00
Iso Menthone 15.00
Iso Bornyl Acetate 21.70
Ionone Beta 8.00
Iso E Super 5.00
100.00
(2) Ozonic
Material Name Amount
Xi Aldehyde 8.00
T 6 Nonadienol 10% In Dpg 5.00
Helional 13.00
Hydroxycitronellal 11.50
Calone 1951 0.50
T 6 - Nonadien-l-al/10% In Dpg 5.00
Lyral 20.00
Melonal 1.00
Iso Menthone 10.00
Floralozone 10.00
Bourgeonal 10.00
Delta Muscenone 962191 1.00
Habanolide 100% 5.00
100.00
(3) Fruity
Material Name Amount
Fruitate 5.00
Orange Terpenes 13.00
Ethyl Acetoacetate 3.00
T 6 Nonadienol 10% In Dpg 1.00
Ethyl Acetate 3.00
Benzaldehyde 2.00
Prenyl Acetate 8.00
Benzyl Acetate 15.00
T 6 - Nonadien-l-al/10% In Dpg 1.00
Ethyl-2-methyl Butyrate 8.00
Amyl Acetate 3.00
Cis 3 Hexenyl Acetate 3.00
Methyl Dihydro Jasmonate 10.00
Ligustral 5.00
Melonal 1.00
Ethyl 2 Methyl Pentanoate 8.00
Hexyl Acetate 8.00
Habanolide 100% 3.00
100.00
(4) Citrus
Material Name Amount
Orange Terpenes 20.00
Lemon Terpenes X5 Fold 20.00
Lime Oil Cf-8-1285-1 (conf.- berje) 10.00
Grapefruit Phase C- Ref. N* 12245 20.00
Italian Orange Phase Oil 22.90
Delta Muscenone 962191 0.50
Oxane 0.30
Iso Menthone 1.00
Rhubafuran 0.30
Habanolide 100% 5.00
100.00
(5) Floral
Material Name Amount
Spike Lavender 5.00
Rosemary 5.00
Helional 10.00
Hydroxycitronellal 10.00
Benzyl Acetate 9.30
Lyral 20.00
Ligustral 2.00
Melonal 0.20
Eucalyptol 2.00
Iso Menthone 8.00
Bourgeonal 20.00
Undecavertol 3.00
Delta Muscenone 962191 0.50
Habanolide 100% 5.00
100.00
In certain cases, fabrics that are laundered will have residual brighteners deposited from detergents with which they are washed. Therefore, it may be desirable for the aliphatic aldehydes to be compatible with brighteners so that the freshening composition will not discolor any fabrics with which it comes into contact. A number of the examples above are compatible with brighteners.
2. Perfume Delivery Systems
The malodor control component may include a perfume delivery system. The consumer who selects and uses such a perfumed product makes critical decisions as to how satisfied he or she is with the product at multiple touch points in the product usage profile. Although numerous touch points are known, Applicants have found that they can be advantageously grouped and expressed as three product moments of truth that are experienced by the typically consumer.
The FMOT is typically at the point of purchase, the SMOT typically begins with the product's application and use, and the TMOT typically begins immediately after the product's
application and use. Applicants have recognized that a consumer's FMOT is negatively impacted because the product packaging inhibits the sensory experience; for example, product packaging may make the product difficult to open or, when open, exposes a product that can spill. In addition, formulation ingredients can suppress and/or distort neat product odor. Furthermore, Applicants have recognized that the consumer's SMOT is negatively impacted as volatile perfume raw materials are lost during product storage, resulting in reduced bloom during use. Compensating for these aforementioned deficiencies by adding high perfume levels for the TMOT can distort in-use scent experience, such that the perfume bloom can be too harsh or strong, and/or the perfume character can become less preferred. Also, Applicants have recognized that a consumer's FMOT is negatively impacted as perfume releases from the treated situs, inter alia a dry fabric over long period of time requires perfume levels in product that would distort the scent experience during the first and second moments of truth. Furthermore, addition of high perfume levels for SMOT & TMOT can distort neat product odor, and still not result in sufficient perfume deposition through the wash. In addition, perfume evaporation that occurs during drying can result in lower perfume levels on fabric; and/or the perfume remaining on dry fabric may provide initial dry fabric odor benefit but such perfume can dissipate too quickly to provide sufficient scent longevity benefits. Furthermore, perfume that is present on fabric may release too slowly from the fabric. As mentioned, the same can be the case with perfume delivery to and release from other situs such as hair and skin. The ability to notice the release of perfume can be impacted by a variety of factors such as hair length, clothing worn over skin, situs wash frequency, and the like. Furthermore, perfume intensity and/or character may be perceived differently on wet situs compared to dry situs that is treated with perfume-containing products. Without wishing to be bound by theory, in addition to loss of perfume by evaporation during drying, perfume can be made less available at certain touch points by being carried into or partitioning into the situs, such as cotton fibers, hair, skin, and the like. Situs moisture level can also serve to alter the release profile or release rate of perfume.
Finally, Applicants recognized that solutions to the problems that are associated with one or two moments of truth can be insufficient to resolve the problems associated with the remaining moment(s) of truth or negatively impact the other moment(s) of truth
The following perfume delivery technologies (PDTs) also known as perfume delivery systems may be used in any combination in any type of consumer product:
a. Polymer Assisted Delivery (PAD)
This PDT uses polymeric materials to deliver perfume materials. Classical coacervation, water soluble or partly soluble to insoluble charged or neutral polymers, liquid crystals, hot melts, hydrogels, perfumed plastics, microcapsules, nano- and micro-latexes, polymeric film formers, and polymeric absorbents, polymeric adsorbents, etc. are some examples. PAD includes but is not limited to the following.
Matrix Systems: The fragrance is dissolved or dispersed in a polymer matrix or particle. Perfumes, for example, may be 1) dispersed into the polymer prior to formulating into the product or 2) added separately from the polymer during or after formulation of the product. Diffusion of perfume from the polymer is a common trigger that allows or increases the rate of perfume release from a polymeric matrix system that is deposited or applied to the desired surface (situs), although many other triggers are know that may control perfume release. Absorption and/or adsorption into or onto polymeric particles, films, solutions, and the like are aspects of this technology. Nano- or micro-particles composed of organic materials (e.g., latexes) are examples. Suitable particles include a wide range of materials including, but not limited to polyacetal, polyacrylate, polyacrylic, polyacrylonitrile, polyamide, polyaryletherketone, polybutadiene, polybutylene, polybutylene terephthalate, polychloroprene, poly ethylene, polyethylene terephthalate, polycyclohexylene dimethylene terephthalate, polycarbonate, polychloroprene, polyhydroxyalkanoate, polyketone, polyester, polyethylene, polyetherimide, polyethersulfone, polyethylenechlorinates, polyimide, polyisoprene, polylactic acid, polymethylpentene, polyphenylene oxide, polyphenylene sulfide, polyphthalamide, polypropylene, polystyrene, polysulfone, polyvinyl acetate, polyvinyl chloride, as well as polymers or copolymers based on acrylonitrile-butadiene, cellulose acetate, ethylene-vinyl acetate, ethylene vinyl alcohol, styrene -butadiene, vinyl acetate-ethylene, and mixtures thereof.
"Standard" systems refer to those that are "pre-loaded" with the intent of keeping the pre- loaded perfume associated with the polymer until the moment or moments of perfume release. Such polymers may also suppress the neat product odor and provide a bloom and/or longevity benefit depending on the rate of perfume release. One challenge with such systems is to achieve the ideal balance between 1) in-product stability (keeping perfume inside carrier until you need it) and 2) timely release (during use or from dry situs). Achieving such stability is particularly important during in-product storage and product aging. This challenge is particularly apparent for aqueous-based, surfactant-containing products, such as heavy duty liquid laundry detergents. Many "Standard" matrix systems available effectively become "Equilibrium" systems when formulated into aqueous-based products. One may select an "Equilibrium" system or a Reservoir
system, which has acceptable in-product diffusion stability and available triggers for release (e.g., friction).
"Equilibrium" systems are those in which the perfume and polymer may be added separately to the product, and the equilibrium interaction between perfume and polymer leads to a benefit at one or more consumer touch points (versus a free perfume control that has no polymer-assisted delivery technology). The polymer may also be pre-loaded with perfume; however, part or all of the perfume may diffuse during in-product storage reaching an equilibrium that includes having desired perfume raw materials (PRMs) associated with the polymer. The polymer then carries the perfume to the surface, and release is typically via perfume diffusion. The use of such equilibrium system polymers has the potential to decrease the neat product odor intensity of the neat product (usually more so in the case of pre-loaded standard system). Deposition of such polymers may serve to "flatten" the release profile and provide increased longevity. As indicated above, such longevity would be achieved by suppressing the initial intensity and may enable the formulator to use more high impact or low odor detection threshold (ODT) or low Kovats Index (KI) PRMs to achieve FMOT benefits without initial intensity that is too strong or distorted. It is important that perfume release occurs within the time frame of the application to impact the desired consumer touch point or touch points. Suitable micro-particles and micro-latexes as well as methods of making same may be found in US 2005/0003980 Al. Matrix systems also include hot melt adhesives and perfume plastics. In addition, hydrophobically modified polysaccharides may be formulated into the perfumed product to increase perfume deposition and/or modify perfume release. All such matrix systems, including for example polysaccarides and nanolatexes may be combined with other PDTs, including other PAD systems such as PAD reservoir systems in the form of a perfume microcapsule (PMC). PAD matrix systems may include those described in the following references: U.S. Patent Publications: 2004/0110648 Al; 2004/0092414 Al; 2004/0091445 Al and 2004/0087476 Al; and US Patents: 6,531,444; 6,024,943; 6,042,792; 6,051,540; 4,540,721 and 4,973,422.
Silicones are also examples of polymers that may be used as PDT, and can provide perfume benefits in a manner similar to the polymer-assisted delivery "matrix system". Such a PDT is referred to as silicone-assisted delivery (SAD). One may pre-load silicones with perfume, or use them as an equilibrium system as described for PAD. Suitable silicones as well as making same may be found in WO 2005/102261; US 20050124530A1; US 20050143282A1; and WO 2003/015736. Functionalized silicones may also be used as described in US
2006/003913 Al. Examples of silicones include polydimethylsiloxane and poly alky ldimethylsiloxanes. Other examples include those with amine functionality, which may be used to provide benefits associated with amine-assisted delivery (AAD) and/or polymer- assisted delivery (PAD) and/or amine-reaction products (ARP). Other such examples may be found in US 4,911,852; US 2004/0058845 Al; US 2004/0092425 Al and US 2005/0003980 Al.
Reservoir Systems: Reservoir systems are also known as a core-shell type technology, or one in which the fragrance is surrounded by a perfume release controlling membrane, which may serve as a protective shell. The material inside the microcapsule is referred to as the core, internal phase, or fill, whereas the wall is sometimes called a shell, coating, or membrane. Microparticles or pressure sensitive capsules or microcapsules are examples of this technology. Microcapsules of the current invention are formed by a variety of procedures that include, but are not limited to, coating, extrusion, spray-drying, interfacial, in-situ and matrix polymerization. The possible shell materials vary widely in their stability toward water. Among the most stable are polyoxymethyleneurea (PMU)-based materials, which may hold certain PRMs for even long periods of time in aqueous solution (or product). Such systems include but are not limited to urea-formaldehyde and/or melamine-formaldehyde. Gelatin-based microcapsules may be prepared so that they dissolve quickly or slowly in water, depending for example on the degree of cross-linking. Many other capsule wall materials are available and vary in the degree of perfume diffusion stability observed. Without wishing to be bound by theory, the rate of release of perfume from a capsule, for example, once deposited on a surface is typically in reverse order of in-product perfume diffusion stability. As such, urea-formaldehyde and melamine-formaldehyde microcapsules for example, typically require a release mechanism other than, or in addition to, diffusion for release, such as mechanical force (e.g., friction, pressure, shear stress) that serves to break the capsule and increase the rate of perfume (fragrance) release. Other triggers include melting, dissolution, hydrolysis or other chemical reaction, electromagnetic radiation, and the like. The use of pre-loaded microcapsules requires the proper ratio of in-product stability and in- use and/or on-surface (on-situs) release, as well as proper selection of PRMs. Microcapsules that are based on urea-formaldehyde and/or melamine-formaldehyde are relatively stable, especially in near neutral aqueous-based solutions. These materials may require a friction trigger which may not be applicable to all product applications. Other microcapsule materials (e.g., gelatin) may be unstable in aqueous-based products and may even provide reduced benefit (versus free perfume control) when in-product aged. Scratch and sniff technologies are yet another example of PAD. Perfume microcapsules (PMC) may include those described in the following references:
US Patent Publications: 2003/0125222 Al; 2003/215417 Al; 2003/216488 Al; 2003/158344 Al; 2003/165692 Al; 2004/071742 Al; 2004/071746 Al; 2004/072719 Al; 2004/072720 Al; 2006/0039934 Al; 2003/203829 Al; 2003/195133 Al; 2004/087477 Al; 2004/0106536 Al; and US Patents: 6,645,479 Bl; 6,200,949 Bl; 4,882,220; 4,917,920; 4,514,461; 6,106,875 and 4,234,627, 3,594,328 and US RE 32713.
b. Molecule- Assisted Delivery (MAD)
Non-polymer materials or molecules may also serve to improve the delivery of perfume. Without wishing to be bound by theory, perfume may non-covalently interact with organic materials, resulting in altered deposition and/or release. Non-limiting examples of such organic materials include but are not limited to hydrophobic materials such as organic oils, waxes, mineral oils, petrolatum, fatty acids or esters, sugars, surfactants, liposomes and even other perfume raw material (perfume oils), as well as natural oils, including body and/or other soils. Perfume fixatives are yet another example. In one aspect, non-polymeric materials or molecules have a CLogP greater than about 2. MAD may also include those described in US 7,119,060 and US 5,506,201.
c. Fiber- Assisted Delivery (FAD)
The choice or use of a situs itself may serve to improve the delivery of perfume. In fact, the situs itself may be a perfume delivery technology. For example, different fabric types such as cotton or polyester will have different properties with respect to ability to attract and/or retain and/or release perfume. The amount of perfume deposited on or in fibers may be altered by the choice of fiber, and also by the history or treatment of the fiber, as well as by any fiber coatings or treatments. Fibers may be woven and non- woven as well as natural or synthetic. Natural fibers include those produced by plants, animals, and geological processes, and include but are not limited to cellulose materials such as cotton, linen, hemp jute, flax, ramie, and sisal, and fibers used to manufacture paper and cloth. FAD may consist of the use of wood fiber, such as thermomechanical pulp and bleached or unbleached kraft or sulfite pulps. Animal fibers consist largely of particular proteins, such as silk, sinew, catgut and hair (including wool). Polymer fibers based on synthetic chemicals include but are not limited to polyamide nylon, PET or PBT polyester, phenol-formaldehyde (PF), polyvinyl alcohol fiber (PVOH), polyvinyl chloride fiber (PVC), polyolefins (PP and PE), and acrylic polymers. All such fibers may be pre-loaded with a perfume, and then added to a product that may or may not contain free perfume and/or one or more perfume delivery technologies. In one aspect, the fibers may be added to a product prior to being loaded with a perfume, and then loaded with a perfume by adding a perfume that may
diffuse into the fiber, to the product. Without wishing to be bound by theory, the perfume may absorb onto or be adsorbed into the fiber, for example, during product storage, and then be released at one or more moments of truth or consumer touch points.
d. Amine Assisted Delivery (AAD)
The amine-assisted delivery technology approach utilizes materials that contain an amine group to increase perfume deposition or modify perfume release during product use. There is no requirement in this approach to pre-complex or pre-react the perfume raw material(s) and amine prior to addition to the product. In one aspect, amine-containing AAD materials suitable for use herein may be non-aromatic; for example, polyalkylimine, such as PEI, or PVam, or aromatic, for example, anthranilates. Such materials may also be polymeric or non-polymeric. In one aspect, such materials contain at least one primary amine. This technology will allow increased longevity and controlled release also of low ODT perfume notes (e.g., aldehydes, ketones, enones) via amine functionality, and delivery of other PRMs, without being bound by theory, via polymer-assisted delivery for polymeric amines. Without technology, volatile top notes can be lost too quickly, leaving a higher ratio of middle and base notes to top notes. The use of a polymeric amine allows higher levels of top notes and other PRMS to be used to obtain freshness longevity without causing neat product odor to be more intense than desired, or allows top notes and other PRMs to be used more efficiently. In one aspect, AAD systems are effective at delivering PRMs at pH greater than about neutral. Without wishing to be bound by theory, conditions in which more of the amines of the AAD system are deprotonated may result in an increased affinity of the deprotonated amines for PRMs such as aldehydes and ketones, including unsaturated ketones and enones such as damascone. In another aspect, polymeric amines are effective at delivering PRMs at pH less than about neutral. Without wishing to be bound by theory, conditions in which more of the amines of the AAD system are protonated may result in a decreased affinity of the protonated amines for PRMs such as aldehydes and ketones, and a strong affinity of the polymer framework for a broad range of PRMs. In such an aspect, polymer-assisted delivery may be delivering more of the perfume benefit; such systems are a subspecies of AAD and may be referred to as Amine- Polymer-Assisted Delivery or APAD. In some cases when the APAD is employed in a composition that has a pH of less than seven, such APAD systems may also be considered Polymer-Assisted Delivery (PAD). In yet another aspect, AAD and PAD systems may interact with other materials, such as anionic surfactants or polymers to form coacervate and/or coacervates-like systems. In another aspect, a material that contains a heteroatom other than nitrogen, for example sulfur, phosphorus or selenium, may be
used as an alternative to amine compounds. In yet another aspect, the aforementioned alternative compounds can be used in combination with amine compounds. In yet another aspect, a single molecule may comprise an amine moiety and one or more of the alternative heteroatom moieties, for example, thiols, phosphines and selenols. Suitable AAD systems as well as methods of making same may be found in US Patent Applications 2005/0003980 Al; 2003/0199422 Al; 2003/0036489 Al; 2004/0220074 Al and USP 6,103,678.
e. Starch Encapsulated Accord (SEA)
The use of a starch encapsulated accord (SEA) technology allows one to modify the properties of the perfume, for example, by converting a liquid perfume into a solid by adding ingredients such as starch. The benefit includes increased perfume retention during product storage, especially under non-aqueous conditions. Upon exposure to moisture, a perfume bloom may be triggered. Benefits at other moments of truth may also be achieved because the starch allows the product formulator to select PRMs or PRM concentrations that normally cannot be used without the presence of SEA. Another technology example includes the use of other organic and inorganic materials, such as silica to convert perfume from liquid to solid. Suitable SEAs as well as methods of making same may be found in USPA 2005/0003980 Al and USP 6,458,754 Bl.
f. Zeolite & Inorganic Carrier (ZIC)
This technology relates to the use of porous zeolites or other inorganic materials to deliver perfumes. Perfume-loaded zeolite may be used with or without adjunct ingredients used for example to coat the perfume-loaded zeolite (PLZ) to change its perfume release properties during product storage or during use or from the dry situs. Suitable zeolite and inorganic carriers as well as methods of making same may be found in US Patent Publications: 2005/0003980 Al and US Patents: 5,858,959; 6,245,732 Bl; 6,048,830 and 4,539,135. Silica is another form of ZIC. Another example of a suitable inorganic carrier includes inorganic tubules, where the perfume or other active material is contained within the lumen of the nano- or micro-tubules. Preferably, the perfume-loaded inorganic tubule (or Perfume-Loaded Tubule or PLT) is a mineral nano- or micro-tubule, such as halloysite or mixtures of halloysite with other inorganic materials, including other clays. The PLT technology may also comprise additional ingredients on the inside and/or outside of the tubule for the purpose of improving in-product diffusion stability, deposition on the desired situs or for controlling the release rate of the loaded perfume. Monomeric and/or polymeric materials, including starch encapsulation, may be used to coat,
plug, cap, or otherwise encapsulate the PLT. Suitable PLT systems as well as methods of making same may be found in US 5,651,976.
g. Pro-Perfume (PP)
This technology refers to perfume technologies that result from the reaction of perfume materials with other substrates or chemicals to form materials that have a covalent bond between one or more PRMs and one or more carriers. The PRM is converted into a new material called a pro-PRM (i.e., pro-perfume), which then may release the original PRM upon exposure to a trigger such as water or light. PP may provide enhanced perfume delivery properties such as increased perfume deposition, longevity, stability, retention, and the like. PP include those that are monomeric (non-polymeric) or polymeric, and may be pre-formed or may be formed in-situ under equilibrium conditions, such as those that may be present during in-product storage or on the wet or dry situs. Non-limiting examples of PP include Michael adducts (e.g., beta- amino ketones), aromatic or non-aromatic imines (Schiff bases), oxazolidines, beta-keto esters, and orthoesters. Another aspect includes compounds comprising one or more beta-oxy or beta-thio carbonyl moieties capable of releasing a PRM, for example, an alpha, beta-unsaturated ketone, aldehyde or carboxylic ester. The typical trigger for perfume release is exposure to water; although other triggers may include enzymes, heat, light, pH change, autoxidation, a shift of equilibrium, change in concentration or ionic strength and others. For aqueous-based products, light-triggered pro-perfumes are particularly suited. Such photo-pro-perfumes (PPPs) include but are not limited to those that release coumarin derivatives and perfumes and/or pro-perfumes upon being triggered. The released PP may release one or more PRMs by means of any of the above mentioned triggers. In one aspect, the PPP releases a nitrogen-based PP when exposed to a light and/or moisture trigger. In another aspect, the nitrogen-based PP, released from the PPP, releases one or more PRMs selected, for example, from aldehydes, ketones (including enones) and alcohols. In still another aspect, the PPP releases a dihydroxy coumarin derivative. The light- triggered pro-perfume may also be an ester that releases a coumarin derivative and a perfume alcohol. In one aspect the pro-perfume is a dimethoxybenzoin derivative as described in US 2006/0020459 Al. In another aspect, the PP is a 3', 5 '-dimethoxybenzoin (DMB) derivative that releases an alcohol upon exposure to electromagnetic radiation. In yet another aspect, the pro- perfume releases one or more low ODT PRMs, including tertiary alcohols such as linalool, tetrahydrolinalool, or dihydromyrcenol. Suitable pro-perfumes and methods of making same can be found in US Patents: 7,018,978 B2; 6,987,084 B2; 6,956,013 B2; 6,861,402 Bl; 6,544,945 Bl; 6,093,691; 6,277,796 Bl; 6,165,953; 6,316,397 Bl; 6,437,150 Bl; 6,479,682 Bl; 6,096,918;
6,218,355 Bl; 6,133,228; 6,147,037; 7,109,153 B2; 7,071,151 B2; 6,987,084 B2; 6,610,646 B2 and 5,958,870, as well as can be found in US Patent Publications: 2005/0003980 Al and 2006/0223726 Al.
Amine Reaction Product (ARP): For purposes of the present application, ARP is a subclass or species of PP. One may also use "reactive" polymeric amines in which the amine functionality is pre-reacted with one or more PRMs to form an amine reaction product (ARP). Typically the reactive amines are primary and/or secondary amines, and may be part of a polymer or a monomer (non-polymer). Such ARPs may also be mixed with additional PRMs to provide benefits of polymer-assisted delivery and/or amine-assisted delivery. Nonlimiting examples of polymeric amines include polymers based on polyalkylimines, such as PEI, or PVam. Nonlimiting examples of monomeric (non-polymeric) amines include hydroxyl amines, such as 2-aminoethanol and its alkyl substituted derivatives, and aromatic amines such as anthranilates. The ARPs may be premixed with perfume or added separately in leave-on or rinse-off applications. In another aspect, a material that contains a heteroatom other than nitrogen, for example oxygen, sulfur, phosphorus or selenium, may be used as an alternative to amine compounds. In yet another aspect, the aforementioned alternative compounds can be used in combination with amine compounds. In yet another aspect, a single molecule may comprise an amine moiety and one or more of the alternative heteroatom moieties, for example, thiols, phosphines and selenols. The benefit may include improved delivery of perfume as well as controlled perfume release. Suitable ARPs as well as methods of making same can be found in US 2005/0003980 Al and US 6,413,920 Bl.
3. Low molecular weight polyols
In addition to perfume materials, the malodor control component may include low molecular weight polyols with relatively high boiling points (compared to water) such as ethylene glycol, diethylene glycol, triethylene glycol, propylene glycol, dipropylene glycol, and/or glycerine. Such polyols may improve odor neutralization of the composition of the present invention. Some polyols, e.g., dipropylene glycol, are also useful to facilitate the solubilization of some perfume ingredients in the composition of the present invention.
The glycol used in the composition of the present invention may be glycerine, ethylene glycol, propylene glycol, dipropylene glycol, polyethylene glycol, propylene glycol methyl ether, propylene glycol phenyl ether, propylene glycol methyl ether acetate, propylene glycol n-butyl ether, dipropylene glycol n-butyl ether, dipropylene glycol n-propyl ether, ethylene glycole phenyl ether, diethylene glycol n-butyl ether, dipropylene glycol n-butyl ether, diethylene glycol
mono butyl ether, dipropylene glycol methyl ether, tripropylene glycol methyl ether, tripropylene glycol n-butyl ether, other glycol ethers, or mixtures thereof. In one embodiment, the glycol used is ethylene glycol, propylene glycol, or mixtures thereof. In another embodiment, the glycol used is diethylene glycol.
Typically, the low molecular weight polyol is added to the composition of the present invention at a level of from about 0.01% to about 5%, by weight of the composition, alternatively from about 0.05% to about 1%, alternatively from about 0.1% to about 0.5%, by weight of the composition. Compositions with higher concentrations may make fabrics susceptible to soiling and/or leave unacceptable visible stains on fabrics as the solution evaporates off of the fabric. The weight ratio of low molecular weight polyol to the malodor binding polymer is from about 500:1 to about 4:1, alternatively from about 1:100 to about 25:1, alternatively from about 1:50 to about 4:1, alternatively about 4:1.
4. Cyclodextrin
In some embodiments, the freshening composition may include solubilized, water- soluble, uncomplexed cyclodextrin. As used herein, the term "cyclodextrin" includes any of the known cyclodextrins such as unsubstituted cyclodextrins containing from six to twelve glucose units, especially, alpha-cyclodextrin, beta-cyclodextrin, gamma-cyclodextrin and/or their derivatives and/or mixtures thereof. The alpha-cyclodextrin consists of six glucose units, the beta-cyclodextrin consists of seven glucose units, and the gamma-cyclodextrin consists of eight glucose units arranged in a donut-shaped ring. The specific coupling and conformation of the glucose units give the cyclodextrins a rigid, conical molecular structure with a hollow interior of a specific volume. The "lining" of the internal cavity is formed by hydrogen atoms and glycosidic bridging oxygen atoms, therefore this surface is fairly hydrophobic. The unique shape and physical-chemical property of the cavity enable the cyclodextrin molecules to absorb (form inclusion complexes with) organic molecules or parts of organic molecules which can fit into the cavity. Many perfume molecules can fit into the cavity.
Cyclodextrin molecules are described in US 5,714,137, and US 5,942,217. Suitable levels of cyclodextrin are from about 0.1% to about 5%, alternatively from about 0.2% to about 4%, alternatively from about 0.3% to about 3%, alternatively from about 0.4% to about 2%, by weight of the freshening composition. Freshening compositions with higher concentrations can make fabrics susceptible to soiling and/or leave unacceptable visible stains on fabrics as the solution evaporates off of the fabric. The latter is especially a problem on thin, colored, synthetic fabrics. In order to avoid or minimize the occurrence of fabric staining, the fabric may be treated
at a level of less than about 5 mg of cyclodextrin per mg of fabric, alternatively less than about 2 mg of cyclodextrin per mg of fabric.
5. Acid Catalyst
The malodor control component of the present invention may include an effective amount of an acid catalyst to neutralize sulfur-based malodors. It has been found that certain mild acids have an impact on aldehyde reactivity with thiols in the liquid and vapor phase. It has been found that the reaction between thiol and aldehyde is a catalytic reaction that follows the mechanism of hemiacetal and acetal formation path. When the present malodor control component contains an acid catalyst and contacts a sulfur-based malodor, the volatile aldehyde reacts with thiol. This reaction may form a thiol acetal compound, thus, neutralizing the sulfur- based odor. Without an acid catalyst, only hemi-thiol acetal is formed.
Suitable acid catalysts have a VP, as reported by Scifinder, in the range of about 0.001 torr to about 38 torr, measured at 25 °C, alternatively about 0.001 torr to about 14 torr, alternatively from about 0.001 to about 1, alternatively from about 0.001 to about 0.020, alternatively about 0.005 to about 0.020, alternatively about 0.010 to about 0.020.
The acid catalyst may be a weak acid. A weak acid is characterized by an acid dissociation constant, Ka> which is an equilibrium constant for the dissociation of a weak acid; the pKa being equal to minus the decimal logarithm of Ka. The acid catalyst may have a pKa from about 4.0 to about 6.0, alternatively from about 4.3 and 5.7, alternatively from about 4.5 to about 5, alternatively from about 4.7 to about 4.9. Suitable acid catalyst include those listed in Table 4.
Table 4
Depending on the desired use of the malodor control component, one may consider the scent character or the affect on the scent of the malodor control component when selecting an acid catalyst. In some embodiments of the malodor control component, it may be desirable to
select an acid catalyst that provides a neutral to pleasant scent. Such acid catalysts may have a VP of about 0.001 torr to about 0.020 torr, measured at 25 °C, alternatively about 0.005 torr to about 0.020 torr, alternatively about 0.010 torr to about 0.020 torr. Non-limiting examples of such acid catalyst include 5-methyl thiophene carboxaldehyde with carboxylic acid impurity, succinic acid, or benzoic acid.
The malodor control component may include about 0.05% to about 5%, alternatively about 0.1% to about 1.0%, alternatively about 0.1% to about 0.5%, alternatively about 0.1% to about 0.4%, alternatively about 0.4% of an acid catalyst by weight of the malodor control component.
In an acetic acid system, the present malodor control component may include about 0.4% of acetic acid (50:50 TC:DPM, 0.4% acetic acid).
Table 5
The malodor control component may have a pH from about 3 to about 8, alternatively from about 4 to about 7, alternatively from about, alternatively from about 4 to about 6.
6. Optional Ingredients
The malodor control component may, optionally, include odor masking agents, and/or odor blocking agents. "Odor blocking" refers to the ability of a compound to dull the human sense of smell. "Odor-masking" refers to the ability of a compound to mask or hide a malodorous compound. Odor-masking may include a compound with a non-offensive or pleasant smell that is dosed such that it limits the ability to sense a malodorous compound. Odor- masking may involve the selection of compounds which coordinate with an anticipated malodor to change the perception of the overall scent provided by the combination of odorous compounds.
The malodor control component may also, optionally, include perfume raw materials that solely provide a hedonic benefit (i.e. that do not neutralize malodors yet provide a pleasant
fragrance). Suitable perfumes are disclosed in US 6,248,135, which is incorporated in its entirety by reference.
For example, the malodor control component may include a mixture of volatile aldehydes for neutralizing a malodor, perfume ionones, and a diluent. Alternatively, the malodor control component may include 100% volatile aldehydes.
C. Buffering agent
The freshening composition of the present invention includes a buffering agent which may be a dibasic acid, carboxylic acid, or a dicarboxylic acid like maleic acid. The acid may be sterically stable, and used in this composition solely for maintaining the desired pH. The freshening composition may have a pH from about 3 to about 8, alternatively from about 4 to about 7, alternatively from about 5 to about 8, alternatively from about 6 to about 8, alternatively about 6 to about 7, alternatively about 7, alternatively about 6.5.
Carboxylic acids such as citric acid may act as metal ion chelants and can form metallic salts with low water solubility. As such, in some embodiments, the freshening composition is essentially free of citric acids. The buffer can be alkaline, acidic or neutral.
Other suitable buffering agents for freshening compositions of this invention include biological buffering agents. Some examples are nitrogen-containing materials, sulfonic acid buffers like 3-(N-morpholino)propanesulfonic acid (MOPS) or N-(2-Acetamido)-2- aminoethanesulfonic acid (ACES), which have a near neutral 6.2 to 7.5 pKa and provide adequate buffering capacity at a neutral pH. Other examples are amino acids such as lysine or lower alcohol amines like mono-, di-, and tri-ethanolamine. Other nitrogen-containing buffering agents are tri(hydroxymethyl)amino methane (HOCH2)3CNH3 (TRIS), 2-amino-2-ethyl-l,3- propanediol, 2-amino-2-methyl-propanol, 2-amino-2-methyl-l,3-propanol, disodium glutamate, N-methyl diethanolamide, 2-dimethylamino-2-methylpropanol (DMAMP), 1,3- bis(methylamine)-cyclohexane, 1,3-diamino-propanol Ν,Ν'-tetra- methyl- l,3-diamino-2- propanol, N,N-bis(2-hydroxyethyl)glycine (bicine) and N-tris (hydroxymethyl)methyl glycine (tricine). Mixtures of any of the above are also acceptable.
The compositions may contain at least about 0%, alternatively at least about 0.001%, alternatively at least about 0.01%, by weight of the composition, of a buffering agent. The composition may also contain no more than about 1%, alternatively no more than about 0.75%, alternatively no more than about 0.5%, by weight of the composition, of a buffering agent.
D. Solubilizer
The freshening composition of the present invention may contain a diluent or solubilizing aid to solubilize any excess hydrophobic organic materials, particularly any perfume materials, and also optional ingredients (e.g., insect repelling agent, antioxidant, etc.) which can be added to the composition, that are not readily soluble in the composition, to form a clear solution. Exemplary solubilizing aids include such as a no-foaming or low-foaming surfactants, nonionic surfactants, cationic surfactants, amphoteric surfactants, zwitterionic surfactants, and mixtures thereof. Exemplary solubilizing aids also include dipropylene glycol methyl ether, 3-methoxy-3- methyl-l-butanol, and mixtures thereof
In some embodiments, the composition contains nonionic surfactants, cationic surfactants, and mixtures thereof. In one embodiment, the freshening composition contains hydrogenated castor oil. One suitable hydrogenated castor oil that may be used in the present composition is Basophor™, available from BASF.
Compositions containing anionic surfactants and/or detergent surfactants may make fabrics susceptible to soiling and/or leave unacceptable visible stains on fabrics as the solution evaporates off of the fabric. In some embodiments, the freshening composition is free of anionic surfactants and/or detergent surfactants.
When the solubilizing agent is present, it is typically present at a level of from about 0.01% to about 3%, alternatively from about 0.05% to about 1%, alternatively from about 0.01% to about 0.05%, by weight of the freshening composition. Freshening compositions with higher concentrations may make fabrics susceptible to soiling and/or leave unacceptable visible stains on fabrics as the solution evaporates off of the fabric.
E. Antimicrobial Compounds
The freshening composition of the present invention may include an effective amount of a compound for reducing microbes in the air or on inanimate surfaces. Antimicrobial compounds are effective on gram negative and gram positive bacteria and fungi typically found on indoor surfaces that have contacted human skin or pets such as couches, pillows, pet bedding, and carpets. Such microbial species include Klebsiella pneumoniae, Staphylococcus aureus, Aspergillus niger, Klebsiella pneumoniae, Steptococcus pyogenes, Salmonella choleraesuis, Escherichia coil, Trichophyton mentagrophytes, and Pseudomonoas aeruginosa. In some embodiments, the antimicrobial compounds are also effective on viruses such Hl-Nl, Rhinovirus, Respiratory Syncytial, Poliovirus Type 1, Rotavirus, Influenza A, Herpes simplex types 1 & 2, Hepatitis A, and Human Coronavirus.
Antimicrobial compounds suitable in the composition of the present invention can be any organic material which will not cause damage to fabric appearance (e.g., discoloration, coloration such as yellowing, bleaching). Water-soluble antimicrobial compounds include organic sulfur compounds, halogenated compounds, cyclic organic nitrogen compounds, low molecular weight aldehydes, quaternary compounds, dehydroacetic acid, phenyl and phenoxy compounds, or mixtures thereof.
In one embodiment, a quaternary compound is used. Examples of commercially available quaternary compounds suitable for use in the freshening composition is Barquat available from Lonza Corporation; and didecyl dimethyl ammonium chloride quat under the trade name Bardac® 2250 from Lonza Corporation.
The antimicrobial compound may be present in an amount from about 500 ppm to about 7000 ppm, alternatively about 1000 ppm to about 5000 ppm, alternatively about 1000 ppm to about 3000 ppm, alternatively about 1400 ppm to about 2500 ppm, by weight of the freshening composition.
F. Preservatives
The composition of the present invention may include a preservative. The preservative is included in the present invention in an amount sufficient to prevent spoilage or prevent growth of inadvertently added microorganisms for a specific period of time, but not sufficient enough to contribute to the odor neutralizing performance of the freshening composition. In other words, the preservative is not being used as the antimicrobial compound to kill microorganisms on the surface onto which the composition is deposited in order to eliminate odors produced by microorganisms. Instead, it is being used to prevent spoilage of the composition in order to increase the shelf-life of the composition.
The preservative can be any organic preservative material which will not cause damage to fabric appearance, e.g., discoloration, coloration, bleaching. Suitable water-soluble preservatives include organic sulfur compounds, halogenated compounds, cyclic organic nitrogen compounds, low molecular weight aldehydes, parabens, propane diaol materials, isothiazolinones, quaternary compounds, benzoates, low molecular weight alcohols, dehydroacetic acid, phenyl and phenoxy compounds, or mixtures thereof.
Non-limiting examples of commercially available water-soluble preservatives for use in the present invention include a mixture of about 77% 5-chloro-2-methyl-4-isothiazolin-3-one and about 23% 2-methyl-4-isothiazolin-3-one, a broad spectrum preservative available as a 1.5% aqueous solution under the trade name Kathon® CG by Rohm and Haas Co.; 5-bromo-5-nitro-
1,3-dioxane, available under the tradename Bronidox L® from Henkel; 2-bromo-2-nitropropane- 1,3-diol, available under the trade name Bronopol® from Inolex; Ι,Γ-hexamethylene bis(5-(p- chlorophenyl)biguanide), commonly known as chlorhexidine, and its salts, e.g., with acetic and digluconic acids; a 95:5 mixture of l,3-bis(hydroxymethyl)-5,5-dimethyl-2,4-imidazolidinedione and 3-butyl-2-iodopropynyl carbamate, available under the trade name Glydant Plus® from Lonza; N-[l,3-bis(hydroxymethyl)2,5-dioxo-4-imidazolidinyl]-N,N'-bis(hydroxy-methyl) urea, commonly known as diazolidinyl urea, available under the trade name Germall® II from Sutton Laboratories, Inc.; N,N"-methylenebis{N'-[l-(hydroxymethyl)-2,5-dioxo-4-imidazolidinyl]urea}, commonly known as imidazolidinyl urea, available, e.g., under the trade name Abiol® from 3V- Sigma, Unicide U-13® from Induchem, Germall 115® from Sutton Laboratories, Inc.; polymethoxy bicyclic oxazolidine, available under the trade name Nuosept® C from Huls America; formaldehyde; glutar aldehyde; polyaminopropyl biguanide, available under the trade name Cosmocil CQ® from ICI Americas, Inc., or under the trade name Mikrokill® from Brooks, Inc; dehydroacetic acid; and benzsiothiazolinone available under the trade name Koralone™ B - 119 from Rohm and Hass Corporation.
Suitable levels of preservative are from about 0.0001% to about 0.5%, alternatively from about 0.0002% to about 0.2%, alternatively from about 0.0003% to about 0.1%, by weight of the freshening composition.
G. Wetting Agent
The composition may include a wetting agent that provides a low surface tension that permits the composition to spread readily and more uniformly on hydrophobic surfaces like polyester and nylon. It has been found that the aqueous solution, without such a wetting agent will not spread satisfactorily. The spreading of the composition also allows it to dry faster, so that the treated material is ready to use sooner. Furthermore, a composition containing a wetting agent may penetrate hydrophobic, oily soil better for improved malodor neutralization. A composition containing a wetting agent may also provide improved "in-wear" electrostatic control. For concentrated compositions, the wetting agent facilitates the dispersion of many actives such as antimicrobial actives and perfumes in the concentrated aqueous compositions.
Nonlimiting examples of wetting agents include block copolymers of ethylene oxide and propylene oxide. Suitable block polyoxyethylene-polyoxypropylene polymeric surfactants include those based on ethylene glycol, propylene glycol, glycerol, trimethylolpropane and ethylenediamine as the initial reactive hydrogen compound. Polymeric compounds made from a sequential ethoxylation and propoxylation of initial compounds with a single reactive hydrogen
atom, such as C12-I8 aliphatic alcohols, are not generally compatible with the cyclodextrin.
Certain of the block polymer surfactant compounds designated Pluronic® and Tetronic® by the BASF- Wyandotte Corp., Wyandotte, Michigan, are readily available.
Nonlimiting examples of cyclodextrin-compatible wetting agents of this type are described in US 5,714,137 and include the Silwet® surfactants available from Momentive
Performance Chemical, Albany, New York. Exemplary Silwet surfactants are as follows
Name Average MW
L-7608 600
L-7607 1,000
L-77 600
L-7605 6,000
L-7604 4,000
L-7600 4,000
L-7657 5,000
L-7602 3,000;
and mixtures thereof.
H. Aqueous carrier
The composition of the present invention may include an aqueous carrier. The aqueous carrier which is used may be distilled, deionized, or tap water. Water may be present in any amount for the composition to be an aqueous solution. In some embodiments, water may be present in an amount of about 85% to 99.5%, alternatively about 90% to about 99.5%, alternatively about 92% to about 99.5%, alternatively about 95%, by weight of said freshening composition. Water containing a small amount of low molecular weight monohydric alcohols, e.g., ethanol, methanol, and isopropanol, or polyols, such as ethylene glycol and propylene glycol, can also be useful. However, the volatile low molecular weight monohydric alcohols such as ethanol and/or isopropanol should be limited since these volatile organic compounds will contribute both to flammability problems and environmental pollution problems. If small amounts of low molecular weight monohydric alcohols are present in the composition of the present invention due to the addition of these alcohols to such things as perfumes and as stabilizers for some preservatives, the level of monohydric alcohol may be less than about 6%, alternatively less than about 3%, alternatively less than about 1%, by weight of the freshening composition.
I. Other Optional ingredients
Adjuvants can be optionally added to the freshening composition herein for their known purposes. Such adjuvants include, but are not limited to, water soluble metallic salts, antistatic agents, insect and moth repelling agents, colorants, antioxidants, and mixtures thereof. II. Method of Making
The composition can be made in any suitable manner known in the art. All of the ingredients can simply be mixed together. In certain embodiments, it may be desirable to make a concentrated mixture of ingredients and dilute by adding the same to an aqueous carrier before dispersing the composition into the air or on an inanimate surface. In another embodiment, the malodor binding polymer may be dispersed in one vessel containing deionized water and ethanol, and low molecular polyols. To this vessel, then, the buffer is added until fully dispersed and visually dissolved. In a separate vessel, the solubilizer and perfume are mixed until homogenous. The solution of solubilizer and perfume are then added to the first mixing vessel, and mixed until homogenous.
III. Methods of Use
The freshening composition of the present invention can be used by dispersing, e.g., by placing the aqueous solution into a dispensing means, such as a spray dispenser and spraying an effective amount into the air or onto the desired surface or article. An effective amount as defined herein means an amount sufficient to neutralize malodor to the point that it is not discernible by the human sense of smell yet not so much as to saturate or create a pool of liquid on an article or surface and so that, when dry, there is no visual deposit readily discernible.
Dispersing can be achieved by using a spray device, a roller, a pad, etc.
The present invention encompasses the method of dispersing an effective amount of the composition for reducing malodor onto household surfaces. The household surfaces are selected from the group consisting of countertops, cabinets, walls, floors, bathroom surfaces, and kitchen surfaces.
The present invention encompasses the method of dispersing a mist of an effective amount of the composition for reducing malodor onto fabric and/or fabric articles. The fabric and/or fabric articles include, but are not limited to, clothes, curtains, drapes, upholstered furniture, carpeting, bed linens, bath linens, tablecloths, sleeping bags, tents, car interior, e.g., car carpet, fabric car seats, etc.
The present invention encompasses the method of dispersing a mist of an effective amount of the composition for reducing malodor impression onto and into shoes wherein the shoes are not sprayed to saturation.
The present invention encompasses the method of dispersing a mist of an effective amount of the composition for reducing malodor impression onto shower curtains.
The present invention relates to the method of dispersing a mist of an effective amount of the composition for reducing malodor impression onto and/or into garbage cans and/or recycling bins.
The present invention relates to the method of dispersing a mist of an effective amount of the composition for reducing malodor impression into the air to neutralize malodor.
The present invention relates to the method of dispersing a mist of an effective amount of the composition for reducing malodor impression into and/or onto major household appliances including, but not limited to, refrigerators, freezers, washing machines, automatic dryers, ovens, microwave ovens, dishwashers, etc., to neutralize malodor.
The present invention relates to the method of dispersing a mist of an effective amount of the composition for reducing malodor impression onto cat litter, pet bedding and pet houses to neutralize malodor.
The present invention relates to the method of dispersing a mist of an effective amount of the composition for reducing malodor impression onto household pets to neutralize malodor.
EXAMPLES
Malodor Reduction
Table 6 show non-limiting examples of freshening compositions according to the present invention.
Table 6
I II III IV V VI VII VIII
(Control
)
Lupasol WF 0.070 0.070 0.015 0.035 0 0.035 0.0525 0.07 CAS 9002-98-6
Diethylene 0.175 0.175 0.070 0.175 0.175 0.175 0.170 0.175 Glycol
Perfume mixture 0.2102 0.4880 0.020 0.236 0.655 0.655 0.655 0.655 comprising (0% (0.012 (0.012 (0.012 (0.012
aliphatic aldealdealdealdealdealdehydes hydes) hydes) hydes) hydes) hydes)
Hydroxypropyl 0.630 0.630 0.630 0 0.630 0.630 0.630 0.630 Beta CD
Basophor ELH 0 0.050 0.050 0.050 0.050 0.050 0.050 0.050 60
Uniquat 2250 0 0.060 0 0.060 0.060 0.060 0.060 0.060
Bardac 2250J 0.139 0.100 0 0 0 0 0
Silwet L-7600 0.100 0.100 0.175 0.100 0.100 0.100 0.100 0.100
Citric Acid 0.045 0.015 0.015 0.015 0.015 0.015 0.15 0.015
Maleic Acid 0 0.050 0.060 0.050 0.050 0.050 0.050 0.050 CAS 110-16-7
ACES 0.100 0 0 0 0 0 0 0
Sodium 0 0 0 0.020 0.020 0.020 0.020 0.020 Hydroxide
Koralone B-119 0 0.0150 0 0.015 0.015 0.015 0.015 0.015
Ethanol 3.000 3.000 3.000 3.000 3.000 3.000 3.000 3.000
Deionized 95.571 95.347 95.865 96.264 95.318 95.180 95.095 95.148 Water
Total 100 100 100 100 100
Formulations VI, VII, and VIII in Table 6 are prepared and compared to Control Formulation V, a composition containing no malodor binding polymer, for their effect on malodor. Fig. 1 shows that when including a malodor binding polymer in a freshening composition in accordance with the present invention, aldehydic malodor evaporating off the treated fabric decreases.
Fabric samples are infused with the malodor of interest. For grease infusion, place 8 ounces of grease in a Presto™ electric skillet and cover with the skillet lid. Place the skillet in a 30 gallon metal garbage can. Run the electric cord from the skillet through a 1.5 inch hole in the garbage can. Heat the skillet to 121°C and allow it to equilibrate for 15 minutes. Remove the lid. Suspend 8 inch by 8 inch fabric swatches from the metal clips on a carousel in the garbage can lid. Measured from the bottom of the swatches, the distance to the top of the skillet is 8 inches. Place lid on garbage can and manually turn the carousel 15 rotations per minute for a period of 40 minutes. After infusion, spray the swatches with the respective freshening compositions that are to be tested. The spray for each swatch consists of two full strokes of the trigger sprayer bottle. The bottle is held 6 inches away from the fabric and the spray is centered on the fabric. Immediately after treatment, cut each swatch in half, roll, and place each into a 125 mL headspace vial. Seal the vials. Allow the vials to equilibrate for at least 2 hours at 100 °C and
then analyze by sampling each vial using a PDMS SPME fiber and analyze by GC/MS. Malodor components, previously identified, are then tracked through all the samples. Data is compiled of total area count of the cumulative area counts of the individual peaks.
Microbe Reduction
Table 7 show non-limiting examples of freshening compositions according to the present invention.
Table 7
Formulation IX, the control formulation containing no malodor binding polymer, and Formulation X are compared for their effect on microbe reduction. Fig. 2 shows the results of formulations with and without PEIs when tested for non-residual fabric sanitizer efficacy against Staphylococcus aureus (ATCC 6538), Aspergillus niger (ATCC 6275), Proteus mirabilis (ATCC 7002) and Pseudomonas aeruginosa (ATCC 15442).
Formulation efficacy was assessed by employing a North American Bactericidal Fabric Spray Test Method that is a quantitative modification of the AO AC Germicidal Spray Products
Test method (961.02). This method is a recognized test standard in accordance to U.S. EPA Pesticide Assessment Guidelines Subdivision G, Series 91-52(b)(l). The referenced AOAC method was applied to fabric surfaces. Fabric swatches (1.5 inch, 100% blue oxford cotton) as can be obtained from Testfabrics Inc. were treated with 2 fully depressed sprays of trigger sprayer bottle containing the respective formulation. A contact time between 10 to 30 minutes at ambient temperature was chosen as a conservative time estimate for sprayed fabric surfaces, as provided in Subdivision G, Series 91-1 (b) (4) (i). Any excess liquid is drained off and then transferred to a jar containing 20 ml neutralizer and/or growth promoting broth. The jar is mixed by vortexing, followed by sonication in a Branson Bransonic Ultrasonic Sonicator for 5 minutes. Within 30 minutes of neutralization, the jar is mixed for 2-3 seconds on a vortex type mixer and serially diluted. All the samples were incubated (48 + 4 hours) under the appropriate conditions and monitored for growth or no growth. Samples are plated and counted to determine mean loglO reduction.
Effect of volatile aldehydes on amine-based and sulfur-based malodors
Malodor standards are prepared by pipeting 1 mL of butylamine (amine-based malodor) and butanethiol (sulfur-based malodor) into a 1.2 liter gas sampling bag. The bag is then filled to volume with nitrogen and allowed to sit for at least 12 hours to equilibrate.
A 1 μΕ sample of each volatile aldehyde listed in Table 6 and each Accord (A, B, and C) listed in Tables 1 to 3 is pipeted into individual 10 mL silanized headspace vials. The vials are sealed and allowed to equilibrate for at least 12 hours. Repeat 4 times for each sample (2 for butylamine analysis and 2 for butanethiol analysis).
After the equilibration period, 1.5 mL of the target malodor standard is injected into each vial containing a volatile aldehyde or Accord sample. For thiol analysis, the samples are held at room temperature for 30 minutes prior to injection into the system. A 1 mL headspace syringe is used to inject 250 μΐ^ of each sample into the system for the thiol samples. For amine analysis, the samples are injected immediately into the system after the malodor is introduced. A 1 mL headspace syringe is used to inject 500 μΐ^ of each sample into the system for the amine samples.
A GC pillow is used for the amine analysis to shorten the run times. Samples are then analyzed using a GC/MS with a DB-5, 20 m, 1 μηι film thickness column with an MPS-2 autosampler equipment with static headspace function. Data is analyzed by ion extraction on each total ion current current (56 for thiol - 30 for amine) and the area is used to calculate the percent reduction from the malodor standard for each sample.
Table 8 shows the effect of certain volatile aldehydes on neutralizing amine-based and sulfur based malodors at 40 seconds and 30 minutes, respectively.
Table 8
Perfume Raw Material (R-CHO) At least 20% At least 20%
butylamine butanethiol reduction at 40 reduction at 30 seconds? minutes?
2,4,5 Trimethoxy Benzaldehyde No No
2,4, 6-Trimethoxy-benzylaldehyde No No
2-ethoxy benzylaldehyde Yes Yes
2-isopropyl-5-methyl-2-hexenal Yes Yes
2-methyl-3-(2-furyl)-propenal No No
3,4,5 Trimethoxy Benzaldehyde No No
3 ,4-Trimethoxy-benzylaldehyde No No
4-tertbutyl benzylaldehyde Yes No
5 -methyl furfural Yes Yes
5-methyl-thiophene-carboxaldehyde No Yes
Adoxal Yes No
Amyl cinnamic aldehyde No No
Benzylaldehyde Yes No
Bourgenal No Yes
Cinnamic aldehyde Yes Yes
Citronelyl Oxyacetaldehyde No No
Cymal Yes No
Decyl aldehyde Yes No
Floral Super Yes Yes
Florhydral Yes Yes
Floralozone No No
Helional Yes No
Hydroxycitronellal No No
Laurie aldehyde Yes No
Ligustral Yes No
Lyral Yes No
Melonal Yes No
Methyl nonyl acetaldehyde No No o-anisaldehyde Yes Yes p-anisaldehyde Yes No
Pino acetaldehyde Yes Yes
P.T. Bucinal Yes No
Thiophene Carboxaldehyde Yes No
Trans-4-decenal Yes Yes
Trans Trans 2,4-Nonadienal Yes No
Undecyl aldehyde Yes No
Table 9 shows the percent reduction of butylamine and butaniethiol at 40 seconds and 30 minutes, respectively, for Accords A, B, and C.
Table 9
Sensory Test - effect of volatile aldehydes on a sulfur-based malodor
Place Presto™ skillet into fume hood and turn on to 250°F. Place 80 grams of Crisco® oil into skillet and cover with skillet lid. Allow 10 minutes for equilibration. Remove skillet lid and check oil temperature with thermometer. Place 50 grams of chopped, commercially prepared garlic in water into skillet. Cover skillet with lid. Cook for 2.5 minutes or until garlic is translucent, with a portion staring to turn brown but not burn. Remove garlic from the skillet. Place 5 grams of garlic in each of 4 Petri dishes. Place covers on each Petri dish.
Place each covered Petri dish into individual test chambers. Each test chamber is 39.25 inches wide, by 25 inches deep, by 21.5 inches high with a volume of 12.2 cubic feet (0.34 cubic meters). The test chamber can be purchased from Electro-Tech Systems, Glenside, PA. Each test chamber is equipped with a fan (Newark catalog #70K9932, 115 VAC, 90CFM) purchased from Newark Electronics, Chicago, IL.
Remove the lids of the Petri dishes to expose the malodor for a dwell time sufficient to provide an initial odor intensity grade of 70-80 (about 1 minute). Once the initial odor intensity grade has been reached in a test chamber, remove the Petri dish from the test chamber.
Next, 3 Noticeables® air freshening devices, marketed by P&G, are each filled with the
Control composition shown in Table 10.
Table 10
Material Name Wt%
Benzaldehyde 0.150
Floralozone 0.097
Helional 1.455
Hydroxycitronellal 3.880
Ligustral Or Triplal 1.028
Esters 12.950
Ethers 50.190
Ketones 3.010
Lactones 0.490
Alcohols 21.610
Terpenes
The devices are set to the low intensity position and plugged into 3 of the 4 test chambers. All doors on chamber are closed.
At 5, 15, 20, 30, 45, and 60 minutes, trained evaluators open each chamber, smell the chamber for malodor intensity, and assign a malodor score, based on the scale in Table 11. The chamber door is closed but not locked between sequential evaluators. The scores are tabulated and the average score for each time interval is recorded.
Table 11
The above protocol is repeated using Prototype I shown in Table 12 (instead of Control composition) in the 3 Noticeables air freshening devices.
Table 12
Material Name Wt. %
Benzaldehyde 0.135
Floralozone 0.087
Helional 1.310
Hydroxycitronellal 3.492
Ligustral Or Triplal 0.925
o-anisaldehyde 2.500
Intreleven Aldehyde 0.500
Florhydral 1.000
Floral Super 2.500
Scentenal 1.000
Cymal 2.500
Esters 11.662
Ethers 45.171
ketones 2.705
lactones 0.437
Alcohols 19.446
Terpenes 4.632
The above protocol is repeated using Prototype 2 shown in Table 13 in the 3 Noticeables® air freshening devices.
Table 13
Fig. 3 shows that the formulation having 10% of the malodor control composition of the present invention reduces the garlic malodor more than the Control composition that lacks such malodor control composition. Sensory test - effect of volatile aldehydes on an amine -based malodor
Separate fresh ocean perch fillets from skin and add to a Magic Bullet™ food chopper. Fish meat is chopped for 35-40 seconds. 25 grams of chopped fish is weighed and fashioned into a patty suitable to fit into a 60 x 15 mm Petri dish. Repeat 3 more times so there is one fish patty in each of 4 Petri dishes. Add 40g of Crisco® oil to Presto™ skillet. Place lid on skillet and turn on to 350°F. Allow 10 minutes for equilibration. Remove lid. Cut a slit in the middle of each patty, place 1 patty into skillet, and begin frying. Replace lid. After 2.5 minutes, flip fish patty and fry an additional 2.5 minutes. Remove fish patty from skillet and blot briefly onto a paper towel for 10 seconds. Fry the remaining 3 patties in the same manner. Place each fish patty into a 60 x 15mm Petri dish and cover with a lid.
Introduce each Petri dish containing a fish patty into individual test chambers. The specifications of the test chamber are the same as those in the above sulfur-based (i.e. garlic) malodor test. Remove the lids to expose the malodor for a dwell time sufficient for providing an initial odor intensity grade of 70-80 (about 1 minute). Once the initial odor intensity grade has been reached in a test chamber, remove the Petri dish from the test chamber.
Next, 3 Noticeables® air freshening devices, marketed by P&G, are each filled with the Control composition outlined in Table 10. The devices are set to the low intensity position and plugged into 3 of the 4 test chambers. All doors on chamber are closed.
At 5, 15, 20, 30, 45, and 60 minutes, trained evaluators open each chamber, smell the chamber for malodor intensity, and assign a malodor score, based on the scale in Table 9. The chamber door is closed but not locked between sequential evaluators. The scores are tabulated and the average score for each time interval is recorded.
The above protocol is repeated using Prototype I shown in Table 10 (instead of Control composition) in the 3 Noticeables® air freshening devices. The above protocol is also repeated using Prototype 2 shown in Table 11 in the 3 Noticeables® air freshening devices.
Fig. 4 shows that the formulation having 10% of the malodor control composition of the present invention reduces the fish malodor more than the Control that lacks such malodor control composition. Effect of acid catalysts on sulfur-based malodors
Malodor standards are prepared by pipeting 1 mL of butanethiol (sulfur-based malodor) into a 1.2 liter gas sampling bag. The bag is then filled to volume with nitrogen and allowed to sit for at least 12 hours to equilibrate.
A 1 μΐ aliquot of each of the following samples are pipeted into individual 10 mL silanized headspace vials in duplicate. The following samples were analyzed: Thiophene carboxyaldehyde as a control, and a 50/50 mixture of Thiophene Carboxaldehyde and each of the following acid catalysts, at 0.04%, at 0.10%, at 0.43% in DPM, at 1.02% in DPM, and at 2.04% in DPM, is pipeted into individual 10 mL silanized headspace vials: phenol, mesitylenic acid, caprylic acid, succinic acid, pivalic acid, tiglic acid, and benzoic acid. The vials are sealed and allowed to equilibrate for at least 12 hours.
After the equilibration period, 1.5 mL of the target malodor standard is injected into each vial containing a sample. The samples are held at room temperature for 30 minutes prior to injection. Samples are then analyzed using a GC/MS with a DB-5, 20 m, 1 μηι film thickness
column with an MPS-2 autosampler utilizing static headspace function. A 1 mL headspace syringe is used to inject 250 μΕ of each sample into the system. As with the samples, a repetition of at least 2 of the malodor standard is run according to the respective method. Data is analyzed by ion extraction on each total ion current current (56 for thiol), and the area is used to calculate the % reduction from the malodor standard for each acid catalyst sample.
Fig. 5 demonstrates that low vapor pressure acid catalysts provide up to 3 times better reduction of sulfur-based malodors in comparison to the control.
All percentages stated herein are by weight unless otherwise specified. It should be understood that every maximum numerical limitation given throughout this specification will include every lower numerical limitation, as if such lower numerical limitations were expressly written herein. Every minimum numerical limitation given throughout this specification will include every higher numerical limitation, as if such higher numerical limitations were expressly written herein. Every numerical range given throughout this specification will include every narrower numerical range that falls within such broader numerical range, as if such narrower numerical ranges were all expressly written herein.
The dimensions and values disclosed herein are not to be understood as being strictly limited to the exact numerical values recited. Instead, unless otherwise specified, each such dimension is intended to mean both the recited value and a functionally equivalent range surrounding that value. For example, a dimension disclosed as "40 mm" is intended to mean "about 40 mm."
Every document cited herein, including any cross referenced or related patent or application, is hereby incorporated herein by reference in its entirety unless expressly excluded or otherwise limited. The citation of any document is not an admission that it is prior art with respect to any invention disclosed or claimed herein or that it alone, or in any combination with any other reference or references, teaches, suggests or discloses any such invention. Further, to the extent that any meaning or definition of a term in this document conflicts with any meaning or definition of the same term in a document incorporated by reference, the meaning or definition assigned to that term in this document shall govern.
While particular embodiments of the present invention have been illustrated and described, it would be obvious to those skilled in the art that various other changes and modifications can be made without departing from the spirit and scope of the invention. It is therefore intended to cover in the appended claims all such changes and modifications that are within the scope of this invention.