Abstract
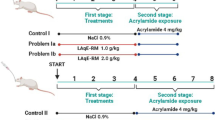
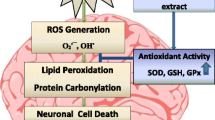
Monosodium glutamate (MSG) is a food additive that can be toxic to multiple organs of the body. It has been demonstrated that Garcinia kola seed has enormous health benefits and protects against exposure to toxicants. This study aimed to investigate the effect of Garcinia kola seed extracts on multi-organ toxicity in MSG-administered rats. Methanol and alkaloid-rich extracts of Garcinia kola seeds (GKME and GKAE, respectively) were prepared and phytochemically characterized using HPLC–DAD. Adult male Wistar rats received 2 g/kg MSG orally for 21 days with or without co-treatment with GKME, GKAE, quercetin, or nicotinic acid. Biochemical analyses were carried out on the plasma, kidney, heart, liver, and the striatum, hippocampus, and cortex of the brain of the rats. Histopathological analysis was also carried out on the tissues and organs. Intoxication with MSG caused marked negative alterations to the levels of liver function markers, kidney function markers, and the lipid profile. MSG administration also produced deleterious effects on biomarkers related to cardiac and neurologic function as echoed by increased atherogenic and coronary risk indices, and altered levels of lactate dehydrogenase, acetylcholinesterase and dopamine. Also, MSG-intoxication evoked oxidative damage in the kidney, heart, liver, and discrete brain regions. These biochemical findings were corroborated by histopathological observation which revealed abnormalities in the tissues of MSG-administered rats. The multiorgan deteriorative and oxidative damage, and the histopathological aberrations of MSG toxicity were mitigated by GKME and GKAE. These findings suggest that Garcinia kola is beneficial in ameliorating MSG-induced toxicity.






Similar content being viewed by others
Availability of data and material
All data generated or analyzed in this study are included in this article and are also available from the corresponding author on reasonable request.
References
Abarikwu SO (2014) Kolaviron, a natural flavonoid from the seeds of Garcinia kola, reduces LPS-induced inflammation in macrophages by combined inhibition of IL-6 secretion, and inflammatory transcription factors, ERK1/2, NF-κB, p38, Akt, p-c-JUN and JNK. Biochim Biophys Acta BBA Gen Subj 1840:2373–2381. https://doi.org/10.1016/j.bbagen.2014.03.006
Abdulsalam H, Adamu S, Sambo SJ, Chiroma MA, Gadzama JJ, Mohzo DL, Atata JA (2018) Monosodium glutamate-induced changes on plasma markers of pancreatic function in adult male Wistar rats. Sokoto J Vet Sci 16(2): 21–27–27. https://doi.org/10.4314/sokjvs.v16i2.3
Ajayi SA, Ofusori DA, Ojo GB, Ayoka OA, Abayomi TA, Tijani AA (2011) The microstructural effects of aqueous extract of Garcinia kola (Linn) on the hippocampus and cerebellum of malnourished mice. Asian Pac J Trop Biomed 1(4):261–265. https://doi.org/10.1016/S2221-1691(11)60039-7
Araujo TR, Freitas IN, Vettorazzi JF, Batista TM, Santos-Silva JC, Bonfleur ML, Balbo SL, Boschero AC, Carneiro EM, Ribeiro RA (2017) Benefits of L-alanine or L-arginine supplementation against adiposity and glucose intolerance in monosodium glutamate-induced obesity. Eur J Nutr 56:2069–2080. https://doi.org/10.1007/s00394-016-1245-6
Benzie IF, Strain J (1996) The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the frap assay. Anal Biochem 239(1):70–76. https://doi.org/10.1006/abio.1996.0292
Beutler E, Duwn O, Kelly, (1985) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Beyreuther K, Biesalski HK, Fernstrom JD, Grimm P, Hammes WP, Heinemann U, Kempski O, Stehle P, Steinhart H, Walker R (2007) Consensus meeting: monosodium glutamate-an update. Eur J Clin Nutr 61:304–313. https://doi.org/10.1038/sj.ejcn.1602526
Bhat JA, Gupta S, Kumar M (2021) Neuroprotective effects of theobromine in transient global cerebral ischemia-reperfusion rat model. Biochem Biophys Res Commun 571:74–80. https://doi.org/10.1016/j.bbrc.2021.07.051
Brosnan JT, Drewnowski A, Friedman MI (2014) Is there a relationship between dietary MSG and obesity in animals? Amino Acids 46:2075–2087. https://doi.org/10.1007/s00726-014-1799-7
Calis IU, Cosan DT, Saydam F, Kolac UK, Soyocak A, Kurt H, Gunes HV, Sahinturk V, Mutlu FS, Koroglu ZO, Degirmenci I (2016) The effects of monosodium glutamate and tannic acid on adult rats. Iran Red Crescent Med J 18(10). https://doi.org/10.5812/ircmj.37912
Chakraborty SP (2018) Patho-physiological and toxicological aspects of monosodium glutamate. Toxicol Mech Met. https://doi.org/10.1080/15376516.2018.1528649
Chaudhary S, Ganjoo P, Raiusddin S, Parvez S (2015) Nephroprotective activities of quercetin with potential relevance to oxidative stress induced by valproic acid. Protoplasma 252(1):209–217. https://doi.org/10.1007/s00709-014-0670-8
Cock IE (2015) The medical properties and phytochemistry of plants of the genus Terminalia (Combretaceae). Inflammopharmacol 23:203–229. https://doi.org/10.1007/s10787-015-0246-z
Collison KS, Maqbool Z, Saleh SM, Inglis A, Makhoul NJ, Bakheet R, Al-Johi M, Al-Rabiah R, Zaidi MZ, Al-Mohanna FA (2009) Effect of dietary monosodium glutamate on trans fat-induced nonalcoholic fatty liver disease. J Lipid Res 50(8):1521–1537. https://doi.org/10.1194/jlr.M800418-JLR200
Costa LG, Garrick JM, Roquè PJ, Pellacani C (2016) Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev. https://doi.org/10.1155/2016/2986796
De Almeida ACA, de-Faia FM, Dunde RJ, Manzo LPB, Souza-Brito ARM, Luiz-Ferreir A, (2017) Recent trends in pharmacological activity of alkaloids in animal colitis: potential use for inflammatory bowel disease. Evid Based Complement Alternat Med. https://doi.org/10.1155/2017/8528210
Dong HV, Robbins WA (2015) Ingestion of monosodium glutamate (MSG) in adult male rats reduces sperm count, testosterone, and disrupts testicular histology. Nutr Bytes 19(1)
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Farombi EO, Onyema OO (2006) Monosodium glutamate induced oxidative damage and genotoxicity in rat: modulatory role of vitamin C, vitamin E and quercetin. Hum Exp Toxicol 25(5):251–259. https://doi.org/10.1191/0960327106ht621oa
Farombi EO, Awogbindin IO, Farombi TH, Oladele JO, Izomoh ER, Aladelokun OB, Ezekiel IO, Adebambo OI, Abah VO (2019) Neuroprotective role of kolaviron in striatal redo-inflammation associated with rotenone model of Parkinson’s disease. Neurotoxicology 73:132–141. https://doi.org/10.1016/j.neuro.2019.03.005
Firgany AEL, Sarhan NR (2020) Quercetin mitigates monosodium glutamate-induced excitotoxicity of the spinal cord motoneurons in aged rats via p38 MAPK inhibition. Acta Histochem 122(5):151554. https://doi.org/10.1016/j.acthis.2020.151554
Frank RJ, Damasio H, Grabowski TJ (1997) Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage 5(1):13–30. https://doi.org/10.1006/nimg.1996.0250
Freeman M (2006) Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract 18(10):482–486. https://doi.org/10.1111/j.1745-7599.2006.00160.x
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Gomina M, Salifou T, Djidonou G, Zinsou S (2020) Effect of the Garcinia kola seed on glycemia, creatininemia and aminotransferases in adult subjects. Int J Biochem Res Rev 29(2):25–32. https://doi.org/10.9734/ijbcrr/2020/v29i230169
Guo L, Zhang Y, Li Q (2009) Spectrophotometric determination of dopamine hydrochloride in pharmaceutical, banana, urine and serum samples by potassium ferricyanide-Fe(III). Anal Sci 25(12):1451–1455. https://doi.org/10.2116/analsci.25.1451
Hassan DA, Alim MA, Sharkawi SM, Nabil S (2020) Detection of cardiac tissue toxicity caused by monosodium glutamate and the protective role of vitamin C by immunohistochemical method, herat tissue oxidative stress biomarkers and cardiac dysfunction biomarkers. Egypt J Forensic Sci Appli Toxicol 20(3):1–9
Hazzaa SM, Abdelaziz SAM, Mabrouk AE, Abdel-Daim MM, Elgarawany GE (2020a) Neuroprotective potential of Allium sativum against monosodium glutamate-Induced excitotoxicity: impact on short-term memory, gliosis, and oxidative stress. Nutrients 12:1028. https://doi.org/10.3390/nu12041028
Hazzaa SM, El-Roghy ES, Abd Eldaim MA, Elgarawany GE (2020b) Monosodium glutamate induces cardiac toxicity via oxidative stress, fibrosis, and P53 proapoptotic protein expression in rats. Environ Sci Pollut Res Int 27(16):20014–20024. https://doi.org/10.1007/s11356-020-08436-6
Ikeuba AI, Okafor PC, Ekpe UJ, Ebenso EE (2013) Alkaloid and non-alkaloid ethanolic extract from seeds of Garcinia kola as green corrosion inhibitors of mild steel in H2SO4 solution. Int J Electrochem Sci 8:7455–7467
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. IJBB 21(2). Retrieved from http://nopr.niscair.res.in/handle/123456789/19932
Khalil RM, Khedr NF (2016) Curcumin protects against monosodium glutamate neurotoxicity and decreasing NMDA2B and mGluR5 expression in rat hippocampus. Neurosignals 24:81–87. https://doi.org/10.1159/000442614
Kushida H, Matsumoto T, Ikarashi Y (2021) Properties, pharmacology, and pharmacokinetics of active indole and oxindole alkaloids in Uncaria Hook. Front Pharmacol 12:688670. https://doi.org/10.3389/fphar.2021.688670
Lee EH, Lee DI (1976) A study of intake of monosodium glutamate in Korea. Korean J Environ Health Soc 12:75–85
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478. https://doi.org/10.1016/0076-6879(90)86141-H
Liu CS, Lin CC, Li TC (1999) The relation of white blood cell count and atherogenic index ratio of LDL-cholesterol to HDL-cholesterol in Taiwan school children. Acta Paediatr Taiwan 40:319–324
Maňourová A, Leuner O, Tchoundjeu Z, Van Damme P, Verner V, Přibyl O, Lojka B (2019) Medicinal potential, utilization and domestication status of bitter kola (Garcinia kola Heckel) in West and Central Africa. Forests 10:124. https://doi.org/10.3390/f10020124
Manuwa TR, Akinmoladun AC, Crown OO, Komolafe K, Olaleye MT (2017) Toxicological assessment and ameliorative effects of Parinari curatellifolia alkaloids on triton-induced hyperlipidemia and atherogenicity in rats. PNAS India Section b: Biol Sci 87(2):611–623. https://doi.org/10.1007/s40011-015-0630-x
McKee RW, Longstaff E (1972) Latner AL (1972) Lactate dehydrogenase in human testes. Clin Chim Acta 39(1):221–227. https://doi.org/10.1016/0009-8981(72)90319-1
Modrau B, Andersen G, Mikkelsen IK, Nielsen A, Hansen MB, Johansen MB, Eskildsen HW, Povlsen JP, Yavarian Y, Mouridsen K, Østergaard L, Bach FW, Hjort N (2020) Theophylline as an add-on to thrombolytic therapy in acute ischemic stroke: a randomized placebo-controlled trial. Stroke 51(7):1983–1990. https://doi.org/10.1161/strokeaha.119.027446
Mondal M, Sarkar K, Nath PP, Paul G (2017) Monosodium glutamate suppresses the female reproductive function by impairing the functions of ovary and uterus in rat. Environ Toxicol 33:198–208. https://doi.org/10.1002/tox.22508
Niaz K, Zaplatic E, Spoor J (2018) Extensive use of monosodium glutamate: a threat to public health? EXCLI J 17: 273–278. https://doi.org/10.17179/excli2018-1092
Nilnumkhum A, Kanlaya R, Yoodee S, Thongboonkerd V (2019) Caffeine inhibits hypoxia-induced renal fibroblast activation by antioxidant mechanism. Cell Adh Migr 13(1):259–271. https://doi.org/10.1080/19336918.2019.163.8691
Nnadozie JO, Chijioke UO, Okafor OC, Olusina DB, Oli AN, Nwonu PC, Mbagwu HO, Chijioke CP (2019) Chronic toxicity of low dose monosodium glutamate in albino Wistar rats. BMC Res Notes 12:593. https://doi.org/10.1186/s13104-019-4611-7
Ojo OB, Amoo ZA, Saliu IO, Olaleye MT, Farombi EO, Akinmoladun AC (2019) Neurotherapeutic potential of kolaviron on neurotransmitter dysregulation, excitotoxicity, mitochondrial electron transport chain dysfunction and redox imbalance in 2-VO brain ischemia/reperfusion injury. Biomed Pharmacother 111:859–872. https://doi.org/10.1016/J.BIOPHA.2018.12.144
Ortiz GG, Bitzer Quintero OK, Zarate CB, Rodriguez Reynoso S, Larios Arceo F (2006) Monosodium glutamate induced damage in liver and kidney: a morphological and biochemical approach. Biomed Pharmacother 60: 86–91. http://hdl.handle.net/20.500.12104/66309
Ortiz-Vilchis P, Ortiz-Flores M, Pacheco M, Ramirez-Sanchez I, Moreno-Ulloa A, Vega L, Ortiz A, Villarreal F, Rubio-Gayosso I, Najera N, Meaney E, Ceballos G (2018) The cardioprotective effects of (-)-Epicatechin are mediated through arginase activity inhibition in a murine model of ischemia/reperfusion. Eur J Pharmacol 818:335–342. https://doi.org/10.1016/j.ejphar.2017.11.007
Othman SI, Bin-Jumah M (2019) Histomorphological changes in mono-sodium glutamate induced hepato-renal toxicity in mice. Int J Pharmacol 15:449–456. https://doi.org/10.3923/ijp.2019.449.456
Oyenihi OR, Brooks NL, Oguntibeju OO (2015) Effects of kolaviron on hepatic oxidative stress in streptozotocin induced diabetes. BMC Complement Altern Med 15:236. https://doi.org/10.1186/s12906-015-0760-y
Pradeep CR, Kuttan G (2004) Piperine is a potent inhibitor of nuclear factor-κB (NF-κB), c-Fos, CREB, ATF-2 and proinflammatory gene expression in B16F–10 melanoma cells. Int Immunopharmacol 4(14):1795–1803
Rhodes J, Titherley AC, Norman JA, Wood R, Lord DW (1991) A survey of the monosodium glutamate content of foods and an estimation of the dietary intake of monosodium glutamate. Food Addit Contam 8(5):663–672. https://doi.org/10.1080/02652039109374021
Rosa SG, Chagas PM, Pesarico AP, Nogueira CW (2018) Monosodium glutamate induced nociception and oxidative stress dependent on time of administration, age of rats and susceptibility of spinal cord and brain regions. Toxicol Appl Pharmacol 351:64–73. https://doi.org/10.1016/j.taap.2018.05.019
Sadek K, Abouzed T, Nasr S (2015) Lycopene modulated cholinergic dysfunction, Bcl-2/Bax balance and antioxidant enzymes gene transcripts in monosodium glutamate (E621) induced neurotoxicity in rat model. Can J Physiol Pharmacol 94:394–401
Saliu IO, Amoo ZA, Khan MF, Olaleye MT, Rema V, Akinmoladun AC (2021) Abatement of neurobehavioral and neurochemical dysfunctions in cerebral ischemia/reperfusion injury by Tetrapleura tetraptera fruit extract. J Ethnopharmacol 264:113284. https://doi.org/10.1016/j.jep.2020.113284
Sharma A (2015) Monosodium glutamate induced oxidative kidney damage and possible mechanisms: a mini-review. J Biomed Sci 2015(22):93. https://doi.org/10.1186/s12929-015-0192-5
Tang Y, Li J, Gao C, Xu Y, Li Y, Yu X, Wang J, Liu L, Yao P (2016) Hepatoprotective effect of quercetin on endoplasmic reticulum stress and inflammation after intense exercise in mice through Phosphoinositide 3-Kinase and Nuclear Factor-Kappa B. Oxid Med Cell Longev. https://doi.org/10.1155/2016/8696587
Terashima K, Takawa Y, Niwa M (2002) Powerful antioxidative agents based on garcinoic acid from Garcinia kola. Bioorg Med Chem 10:1619–1625. https://doi.org/10.1016/s0968-0896(01)00428-x
Unaeze HN (2010) Consumer knowledge attitude and practice towards the use of monosodium glutamate and food grade bouillon cubes as dietary constituents. Pak J Nutr 9(1):76–80. https://doi.org/10.3923/pjn.2010.76.80
Varshney R, Kale RK (2009) Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Rad Biol 58(5):733–743. https://doi.org/10.1080/09553009014552121
Wang LT, He PC, Li AQ, Cao KX, Yan JW, Guo S, Jiang L, Yao L, Dai XY, Feng D, Xu YM, Tan N (2021) Caffeine promotes angiogenesis through modulating endothelial mitochondrial dynamics. Acta Pharmacol Sin. https://doi.org/10.1038/s41401-021-00623-6
Wilson TA, Meservey CM (1998) Nicolosi RJ (1998) Soy lecithin reduces plasma lipoprotein cholesterol and early atherogenesis in hypercholesterolemic monkeys and hamsters: beyond linoleate. Atherosclerosis 140(1):147–153. https://doi.org/10.1016/S0021-9150(98)00132-4
Zhang Y, Lu X, Bhavnani BR (2003) Equine estrogens differentially inhibit DNA fragmentation induced by glutamate in neuronal cells by modulation of regulatory proteins involved in programmed cell death. BMC Neurosci 4:32. https://doi.org/10.1186/1471-2202-4-32
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not supported by any funding.
Conflict of interest
The authors declare no competing interests.
Ethics approval
This experiment was approved by the animal research ethical committee of the Federal University of Technology, Akure. Animals were handled and used following the ethical principles established by the National Institute of Health Guide for the Care and Use of Laboratory Animals (National Institute of Health, NIH Publication No. 8523, 1978, revised 2011).
Informed consent
For this type of study informed consent is not required.
Consent for publication
For this type of study consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kareem, A., Josiah, S.S., Saliu, I.O. et al. Ameliorative influence of Garcinia kola seed extracts against multiple organ toxicity in monosodium glutamate-administered Wistar rats. Comp Clin Pathol 31, 987–1004 (2022). https://doi.org/10.1007/s00580-022-03406-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-022-03406-5