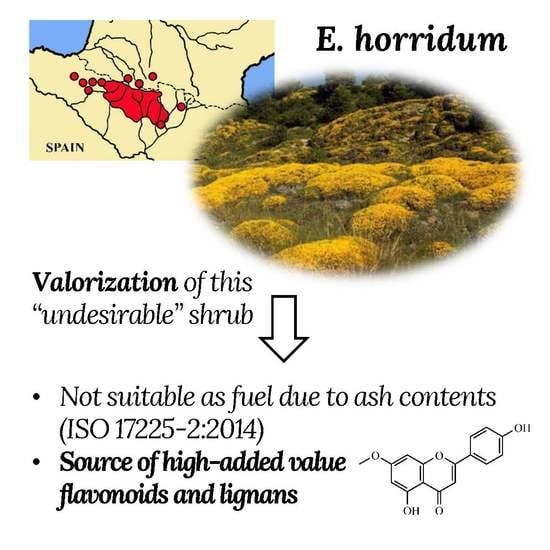
On the Physicochemical Characteristics and Applications of an “Undesirable” Pyrenean Thorny Cushion Dwarf: Echinospartum horridum (Vahl) Roth
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fuel Characterization and Biomass Combustion Properties
2.1.1. Elemental Analysis
2.1.2. Higher Heating Values Derived from Elemental Analysis Data
2.1.3. Component Percentages
2.1.4. HHV Values Derived from Component Percentages
2.1.5. Thermal Analysis
2.1.6. Ash Content
2.1.7. Lower Heating Values
2.2. Vibrational Spectroscopy Characterization
2.3. High Added-Value Products Characterization and Quantification
2.3.1. Identification
2.3.2. Genkwanin Quantification
2.4. Opportunities for Valorization
3. Material and Methods
3.1. Sampling
3.2. Fuel Characterization and Biomass Combustion Properties
Calorific Values Calculation
3.3. Vibrational Characterization
3.4. Extraction Process and High-Added Value Products Characterization and Quantification
3.4.1. Extraction Process
3.4.2. Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry (ESI-Q-TOF MS) Characterization
3.4.3. Genkwanin Quantification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gómez, D.; Mateo, G.; Mercadal, N.; Montserrat, P.; Sesé, J.A. Atlas de la Flora de Aragón. Available online: http://floragon.ipe.csic.es/index.php (accessed on 11 July 2020).
- Palacio, S.; Millard, P.; Montserrat-Martí, G. Aboveground biomass allocation patterns within Mediterranean sub-shrubs: A quantitative analysis of seasonal dimorphism. Flora—Morphol. Distrib. Funct. Ecol. Plants 2006, 201, 612–622. [Google Scholar] [CrossRef]
- Palacio, S.; Montserrat-Martí, G. Above and belowground phenology of four Mediterranean sub-shrubs. Preliminary results on root–shoot competition. J. Arid Environ. 2007, 68, 522–533. [Google Scholar] [CrossRef]
- Komac, B.; Kefi, S.; Nuche, P.; Escós, J.; Alados, C.L. Modeling shrub encroachment in subalpine grasslands under different environmental and management scenarios. J. Environ. Manag. 2013, 121, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badía, D.; Armas Herrera, C.M.; Mora Hernández, J.L.; Gómez, D.; Montserrat Martí, G.; Palacios, S. ¿Podemos controlar la expansión del erizón mediante quemas? Lucas Mallada Rev. Cienc. 2017, 19, 69–94. [Google Scholar]
- Badía, D.; López-García, S.; Martí, C.; Ortíz-Perpiñá, O.; Girona-García, A.; Casanova-Gascón, J. Burn effects on soil properties associated to heat transfer under contrasting moisture content. Sci. Total Environ. 2017, 601–602, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Palacio, S.; Milla, R.; Albuixech, J.; Pérez-Rontomé, C.; Camarero, J.J.; Maestro, M.; Montserrat-Martí, G. Seasonal variability of dry matter content and its relationship with shoot growth and nonstructural carbohydrates. New Phytol. 2008, 180, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Marinas, A.; García-González, R.; Fondevila, M. The nutritive value of five pasture species occurring in the summer grazing ranges of the Pyrenees. Anim. Sci. 2016, 76, 461–469. [Google Scholar] [CrossRef] [Green Version]
- Mora, J.L.; Armas, C.; Badía, D.; Montserrat, G.; Palacio, S.; Gómez, D. Evaluación de la calidad bromatológica del erizón tras el uso de fuego prescrito para la mejora de pastos en el Pirineo Central. In Proceedings of 57° Reunión Científica de la Sociedad Española de Pastos; Roig, S., Barrantes, O., Eds.; Sociedad Española de Pastos: Teruel, Spain, 2018; pp. 142–150. [Google Scholar]
- Harborne, J. Chemosystematics of the Leguminosae. Flavonoid and isoflavonoid patterns in the tribe Genisteae. Phytochemistry 1969, 8, 1449–1456. [Google Scholar] [CrossRef]
- Grayer-Barkmeijer, R.J.; Ingham, J.L.; Dewick, P.M. 5-O-methylbiochanin a, a new isoflavone from Echinospartum horridum. Phytochemistry 1978, 17, 829–830. [Google Scholar] [CrossRef]
- Carrión-Prieto, P.; Hernández-Navarro, S.; Martín-Ramos, P.; Sánchez-Sastre, L.F.; Garrido-Laurnaga, F.; Marcos-Robles, J.L.; Martín-Gil, J. Mediterranean shrublands as carbon sinks for climate change mitigation: New root-to-shoot ratios. Carbon Manag. 2017, 8, 67–77. [Google Scholar] [CrossRef]
- García Rosa, M. Estudio de la Biomasa de Cistus ladanifer L. y Retama sphaerocarpa L. como Sumidero de CO2: Existencias y Potencialidad. Ph.D. Thesis, Universidad de Extremadura, Badajoz, Spain, 2013. [Google Scholar]
- Carrión-Prieto, P.; Martín-Ramos, P.; Hernández-Navarro, S.; Sánchez-Sastre, L.F.; Marcos-Robles, J.L.; Martín-Gil, J. Valorization of Cistus ladanifer and Erica arborea shrubs for fuel: Wood and bark thermal characterization. Maderas Cienc. Tecnol. 2017, 19, 443–454. [Google Scholar] [CrossRef]
- European Pellet Council. Handbook for the Certification of Wood Pellets for Heating Purposes, Based on EN 14961-2; European Pellet Council: Brussels, Belgium, 2011; 33p. [Google Scholar]
- International Organization for Standardization. Solid Biofuels, Fuel Specifications and Classes. Part 2: Graded Wood Pellets; International Organization for Standardization: Geneva, Switzerland, 2014; Volume 17225-2:2014, 9p. [Google Scholar]
- Energy Research Centre of the Netherlands. ECN Phyllis2 Database for Biomass and Waste. Available online: https://www.ecn.nl/phyllis2 (accessed on 6 August 2017).
- Reed, T.B.; Das, A. Handbook of Biomass Downdraft Gasifier Engine Systems; U.S. Department of Energy, Solar Energy Research Institute: Golden, CO, USA, 1988; 149p.
- ENplus. ENplus Handbook Version 3.0; European Biomass Association AEBIOM: Brussels, Belgium, 2015; 103p. [Google Scholar]
- Jose, C.I.; Phadke, P.S.; Rao, A.V.R. Infrared spectra of flavones and isoflavones: Effect of iodine and boron trifluoride on carbonyl frequencies. Spectrochim. Acta Part A Mol. Spectrosc. 1974, 30, 1199–1206. [Google Scholar] [CrossRef]
- Emmanuel, V.; Odile, B.; Céline, R. FTIR spectroscopy of woods: A new approach to study the weathering of the carving face of a sculpture. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Faust, B. Modern Chemical Techniques: An Essential Reference for Students and Teachers; Royal Society of Chemistry: London, UK, 1997. [Google Scholar]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Traoré, M.; Kaal, J.; Martínez Cortizas, A. Application of FTIR spectroscopy to the characterization of archeological wood. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 63–70. [Google Scholar] [CrossRef]
- Fan, X.; Bai, J.; Zhao, S.; Hu, M.; Sun, Y.; Wang, B.; Ji, M.; Jin, J.; Wang, X.; Hu, J.; et al. Evaluation of inhibitory effects of flavonoids on breast cancer resistance protein (BCRP): From library screening to biological evaluation to structure-activity relationship. Toxicol. In Vitro 2019, 61, 104642. [Google Scholar] [CrossRef]
- Hakobyan, A.; Arabyan, E.; Kotsinyan, A.; Karalyan, Z.; Sahakyan, H.; Arakelov, V.; Nazaryan, K.; Ferreira, F.; Zakaryan, H. Inhibition of African swine fever virus infection by genkwanin. Antivir. Res. 2019, 167, 78–82. [Google Scholar] [CrossRef]
- Hemmati, S.; Seradj, H. Justicidin B: A promising bioactive lignan. Molecules 2016, 21, 820. [Google Scholar] [CrossRef]
- MacRae, W.; Hudson, J.; Towers, G. The antiviral action of lignans. Planta Med. 2007, 55, 531–535. [Google Scholar] [CrossRef]
- Du, M.; Ouyang, Y.; Meng, F.; Zhang, X.; Ma, Q.; Zhuang, Y.; Liu, H.; Pang, M.; Cai, T.; Cai, Y. Polymer-lipid hybrid nanoparticles: A novel drug delivery system for enhancing the activity of Psoralen against breast cancer. Int. J. Pharm. 2019, 561, 274–282. [Google Scholar] [CrossRef]
- Qi, X.-L.; Peng, X.; Huang, Y.-Y.; Li, L.; Wei, Z.-F.; Zu, Y.-G.; Fu, Y.-J. Green and efficient extraction of bioactive flavonoids from Equisetum palustre L. by deep eutectic solvents-based negative pressure cavitation method combined with macroporous resin enrichment. Ind. Crop. Prod. 2015, 70, 142–148. [Google Scholar] [CrossRef]
- Lin, J.H.; Lin, Y.T.; Chiou, Y.N.; Wen, K.C.; Liao, C.H. Determination of flavonoids in Daphnis Genkwae Flos by high performance liquid chromatography. J. Food Drug Anal. 2020, 9, 1–5. [Google Scholar] [CrossRef]
- Pereira, S.V.; Reis, R.A.S.P.; Garbuio, D.C.; Freitas, L.A.P.d. Dynamic maceration of Matricaria chamomilla inflorescences: Optimal conditions for flavonoids and antioxidant activity. Rev. Bras. Farmacogn. 2018, 28, 111–117. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Liang, Y.; Ito, Y.; Wang, X.; Chen, R.; He, J.; Li, H.; Zhang, T. Preparative isolation and purification of four flavonoids from Daphne genkwa Sieb. et Zucc. by high-speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2360–2372. [Google Scholar] [CrossRef]
- Wang, F.; Hu, L.J.; Zheng, Y.W.; Huang, Y.B.; Yang, X.Q.; Liu, C.; Kang, J.; Zheng, Z.F. Regulation for Optimal Liquid Products during Biomass Pyrolysis: A Review. IOP Conf. Ser. Earth Environ. Sci. 2016, 40, 012047. [Google Scholar] [CrossRef] [Green Version]
- Talwalkar, A.T.; U.S. Department of Energy, Institute of Gas Technology. IGT/DOE Coal-Conversion Systems Technical Data Book; Institute of Gas Technology: Chicago, IL, USA, 1981; 23p. [Google Scholar]
- CHPQA. Guidance Note 29: Alternative Fuels—Energy Inputs; UK Combined Heat & Power Quality Assurance Programme: London, UK, 2008; 6p.
- Kozłowska, J.; Grela, E.; Baczyńska, D.; Grabowiecka, A.; Anioł, M. Novel O-alkyl derivatives of naringenin and their oximes with antimicrobial and anticancer activity. Molecules 2019, 24, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Sample | %C | %H | %N | %S | C/N Ratio |
|---|---|---|---|---|---|
| Leaflets/green stems | 48.27 | 6.53 | 2.08 | 0.00 | 23.2 |
| Brown stems | 49.88 | 6.51 | 0.90 | 0.00 | 55.4 |
| Little branches (twigs) | 50.27 | 6.56 | 1.05 | 0.00 | 47.9 |
| Sulfamethazine standard | 51.86 | 5.05 | 20.13 | 11.41 | |
| Sulfamethazine sample | 51.75 | 5.09 | 20.49 | 11.83 |
| Sample | Fraction | Mass Fraction (wt%) | Fresh Weight (g) | Dry Weight (g) | Moisture (g) | Moisture Content (wt%) | Moisture Content (wt%, Weighted Average) |
|---|---|---|---|---|---|---|---|
| 1 | Green stems | 15 | 73.94 | 55.07 | 18.87 | 34.27 | 18.50 |
| Brown stems | 80 | 119.1 | 103.33 | 15.78 | 15.27 | ||
| Twigs | 5 | 51.66 | 42.07 | 9.59 | 22.80 | ||
| 2 | Green stems | 21 | 63.17 | 48.73 | 14.44 | 29.63 | 16.40 |
| Brown stems | 75 | 97.68 | 86.73 | 10.95 | 12.63 | ||
| Twigs | 4 | 37.41 | 31.78 | 5.63 | 17.72 | ||
| 3 | Green stems | 28 | 30.21 | 25.12 | 5.09 | 20.26 | 13.50 |
| Brown stems | 70 | 69.50 | 62.75 | 6.75 | 10.76 | ||
| Twigs | 2 | 15.51 | 13.51 | 2.00 | 14.80 | ||
| 4 | Green stems | 27 | 30.80 | 25.75 | 5.05 | 19.61 | 13.22 |
| Brown stems | 70 | 64.21 | 58.09 | 6.12 | 10.54 | ||
| Twigs | 3 | 20.38 | 17.24 | 3.14 | 18.21 |
| Fraction | Purge Gas | Weight Loss (%) | DTG (°C) | DSC (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaves/ green stems | N2 | 24.9 | 75.4 | 350.4 | 420 | 458 | 75.4 endo | 354 exo | ||||
| O2 | 3.65 | 78.5 | 339.1 | 460 | 73.5 | 347 exo | 483 exo | |||||
| Brown stems | N2 | 23.3 | 70 | 374.7 | 425.5 | 463.3 | 76.6 endo | 405 exo | ||||
| O2 | 2.7 | 70 | 347.3 | 437 | 450.5 | 512 | 68 | 365 exo | 465 exo | 515.5 exo | ||
| Little branches (twigs) | N2 | 58.9 | 66 | 374.5 | 422 | 462.7 | 69 endo | |||||
| O2 | 4.5 | 73 | 333.0 | 441 | 505 | 352 exo | 448 exo | 505 exo | ||||
| Species, Fraction, Purge Gas | Holocellulose (Cellulose + Hemicellulose) Tpeak (°C) | Lignin Tpeak (°C) |
|---|---|---|
| E. horridum, green stems, O2 | 347 | 483 |
| E. horridum, brown stems, O2 | 365 | 465 |
| E. horridum, twigs, O2 | 352 | 448 |
| C. ladanifer, air | 365 | 455 |
| E. arborea, air | 376 | 527 |
| Wavenumber (cm−1) | Vegetal Component | Bonds | Assignments |
|---|---|---|---|
| 3288–3308 | OH | ||
| 2917–2927 | Cellulose, hemicellulose, lignin | CH stretch | |
| 2849–2850 | Cellulose, hemicellulose, lignin | CH stretch | Symmetric CH2 valence vibration |
| 1727–1733 | Hemicellulose, lignin | C=O | Ester linkage of the carboxylic group of p-coumaric and ferulic acids |
| 1648–1652 | Flavone nucleus | Conjugated double bond | |
| 1621–1625 | Cellulose, lignin | O−H, C−O | O–H and conjugated C–O |
| 1612–1614 | Flavone nucleus | C=O | Conjugated and chelated γ-pyrone carbonyl |
| 1541–1558 | Aromatic ring | ||
| 1514–1516 | Lignin | C=C | Stretching vibrations of aromatic structure |
| 1437–1443 | Saccharide backbone | C−H | Alkane deformation relating to CH and CH2, consistent with the saccharide backbone |
| 1417 | C=C | C=C ring stretching | |
| 1363–1370 | Cellulose | C−H | In-plane bending vibration of the C−H and C−O groups of the hexose ring |
| 1316 | Cellulose, hemicellulose, lignin | C-O, CH2 | Condensation of the guaiacyl unit and syringyl unit; syringyl unit and CH2 bending stretching; CH2 rocking vibration |
| 1237–1240 | Lignin | C−H | C−C plus C−O plus C=O stretching (OH plane deformation, also COOH) |
| 1178 | C−H | ||
| 1151 | Cellulose | C−H | C–O–C asymmetric valence vibration, C=O stretching in aliphatic group |
| 1027–1035 | Cellulose, hemicellulose, lignin | C−O, C=C and C−C−O | Aromatic C–H in plane deformation; plus C−O deformation in alcohols; plus C=O stretch (unconjugated) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Ramos, P.; Martín-Gil, J.; Gómez-García, D.; Cuchí-Oterino, J.A. On the Physicochemical Characteristics and Applications of an “Undesirable” Pyrenean Thorny Cushion Dwarf: Echinospartum horridum (Vahl) Roth. Plants 2020, 9, 1180. https://doi.org/10.3390/plants9091180
Martín-Ramos P, Martín-Gil J, Gómez-García D, Cuchí-Oterino JA. On the Physicochemical Characteristics and Applications of an “Undesirable” Pyrenean Thorny Cushion Dwarf: Echinospartum horridum (Vahl) Roth. Plants. 2020; 9(9):1180. https://doi.org/10.3390/plants9091180
Chicago/Turabian StyleMartín-Ramos, Pablo, Jesús Martín-Gil, Daniel Gómez-García, and José Antonio Cuchí-Oterino. 2020. "On the Physicochemical Characteristics and Applications of an “Undesirable” Pyrenean Thorny Cushion Dwarf: Echinospartum horridum (Vahl) Roth" Plants 9, no. 9: 1180. https://doi.org/10.3390/plants9091180